Abstract
Background
Indigenous people in Canada often face barriers to access specialized care, with limited data in evaluating health care utilization among Indigenous people with inflammatory bowel disease (IBD). We aimed to compare health care utilization between First Nations patients and those in the general population diagnosed with IBD in Saskatchewan.
Methods
We conducted a patient-oriented, population-based, retrospective cohort study by linking administrative health databases of Saskatchewan between fiscal years 1998/99 and 2017/18. We designed and completed this study in partnership with Indigenous patients and family advocates. We applied a validated algorithm to identify IBD incident cases and then used the self-declared First Nations status variable to divide those cases. We applied a 1:5 ratio for age and sex matching and used Cox proportional models to assess associations. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported.
Results
We created a matched cohort with 696 IBD incident cases: 116 First Nations patients and 580 patients in the general population. We observed differences between the groups for IBD-specific hospital admissions (HR 1.33, 95% CI 1.01–1.75), IBD-related hospital admissions (HR 1.55, 95% CI 1.20–2.01), medication claims for IBD (HR 0.52, 95% CI 0.41–0.65) and 5-aminosalicylic acid claims (HR 0.56, 95% CI 0.45–0.71) adjusting by rural or urban residence and diagnosis type. There were no significant differences in the hazard rate of outpatient gastroenterology visits (HR 1.13, 95% CI 0.90–1.41), colonoscopies (HR 1.14, 95% CI 0.92–1.41) and surgeries for IBD (HR 1.14, 95% CI 0.80–1.64).
Interpretation
We identified that First Nations patients diagnosed with IBD had a higher rate of hospital admissions owing to IBD than patients in the general population diagnosed with IBD. We also found an inverse association between First Nations status and having prescription medication claims for IBD.
Oppression and racism are ongoing problems faced by Indigenous people,1 a population that continues to have inequitable health outcomes compared with the Canadian general population.2 Indigenous people often access health care when they are experiencing more severe or complex health care challenges.3 Health care disparities among the Indigenous community is a problem previously studied;4–7 however, little is known about access to care for inflammatory bowel disease (IBD) among Indigenous people. Inflammatory bowel disease, including Crohn disease and ulcerative colitis, is a chronic, idiopathic and incurable disorder, causing inflammation of the gastrointestinal tract.8 Canada has one of the highest prevalence and incidence rates of IBD in the world.9,10 In 2018, 0.7% of Canadians lived with IBD.11 By 2030, researchers estimate that 1% of the Canadian population will have IBD.11 Our research group (IBD Among Indigenous Peoples Research Team) reported that the prevalence of IBD among First Nations patients, the largest Indigenous subgroup in Canada, doubled between 1999 and 2016 (from 64 per 100 000 to 142 per 100 000 population) in Saskatchewan and estimated an annual average increase of 4.2%.12 We also observed IBD annual incidence rates among First Nations patients, at around 11 per 100 000 inhabitants, and identified a predominance of ulcerative colitis relative to Crohn disease among First Nations patients.12
Indigenous patient and family advocates (Indigenous people or family members of an Indigenous person living with IBD) have manifested concerns about the access to IBD care,13 as data are lacking comparing IBD health care utilization between First Nations patients and the general population. Furthermore, one-half of First Nations patients live on-reserve in Saskatchewan.14 People with IBD in rural areas may not receive gastroenterologist care as often as those living with IBD in urban centres.15 This issue may also affect the health of First Nations patients living with IBD.15,16 We aimed to compare health care utilization (i.e., outpatient gastroenterologist visits, colonoscopies, IBD medication claims, IBD-specific and -related hospital admissions and surgeries for IBD) between First Nations patients and those from the general population living with IBD in Saskatchewan.
Methods
We conducted a population-based retrospective cohort study in Saskatchewan between fiscal years 1998/1999 and 2017/2018. This project was part of the “Understanding and advocating for miyo-māhcihowin among Indigenous Peoples living with IBD” project, a patient-oriented research initiative of our IBD Among Indigenous Peoples Research Team.12
Patient engagement
Indigenous patient and family advocates contributed to each stage of this project (study conception and design to data analysis and knowledge sharing phases of our research) providing opinions and perspectives which had a considerable weight on the decision-making toward the research process. For example, the study outcomes were chosen in close collaboration with Indigenous patient and family advocates, with 1 advocate having co-presented this work at 3 scientific conferences. The Indigenous patient and family advocates received periodic reports and offered feedback on results and interpretations. We also held research team meetings, had meals and coffee time together and visited their communities.
Data sources
We used administrative health databases from Saskatchewan, including the Person Health Registration System, hospital discharge abstracts, physician claims and prescription medication claims. These databases captured all hospital admissions, outpatient physician visits (except those on-reserve) and prescription medication claims in the province (regardless of the payer). Data were linked using the encrypted unique identifiers, and extracted and analyzed at the Saskatchewan Health Quality Council.
