Abstract
Little is known about bacteria associated with asymptomatic bacteriuria (ABU) with regard to urinary tract colonization mechanisms. In this study, virulence properties of Escherichia coli 83972, a strain that was isolated from a clinical ABU episode, were examined. The genetic potential for expression of P and type 1 pili was demonstrated, and DNA sequences related to type 1C and G (UCA) pilus genes were also detected. However, E. coli 83972 did not express d-mannose-resistant or d-mannose-sensitive hemagglutination after growth under standard conditions in vitro or upon isolation from the urine of colonized test subjects. Limited uroepithelial cell adherence was observed in vivo, and weak d-mannose-sensitive hemagglutination was detected after extended growth in urine in vitro.
Urine of people who have structural or functional abnormalities of the urinary tract is likely to contain bacteria (38). Urine colonization often occurs in the absence of clinical symptoms and is called asymptomatic bacteriuria (ABU). In some patient groups, treatment of ABU is often not warranted and the benign bacteria causing asymptomatic colonization may even be beneficial in preventing infection by more antibiotic-resistant or virulent organisms (8–10, 22, 26–29, 34, 37, 38).
Unfortunately, the ABU-associated bacterial strain that colonizes the bladder is self-selecting, and physicians have little knowledge of the potential for urovirulence of the organism. Usually the strain is allowed to persist until it, or another invading strain, produces symptoms of urinary tract infection (UTI). The inevitable variation in virulence among such strains may account for the disparate reports regarding the outcomes of treatment and nontreatment of ABU. A better understanding of the virulence potential of the ABU-associated organism and of its potential for long-term asymptomatic bladder colonization would reduce the uncertainty involved in treatment decisions.
Studies by Andersson et al. (1) have identified an Escherichia coli strain capable of long-term asymptomatic bladder colonization. These researchers used E. coli 83972 to colonize eight volunteers who had a variety of underlying illnesses. E. coli 83972 persisted in the urine of the volunteers between 1 and 232 days (mean, 88 days). None of the volunteers developed fever or any other symptom of systemic illness. A recent study by Wullt et al. (40) confirmed this observation.
We have examined the prototypic ABU-associated bacterium, E. coli 83972, with respect to genetic and phenotypic properties that may contribute to bacterial persistence in the urinary tract. E. coli 83972 was selected for study because of its demonstrated capacity for long-term asymptomatic human bladder colonization.
Adherence genotype of E. coli 83972.
E. coli 83972, serotype O nt/K5 (nt, nontypeable), is a wild-type ABU-associated isolate that colonized the urinary tract of a girl in Gothenburg, Sweden, for 3 years. Colony blot and Southern blot hybridization analyses were used to identify adherence gene sequences in E. coli 83972 that are associated with extraintestinal colonizations (23). Specific hybridization probes used for detection of draABC, papEFG, and pilA to -G genes have been described elsewhere (4, 12, 30). Probes specific for focH and sfaS were prepared by PCR amplification of recombinant-DNA-containing strains HB101(pPIL110-54) and HB101(pANN801-13) kindly provided by J. Hacker. The sequences of PCR primers used were 5′ GACGTGGATACGACGATTACTG 3′ and 5′ TACGCATAGGTATAGGTGAC 3′. The probe for ucaA was prepared by PCR amplification of Proteus mirabilis HU1069 (6). Primer sequences were 5′ CTCATAAGCGATGGTGTAATGAACTGTAGC 3′ and 5′ TATGACGGTACAATTACTTTTACTGGAAA 3′. The ucaA probe was used for detection of genetically related E. coli G pilus genes. Hybridizations were conducted at high stringency (1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 68°C). The results are shown in Table 1. DNA sequences related to four of six adherence gene families tested, including pap, pil, foc, and uca, were present.
TABLE 1.
Hybridization of E. coli 83972 with probes for adhesin genes associated with E. coli causing extraintestinal infections
| Adhesin gene | Pilus | Hybridization | HAa | Infection associated with adhesin |
|---|---|---|---|---|
| pil | Type 1 | +b | −/weak | UTI (43) |
| pap | P | +b | −/− | Pyelonephritis (38) |
| uca | G | + | −/− | UTI (6, 39) |
| foc | Type 1C | + | NT | UTI (37) |
| dra | DR | − | − | Cystitis (34) |
| sfa | S | − | − | Meningitis (36) |
Results are shown for bacteria passaged in broth and urine (broth/urine); HA was observed only after 10 passes. NT, not tested (this adhesin does not mediate HA).
DNA sequences homologous with pap and pil were present as single copies on the E. coli 83972 chromosome.
In vitro adherence phenotype of E. coli 83972.
