Abstract
Mucosal vaccination is regarded as a promising alternative to classical, intramuscular vaccine delivery. However, only a limited number of vaccines have been licensed for mucosal administration in humans. Here we propose Leishmania tarentolae, a protozoan parasite, as a potential antigen vehicle for mucosal vaccination, for administration via the rectal or oral routes. To test this hypothesis, we exploited L. tarentolae for the production and delivery of SARS-CoV-2 antigens. Two antigens were assayed in BALB/c mice: Lt-spike, a L. tarentolae clone engineered for the surface expression of the SARS-CoV-2 spike protein; RBD-SD1, a purified portion of the spike protein, produced by another engineered clone of the protozoon. Immune response parameters were then determined at different time points. Both antigens, administered either separately or in combination (Lt-spike + RBD-SD1, hereafter LeCoVax-2), determined significant IgG seroconversion and production of neutralizing antibodies after subcutaneous administration, but only in the presence of adjuvants. After rectal administration, the purified RBD-SD1 antigen did not induce any detectable immune response, in comparison with the intense response observed after administration of LeCoVax-2 or Lt-spike alone. In rectal administration, LeCoVax-2 was also effective when administered without adjuvant. Our results show that L. tarentolae is an efficient and safe scaffold for production and delivery of viral antigens, to be used as vaccines. In addition, rectal vaccination experiments prove that L. tarentolae is suitable as a vaccine vehicle and adjuvant for enteral vaccination. Finally, the combined preparation LeCoVax-2 can be considered as a promising candidate vaccine against SARS-CoV-2, worthy of further investigation.
Keywords: Immunity to infection, Leishmania, Mucosal vaccines, Adjuvants, Parasites, SARS-CoV-2
Graphical Abstract
1. Introduction
Mucosal epithelia represent both an important colonization site and the main access route to the human body for most infectious agents, from respiratory viruses to intestinal helminths [1], [2]. For these types of infections, mucosal immunity is a first shield, that protects the body during the initial phases of pathogen colonization and spread [2], [3], [4]. Mucosal vaccine administration (e.g., through the oral or nasal routes) is regarded as an ideal means to stimulate an effective immune response at the mucosal level, whose major effectors against a vast array of pathogens are secretory IgA antibodies [5]. In addition, mucosal vaccination, particularly through the oral route, holds several potential practical advantages compared to classic intramuscular injection, which includes a simplification of vaccine production procedures (in terms of sterility and purity of the components), easier administration and the possibility of partially overcoming vaccination hesitancy. Despite the advantages of mucosal vaccination, less than ten mucosal vaccines have been licensed for human use, to date, and are limited to intranasal influenza vaccines and to a few oral vaccines against intestinal pathogens [1]. This issue is also relevant to currently licensed COVID-19 vaccines, all designed for intramuscular administration [6], [7], [8]. These vaccines have indeed been very effective in reducing the incidence of severe disease and hospitalization [9], [10] but have had less effects in the containment of asymptomatic or mild infections, and thus on viral circulation [3], [11].
Considering the relevance of mucosal vaccination to confer protection against a vast array of infectious agents, we focused on an alternative vaccine platform, the protozoan parasite Leishmania tarentolae, which is possibly suitable for delivery through the enteral route. L. tarentolae is a reptile parasite and is not pathogenic to humans and other mammals [12], [13]. This microorganism has already been manipulated for the expression of viral antigens and has been tested as a living vaccine vehicle in murine models, following classical subcutaneous injection (e.g., [14], [15], [16], [17]). The rationale that led us to hypothesize that this parasite is suitable for enteral administration, as a vehicle for antigen delivery and vaccination, is as follows: (1) Leishmania cells, including the promastigote stage, are resistant to osmotic shock and chemical and mechanical insults, owing to the presence of interlinked microtubules beneath the plasma membrane [18] the robustness of these cells thus makes them suitable for administration through alternative routes, e.g. through the enteral route; (2) the size of Leishmania promastigotes lies in the range of the particles that are expected to be internalized by microfold (M) cells in the intestine, which are involved in the transfer of antigens from the gut lumen to mononuclear phagocytes in Peyer’s patches [19]; (3) both classical and recent studies prove that infection by Leishmania parasites can be acquired by oral ingestion, in addition to the common and well-established transmission through the bite of sand flies [13], [20], [21]. The above issues, in particular the evidence of oral transmission in the natural cycle of L. tarentolae, strengthen the potential of this microorganism as a vehicle for the administration of antigens through an enteral route.
A further major feature that makes L. tarentolae a suitable vaccine vehicle is that, upon its inoculation into mammalian tissues, it is expected to target dendritic cells (DCs) and other phagocytic cells [14], [22]. Indeed, the major niche for survival and replication of Leishmania species infecting mammals is represented by phagocytic myeloid cells, including DCs and macrophages [23], [24]. This propensity to be phagocytized by mammalian DCs and macrophages has also been demonstrated for L. tarentolae [25], [26]. In addition, commercial kits are available for the genetic transformation of L. tarentolae, for the production of recombinant proteins [12], [13]. Finally, protein glycosylation in L. tarentolae mimics that of mammals, which is an interesting feature for the production of viral antigens to be used as vaccines [12], [13].
In a recent in vitro study, we showed that the Lt-spike clone of L. tarentolae, engineered for the surface expression of the spike antigen of SARS-CoV-2, effectively delivers the spike antigen into DCs [26]. This L. tarentolae clone thus represents a potential COVID-19 vaccine candidate, suitable for further testing in in vivo assays. Here, we present the results of a murine model study that assesses the capacity of Lt-spike to induce an effective and neutralizing antibody response, when administered either alone, or in combination with the receptor binding domain of the spike protein [27]. In addition to the classical, subcutaneous route of administration (which represents, in the mouse model, the surrogate for intramuscular delivery), we investigated the rectal route, with the aim of determining the potential of L. tarentolae as a vehicle for mucosal vaccination through the enteral route.