The algorithm developed by Bernstein and colleagues17 was applied to identify IBD cases using the International Classification of Diseases (ICD) codes (for Crohn disease, ICD-10-CA: K50 and ICD-9: 555; for ulcerative colitis, ICD-10-CA: K51 and ICD-9: 556). An IBD case had 5 or more separate IBD contacts within 2 years of health care coverage, or 3 or more IBD contacts when having less than 2 years of health care coverage. This algorithm showed evidence of good sensitivity (74.4%–89.2%) and specificity (89.8%–93.7%) to identify Crohn disease and ulcerative colitis cases.17,18 In addition, we previously used this case definition to determine the epidemiology of IBD among First Nations patients in Saskatchewan.12 The cases were classified as Crohn disease or ulcerative colitis according to the most frequent diagnosis of IBD.12,19,20
People aged 18 years and older with Saskatchewan Health coverage and meeting the IBD case definition were considered. Only incident IBD cases were included in this study, which were distinguished from prevalent ones by using an 8-year washout period. This 8-year washout period was chosen based on previous IBD epidemiological studies using administrative health data.20,21 The self-declared First Nations status variable in the Person Health Registration System was used to classify people with a diagnosis of IBD in 2 groups: patients with First Nations status and those from the general population.22 Previous studies using Saskatchewan administrative databases have used this method to include First Nations patients.12,23,24
Our study outcome was IBD health care utilization assessed by measuring outpatient gastroenterologist visits, colonoscopy, prescription medication claims for IBD, IBD-specific hospital admissions, IBD-related hospital admissions and surgeries for IBD. These outcomes were measured after diagnosis of IBD (i.e., first eligible health care contact of the case definition). We considered the codes of surgeries for IBD in the Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures and the Canadian Classification of Health Interventions,15,25 as well as the codes for colonoscopies. We used the drug identification numbers of IBD medications, including biologic (e.g., infliximab, adalimumab, golimumab, certolizumab, vedolizumab and ustekinumab), immunomodulator (e.g., azathioprine, mercaptopurine and methotrexate) and 5-aminosalicylic acid ([5-ASA]; e.g., mesalamine, sulfasalazine and olsalazine sodium) in the prescription claims data set. Furthermore, we measured the time from the date of diagnosis of IBD to each of the study outcomes. The list of databases and codes can be found in Appendix 1, available at www.cmajopen.ca/content/10/4/E964/suppl/DC1.
Statistical analysis
We matched First Nations patients and those in the general population to a 1:5 ratio based on age and sex; the caliper for age was 5 years. Unadjusted and multivariable Cox proportional regression models were used to identify differences between these groups in outpatient gastroenterologist visits, colonoscopy, medication claims, IBD-specific and -related hospital admissions and surgeries for IBD. Time was measured in years from diagnosis of IBD and terminated by either the time of the first event or censoring. Hazard ratios (HRs) and corresponding 95% confidence intervals (CI) were reported.
Models were adjusted by rural or urban status and diagnosis type. We labelled IBD cases as having urban status with a residential postal code at the date of diagnosis within a census metropolitan area or census agglomeration of 15 000 or more inhabitants.20,26 Income quintile, region of residence (i.e., Regina or Saskatoon and surrounding areas, Northern and Southern Saskatchewan), age at diagnosis of IBD and sex were evaluated as confounding variables. A set of health care utilization variables (i.e., number of visits to a general practitioner, outpatient visits with specialists and IBD medication claims),19,27 corticosteroid dependency19,28 and Charlson Comorbidity Index29 were measured in the year before a diagnosis of IBD and evaluated as potential confounding variables. A potential confounder was retained in the final model if its inclusion changed coefficients of the First Nations status variable or the other variables in the model by more than 10%.30,31 Corticosteroid dependency was defined as having 2 or more prescriptions of oral corticosteroids within 6 months.19,28 A stratified analysis was completed by type of disease (ulcerative colitis and Crohn disease).
We used 2 different case definitions of IBD, as a sensitivity analysis, to evaluate variations in the identified associations. Two additional IBD incident cohorts were created using the case definition of Rezaie and colleagues,32 and Benchimol and colleagues21 (Appendix 2, available at www.cmajopen.ca/content/10/4/E964/suppl/DC1). After applying the matching procedure used in the main cohort, adjusted HRs were calculated for each study outcome in the different cohorts.
A 2-sided p value of less than 0.05 was considered significant. Data analyses were conducted using SAS version 9.4 (SAS Institute).
Ethics approval
Anonymized data from the Saskatchewan Ministry of Health and eHealth were accessed at a Saskatchewan Health Quality Council secure location. Aggregated results were transferred. This study received approval from the Research Ethics Board of the University of Saskatchewan (Beh-REB 977).
