Aims
The purpose of this study is to evaluate the accuracy of the Senbiosys device in measuring blood pressure (BP) by photoplethysmography (PPG) in patients undergoing coronary angiography.
Methods
This is a substudy within the Senbiosys trial, which is a prospective, single-arm, single-center study, evaluating the accuracy of BP estimation of the Senbiosys device compared to invasive BP. Patients referred for coronary angiography underwent invasive BP measurement and simultaneously wore the Senbiosys ring. SBP and DBP estimations measured by the Senbiosys device were compared with invasive measurements.
Results
A total of 25 patients were included. Overall, 708 epochs with adequate PPG signal belonging to 17 patients were analyzed. A total of 84% of the SBP estimates and 99% of the DBP estimates have an absolute error of less than 10 mmHg compared with the invasive measurements. Mean difference was 2.3 ± 7.0 mmHg and 0.5 ± 3.5 mmHg for SBP and DBP, respectively.
Conclusion
The Senbiosys device is accurate enough to determine BP in a selected population undergoing coronary angiography.
Keywords: blood pressure, coronary angiography, monitoring, Senbiosys device
Introduction
During the last decade, a growing number of wearable devices evolved to provide continuous and noninvasive monitoring of vital parameters using photoplethysmography (PPG) [1–4]. PPG records the volumetric pulsations of blood, associated with the arterial pressure pulse [5]. This technology is inexpensive and comfortable, allowing long-term continuous monitoring during regular daily activities [6]. However, reliable devices are essential for proper BP measurement, as their inaccuracy may lead to patient mismanagement [7]. For the clinical validation of BP measuring devices, a universal standard has been developed by scientific organizations and approved into a single protocol that had global acceptance [8].
In this regard, the Senbiosys device (Senbiosys SA, Neuchâtel, Switzerland), named SBF2003, is an investigational device that records PPG signals from patients and extracts BP values, both SBP and DBP. The location of the PPG sensor on the human body is an important issue that affects the quality of the signal and its performance in BP estimation. The Senbiosys device provides PPG signals from the finger, the location of which was chosen to optimize the performance of the sensor because of the larger signal amplitude that can be obtained on the finger compared to other sites [9,10].
The purpose of this study is to evaluate the accuracy of BP estimation using the Senbiosys device. Accordingly, we included a substudy to determine the precision of the Senbiosys ring in measuring SBP and DBP compared to gold standard invasive BP measurements in patients undergoing coronary angiography.
Methods
Study population
As previously described, the Senbiosys trial is a prospective, single-arm, single-center study trial aiming at assessing the accuracy of the Senbiosys device to estimate BP versus the invasive blood pressure (BP) measurement [11]. The total study population consists of 35 adult patients including patients undergoing coronarography and patients in the ICU with an arterial catheter in place.
The present substudy is nested within the Senbiosys trial and is focused on patients referred for coronary angiography at the cardiac catheterization laboratory of the University & Hospital, Fribourg. All 18 years or older patients referred for coronarography, requiring invasive BP monitoring and with an arterial catheter in place, were eligible for the study. Patients with myocardial infarction, atrial fibrillation, COVID-19 infection, and/or intracardiac monitoring and unable or unwilling to provide written informed consent, were excluded. All patients provided a written informed consent. The study was in accordance with the Declaration of Helsinki and approved by the local ethics committee (CER-VD-ID 2020-00996, ClinicalTrials-ID: NCT04379986, first registration on 8 May 2020).