2. Materials and methods
2.1. Tested antigens and the LeCoVax-2 vaccine candidate
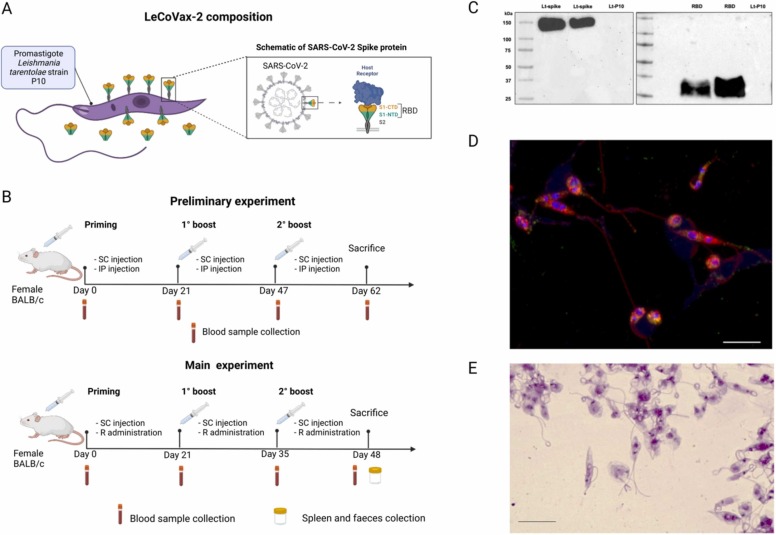
Three types of antigen preparations were assayed in this study: Lt-spike; RBD-SD1 and LeCoVax-2 ( Fig. 1A). Lt-spike is a clone of the L. tarentolae P10 strain, designed to express the whole spike protein of the SARS-CoV-2 virus at its surface. RBD-SD1 (hereafter RBD) is a purified polypeptide that includes both the receptor binding domain (RBD) and the SD1 portion of the spike protein. RBD was obtained by engineering the L. tarentolae P10 strain for secretory expression of RBD (Lt-RBD clone). In both cases, the source of the sequence to be cloned into L. tarentolae was the Whuan SARS-CoV-2 strain. LeCoVax-2 is composed by the two antigens described above, Lt-spike and RBD. For more details see [26], [27]. Briefly, Lt-spike was obtained using the codon-optimized DNA sequence of the spike gene (GenBank: MN908947) with a GSAS substitution at the furin cleavage site (residues 682–685) and a C-terminal 6xHis-tag. The sequence was synthesized and cloned into the pLEXSY-sat2 vector (Jena Bioscience, Jena, Germany); engineered Lt-spike was grown in Brain Heart Infusion (BHI) liquid medium supplemented with 5 μg/ml porcine hemin, 50,000 U/l Penicillin, 50 mg/l Streptomycin and 100 μg/ml Nourseothricin (Jena Bioscience, Jena, Germany) at 26 °C in the dark under aerated conditions. Target protein expression was confirmed by Western blotting and immunofluorescence assays, as described in Varotto-Boccazzi et al. [26]. To obtain the Lt-RBD clone, the gene coding for RBD-SD1 was codon-optimized, synthesized and subcloned into the pLEXSY-sat2 vector (Jena Bioscience, Jena, Germany) for constitutive, secreted expression. Cloning results in the incorporation of a C-terminal 6xHis-tag with a signal sequence from Leishmania mexicana, which allows the secretion of the target protein into the culture medium. To obtain the purified RBD-SD1 antigen, Lt-RBD were cultured (2.5 L) for 4 days at 26 °C in the dark, with constant agitation under aerated conditions. Purification of the secreted protein is reported in detail in Varotto-Boccazzi and colleagues [27]. Protein concentration was measured spectrophotometrically using a NanoDrop spectrophotometer, using the theoretically calculated A280 nm for 1 mg/ml Lt-RBD of 1.077 and with the BCA Protein Assay Kit (Merck). Finally, the L. tarentolae P10 (non-engineered strain; Lt-P10) was used as a control strain for all experiments. Both antigens (Lt-spike and RBD) were tested in BALB/c mice either separately, or as the combined preparation LeCoVax-2.
Fig. 1.
Characterization of the LeCoVax-2 vaccine. (A) The LeCoVax-2 vaccine formulation is defined as the combination of the protozoan parasite Leishmania tarentolae, engineered to express the SARS-CoV-2 virus spike protein, and the purified recombinant protein RBD-SD1 overexpressed in L. tarentolae. (B) Schematic diagram of preliminary and main immunization experiments and sample collection, performed on BALB/c female mice. SC: subcutaneous injection; IP: intraperitoneal injection; R: rectal administration. (C) Expression of spike and RBD proteins were confirmed by Western blotting using a SARS Coronavirus spike protein polyclonal antibody. Lt-P10: L. tarentolae unengineered, control strain. (D) Evaluation of spike protein production by engineered L. tarentolae by immunofluorescence assay. Green dots show the presence of spike protein in the Leishmania cytoplasm, while orange/yellow signals represent the colocalization of spike protein to the membranes, which are labeled in red using Concanavalin A staining. Nuclear and kinetoplastid DNA were stained with DAPI (blue). (E) Giemsa staining of L. tarentolae engineered for the expression of spike protein of SARS-CoV-2 virus after treatment with formalin. The scale bar represents 10 µm.
2.2. Mouse study designs
A preliminary experiment was performed to evaluate the immunogenicity and efficacy of the two Lt-spike and RBD antigens, administered alone, or in combination (LeCoVax-2), in the absence of adjuvant, testing two classical routes of administration: subcutaneous (SC) and intraperitoneal (IP). A second experiment was carried out testing the immunogenicity of LeCoVax-2 with adjuvants (AddaVax, Alum, R848; InvivoGen) with two routes of administration, rectal (R) or SC.
2.2.1. Mouse immunization
2.2.1.1. Animals and animal ethics statement
Female BALB/c mice (not germ-free), 8–10 weeks old, were housed at Farefarma’s animal facility Emozoo (Casole d’Elsa, Siena). Each group of animals was housed in a cage in an animal facility (with limited access) on a 12-h light/dark cycle at room temperature (24 ± 2 °C) with constant humidity (40 ± 15%); animals were allowed free access to food and water. The animals were used according to Directive 2010/63/UE regarding the protection of animals used for experimental or other scientific purposes, enforced by the Italian Legislative Decree n° 26 of 2014 and Ministerial. Decree. Project authorizations: 859/2020-PR, phase B authorizations: D4A18.B.1YW; D4A18.B.FX6. At the end of the experiment the animals of all groups were anesthetized by isoflurane then sacrificed by cervical dislocation.
2.2.1.2. Preliminary immunization experiment
Animals were divided into eight groups (n = 5 mice per group) and received three prime-booster immunizations on days 0, 21, 47, according to the modalities described in Table 1 and Fig. 1B. 62 days post-immunization, sera from the immunized mice were collected for subsequent analysis.
Table 1.