Results
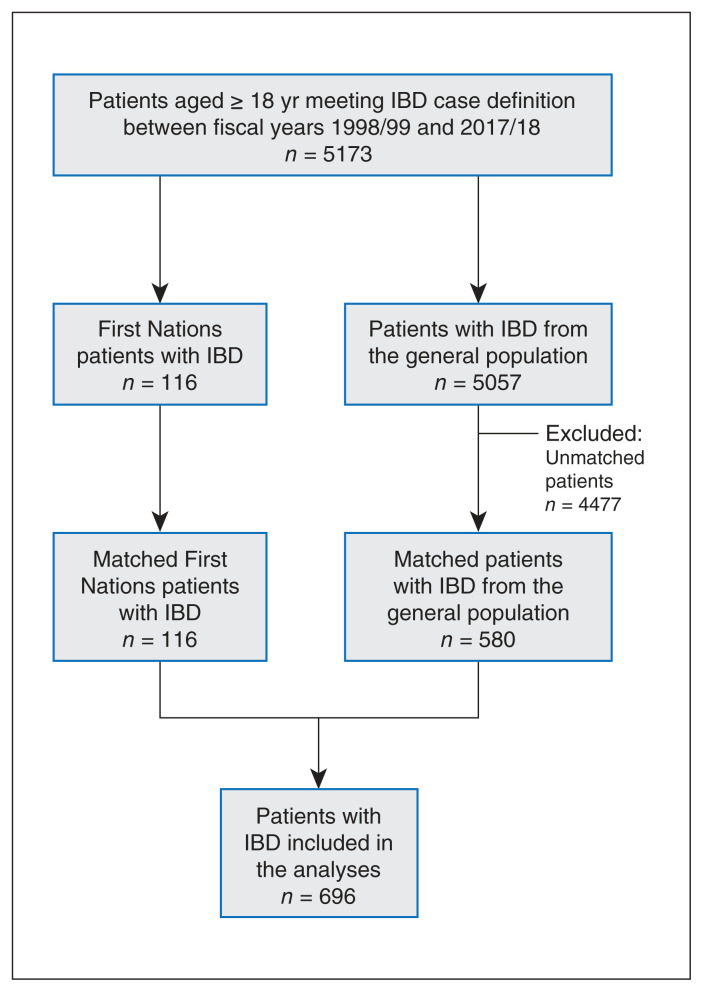
We created a matched cohort of 696 incident cases, with 580 IBD cases from the general population and 116 from the First Nations group (Figure 1). Compared with the general population patients diagnosed with IBD, First Nations patients tended to be in the lower income quintiles, live in rural and Northern Saskatchewan and diagnosed with ulcerative colitis (Table 1). The number of events of each outcome are reported in Table 2.
Figure 1:
Flow diagram of the study cohort. The caliper used for age was 5 years. Note: IBD = inflammatory bowel disease.
Table 1:
Sample characteristics
| Characteristic | No. (%) of patients* | ||
|---|---|---|---|
| Total n = 696 |
General population n = 580 |
First Nations n = 116 |
|
| Age at diagnosis of IBD, yr, mean ± SD | 41.4 ± 14.8 | 41.5 ± 14.7 | 41.2 ± 15.1 |
| Age group, yr | |||
| ≤ 30 | 157 (22.6) | 130 (22.4) | 27 (23.3) |
| 31–49 | 346 (49.7) | 287 (49.5) | 59 (50.9) |
| ≥ 50 | 193 (27.7) | 163 (28.1) | 30 (25.9) |
| Sex | |||
| Female | 414 (59.5) | 345 (59.5) | 69 (59.5) |
| Male | 282 (40.5) | 235 (40.5) | 47 (40.5) |
| Income quintile† | |||
| 1 (lowest) | 101 (15.4) | 62 (11.3) | 39 (36.1) |
| 2 | 150 (22.8) | 130 (22.6) | 20 (18.5) |
| 3 | 130 (19.8) | 113 (20.6) | 17 (15.7) |
| 4 | 156 (23.7) | 137 (24.9) | 19 (17.6) |
| 5 (highest) | 121 (18.4) | 108 (19.6) | 13 (12.0) |
| Residence‡ | |||
| Rural | 239 (34.6) | 190 (33.0) | 49 (42.2) |
| Urban | 452 (65.4) | 385 (67.0) | 67 (57.8) |
| Region of residence§ | |||
| Regina, Saskatoon and surrounding | 353 (50.8) | 305 (52.7) | 48 (41.4) |
| Northern Saskatchewan | 146 (21.0) | 97 (16.8) | 49 (42.2) |
| Southern Saskatchewan | 196 (28.2) | 177 (30.6) | 19 (16.4) |
| Diagnosis type | |||
| Crohn disease | 342 (49.1) | 300 (51.7) | 42 (36.2) |
| Ulcerative colitis | 354(50.9) | 280 (48.3) | 74 (63.8) |
| Length of follow-up, yr, mean ± SD | 10.7 ± 5.5 | 11.1 ± 5.4 | 8.8 ± 5.4 |
Note: IBD = inflammatory bowel disease, SD = standard deviation.