The investigational device
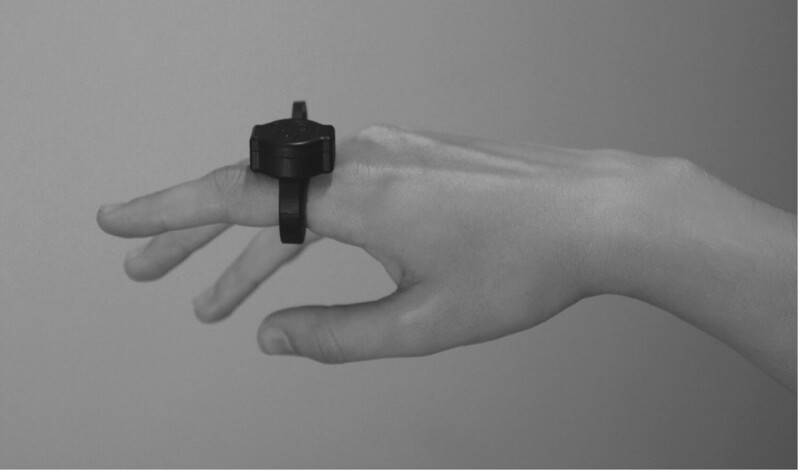
The SBF2003, shown in Fig. 1, is a wearable ring that measures the PPG signal on the finger. The ring is controlled via Bluetooth low energy (BLE) by Senbiosys proprietary firmware installed on a laptop. Once the SBF2003 is correctly placed on the patient’s index finger, it records and transfers the PPG data via the BLE to the computer. The recorded PPG measurements are fed into the PPG-based BP monitoring (PPG-BPM) algorithm [12] that runs in offline mode to generate the SBP and DBP values.
Fig. 1.
The SBF2003 on the index finger.
Standardized measures in the cardiac catheterization laboratory
The investigator places the ring on the patient’s index finger. The device is placed prior to the placement of the sterile field. The arterial puncture is performed either via the radial or femoral access. Intra-arterial BP measurements are recorded using a fluid-filled catheter after leveling and zeroing the transducer and checking the quality of the BP waveform. The study protocol involves three 3-min measurements: (a) upon the positioning of the catheter in the aorta for an initial stable BP recording, (b) immediately after the administration of 300 μg of nitroglycerin (NTG), and (c) at the end of the coronary angiography for a final stable BP recording. All the intra-arterial recordings were performed in the aorta.
Outcomes
The primary outcome is the assessment of mean bias (95% confidence interval or precision of bias) for SBP, DBP, and mean BP (MBP) between invasive and noninvasive BP measurements. The SD of the bias (95% limits of agreement) is assessed for SBP, DBP, and MBP measurements.
Data processing
The data processing is done offline upon the completion of all recordings. Recordings without significant arrhythmias are processed by the Senbiosys proprietary beat-to-beat detection algorithm to identify the PPG pulses and generate a signal quality index (SQI) for each of the pulses. The SQI index is a value between 0 and 1 that indicates the reliability of the beat estimation, with larger index values indicating higher reliability. The reliability index of each epoch is based on the number of PPG pulses with high SQI values and the number of PPG pulses that have the proper morphology and the necessary fiduciary points required for feature extraction for an accurate pulse wave analysis (PWA) [12]. The SQI value is based on: (a) the eligibility of the detected beat length, (b) how much the detected beat interval deviates from the previous beat intervals, (c) the skewness of the corresponding PPG pulse. It is important to have PPG pulses with high SQI values for a reliable and accurate PWA. The PPG pulses with high SQI values are eligible to be processed by the PWA block of the Senbiosys proprietary PPG-BPM algorithm [12]. The PWA block extracts several time-related and amplitude-related features, as proposed in the state-of-the-art [13,14], from the qualified PPG pulses. The extracted features are then mapped to two values using a multiple linear regression (MLR) model. The two values generated by the MLR model represent the uncalibrated SBP and DBP estimates of the PPG-BPM algorithm, corresponding to a specific PPG pulse.
The generated beat-to-beat BP estimates are then grouped into intervals/epochs of 10 s each. Epochs containing enough BP estimates, called clean epochs, are kept, and the remaining epochs are discarded. Finally, the algorithm generates uncalibrated SBP and DBP estimates for each clean epoch using the beat-to-beat BP estimates available in the given epoch. For the reference BP values, we average the SBP and the DBP values from the arterial line within each epoch. The SBP and DBP estimates from the algorithm are uncalibrated. We adopt the starting-point calibration method to generate the final SBP and DBP estimates [12]. The entry-point calibration is based on the first BP reference value. An offset value is calculated for each subject based on the difference between the first BP reference value (for SBP and DBP) and the corresponding PPG-based BP estimate.