Summary of the preliminary immunization experiment according to vaccine administration mode (n = 5 female mice per experimental group).
| Experimental group | Formulation | Dose/volume |
|---|---|---|
| Subcutaneous injection | ||
| PBS-SC | PBS solution (control) | 100 µl |
| Lt-spike-SC | 2 × 107 cells Lt-spike | 100 µl |
| Lt-spike+RBD-SC | 2 × 107 cells Lt-spike + 10 μg RBD (LeCoVax-2) | 100 µl |
| RBD-SC | 10 μg RBD | 100 µl |
| Lt-P10-SC | 2 × 107 cells Lt-P10 | 100 µl |
| Intraperitoneal injection | ||
| Lt-spike-IP | 5 × 106 cells Lt-spike | 150 µl |
| Lt-spike-RBD-IP | 5 × 106 cells Lt-spike + 10 μg RBD (LeCoVax-2) | 150 µl |
| RBD-IP | 10 μg RBD | 150 µl |
| Lt: Leishmania tarentolae; RBD: Receptor Binding Domain | ||
2.2.1.3. Main immunization experiment
Prior to immunization, Leishmania parasites were inactivated overnight with 0.15% formalin at 4 °C, then the cells were washed three times with PBS and finally stored at − 80 °C. To evaluate the integrity of the Leishmania cells after inactivation, a morphological evaluation was performed by Giemsa staining. Endotoxin determination was performed on recombinant RBD protein and Lt-spike with the Pierce Chromogenic Endotoxin Quant kit (ThermoFisher, USA). Animals were divided into ten groups (n = 10 mice per group) and were immunized by SC injection or rectal administration with different vaccine compositions, as reported in Table 2 and Fig. 1B. Briefly, five groups were subcutaneously immunized in the dorsal region of the mouse (three prime-boost immunization) on day 0, 21 and 35 with different treatments: i) Lt-spike and recombinant RBD antigen, mixed with AddaVax adjuvant (AddaVax; InvivoGen) at a 1:1 ratio (v:v); ii) Lt-spike and recombinant RBD antigen, mixed with aluminium phosphate gel adjuvant (Alum) (Adju-Phos 2%; InvivoGen) at a 1:1 ratio (v:v); iii) recombinant RBD antigen and AddaVax at a 1:1 ratio (v:v); iv) recombinant RBD antigen mixed with Alum at a 1:1 ratio (v:v); v) PBS as placebo. Five groups were rectally immunized on day 0, 21 and 35 with different formulation as follows: i) Lt-spike and recombinant RBD antigen mixed with 10 µg Resiquimod (R848, Invivogen); ii) Lt-spike with recombinant RBD antigen and 25 µg R848; iii) Lt-spike and recombinant RBD antigen without the addition of adjuvant; iv) recombinant RBD antigen mixed with 25 µg R848; v) PBS as placebo. Sera were collected on day 0 (prior to immunization), 21, 35 and 48 in 95 animals for characterization of the antibody response. The body weight of each mouse was measured on day 0 and every seven days. On the day of sacrifice (day 48), the spleens were collected and used to isolate splenocytes for peptide stimulation and subsequent ELISpot analysis, whereas the faeces were immediately frozen at − 80 °C for IgA antibody response determination. Selected organs/tissues were sampled for histological examination.
Table 2.
Summary of the main immunization experiment (n = 10 mice per experimental group) according to vaccine administration mode.
| Experimental group | Formulation | Dose/volume |
|---|---|---|
| Subcutaneous injection | ||
| PBS-SC | PBS solution (control) | 100 µl |
| LeCoVax-2 +Alum-SC | 2 × 107 cells Lt-spike + 10 μg RBD + Alum | 100 µl |
| LeCoVax-2 +AddaVax-SC | 2 × 107 cells Lt-spike + 10 μg RBD + AddaVax | 100 µl |
| RBD+Alum-SC | 20 μg RBD + Alum | 100 µl |
| RBD+AddaVax-SC | 20 μg RBD + AddaVax | 100 µl |
| Rectal administration | ||
| PBS-R | PBS solution (control) | 50 µl |
| LeCoVax-2-R | 1 × 108 cells Lt-spike + 20 μg RBD | 50 µl |
| LeCoVax-2 +R84810-R | 1 × 108 cells Lt-spike + 20 μg RBD + 10 μg R848 | 50 µl |
| LeCoVax-2 +R84825-R | 1 × 108 cells Lt-spike + 20 μg RBD + 25 μg R848 | 50 µl |
| RBD+R84825-R | 20 μg RBD + 25 μg R848 | 50 µl |
| Lt: Leishmania tarentolae; RBD: Receptor Binding Domain; SC: subcutaneous; R: rectal; Alum: aluminium phosphate gel; AddaVax: squalene-based oil-in-water (w/o) nano-emulsion; R848: Resiquimod. | ||
2.3. In-house enzyme-linked immunosorbent assay (ELISA)
2.3.1. Serum IgG determination
An ELISA assay was used to determine the IgG antibody response in the sera of immunized mice. Briefly, 96-well ELISA plates were coated with 1 µg/ml recombinant SARS-CoV-2 Spike-RBD protein (Sino Biological) at 4 °C overnight. After three washes with Tris Buffered Saline (TBS) containing 0.05% (v/v) Tween 20 (T-TBS), coated plates were blocked for 1 h at 37 °C with a solution of T-TBS containing 5% (w/v) Non-Fat Dry Milk (NFDM, Euroclone). Murine serum samples were serially diluted from 1/50 up to 1/3200 in two-fold dilutions in T-TBS containing 5% NFDM. Plates were washed three times with T-TBS and 100 µl of each serial dilution were added to the plates and incubated for 1 h at 37 °C. Plates were washed three times and 100 µl of goat anti-mouse IgG1 HRP-conjugated (Bethyl Laboratories) diluted 1:50,000 in 5% NFDM/T-TBS were added to each well and the plate was incubated at 26 °C for 30 min. Following incubation, plates were washed three times and 100 µl/well of 3,3′,5,5′ -Tetramethylbenzidine (TMB) substrate (Bethyl Laboratories) was added and incubated in the dark at room temperature (RT) for 20 min. The reaction was stopped by adding 100 µl of hydrochloric acid solution 0.5 M (Fisher Chemical) and read within 20 min at 450 nm with SpectraMax ELISA plate (Medical Device) reader. A cut-off value was obtained for each plate by multiplying by three the average of the blank optical density (OD) signal derived from wells not containing analyte (background) [28].
2.3.2. IgA determination in faeces
For the measurement of mucosal IgA, fresh fecal pellets were weighed and dissolved in a protease inhibitor solution (Roche), as reported in Tonti and colleagues [29]. After 10 min at RT, samples were vigorously vortexed; these two steps were repeated once more. Finally, samples were centrifuged at 13000 g for 5 min at 4 °C, the supernatants were collected and stored at − 20 °C. Supernatants were tested in ELISA assay, as described above with minor modifications. Samples were serially diluted starting from 1/2–1/16 in two-fold dilutions in the blocking buffer (NFDM/T-TBS) and, after the washing step, the plates with the serial dilutions were incubated for 2 h at RT. After three washes, goat anti-mouse IgA Heavy Chain Antibody HRP (Bethyl Laboratories) diluted 1:5000 was added to each well and the plates were incubated at RT for 1 h. A cut-off value was determined for each plate by multiplying by three the average of the blank OD signal derived from wells not containing murine sample [28].