Unless otherwise stated.
Data of income quintile not available for all participants (missing values = 38).
Data of rural or urban residence not available for all participants (missing values = 5).
Data of region of residence not available for all participants (missing values = 1).
Table 2:
Number of people who ever had the study outcome observed during the approximate 10-year follow-up period*
| Outcome | Total n = 696 |
General population n = 580 |
First Nations n = 116 |
|---|---|---|---|
| Outpatient gastroenterologist visit | 579 | 485 | 94 |
| Colonoscopy | 610 | 504 | 106 |
| Prescription claim for IBD | 610 | 518 | 92 |
| Prescription claim of a biologic | 160 | 145 | 15 |
| Prescription claim of an IM | 264 | 232 | 32 |
| Prescription claim of a 5-ASA | 560 | 472 | 88 |
| IBD-specific hospitalization | 349 | 286 | 63 |
| IBD-related hospitalization | 380 | 306 | 74 |
| Surgery for IBD | 230 | 194 | 36 |
Note: 5-ASA = 5-aminosalicylic acid, IBD = inflammatory bowel disease, IM = immune modulator.
No recurrent events were included.
The unadjusted models revealed differences between First Nations patients and patients in the general population in having prescription medication claims for biologic (HR 0.58, 95% CI 0.34–0.99) and 5-ASA (HR 0.68, 95% CI 0.54–0.85) therapies, and differences in having a colonoscopy (HR 0.58, 95% CI 0.47–0.73) and an IBD-related hospital admission (HR 1.45, 95% CI 1.12–1.87) (Table 3).
Table 3:
Measures of association between First Nation status (reference general population) and each of the study outcomes*
| Outcomes | Full-group analysis n = 696 |
Stratified analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Crohn disease n = 342 |
Ulcerative colitis n = 354 |
|||||
|
|
|
|
||||
| Unadjusted HR (95% CI) | Adjusted HR (95% CI)† | Unadjusted HR (95% CI) | Adjusted HR (95% CI)‡ | Unadjusted HR (95% CI) | Adjusted HR (95% CI)§ | |
| Outpatient gastroenterologist visit | 1.10 (0.88–1.37) | 1.13 (0.90–1.41) | 0.95 (0.65–1.39) | 0.99 (0.67–1.45) | 1.22 (0.92–1.61) | 1.20 (0.91–1.59) |
|
| ||||||
| Colonoscopy | 1.25 (1.01–1.54) ¶ | 1.14 (0.92–1.41) | 1.19 (0.83–1.72) | 1.20 (0.83–1.73) | 1.11 (0.86–1.45) | 1.11 (0.85–1.44) |
|
| ||||||
| Prescription claim for IBD | 0.58 (0.47–0.73) ¶ | 0.52 (0.41–0.65) ¶ | 0.53 (0.35–0.78) ¶ | 0.51 (0.34–0.76) ¶ | 0.52 (0.39–0.68) ¶ | 0.51 (0.39–0.68) ¶ |
|
| ||||||
| Prescription claim of a biologic | 0.58 (0.34–0.99) ¶ | 0.65 (0.38–1.11) | 0.67 (0.33–1.38) | 0.67 (0.32–1.37) | 0.61 (0.28–1.35) | 0.62 (0.28–1.36) |
|
| ||||||
| Prescription claim of an IM | 0.70 (0.48–1.01) | 0.79 (0.55–1.15) | 0.68 (0.40–1.15) | 0.69 (0.40–1.17) | 0.93 (0.55–1.57) | 0.93 (0.55–1.58) |
|
| ||||||
| Prescription claim of a 5-ASA | 0.68 (0.54–0.85) ¶ | 0.56 (0.45–0.71) ¶ | 0.60 (0.39–0.92) ¶ | 0.56 (0.36–0.86) ¶ | 0.54 (0.41–0.72) ¶ | 0.54 (0.41–0.72) ¶ |
|
| ||||||
| IBD-specific hospitalization | 1.24 (0.94–1.63) | 1.33 (1.01–1.75) ¶ | 1.55 (1.04–2.30) ¶ | 1.50 (1.00–2.23) | 1.18 (0.80–1.72) | 1.17 (0.80–1.71) |
|
| ||||||
| IBD-related hospitalization | 1.45 (1.12–1.87) ¶ | 1.55 (1.20–2.01) ¶ | 1.74 (1.19–2.54) ¶ | 1.68 (1.14–2.46) ¶ | 1.42 (1.00–2.01) | 1.41 (1.00–2.00) |
|
| ||||||
| Surgery for IBD | 1.13 (0.79–1.62) | 1.14 (0.80–1.64) | 0.95 (0.52–1.72) | 0.93 (0.51–1.70) | 1.32 (0.84–2.07) | 1.30 (0.83–2.05) |
Note: 5-ASA = 5-aminosalicylic acid, CI = confidence interval, HR = hazard ratio, IBD = inflammatory bowel disease, IM = immune modulator.