E. coli 83972 was tested for expression of adherence by standard hemagglutination (HA) assays following growth in vitro. Prior to testing, strains were grown under conditions thought to optimize expression of the specific adhesion to be tested; i.e., bacteria were passaged on L agar plates prior to assay of P or G pilus adherence or were passaged as 48-h static cultures of broth or urine up to 10 times prior to the assay of type 1 pilus adherence (15). E. coli G pili are genetically related to P. mirabilis Uca pili but are also hemagglutinins (6, 32). Bacteria were collected from agar plates or liquid cultures and suspended in buffered saline-gelatin (BSG) (8.5 g of NaCl, 0.3 g of KH2PO4, 0.6 g of Na2HPO4, 10 ml of 1% gelatin, 990 ml of distilled water [pH 7.0]) at a concentration of 109 to 1010 per ml. Thirty-microliter samples of bacteria were mixed with 30 μl of washed erythrocytes in BSG on a chilled glass plate. The plate was incubated over ice with occasional rocking for 10 min or until HA was observed. Bacteria were tested for HA of sheep and human erythrocytes in the presence of d-mannose for P and G pili or HA of guinea pig erythrocytes in the absence of d-mannose (MSHA) for type 1 pili. The results are also shown in Table 1. MSHA of guinea pig erythrocytes was observed after bacteria were passaged extensively in urine. No other HA phenotype was detected.
In vivo adherence phenotype of E. coli 83972.
As part of an ongoing human bladder colonization study, the urine of seven male volunteers who had neurogenic bladders subsequent to spinal cord injury, and who had a history of recurrent UTI, was deliberately colonized with E. coli 83972. The inoculation protocol has been described previously (1). Study participants remained bacteriuric with E. coli 83972 as the only colonizing bacterium for extended periods (>6 months). E. coli 83972 bacteria collected from the urine of volunteers were tested directly for adherence phenotype, and exfoliated uroepithelial cells of colonized volunteers were observed for attached bacteria. Urine (200 to 400 ml) was collected from test subjects who had ABU with E. coli 83972 for a minimum of 1 month. An additional urine sample collected at the same time was sent to the clinical microbiology laboratory for culture and sensitivity. If the results from the clinical workup indicated that E. coli 83972 was present in a mixed culture with any other bacteria, results from that experiment were discarded. Within 1 h of collection, urine specimens were centrifuged at 5,000 × g to collect suspended bacteria and uroepithelial cells. The urine was discarded, and the pellet was resuspended in 30 ml of BSG. The suspension was centrifuged at 1,500 × g for 10 min, and the supernatant containing bacteria was transferred to a fresh tube. The pellet containing uroepithelial cells was resuspended in 30 ml of BSG and centrifuged again at 1,500 × g for 10 min (first wash). The supernatant was discarded, and the pellet was washed three more times with BSG. Washed cells were then examined for attached bacteria in a phase-contrast microscope.
Bacteria in the first supernatant were collected by centrifugation at 5,000 × g and resuspended in BSG at a concentration of 109 to 1010 per ml. A 10-μl sample was removed for determination of bacterial titer and to confirm that E. coli 83972 was the only organism present. E. coli 83972 was differentiated from other potentially contaminating E. coli strains based upon its unique colony morphology on MacConkey agar. A minimum of 100 colonies were visually observed for each experiment. In addition, whole-cell DNA samples obtained from five typical colonies were compared with E. coli 83972 DNA by restriction fragment length polymorphism analysis. The remainder of the sample was used for HA tests by using the method described for in vitro HA assays. Six separate experiments typically yielded negative results for HA phenotypes for sheep, humans, and guinea pigs. Moderate uroepithelial cell adherence was observed (4 of 40 cells had 1 to 10 bacteria attached). These data suggest that although E. coli 83972 possesses genes associated with three different hemagglutinins, the phenotypes associated with these agglutinins are expressed weakly or not at all. The negative adherence phenotypes may result from incomplete or mutant adhesin genes in E. coli 83972 or from down-regulation of adhesin gene expression. The following experiments were done to determine if E. coli 83972 adherence genes are potentially functional.
Adherence characteristics of cloned adherence genes.
We have prepared recombinant DNA cosmid clones that contain the entire genetic region representing each of the four E. coli 83972 adhesin gene clusters (pil, pap, uca, and foc) (13). The recombinant molecules were transferred to a laboratory E. coli strain that does not itself possess any of the adherence genes tested. Clones encoding pap, pil, or uca were then tested for function by using a standard HA assay. As shown in Table 2, the recombinant DNA strains containing pap or pil genes expressed adherence (HA) in vitro. The uca clone did not express G pilus adherence. These results show that E. coli 83972 possesses functional copies of both P and type 1 pilus genes.