2.4. ELISpot assay
Splenocytes were isolated from the murine spleen by gently pressing it through a 70 µm cell strainer using a syringe plunger. Cells were washed with RPMI-1640 medium with L-glutamine and 25 mM HEPES (Gibco) supplemented with 10% FBS (Euroclone) and 100X Penicillin/Streptomycin (Euroclone) and centrifuged at 1300 rpm for 10 min. Red blood cells were lysed in 10X RBC Lysis buffer (BioLegend) and centrifuged at 1300 rpm for 10 min and the pellet was resuspended in FBS with 7.5% Dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored in the liquid nitrogen tank until use. The T cell responses of immunized mice were analyzed using a mouse IFN-γ/IL-4 Double-Color ELISPOT kit (CTL ImmunoSpot) and a pre-coated mouse TNF-α Single-Color ELISPOT kit (CTL ImmunoSpot), following the manufacturer's protocol.
Briefly, the day before starting the assay, membrane-bottomed 96-well plates were coated with anti-IL-4 and IFN-γ monoclonal capture antibodies and kept at 4 °C overnight, while the membrane-bottomed 96-well plate for TNF-α was ready-to use. The day after, 3 × 105 cells/well splenocytes were seeded and stimulated in triplicate with the PepMix™ SARS-CoV-2 Spike Glycoprotein (1 µg/ml, JPT); complete CTL medium alone and phorbol myristate acetate (PMA)/Ionomycin (Invitrogen) were used as negative and positive control, respectively. The plates were incubated for 24 h in a humidified 5% CO2 incubator at 37 °C. Following incubation, plates were washed and biotinylated IL-4, TNF-α and IFN-γ antibodies were added for 2 h at RT. Finally, streptavidin-alkaline phosphatase was added and after 1 h of incubation substrate solutions were added. The reaction was stopped by extensively washing the plates with tap water, then the plates were left to dry at least 24 h in darkness. Wells were imaged with ImmunoSpot® Analyzers (CTL-Immunospot) and spot-forming units (SFUs) were determined with the ImmunoSpot® Double and Single Color Enzymatic Software Suite (CTL-Immunospot).
2.5. Micro-neutralization assay
The micro-neutralization (MN) assay was performed, as previously reported by Manenti and colleagues [30]. In brief, VERO E6 C1008 cells (CRL-1586) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), High Glucose (Euroclone), supplemented with 2 mM L-glutamine (Lonza), 100 units/ml Penicillin–Streptomycin mixture (Lonza) and 10% Fetal Bovine serum (FBS) (Euroclone), in a 37 °C and 5% CO2 humidified incubator. Serial two-fold dilution of murine serum samples, starting from 1:10, were incubated with an equal volume of SARS-CoV-2 (Wuhan Strain) viral solution containing 25 tissue culture infective dose 50% (TCID50) for 1 h at RT [31]. After incubation, 100 µl of the serum-virus mixture was transferred to a 96-well plate containing an 80% sub-confluent Vero E6 cell monolayer containing 2% FBS. The plates were incubated for three days at 37 °C and 5% CO2. At the end of incubation, the presence/absence of cytopathic effect (CPE) was assessed by means of an inverted optical microscope. A CPE higher than 50% was indicative of infection. The MN titre was expressed as the reciprocal of the highest serum dilution showing protection from viral infection and CPE.
2.6. Histopathological examination
At sacrifice, mice underwent complete necropsy, and any gross change was recorded. The following organs/tissues were sampled and fixed in 10% neutral buffered formalin for histological examination: liver, kidneys, spleen (when available), lungs, lumbar skin and subcutis, inguinal lymph nodes, para-aortic lymph nodes, mesenteric lymph nodes, ileum-ceco-colic junction, and any other organ/tissue with grossly detectable lesions. Tissues were routinely processed for paraffin embedding, and 4 µm sections from each paraffin block were stained with hematoxylin-eosin (H&E) and evaluated under a light microscope. Findings were recorded according to the following grading system: 0 =absent; 1 =minimal; 2 =slight; 3 =moderate; 4 =marked; 5 =severe. Additionally, the number of intestinal lymphoid structures (part of the gut-associated lymphoid tissue, GALT) (small < 0.5 mm in diameter; medium: 0.5–2 mm in diameter; large > 2 mm in diameter) were recorded per each examined mouse. The total number of structures and the GALT total score (no. of small structures*1 + no. of medium structures*2 + no. of large structures*3) were then calculated. For the digital image analysis, digital slides were obtained from 4 µm H&E-stained sections of the ileum-cecum-colon portion by using the NanoZoomer-XR Digital slide scanner (Hamamatsu, C12000), and images were captured by using the NDP.view2 Viewing software (Hamamatsu, U12388–01). The area of each lymphoid structure identified in the intestinal mucosa was measured using the freehand region selection tool of the NDP.view2 Viewing software. The mean area of lymphoid structures and the total area were then calculated.
2.7. Statistical analysis
Statistics analyses were performed only for data collected within the framework of the main experiment, whereas, due to the limited sample size, only a qualitative assessment was performed for the preliminary experiment.
To investigate the effect of different immunization treatments on IgG levels, we relied on gamma generalized linear mixed models (GLMM) with log-link function, accounting for heterogeneity of variances in IgG values between time points (days 21, 35 and 48 post-vaccination) and experimental groups (listed in Table 2). Mouse identity was included as a random intercept effect to account for repeated measures of the same individual across time points, whereas experimental groups, time point, and their interaction were included as fixed factors. The effects of immunization treatments on IgA levels at day 48 post-vaccination were assessed by gamma generalized linear models (GLM) accounting for heterogeneity of variances among experimental groups. Separate models were fitted for treatment groups subjected to subcutaneous or rectal vaccine administration. For both IgG and IgA levels, significance of main effects was tested by Wald χ2 tests. Post-hoc pairwise comparisons were computed (on the log-scale) to investigate differences in IgG/IgA values between experimental groups (within each time point for IgG models), adjusting p-values according to the Tukey method (on families of 5 estimates). Checks of model diagnostics (performed using the DHARMa R package, ver. 0.4.4) indicated that the models fitted the data adequately (details not shown for brevity).
The number of IFN-γ-, IL-4-, TNF-ɑ-secreting cells were compared among mice from different treatment groups using Mann-Whitney tests. Because sample size was very small (< 5) for several groups (see Results), biologically relevant pairwise statistical comparisons between treatment groups were performed only when sample size for both groups was > = 5.