Unless stated otherwise.
Models adjusted by rural or urban status, and diagnosis type (n = 691).
Crohn disease group, models adjusted by rural/urban status (n = 339).
Ulcerative colitis group, models adjusted by rural/urban status (n = 352).
Bold values denote significant results.
In the adjusted analyses by rural or urban residence at diagnosis of IBD and diagnosis type, the HR was 48% lower to the first IBD medication claims (HR 0.52, 95% CI 0.41–0.65) and 44% lower to the first 5-ASA medication claims in the First Nations group (HR 0.56, 95% CI 0.45–0.71) compared with the general population. In addition, First Nations patients had 33% higher hazard rates of having an IBD-specific hospital admission (HR 1.33, 95% CI 1.01–1.75) and 55% of having an IBD-related hospital admission (HR 1.55, 95% CI 1.20–2.01). None of the potential confounders changed regression estimates to more than 10% and were not retained in the final model.30,31 The distribution of variables measured the year before the diagnosis of IBD and evaluated as potential confounders are presented in Appendix 3, available at www.cmajopen.ca/content/10/4/E964/suppl/DC1.
In the stratified analysis by disease type (Table 3), First Nations patients with Crohn disease and ulcerative colitis had fewer medication claims for IBD (Crohn disease: HR 0.51, 95% CI 0.34–0.76; ulcerative colitis: HR 0.51, 95% CI 0.39–0.68) and 5-ASA claims (Crohn disease: HR 0.56, 95% CI 0.36–0.86; ulcerative colitis: HR 0.54, 95% CI 0.41–0.72) than people with IBD from the general population. Also, differences were observed for IBD-related hospital admissions (HR 1.68, 95% CI 1.14–2.46) in the Crohn disease group.
The sensitivity analysis with the matched cohort using Rezaie and colleagues’32 case definition (matched cohort no. 2) included 990 IBD incident cases with 165 from the First Nations group and 825 from the general population. The HRs from matched cohort no. 2 showed similar strengths and directions of associations to those in the main analysis (Appendix 4, available at www.cmajopen.ca/content/10/4/E964/suppl/DC1). The matched cohort using Benchimol and colleagues’21 case definition (matched cohort no. 3) obtained 708 IBD incident cases, with 118 from the First Nations group and 590 from the general population. The analysis using this case definition also attested to the robustness of the study findings (Appendix 5, available at www.cmajopen.ca/content/10/4/E964/suppl/DC1).
Interpretation
First Nations patients had a higher hazard rate of having an IBD-specific and -related hospital admission than patients in the general population. In addition, First Nations patients had fewer prescription claims for IBD medication and 5-ASA compared with patients in the general population.
In agreement with the evidence of increased hospital admission risks for First Nations patients with other chronic conditions (myocardial infarction and congestive heart failure,6 and chronic kidney disease7), we identified higher hazard rates for hospital admission owing to IBD.
First Nations patients had lower IBD prescription claims than patients in the general population. Some hypotheses to explain this finding could include a shorter follow-up time in the First Nations group which could result in lower medication claims, an association confounded by disease severity, a preference to use traditional medicines, barriers owing to differences in access or coverage criteria for prescription medications and experiences related to systemic racism. Given that we also identified higher IBD-specific and -related hospital admission hazard rates among First Nations patients, these results may suggest that those living with IBD are not receiving appropriate treatments for their disease, resulting in increased rates of hospital admissions. Perhaps owing to lack of trust and racism in the health care systems, Indigenous people often access health care when they are experiencing more severe and complex conditions.3 This late health care access is also related to delays in diagnosis and lack of follow-up, leading Indigenous people to have worse health outcomes.3
These results call for a change in the context of the social determinants of health, the inequities that exist within the health care systems and who has and who does not have access to a more streamlined, optimal health care.33,34 A potential explanation for First Nations patients not having prescription medication claims for IBD involves systemic challenges that are embedded in racist protocols and processes in health care. For example, First Nations patients may have faced a lack of coverage by the Non-Insured Health Benefits program, lack of understanding about the coverage and claim process, or simply rejection of coverage,35 which is more limited and restrictive than provincial coverage for the general population. The Non-Insured Health Benefits program generally has more restrictive criteria to access biologics both in terms of the number of conventional therapies that were unsuccessful, and the duration of time on other therapies before failure of treatment or intolerance can be documented, compared with provincial coverage criteria for the general population.