Statistical methods
Categorical variables were reported as counts and percentages; continuous variables were reported as mean and SD or as median with 25–75% IQR according to their distribution as root mean square error. Normality was assessed by visual inspection of histograms, the computation of Q–Q plots, and the Shapiro–Wilk test. Categorical variables were compared using Chi-square or Fisher exact test as appropriate. Continuous variables were analyzed using the Student t-test or the Wilcoxon rank sum test according to their distribution. Categorical variables fall into three groups representing the limit of the absolute BP differences: ≤5, ≤10, and ≤15 mmHg. Both arterial line and Senbiosys device signals were segmented to epochs of duration (10 s). BP values were computed for each epoch. Furthermore, for each epoch, we computed reliability index. Epochs with reliability indexes above a given threshold were qualified for our study. Bland–Altman plots for repeated measures were used to analyze SBP, DBP, and MBP data collected from the Senbiosys device and the arterial line [15]. Mean difference in scores (bias) and 95% limits of agreement, including the differences between noninvasive and invasive measurements (bias ± 1.96 × SD), were computed. Pearson correlation was used to characterize the relation between noninvasive and invasive BP measurements. The acceptable bias and precision for arterial pressure measurements were fixed a priori at <5 and 8 mmHg, respectively. All statistical analyses were performed using Matlab R2019a (Mathworks, Inc., Natick, Massachusetts, USA).
Results
Patient characteristics
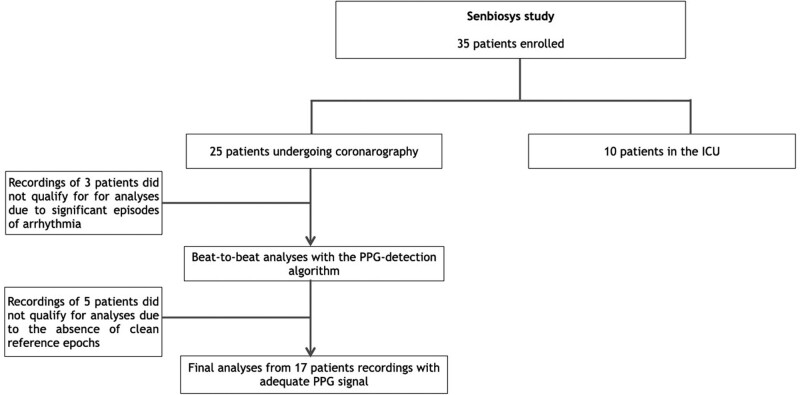
In May 2021, we enrolled 25 consecutive patients. Disposition of substudy patients is depicted in Fig. 2. Patient characteristics are summarized in Table 1. Mean age was 68.9 ± 6.4 years, and 15 patients (60%) were male. Twenty-two patients (88%) were known to have arterial hypertension.
Fig. 2.
Study flow chart. PPG, photoplethysmography.
Table 1.
Patient characteristics
| N = 25 | |
|---|---|
| Male, n (%) | 15 (60) |
| Age (years) | 68.9 ± 6.4 |
| BMI (kg/m2) | 29.1 ± 4.6 |
| Hypertension | 22 (88) |
| Diabetes | 7 (28) |
| Dyslipidaemia | 20 (80) |
| Current smoking | 6 (24) |
| Family history of CVD | 4 (16) |
| Vascular disease | 0 (0) |
| Previous stroke | 1 (4) |
Variables are expressed as mean ± SD or n (%)
CVD, cardiovascular disease.