Variation between treatment groups in total number of lymphoid structures, GALT total score and total area of lymphoid structures in the intestinal tract of R vaccinated mice was assessed by Kruskal-Wallis tests. In case the Kruskal-Wallis test highlighted significant variation among groups, subsequent pairwise comparisons were performed by Mann-Whitney tests.
Variation in body mass among experimental groups from the main experiment between two time points (day 7 and day 42 post-vaccination) was assessed by means of linear mixed models (LMM), fitted separately for treatment groups subjected to subcutaneous or rectal vaccine administration. Mouse identity was included as a random intercept effect to account for repeated measures of the same individual across time points, whereas experimental groups, time point, and their interaction were included as fixed factors. Significance of fixed effects was assessed by F-tests, with degrees of freedom estimated according to the Kenward-Roger approximation. Post-hoc pairwise comparisons were computed to investigate differences in body weight between experimental groups (within each time point), adjusting p-values according to the Tukey method (on families of 5 estimates).
Gamma GL(M)Ms were fitted using the GLMMtmb R package (ver. 1.1.3, [32]), while LMMs were fitted using the lme4 R package (ver. 1.1–26, [33]). Pairwise comparisons were performed using the emmeans and multcomp R packages (ver. 1.7.1 and 1.4, respectively). All statistical analyses were performed using the R software (ver. 4.0.4; [34]).
3. Results
3.1. LeCoVax-2 vaccine design and production
The two clones of L. tarentolae (Lt-spike and Lt-RBD) expressing the full-length (1240 amino acids) SARS-CoV-2 virus spike protein sequence or the RBD-SD1 domain (273 aminoacids), were selected for the successive phases of the study. Before preparing the vaccine doses, the production of both proteins by the recombinant clones of Leishmania was verified by Western blotting, which allowed to specifically stain two bands, at approximately 180 and 35 kDa; this confirms the expression of the whole spike and of the RBD (Fig. 1 C; Supplementary Fig. 1–2). In addition, as shown in Fig. 1D, the presence of the spike protein on the membrane of Leishmania was revealed by immunofluorescence; the yellow/orange dots indicate the colocalization of the spike protein and the external membrane of the protozoan parasite, while green dots indicate that the spike protein was present also in the cytoplasm (see also [26]). Prior to mice immunizations with the different candidate vaccine formulations, spike-protein producing Leishmania cells were inactivated with formalin. As reported in Fig. 1E, the treatment did not alter the morphology of Leishmania cells; in fact, the parasites possessed an intact membrane and maintained their structure (Fig. 1E).
3.2. Mouse IgG responses to preliminary immunization
To determine the immunogenicity of the candidate vaccines, three groups of BALB/c mice were intraperitoneally immunized, and three groups of mice were subcutaneously administered with different compositions, without adjuvants.
Supplementary File 1 reports the specific anti-RBD IgG signals observed in sera samples; for some samples belonging to the groups of mice immunized with LeCoVax-2, subcutaneously or intraperitoneally, or intraperitoneally immunized with 10 µg RBD, the OD value was high (more than 1), corresponding to a high concentration of anti-RBD specific antibodies, with a linear decrease upon dilution, indicating the stability of the signal. No specific antibodies were detected in animals immunized with Lt-P10 (control strain) or PBS. Moreover, the serum of one out five intraperitoneally LeCoVax-2 vaccinated mice that tested positive in ELISA assay, displayed neutralizing activity against SARS-CoV-2 in in vitro assays, indicating the presence of neutralizing antibodies. No significant differences in animal body weight, between vaccinated groups and control groups, were observed, and no local inflammation response at the injection site or other adverse effects were observed from the start of the study until sacrifice (data not shown).
3.3. Subcutaneous and rectal administration induced RBD-specific IgG and IgA responses in vaccinated mice
To test the immunogenicity and the induction of a humoral immune response, BALB/c mice were subcutaneously or intrarectally primed on day 0 and boosted on days 21 and 35 with different vaccine preparations, as described in Table 2.
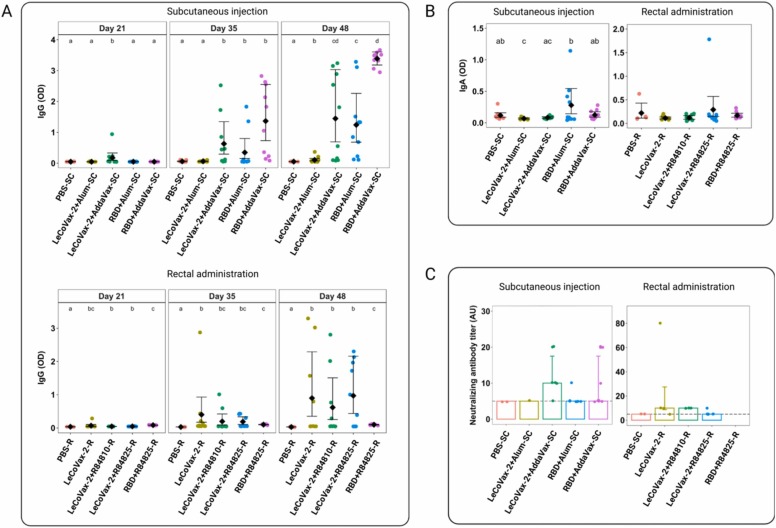
Serum samples were collected from mice before each immunization and the IgG levels were determined. IgG values showed a statistically significant differential variation across time points among different treatment groups (gamma GLMM, treatment × time point interaction, χ2 = 107.2, d.f. = 8, p < 0.0001). As shown in Fig. 2 A, mice groups that were subcutaneously immunized showed high levels of SARS-CoV-2 RBD specific IgG antibodies, with a noticeable increase of OD values after the boosts (Fig. 2). In detail, after 21 days, the mice group vaccinated with LeCoVax-2 plus AddaVax showed significantly higher levels of IgG antibodies compared to other vaccinated groups and the PBS control (gamma GLMM, post-hoc tests correcting for multiple comparisons, p < 0.002 for all comparisons). After the first and second boost (day 48), the IgG levels increased in the mice groups vaccinated with LeCoVax-2 with AddaVax and in mice immunized with recombinant RBD and the AddaVax or Alum adjuvants, showing statistically significant differences compared to the control group (gamma GLMM, post-hoc tests correcting for multiple comparisons, p < 0.0001 for all comparisons) (Fig. 2 A). Among all treated groups, mice immunized with recombinant RBD plus AddaVax showed the highest levels of specific IgG antibodies (Fig. 2 A).
Fig. 2.