TABLE 2.
Hemagglutination phenotype of recombinant-DNA-containing E. coli carrying cloned adherence genes from E. coli 83972
| E. coli straina | Cloned E. coli 83972 gene | HA phenotype
|
||
|---|---|---|---|---|
| Sheep | Human | Guinea pig | ||
| HU1958 | pap | + | + | − |
| HU1886 | pil | − | − | + |
| HU1903 | uca | − | − | − |
| P678-54 | None | − | − | − |
Strains designated HU are recombinant plasmid containing derivatives of the laboratory E. coli strain P678-54. Our previous studies have shown that strain P678-54 does not contain its own genes for pap, pil, or uca (G) pili.
DNA sequence analysis of the papG allele of strain 83972.
Several reports have suggested that different alleles of the gene encoding the P adhesin, papG, may be associated with different clinical syndromes (14, 16). We used DNA sequence analysis of the papG83972 allele to determine its similarity with the pap-1 (class I), pap-2 (prs) (class III), and pap-3 (class II) families of alleles. The results presented in Fig. 1 show that papG 83972 has 97% DNA sequence homology with the pap-2 allele, and the products exhibit 95% protein sequence homology.
FIG. 1.
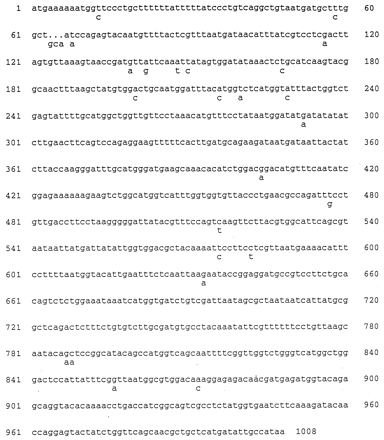
DNA sequence of the papG allele of strain 83972. Differences in the sequence of papG2 (prs) are shown below the sequence.
E. coli 83972 is a bacterial isolate that has been shown clinically and experimentally to persist in the human bladder for extended periods without producing overt symptoms of infection. In 1991, E. coli 83972 was used to evaluate the contribution of bacterial adherence to colonization of the human urinary tract (1). Initial characterization suggested that E. coli 83972 did not express P or type 1 pili in vitro and did not adhere in vitro to human uroepithelial cells. It was thought to contain DNA sequences related to pil but not pap genes. Moreover, the investigators found that upon introduction into E. coli 83972 of functional pil or pap adherence gene clusters as recombinant plasmids, the recombinant plasmid-containing derivatives exhibited reduced persistence in the bladder. They concluded that persistence of E. coli bacteriuria was not determined by bacterial adherence. Results of work presented here, and of other published studies demonstrating reduced in vivo colonization by E. coli strains that overexpress adhesin genes (11, 25), suggest that other conclusions are possible.
Upon reexamination of the genetic potential for adherence of E. coli 83972, we found that it possesses DNA sequences related to four bacterial adhesins associated with extraintestinal colonization. Genes for P and type 1 pili were expressed poorly or not at all when tested in the E. coli 83972 genetic background but were fully functional when tested as recombinant clones in a different host. It is possible that genetically unlinked regulatory elements that reduce expression of these adhesins under the in vitro and in vivo test conditions used exist in E. coli 83972. Several regulatory mechanisms that may control expression of either P or type 1 pili in E. coli 83972 have been described (2, 3, 7, 20, 24, 31, 33, 39). Additional studies will be needed to identify specific mechanisms regulating in vitro and in vivo pilus adherence gene expression by E. coli 83972 and of the role of these regulatory systems in promoting long-term asymptomatic bladder colonization.
The pap-83972 genes are most related to the pap allele that encodes class III specificity group P adhesin. P pilus alleles have been divided into three groups based upon receptor specificity of the adhesin they encode. Class I P adhesins use the Pk antigen as a human tissue receptor while class III P adhesins bind to the LKE (Luke) antigen (17, 18). Alleles encoding class III pili are most often associated with E. coli from cystitis or ABU (14, 16), suggesting a potential role for class III pili in bladder colonization.
Type 1 pili also promote bladder colonization in animal model studies. In a murine model of ascending UTI, E. coli mutants defective in synthesis of type 1 pili exhibited significantly reduced persistence in the bladder and agents that prevent type 1 pilus specific attachment also reduced colonization (5, 19, 21, 35, 36). Based upon these observations and upon the genetic potential of E. coli 83972 for adhesin synthesis, it is possible that P pili, type 1 pili, or both are required for persistence of E. coli 83972 in the human bladder. We are currently preparing mutant derivatives of E. coli 83972 that lack the genetic capacity for expression of either pilus type so that the role of each in ABU may be determined in direct in vivo human colonization studies.