These results speak to more action in the light of the Truth and Reconciliation Commission of Canada Calls to Action, antiracist practices in the health care system and proper addressing of the root causes of the health care inequities for First Nations patients with IBD. Further studies should continue evaluating access to IBD care (including navigation and cultural safety in health care systems), medication use and disease severity among First Nations patients with IBD. Despite the Royal Commission on Aboriginal Peoples and the Truth and Reconciliation Commission of Canada that aimed to improve the health of Indigenous people, there is still a long way to go to promote cultural safety in health care.36–38 Patient navigation could also help First Nations patients to obtain early access to health care services and help reduce health care disparities.39,40
According to the study results, Indigenous patient and family advocates in the research team highlighted the need to improve access to specialized care for First Nations patients living with IBD. The Indigenous patient and family advocates considered that First Nations communities were more exposed to a westernized diet and need more traditional and spiritual care along with westernized medicine. Indigenous patient and family advocates also suggested that poor housing and water and food insecurity on reserves should be considered when studying IBD among First Nations patients. Indigenous patient and family advocates stressed that racism and poor living conditions leading to poorer mental health (e.g., stress, anxiety and depression) might also place First Nations patients living with IBD at a greater disadvantage. First Nations patients with IBD could advocate for themselves with more awareness and education about this disease.
Lessons learned from working with Indigenous patient and family advocates
We evidenced the promotion of reconciliation and healing through bonding with the Indigenous patient and family advocates, appreciation and learning about Indigenous cultures and the prioritization of the Indigenous patient and family advocates’ perspectives and recommendations. We also ensured an inclusive and collaborative research environment. The research team learned that there are different ways forward in advocating for the health of First Nations patients and that the study results have application in the light of systematic racism and oppression. The COVID-19 pandemic restrictions limited in-person interactions; however, research team members communicated regularly by way of videoconference, telephone calls and text messages.
Limitations
Misclassification bias can be a potential issue when using health administrative data to study chronic diseases, such as IBD,20,41 with mistakes originating from data entry.41 A validated case definition that required multiple health care contacts with the diagnosis of IBD was applied to address this potential issue.17 A sensitivity analysis was also completed using 2 additional validated IBD case definitions.21,32 Moreover, the First Nations status variable could account for self-declared First Nations patients in the administrative health databases. Another limitation comes from using Indigenous-specific health information in health systems. Data may not have been collected in a culturally appropriate way and may be compromised owing to factors such as misclassification errors and nonresponse bias, leading to an underestimation of Indigenous health issues.42 Finally, disease severity and disease management data were not available in administrative health data and may confound IBD health care utilization and medication claims. We considered multiple health care utilization variables in the year before diagnosis of IBD as proxy measures of disease severity;19,27 however, these variables did not have an impact on the observed associations.
Conclusion
We identified First Nations patients diagnosed with IBD had higher hazard rates of IBD-specific and -related hospital admissions than patients in the general population diagnosed with IBD. Additionally, inverse associations between First Nations status and having prescription medication claims for IBD and for 5-ASA were found. These associations might reflect a barrier to access IBD medications, contributing to a higher hazard rate for IBD-specific or -related hospital admissions in the First Nations group. Further studies should continue to evaluate access to IBD care among First Nations patients and other Indigenous people living with IBD to improve care and address health disparities.
Supplementary Material
Acknowledgements
The authors express their gratitude to the College of Medicine of the University of Saskatchewan (CoMGRAD and CoMRAD awards), Saskatchewan Health Research Foundation (Sprout grant) and the Saskatchewan Centre for Patient-Oriented Research (Sprout grant) for supporting this study. The authors also thank the staff of Saskatchewan Health Quality Council for supporting the development of this project. In addition, special thanks to Heather McWhinney, Dr. Sarah Oosman, Dr. Xinya Lu and the members of the IBD Among Indigenous Peoples Research Team.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: José Diego Marques Santos and Juan Nicolás Peña-Sánchez designed the study and contributed to the drafting of the manuscript. José Diego Marques Santos completed the analysis of the data. Juan Nicolás Peña-Sánchez contributed to the research methodology and led the data extraction. All authors contributed to the study conception, data interpretation, revisions to the manuscript for important intellectual content, approved the final version for publication and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the Saskatchewan Health Research Foundation (SHRF) and Saskatchewan Centre for Patient-Oriented Research (SCPOR) Sprout Grant award. This research project was also supported by the College of Medicine Graduate Award (CoMGRAD), University of Saskatchewan. The funding agencies had no role in the study or the preparation of the manuscript for publication.