Immune responses after immunization with LeCoVax-2 vaccine. (A-D) Antibody responses in sera of immunized mice were evaluated. (A) Variation of IgG levels detected at days 21, 35 and 48 and IgA levels at day 48 (B) after SC or R administration of different vaccine formulations in mice (PBS administered as control; n = 10 mice per experimental group, except for PBS-R, n = 5). Dots represent original data points, diamonds represent estimated mean values (with 95% CI) from a gamma GLMM accounting for heterogeneity of variances (see Methods). Different letters denote significant (p < 0.05) differences at post-hoc tests (after Tukey correction for multiple testing) within each time point (for IgA determination upon rectal administration, no pairwise comparison was significant after correction for multiple testing). (C) Neutralizing antibody titers at day 48 post-administration of vaccines (left: subcutaneous; right: rectal; note the different y-axis scales). Bars represent median values, error bars 25th and 75th percentiles. Random jittering was added to datapoint to show overlapping data. The horizontal dashed line represents the threshold over which samples were considered as the limit of detection (LOD). Antibody titers were determined only for samples that were IgG positive at day 48 post-administration. The PBS-SC group was included as a control for both subcutaneous and rectal administration; see Methods).
Similarly to subcutaneous administration, IgG values of intrarectally immunized mice showed a statistically significant differential variation across time points among different treatment groups (gamma GLMM, treatment × time point interaction, χ2 = 113.77, d.f. = 8, p < 0.0001). As shown in Fig. 2 A, after R immunization, the highest levels of specific IgG antibodies were detected after the third administration; in particular, mice immunized with LeCoVax-2 showed significantly higher levels of IgG antibodies compared to the control group (gamma GLMM, post-hoc tests correcting for multiple comparisons, p < 0.001 for both comparisons) (Fig. 2 A). On the other side, mice intrarectally vaccinated with the purified RBD antigen plus R848 displayed only a limited IgG response compared to the control group (Fig. 2 A).
The ability of the candidate vaccines to induce mucosal IgA was evaluated by performing ELISA against RBD, collecting faeces from vaccinated mice at day 48. A statistically significant treatment effect was observed both in the subcutaneously and intrarectally vaccinated mice (gamma GLMs, χ2 = 37.6, d.f. = 4, p < 0.0001 and χ2 = 23.5, d.f. = 4, p < 0.0001, respectively). However, post-hoc tests (correcting for multiple comparisons) revealed only a non-significant increase of OD values in the mice group subcutaneously immunized with the RBD protein plus Alum compared to the control group (Fig. 2B). Furthermore, none of the pairwise comparisons between treatment groups subjected to rectal vaccination was statistically significant, although values for the LeCoVax-2 +R848 group were somewhat higher than values for other experimental groups.
3.4. Antibodies induced by vaccination are capable of neutralizing SARS-CoV-2
To evaluate viral neutralization capability, sera from vaccinated mice were tested in a MN neutralization assay. To this end, sera from immunized mice were assessed for their ability to prevent cytopathic effects of SARS-CoV-2 on Vero cells in vitro. Antibody titers, expressed as the reciprocal of the highest serum dilution showing protection from viral infection, were only determined for samples that were IgG positive at day 48.
Although no statistical comparison was performed because of the limited sample size for several experimental groups, as shown in Fig. 2 C and in Table 3, some animals which subcutaneously received LeCoVax-2 plus AddaVax or RBD plus AddaVax were able to neutralize the SARS-CoV-2 with similarly high neutralization titers. Moreover, mice intrarectally immunized with LeCoVax-2 or LeCoVax-2 plus adjuvant (R848) were also able to neutralize the virus with higher neutralization titers, reaching values of up 1:80. Importantly, 75% of the mice that seroconverted to RBD, after immunization with both LeCoVax-2 or LeCoVax-2 plus 10 µg R848 (overall, 6 out of 8 mice), displayed neutralizing activity to SARS-CoV-2 (Table 3). In contrast, no neutralizing capacity was detected in sera from non-vaccinated mice.
Table 3.
IgG positive and SARS-CoV-2 neutralizing samples at day 48 in each tested group of the main experiment.
| Formulation | IgG positive samples (Day 48) |
SARS-CoV-2 neutralizing samples (Day 48) |
|---|---|---|
| LeCoVax-2 + AddaVax-SC | 7/10 (70%) | 5/7 (71%) |
| LeCoVax-2 + Alum-SC | 2/10 (20%) | 0/2 (0%) |
| 20 µg RBD + AddaVax-SC | 10/10 (100%) | 4/10 (40%) |
| 20 µg RBD + Alum-SC | 8/10 (80%) | 1/8 (13%) |
| LeCoVax-2 + 10 µg R848-R | 4/10 (40%) | 3/4 (75%) |
| LeCoVax-2 + 25 µg R848-R | 6/10 (60%) | 1/6 (17%) |
| LeCoVax-2-R | 4/10 (40%) | 3/4 (75%) |
| 20 µg RBD + 25 µg R848-R | 0/10 (0%) | 0/10 (0%) |
| PBS-SC | 0/10 (0%) | 0/2 (0%) |
| PBS-R | 0/10 (0%) | 0/10 (0%) |
RBD: Receptor Binding Domain; SC: subcutaneous; R: rectal; Alum: aluminium phosphate gel; AddaVax: squalene-based oil-in-water (w/o) nano-emulsion.