Nucleotide sequence accession number.
The sequence of papG of E. coli 83972 has been deposited in GenBank under accession no. AF097355.
Acknowledgments
This work was supported by a grant from the Veterans Administration and by a grant from the Paralyzed Veterans of America Spinal Cord Research Foundation.
REFERENCES
- 1.Andersson P, Engberg I, Lidin-Janson G, Lincoln K, Hull R, Hull S, Svanborg C. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect Immun. 1991;59:2915–2921. doi: 10.1128/iai.59.9.2915-2921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Båga M, Goransson M, Normark S, Uhlin B E. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell. 1988;52:197–206. doi: 10.1016/0092-8674(88)90508-9. [DOI] [PubMed] [Google Scholar]
- 3.Braaten B A, Platco J V, van der Woude M W, Simons B H, de Graaf F K, Calvo J M, Low D A. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:4250–4254. doi: 10.1073/pnas.89.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan K, Falkow S, Hull R A, Hull S I. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol. 1985;162:799–803. doi: 10.1128/jb.162.2.799-803.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connell H, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook S W, Mody M, Valle J, Hull R. Molecular cloning of Proteus mirabilis uroepithelial cell adherence (uca) genes. Infect Immun. 1995;63:2082–2086. doi: 10.1128/iai.63.5.2082-2086.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goransson M, Forsman K, Nilsson P, Uhlin B E. Upstream activating sequences that are shared by two divergently transcribed operons mediate cAMP-CRP regulation of pilus-adhesin in Escherichia coli. Mol Microbiol. 1989;3:1557–1565. doi: 10.1111/j.1365-2958.1989.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Hansson S, Cougant D, Jodal U, Svanborg-Eden C. Untreated asymptomatic bacteriuria in girls: I. Stability of urinary isolates. Br Med J. 1989;298:853–855. doi: 10.1136/bmj.298.6677.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson S, Jodal U, Lincoln K, Svanborg-Eden C. Untreated asymptomatic bacteriuria in girls: II. Effect of phenoxymethylpenicillin and erythromycin given for intercurrent infections. Br Med J. 1989;298:856–859. doi: 10.1136/bmj.298.6677.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansson S, Jodal U, Noren L, Bjure J. Untreated bacteriuria in asymptomatic girls with renal scarring. Pediatrics. 1989;84:964–968. [PubMed] [Google Scholar]
- 11.Hull R A, Nowicki B, Kaul A, Runyan R, Svanborg C, Hull S I. Effect of pap copy number and receptor specificity on virulence of fimbriated Escherichia coli in a murine urinary tract colonization model. Microb Pathog. 1994;17:79–86. doi: 10.1006/mpat.1994.1054. [DOI] [PubMed] [Google Scholar]
- 12.Hull S I, Hull R A. Linkage and duplication of copies of genes encoding P fimbriae and hemolysin in the chromosome of a uropathogenic Escherichia coli isolate. In: Kass E H, Svanborg-Eden C, editors. Host parasite interactions in urinary tract infections. Chicago, Ill: The University of Chicago Press; 1986. pp. 157–163. [Google Scholar]
- 13.Hull S I, Hull R A. Molecular cloning of adherence genes. Methods Enzymol. 1995;253:258–269. doi: 10.1016/s0076-6879(95)53024-x. [DOI] [PubMed] [Google Scholar]
- 14.Johanson I-M, Plos K, Marklund B-I, Svanborg C. Pap, papG and prsG DNA sequences in Escherichia coli from the fecal flora and the urinary tract. Microb Pathog. 1993;15:121–129. doi: 10.1006/mpat.1993.1062. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J R, Russo T A, Brown J J, Stapleton A. papG alleles of Escherichia coli strains causing first episode or recurrent acute cystitis in adult women. J Infect Dis. 1998;177:97–101. doi: 10.1086/513824. [DOI] [PubMed] [Google Scholar]
- 17.Källenius G, Möllby R, Svenson S B, Winberg J, Lundblad A, Svensson S, Cedergren B. The Pk antigen as receptor for the hemagglutinin of pyelonephritogenic Escherichia coli in urinary tract infections. Lancet. 1980;ii:1369–1372. [Google Scholar]