Data collection
Data processing was done offline upon the completion of all the 25 recordings. Three recordings, containing uninterrupted episodes of arrhythmia were subsequently discarded. The remaining 22 recordings were processed by the Senbiosys proprietary beat-to-beat detection algorithm [16] to identify the PPG pulses and generate an SQI for each of the pulses. Five of the remaining 22 recordings were discarded due to the absence of clean epochs. In total, out of 17 patients with a total of 918 epochs, the PPG-BPM algorithm generates 708 clean 10-s epochs. The main reasons for discarding recordings were: (a) a noisy BP signal, (b) noisy PPG signal (due to very cold fingers of the patient, motion artifacts, or poor hand positioning), and (c) missing fiducial points, which prevents feature extraction.
Blood pressure estimation
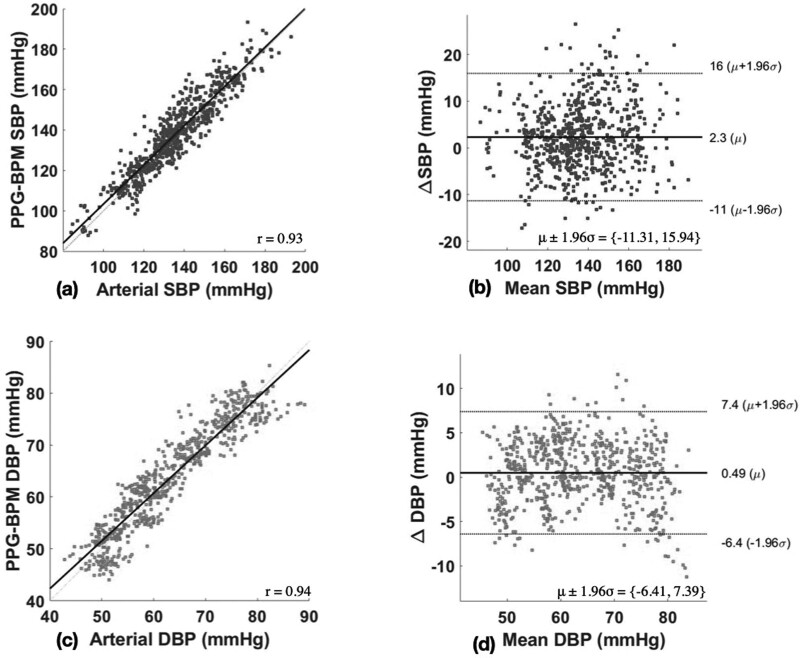
Table 2 reports BP measures in 17 patients with clean PPG data analyzed by the PPG-BPM algorithm. Mean number of clean epochs per patient was 41.6 ± 14.0. Median SBP was 134.2 (83.9−193.0) and median DBP was 61.3 (42.9−89.1). The performance of the investigational device is summarized in Table 3. Mean difference was 2.3 ± 7.0 and 0.5 ± 3.5 mmHg for SBP and DBP, respectively. More than 84 and 99% of the SBP and DBP estimates have an absolute error of less than 10 mmHg compared with invasive measurements. Figure 3 illustrates Bland–Altman and Pearson’s correlation plots between the PPG-based BP values and corresponding arterial BP values. There was a high correlation between SBP (r = 0.93) and DBP (r = 0.94) measurement between PPG-based BP values and corresponding arterial BP values (both P < 0.001). Bias was 2.3 (−11.3 to 15.9) for SBP and 0.5 (−6.4 to 7.4) for DBP.
Table 2.
Blood pressure estimation from the 17 patients with adequate PPG signal
| N = 17 | |
|---|---|
| Number of clean epochs per patient | |
| Mean ± Std. Dev. | 41.6 ± 14.0 |
| Median (IR) | 41 (16−61) |
| SBP (mmHg) | |
| Mean ± Std. Dev. | 134.9 ± 18.3 |
| Median (IR) | 134.2 (83.9−193.0) |
| DBP (mmHg) | |
| Mean ± Std. Dev. | 63.0 ± 10.0 |
| Median (IR) | 61.3 (42.9−89.2) |
| ∆SBP (mmHg) | |
| Mean ± Std. Dev. | 37.4 ± 15.1 |
| ∆DBP (mmHg) | |
| Mean ± Std. Dev. | 11.4 ± 4.6 |
Variables are expressed as mean ± SD or median (interquartile range).