Data sharing: The data for this study are held securely by Saskatchewan Ministry of Health and eHealth. Databases were accessed at a secure location through the Saskatchewan Health Quality Council. Permissions and affiliations are required for data access. The data are not immediately available for sharing, although the analysis could be replicated.
Disclaimer: This study is based on de-identified data provided by the Saskatchewan Ministry of Health and eHealth Saskatchewan. The interpretation and conclusions contained herein do not necessarily represent those of the Government of Saskatchewan, the Saskatchewan Ministry of Health or eHealth Saskatchewan.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/4/E964/suppl/DC1.
References
- 1.Paradies Y. Colonisation, racism and indigenous health. J Popul Res. 2016;33:83–96. [Google Scholar]
- 2.Morrison TG, Morrison MA, Borsa T. A legacy of derogation: prejudice toward Aboriginal persons in Canada. Psychology (Irvine) 2014;5:1001–10. [Google Scholar]
- 3.Horrill T, McMillan DE, Schultz ASH, et al. Understanding access to healthcare among Indigenous peoples: a comparative analysis of biomedical and postcolonial perspectives. Nurs Inq. 2018;25:e12237. doi: 10.1111/nin.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeates K, Tonelli M. Chronic kidney disease among Aboriginal people living in Canada. Clin Nephrol. 2010;74(Suppl 1):S57–60. doi: 10.5414/cnp74s057. [DOI] [PubMed] [Google Scholar]
- 5.Lafond G, Haver CRA, McLeod V, et al. Characteristics and residence of First Nations patients and their use of health care services in Saskatchewan, Canada: informing First Nations and Métis health services. J Eval Clin Pract. 2017;23:294–300. doi: 10.1111/jep.12601. [DOI] [PubMed] [Google Scholar]
- 6.Schultz A, Dahl L, McGibbon E, et al. Differences in coronary artery disease complexity and associations with mortality and hospital admissions among First Nations and non–First Nations patients undergoing angiography: a comparative retrospective matched cohort study. CMAJ Open. 2020;8:E685–94. doi: 10.9778/cmajo.20190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao S. Chronic kidney disease among First Nations people in Alberta: prevalence, health services utilization and access to quality care [masters thesis] Calgary: University of Calgary; 2006. [accessed 2021 Mar. 3]. pp. 1–104. Available: https://prism.ucalgary.ca/bitstream/handle/1880/44851/Gao_MSc_2006_Med.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 8.Kappelman MD, Porter CQ, Galanko JA, et al. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:62–8. doi: 10.1002/ibd.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 10.Benchimol EI, Walters TD, Kaufman M, et al. Assessment of knowledge in adolescents with inflammatory bowel disease using a novel transition tool. Inflamm Bowel Dis. 2011;17:1131–7. doi: 10.1002/ibd.21464. [DOI] [PubMed] [Google Scholar]
- 11.Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156:1345–53e4. doi: 10.1053/j.gastro.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Peña-Sánchez JN, Osei JA, Marques Santos JD, et al. Increasing prevalence and stable incidence rates of inflammatory bowel disease among First Nations: population-based evidence from a Western Canadian province. Inflamm Bowel Dis. 2022;28:514–22. doi: 10.1093/ibd/izab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques Santos JD. Storytelling: amplifying the voices of Indigenous people in the search for IBD care. Saskatoon: Saskatchewan Centre for Patient-Oriented Research; 2021. [accessed 2021 Mar. 13]. Available: https://www.youtube.com/watch?v=N7iNft90m4I&t=0s. [Google Scholar]
- 14.Focus on geography series, 2016 Census: Aboriginal peoples — province of Saskatchewan. Ottawa: Statistics Canada; [accessed 2021 Mar. 3]. modified 2019 Apr 10. Available: https://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-PR-Eng.cfm?TOPIC=9&LANG=Eng&GK=PR&GC=47. [Google Scholar]
- 15.Benchimol EI, Kuenzig ME, Bernstein CN, et al. Canadian Gastro-Intestinal Epidemiology Consortium. Rural and urban disparities in the care of Canadian patients with inflammatory bowel disease: a population-based study. Clin Epidemiol. 2018;10:1613–26. doi: 10.2147/CLEP.S178056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brierley CK, Suarez N, Arora G, et al. Healthcare access and health beliefs of the indigenous peoples in remote Amazonian Peru. Am J Trop Med Hyg. 2014;90:180–3. doi: 10.4269/ajtmh.13-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149:916–24. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–68. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Peña-Sánchez JN, Lix LM, Teare GF, et al. Impact of an integrated model of care on outcomes of patients with inflammatory bowel diseases: evidence from a population-based study. J Crohns Colitis. 2017;11:1471–9. doi: 10.1093/ecco-jcc/jjx106. [DOI] [PubMed] [Google Scholar]