- 18.Karr J F, Nowicki B J, Truong L D, Hull R A, Moulds J J, Hull S I. pap-2-encoded fimbriae adhere to the P blood group-related glycosphingolipid stage-specific embryonic antigen 4 in the human kidney. Infect Immun. 1990;58:4055–4062. doi: 10.1128/iai.58.12.4055-4062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keith B R, Maurer L, Spears P A, Orndorff P F. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986;53:693–696. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986;5:1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langermann S, Palaszynski S, Branhart M, Auguste G, Parker J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg U, Hanson L A, Jodal U, Lincoln K, Olling S. Asymptomatic bacteriuria in schoolgirls: II. Differences in Escherichia coli causing asymptomatic and symptomatic bacteriuria. Acta Paediatr Scand. 1975;64:432–436. doi: 10.1111/j.1651-2227.1975.tb03860.x. [DOI] [PubMed] [Google Scholar]
- 23.Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983;10:296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- 24.McClain M S, Blomfield I C, Eberhardt K J, Eisenstein B I. Inversion-independent phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1993;175:4335–4344. doi: 10.1128/jb.175.14.4335-4344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick B A, Klemm P, Krogfelt K A, Burghoff R L, Pallesen L, Laux D C, Cohen P S. Escherichia coli F18 locked ‘on’ for expression of type 1 fimbriae is a poor colonizer of the streptomycin-treated mouse large intestine. Microb Pathog. 1993;14:33–43. doi: 10.1006/mpat.1993.1004. [DOI] [PubMed] [Google Scholar]
- 26.Mohler I L, Cowen D L, Flanigan R C. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol. 1987;138:336–340. doi: 10.1016/s0022-5347(17)43138-7. [DOI] [PubMed] [Google Scholar]
- 27.Montgomerie J Z. The NIDRR Consensus Validation Conference resource papers: prevention and management of urinary tract infections among people with spinal cord injuries. Washington, D.C: The National Institute on Disability and Rehabilitation Research; 1992. Treatment of urinary tract infection in patients with spinal cord injury; pp. 1–13. [Google Scholar]
- 28.Nicolle L E. Urinary tract infections in the elderly. J Antimicrob Chemother. 1994;33:99–109. doi: 10.1093/jac/33.suppl_a.99. [DOI] [PubMed] [Google Scholar]
- 29.Nicolle L E, Bjornson J, Harding G K M, MacDonell J A. Bacteriuria in elderly institutionalized men. N Engl J Med. 1983;309:1420–1425. doi: 10.1056/NEJM198312083092304. [DOI] [PubMed] [Google Scholar]
- 30.Nowicki B J, Svanborg-Eden C, Hull R, Hull S. Molecular analysis and epidemiology of the DR hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989;57:446–451. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orndorff P, Falkow S. Identification and characterization of a gene product that regulates type 1 piliation in Escherichia coli. J Bacteriol. 1984;160:61–66. doi: 10.1128/jb.160.1.61-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhen M, Klemm P, Korhonen T K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-d-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986;168:1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwan W R, Seifert H S, Duncan J L. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J Bacteriol. 1992;174:2367–2375. doi: 10.1128/jb.174.7.2367-2375.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stover S L, Lloyd K, Waites K B, Jackson A B. Urinary tract infection in spinal cord injury. Arch Phys Med Rehabil. 1989;70:47–54. [PubMed] [Google Scholar]
- 35.Svanborg-Eden C, Freter R, Hagberg L, Hull R, Hull S, Leffler H, Schoolnik G. Inhibition of experimental urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982;298:560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- 36.Svanborg-Eden C, Hagberg L, Hull R, Hull S, Magnusson B, Ohman L. Bacterial virulence versus host resistance in the urinary tract of mice. Infect Immun. 1987;55:1224–1232. doi: 10.1128/iai.55.5.1224-1232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tencer J. Asymptomatic bacteriuria—a long term study. Scand J Urol Nephrol. 1988;22:31–34. doi: 10.1080/00365599.1988.11690380. [DOI] [PubMed] [Google Scholar]
- 38.Warren J W. Catheter-associated bacteriuria in long term care facilities. Infect Control Hosp Epidemiol. 1994;15:557–562. doi: 10.1086/646977. [DOI] [PubMed] [Google Scholar]
- 39.White-Ziegler C, Blyn L B, Braaten B, Low D A. Identification of an Escherichia coli locus involved in thermoregulation of the pap operon. J Bacteriol. 1990;172:1775–1782. doi: 10.1128/jb.172.4.1775-1782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wullt B, Connell H, Rollano P, Mansson W, Colleen S, Svanborg C. Urodynamic factors influence the duration of Escherichia coli bacteriuria in deliberately colonized cases. J Urol. 1998;159:2057–2062. doi: 10.1016/S0022-5347(01)63246-4. [DOI] [PubMed] [Google Scholar]