Table 3.
Overall performance of the Senbiosys device over the 10-S epochs
| SBP | DBP | MBP | |
|---|---|---|---|
| N = 708 | N = 708 | N = 708 | |
| MAE (mmHg) | 5.6 | 2.8 | 2.9 |
| ME (mmHg) | 2.3 | 0.5 | 1.1 |
| SDE (mmHg) | 7.0 | 3.5 | 3.5 |
| ≤5 mmHg (%) | 55 | 86 | 85 |
| ≤10 mmHg (%) | 84 | 99 | 99 |
| ≤15 mmHg (%) | 94 | 100 | 100 |
| R | 0.93 | 0.94 | 0.94 |
| RMSE (mmHg) | 7.3 | 3.6 | 3.6 |
MAE, mean absolute error; MBP, mean blood pressure; ME, mean error; r, correlation; RMSE, root mean square error; SDE, standard deviation of error.
Fig. 3.
Bland–Altman and Pearson’s correlation plots. (a) Pearson’s correlation of SBP measurements; (b) Bland–Altman plots of SBP measurements; (c) Pearson’s correlation DBP measurements (d); Bland–Altman plots of DBP measurements. μ, bias; r, correlation coefficient.
Discussion
This study reports accurate is a high correlation and agreement between PPG-based BP values and corresponding arterial BP values in patients undergoing coronary angiography.
In total, the PPG-BPM algorithm generated 708 clean 10-s epochs from 17 patients. BP measurement using a ring-based PPG sensor showed good accuracy in patients undergoing coronary angiography, with errors of ±7.0 and ±3.5 mmHg, which shows the satisfactory performance of the BP sensor.
The implementation of these devices allows continuous, noninvasive monitoring with longer monitoring periods than are currently implemented. Other advantages of these devices are that they are wireless, cuffless, and easy to use, overcoming the two main drawbacks of other measurement techniques that are sometimes cumbersome and uncomfortable. The main challenge remains that of the motion artifacts that we are confronted with when the patient leaves the inhospital environment.
Despite its advantages, this technique should be used with caution. When applying the technique, operators should know the limiting factors, and interpret the results correctly. Since the PPG-based device is dependent on pulse wave, it will not suit certain patient populations. In patients with stage III hypertension or arterial stiffness, such as in the elderly, the PPG waveform may lose the dicrotic notches, thus the estimation may be less precise [17,18]. Moreover, the device does not provide BP estimates for patients with very frequent irregular beats and/or PPG morphology lacking the necessary fiduciary points. It should be noted, however, that the good performance of the PPG-based Senbiosys device has been obtained in a population composed of elderly patients with several comorbidities.
Future studies should focus on the advanced monitoring capabilities of PPG devices in a variety of clinical settings, from hospital-based to ambulatory settings.
Limitations
This study had several limitations. The number of patients was small, and the study was based on a single-center experience. The study is performed in a specific population of patients undergoing coronary angiography and cannot necessarily be extrapolated to other populations. SBP variations were higher than DBP variations after NTG administration (37.4 ± 15.1 mmHg vs. 11.4 ± 4.6 mmHg per recording). Finally, the quality of the signal depends on certain characteristics of the patient, and the device does not suit certain patient populations and distinct clinical situations.
Conclusion
In this prospective clinical study, we report accurate estimates of SBP and DBP generated by the PPG-BPM algorithm using the PPG from the SBF2003 ring compared with the existing standard invasive technique in patients undergoing coronarography. This PPG-based technology enables continuous, noninvasive remote BP measurements and may help clinicians to monitor BP in specific patient populations. Finally, outcome analyses in our study should be interpreted with caution due to the small number of patients. Data from larger population are needed to further confirm these results.