3.5. Vaccinated mice show antigen-specific T cell responses
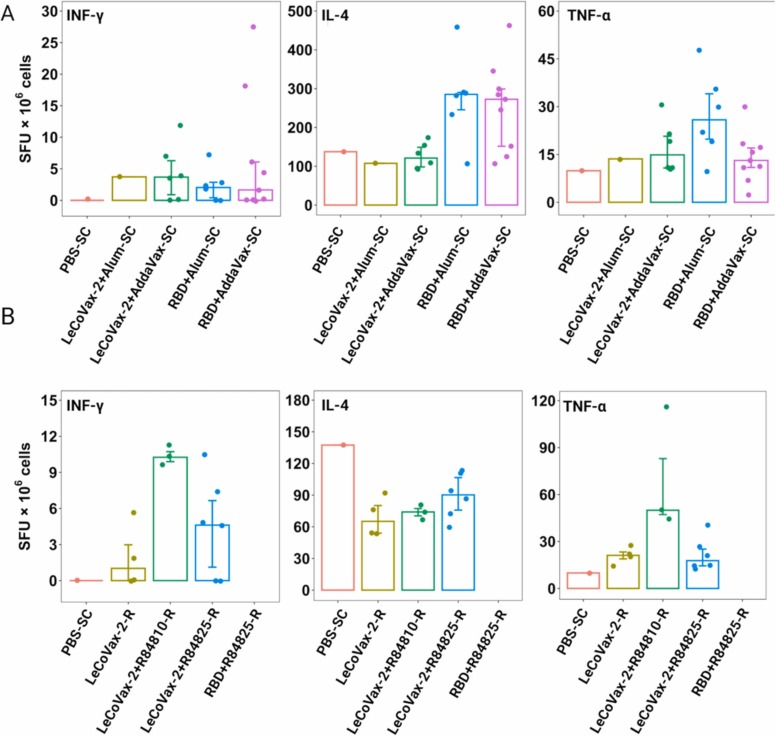
RBD-specific T cell responses were measured through ELISpot analysis, using spleens from immunized mice after three vaccine doses. Quantification of IFN-γ-, IL-4-, TNF-ɑ-secreting cells after stimulation were determined and expressed as SFU/106 splenocytes. As reported in Fig. 3 A, splenocytes from mice vaccinated by SC injection with RBD plus adjuvants (AddaVax or Alum) and re-stimulated with RBD peptides yielded sensibly higher IL-4 secretion (compared to the control value), with significantly higher value in the RBD+AddaVax-SC group compared to LeCoVax-2 +AddaVax-SC (Mann-Whitney test, p = 0.036). For both IFN-γ and TNF-ɑ-secreting T cells, no significant differences in response to RBD stimulation were obtained by analyzing the splenocytes derived from the same mice (Fig. 3 A). Only the group subcutaneously immunized with RBD plus Alum showed a statistically higher frequency of TNF-ɑ-secreting cells compared to the group treated with RBD plus AddaVax (Mann-Whitney test, p = 0.036). Regarding the analyses of splenocytes derived from mice vaccinated through the R route, some secretion of TNF-ɑ and IFN-γ was observed in groups treated with LeCoVax-2 plus adjuvant, compared with other groups or PBS treated mice. No IL-4 secretion was detected in vaccinated mice compared to PBS treated mice (Fig. 3B). We emphasize that T cell response was determined only on animals with positive IgG titers at day 48 and that statistical analyses on T cell responses were performed only for the groups for which the number of IgG-positive animals was ≥ 5 for both groups being compared in pairwise tests.
Fig. 3.
Evaluation of antigen-specific cytokine-producing T cells capacity. The number of IFN-γ, IL-4 and TNF-α secreting cells per million splenocytes (spot forming units, SFU) was determined by ELISpot analysis at day 48 post-administration of LeCoVax-2 through the SC route (A) and R route (B). Only samples that were IgG positive at day 48 post-administration were evaluated. Bars represent median values, error bars 25th and 75th percentiles. For rectal administration, we lacked PBS treated individuals (see Methods): for comparison, we thus reported the values obtained from the single individual which received a SC injection.
3.6. LeCoVax-2 does not induce adverse effects
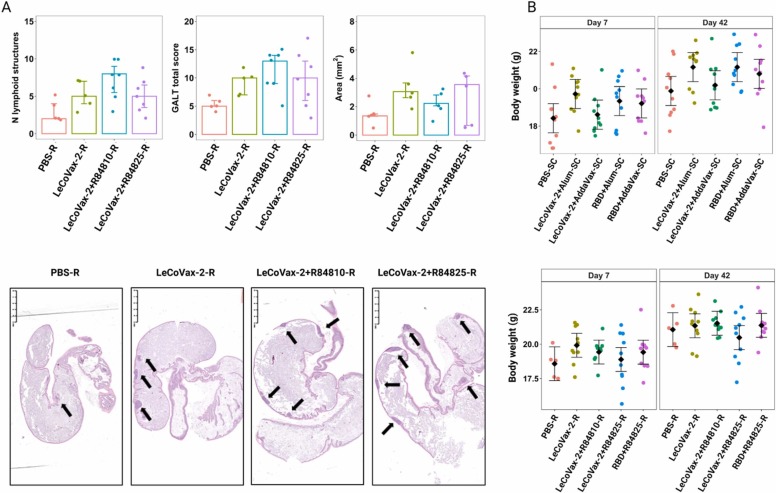
Histological examination revealed that SC and R administration of the different formulations in BALB/c mice induced no adverse effects in liver, kidney, and lung. We observed the development of germinal centers in secondary lymphoid organs (spleen and LNs) as result of an antigenic stimulation; in particular, it was detected in periaortic LNs after SC and R administration of different tested formulations, and in one single spleen after R administration of LeCoVax-2 plus 25 μg R848. However, no obvious association with production of IgG or neutralizing antibody was observed. Histological and digital image analyses of the GALT revealed a significant variation in the number of lymphoid structures between groups (Kruskal-Wallis test, χ2 = 8,02, d.f. = 3, p = 0.045) and a marginally non-significant variation in the GALT total score (Kruskal-Wallis test, χ2 = 7.06, d.f. = 3, p = 0.07), whereas the GALT total area did not significantly vary among treatment groups (Kruskal-Wallis test, χ2 = 4.6, d.f. = 3, p = 0.21) ( Fig. 4 A). For the number of lymphoid structures, subsequent pairwise comparisons revealed that LeCoVax-2-R848 group showed significantly higher values compared to the control group (Mann-Whitney test, p = 0.017). Similarly, to the development of germinal centers observed in LNs, no association between increase in GALT and production of IgG or neutralizing antibody was observed.
Fig. 4.
Histological analyses for adverse effects determination. (A) Total number of lymphoid structures, GALT total score and total area of lymphoid structures in the intestinal tract of R-vaccinated mice in proximity of the ileo-cecal-colic junction are shown. Box plots represent median, 25th and 75th percentiles, minimum and maximum values. Arrows indicate lymphoid structures of gut-associated lymphoid tissue. (B) Body weight variation of mice subjected to subcutaneous (upper panel) or rectal (lower panel) administration of different vaccine formulations (including PBS administration as a control group) in mice (n = 10 mice per experimental group, with the exception of PBS-R, n = 5). Dots represent original datapoints, diamonds represent estimated mean values (with 95% CI) from a LMM (see Methods). No pairwise comparison (within each time point) was significant upon correction for multiple testing.
All the animals immunized with the different vaccine formulations were monitored daily for adverse events, including body weight loss. As shown in Fig. 4B, the body weight of the animals, both immunized subcutaneously and rectally, increased from the first week of observation to the last one before the sacrifice. The change in body weight was not statistically significant different between groups for subcutaneous administration (treatment × time point, F4,45 = 0.83, p = 0.52), whereas there were some differences between groups for the rectal administration (F4,40 = 3.63, p = 0.013). However, for the latter, post-hoc pairwise comparisons revealed no significant differences in body weight between groups within each time point (all p > 0.45, Fig. 4B).