- 20.Osei JA, Peña-Sánchez JN, Fowler SA, et al. Population-based evidence from a western Canadian province of the decreasing incidence rates and trends of inflammatory bowel disease among adults. J Can Assoc Gastroenterol. 2020;4:186–93. doi: 10.1093/jcag/gwaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol. 2014;67:887–96. doi: 10.1016/j.jclinepi.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 22.About Indian status. Gatineau (QC): Indigenous Services Canada; [accessed 2021 Mar. 3]. modified 2022 Feb. 25. Available: https://www.sac-isc.gc.ca/eng/1100100032463/1572459644986. [Google Scholar]
- 23.Dyck R, Osgood N, Lin TH, et al. Epidemiology of diabetes mellitus among First Nations and non-First Nations adults. CMAJ. 2010;182:249–56. doi: 10.1503/cmaj.090846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Ronquillo L, Thorpe L, Pahwa P, et al. Secular trends and population differences in the incidence of epilepsy. A population-based study from Saskatchewan, Canada. Seizure. 2018;60:8–15. doi: 10.1016/j.seizure.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Ma C, Crespin M, Proulx M-C, et al. Postoperative complications following colectomy for ulcerative colitis: a validation study. BMC Gastroenterol. 2012;12:39. doi: 10.1186/1471-230X-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benchimol EI, Kaplan GG, Otley AR, et al. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: a population-based inception and birth cohort study. Am J Gastroenterol. 2017;112:1412–22. doi: 10.1038/ajg.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melesse DY, Lix LM, Nugent Z, et al. Estimates of disease course in inflammatory bowel disease using administrative data: a population-level study. J Crohns Colitis. 2017;11:562–70. doi: 10.1093/ecco-jcc/jjw201. [DOI] [PubMed] [Google Scholar]
- 28.Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut. 1994;35:360–2. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Needham DM, Scales DC, Laupacis A, et al. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12–9. doi: 10.1016/j.jcrc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 32.Rezaie A, Quan H, Fedorak RN, et al. Development and validation of an administrative case definition for inflammatory bowel diseases. Can J Gastroenterol. 2012;26:711–7. doi: 10.1155/2012/278495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Social determinants of health: access to health services as a social determinant of First Nations, Inuit and Métis Health. Prince George (BC): National Collaborating Centre for Indigenous Health; 2019. [accessed 2021 Mar. 3]. Available: https://www.nccih.ca/docs/determinants/FS-AccessHealthServicesSDOH-2019-EN.pdf. [Google Scholar]
- 34.Douglas V. An introduction to Indigenous Health and healthcare in Canada: bridging health and healing. 2nd ed. New York: Springer Publishing Company; 2020. [accessed 2021 Mar. 3]. Available http://connect.springerpub.com/lookup/doi/10.1891/9780826164131. [Google Scholar]
- 35.National report of the First Nations Regional Health Survey phase 3: volume two. Ottawa: First Nations Information Governance Centre; 2018. [accessed 2021 Mar. 3]. Available: https://fnigc.ca/wp-content/uploads/2020/09/fnigc_rhs_phase_3_volume_two_en_final_screen.pdf. [Google Scholar]
- 36.Reading J, Loppie C, O’Neil J. Indigenous health systems governance: from the Royal Commission on Aboriginal Peoples (RCAP) to Truth and Reconciliation Commission. International Journal of Health Governance. 2016;21:222–8. [Google Scholar]
- 37.Barlow K, Loppie C, Jackson R, et al. Culturally competent service provision issues experienced by Aboriginal people living with HIV/AIDS. Pimatisiwin. 2008;6:155–80. [PMC free article] [PubMed] [Google Scholar]
- 38.Hammond C, Thomas R, Gifford W, et al. Cycles of silence: First Nations women overcoming social and historical barriers in supportive cancer care. Psychooncology. 2017;26:191–8. doi: 10.1002/pon.4335. [DOI] [PubMed] [Google Scholar]
- 39.Soto-Perez-de-Celis E, Chavarri-Guerra Y, Ramos-Lopez WA, et al. Patient navigation to improve early access to supportive care for patients with advanced cancer in resource-limited settings: a randomized controlled trial. Oncologist. 2021;26:157–64. doi: 10.1002/onco.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natale-Pereira A, Enard KR, Nevarez L, et al. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(Suppl):3543–52. doi: 10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manuel DG, Rosella LC, Stukel TA. Importance of accurately identifying disease in studies using electronic health records. BMJ. 2010;341:c4226. doi: 10.1136/bmj.c4226. [DOI] [PubMed] [Google Scholar]
- 42.Smylie J, Firestone M. Back to the basics: identifying and addressing underlying challenges in achieving high quality and relevant health statistics for Indigenous populations in Canada. Stat J IAOS. 2015;31:67–87. doi: 10.3233/SJI-150864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.