Acknowledgements
The authors would like to thank the Department of Cardiology of the University and Hospital of Fribourg.
The clinical trial was funded by the Fonds Scientifique Cardiovasculaire Fribourg.
Conflicts of interest
A.C. is the cofounder and CTO of Senbiosys. A.B. is the cofounder and CSO of Senbiosys. S.H. is a system engineer at Senbiosys. The Swiss company Senbiosys SA is a nonprofit company that produces PPG sensors (https://www.senbiosys.com/about-us/). For the remaining authors, there are no conflicts of interest.
Footnotes
Sara Schukraft and Serj Haddad contributed equally to the writing of this article.
References
- 1.Caizzone A. An ultra low-noise micropower PPG sensor. 2021. [Google Scholar]
- 2.Hosanee M, Chan G, Welykholowa K, Cooper R, Kyriacou PA, Zheng D, et al. Cuffless single-site photoplethysmography for blood pressure monitoring. J Clin Med 2020; 9:E723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng XF, Zhang YT. Continuous and noninvasive estimation of arterial blood pressure using a photoplethysmographic approach. In: Proceedings of the 25th annual international conference of the IEEE engineering in medicine and biology society, vol. 4; 2003. pp. 3153–3156. [Google Scholar]
- 4.Choudhury AD, Banerjee R, Sinha A, Kundu S. Estimating blood pressure using Windkessel model on photoplethysmogram. Annu Int Conf IEEE Eng Med Biol Soc 2014; 2014:4567–4570. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Chen Z, Ward R, Elgendi M. Hypertension assessment via ECG and PPG signals: an evaluation using MIMIC database. Diagnostics (Basel) 2018; 8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Kim E, Lee Y, Kim H, Lee J, Kim M, et al. Toward all-day wearable health monitoring: an ultralow-power, reflective organic pulse oximetry sensing patch. Sci Adv 2018; 4:eaas9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine J, Branan KL, Rodriguez AJ, Boonya-Ananta T, Ajmal, Ramella-Roman JC, et al. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors (Basel) 2021; 11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. Hypertension 2018; 71:368–374. [DOI] [PubMed] [Google Scholar]
- 9.Rajala S, Lindholm H, Taipalus T. Comparison of photoplethysmogram measured from wrist and finger and the effect of measurement location on pulse arrival time. Physiol Meas 2018; 39:075010. [DOI] [PubMed] [Google Scholar]
- 10.Castaneda D, Esparza A, Ghamari M, Soltanpur C, Nazeran H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelectron 2018; 4:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schukraft S, Boukhayma A, Cook S, Caizzone A. Remote blood pressure monitoring with a wearable photoplethysmographic device (Senbiosys): protocol for a single-center prospective clinical trial. JMIR Res Protoc 2021; 10:e30051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad S, Boukhayma A, Caizzone A. Continuous PPG-Based blood pressure monitoring using multi-linear regression. IEEE J Biomed Health Inform 2022; 26:2096–2105. [DOI] [PubMed] [Google Scholar]
- 13.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 2002; 103:371–377. [DOI] [PubMed] [Google Scholar]
- 14.Takazawa K, Tanaka N, Fujita M, Matsuoka O, Saiki T, Aikawa M, et al. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension 1998; 32:365–370. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17:571–582. [DOI] [PubMed] [Google Scholar]
- 16.Haddad S, Assim B, Caizzone A. Beat-to-beat detection accuracy using the ultra low power senbiosys PPG sensor. Springer; 2020. pp. 178–188. [Google Scholar]
- 17.Xing X, Ma Z, Zhang M, Zhou Y, Dong W, Song M. An unobtrusive and calibration-free blood pressure estimation method using Photoplethysmography and Biometrics. Sci Rep 2019; 9:8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen J, Murray A. Age-related changes in the characteristics of the photoplethysmographic pulse shape at various body sites. Physiol Meas 2003; 24:297–307. [DOI] [PubMed] [Google Scholar]