4. Discussion
The aim of the present study was to assess, as proof-of-principle, the potential of the parasitic protozoan L. tarentolae as a vehicle for enteral vaccination. To avoid the possible pitfalls associated with oral administration, we focused on the rectal route. Indeed, oral administration would have required ad hoc solutions to protect the vaccine vehicle and the antigen from gastro-duodenal digestion, which was out of the scope of this study. In the selection of SARS-CoV-2 as a virus model, we considered the still wide circulation of this virus in human populations, and the possibility that additional booster vaccine doses could still be required in the future, for the control of COVID-19 pandemic. In this context, a vaccine preparation suitable for mucosal administration may represent an important alternative to current types of vaccines. Several COVID-19 vaccine candidates for nasal or inhalation delivery are currently being tested and have already proved effective in determining a mucosal IgA-based immunity, in addition to cellular immunity and IgG-based humoral responses [1], [35]. The rectal route remains however to be investigated, and our study presents a first example of a candidate COVID-19 vaccine tested in rectal administration. Notably, antigen administration through the enteral route can stimulate B-cell clones that home to the respiratory apparatus, in addition to the intestine [36], [37]. This would thus guarantee specific mucosal responses, at both the gut and respiratory levels.
In addition to the use of L. tarentolae as a vaccine platform, a further peculiarity of our study is that we assayed a preparation (LeCoVax-2), containing both L. tarentolae expressing the spike protein (Lt-spike) and the purified RBD domain of this protein, produced by another engineered clone of the protozoon. The rationale of this strategy is to immunize the host with both a subunit antigen (the recombinant RBD) as well as with a particulate microbial antigen (Lt-spike, where the spike protein includes the RBD domain). The particulate Lt-spike component of LeCoVax-2 is expected to be internalized with high efficacy by DCs [26], [25], and then processed and presented to T CD4 + lymphocytes, which should result in a more effective immune response [38].
Our study consisted of two experiments in the murine model. In the first, preliminary experiment, three types of antigen preparations (Lt-spike; RBD; LeCoVax-2) were administered without adjuvants. As detailed in the results section, IgG response without adjuvants was limited, and was evident only after the third administration (second booster dose). Moreover, only one of the 30 animals vaccinated with the three antigen preparations, at the different dosages and combinations, developed a neutralizing response. Despite the failure in term of the production of a neutralizing response, this first experiment provided encouraging results, and useful information for the prosecution of the study: 1) none of the animals, at any antigen dosage and combination, displayed any detectable adverse reaction; 2) the potential of L. tarentolae as a vaccine platform and vehicle was confirmed, considering the generation of the IgG response even in the absence of adjuvants; 3) the LeCoVax-2 preparation was well tolerated even after SC inoculation of 2 × 107 Lt-spike Leishmania cells in combination with 10 µg of purified RBD antigen, or after IP administration.
In the second experiment, we introduced three types of adjuvants and the rectal route of administration (while the IP route was not further assayed, considering its limited potential in terms of medical translation). Comparison with the results of the first experiment demonstrates that even a vaccine vehicle such as L. tarentolae, which is expected to target DCs and secondary lymphoid tissues [26], benefits from co-administration with adjuvants in SC administration. Indeed, some animals in different groups seroconverted even after the first booster dose, and the percentage of seropositive animals increased after the second boost (while in the first experiment, in the absence of adjuvants, most of the response was observed only after the second boost). Considering the neutralizing properties of the sera from SC immunized animals, the AddaVax adjuvant appears more effective than Alum, in agreement with ELISA assay data assessing IgG responses.
The most important result of the second experiment was the seroconversion in animals immunized through the rectal route. Indeed, while seroconversion after SC antigen administration was not surprising, particularly in the presence of adjuvants, the rectal route for antigen administration has, to date, only been explored in a limited number of studies. In addition, Leishmania or any other Trypanosomatidae species have never been investigated as potential rectal vaccines or vaccine vehicles, against COVID-19 or other pathogens. Comparing the results of SC and rectal antigen administration, while in SC administration the purified RBD antigen, co-administered with AddaVax, was very effective in determining seroconversion, in rectal administration this purified antigen failed to induce any significant IgG production. On the contrary, the LeCoVax-2 preparation, which includes Leishmania cells expressing the spike antigen, determined significant seroconversion after rectal administration. As for the results of the viral neutralization assays, almost all groups produced sera that neutralized SARS-CoV-2, albeit with varying percentages of neutralizing animals in the different groups (see Table 3). We emphasize that the number of neutralizing animals in SC administration was notably higher after the use of AddaVax as adjuvant, as compared to alum-adjuvanted animals (e.g., see Table 3, groups 2 and 4). In the case of rectal delivery, the percentage of neutralizing animals was variable (see discussion below), but the administration of the LeCoVax-2 preparation did not appear to differ in efficacy after administration with or without the mucosal adjuvant R848.
As already emphasized, a rather notable result that we observed after rectal administration was the absence of any detectable IgG responses induced by the purified RBD antigen, which was in contrast very effective in determining the IgG response after SC administration. This contrasts with the results obtained using LeCoVax-2, which was very effective in rectal administration, even in the absence of adjuvants. To summarize, while in SC administration the use of an adjuvant is required for the generation of a significant IgG response (consider the limited and delayed IgG response observed in the first, non-adjuvanted, experiment), in rectal administration the presence of the adjuvant does not appear to be required. In addition, while in SC administration the response induced by LeCoVax-2 (which include Lt-spike cells) did not differ significantly from that induced by the purified RBD antigens, in rectal administration we did not observe a significant response after the use of the purified RBD antigen alone, even in the presence of the adjuvant. The fact that the response to RBD was observed, in rectal administration, only when Lt-spike cells were present (i.e., in the LeCoVax-2 preparation) provides strong evidence for the role played by Leishmania cells in the generation of the immune response.
A major problem that we encountered during our experiments was that some mice defecated within a relatively short time after rectal administration of the antigen preparations. For logistic reasons we could not keep the mice under observation for over 20 min after rectal vaccination, but the fact that one to three mice per group expelled a fecal pellet within this short time suggests that the retention time of the antigen preparation (or part of it) in the mice gut could have been on average rather short, possibly limited to a few tens of minutes, with unpredictable variation of retention times among the animals. In addition, some leakage of the preparation from the anus cannot be excluded. This could possibly explain the variability of the results obtained after rectal administration, and the limited response in terms of IgA production. More in general, both oral and rectal drug administration are expected to imply rather variable interindividual adsorption of the active compound [39], [40]. We should also consider that the gavage we used for rectal administration was calibrated to ensure the delivery of the preparation in the upper part of the colon, without reaching the ileo-cecal valve (to avoid the risk of determining any lesion). Therefore, we assume that the antigen had not been delivered into the small intestine, and did not reach the ileum, where most Peyer’s patches are located.
Considering the limitations of rectal antigen administration, particularly in animal models, where defecation cannot be controlled, we believe that the variations we observed within and between the groups, in terms of IgG and neutralizing response, do not affect the importance of the results that we obtained with the candidate vaccine LeCoVax-2, in terms of a future development of L. tarentolae as a vehicle for mucosal vaccination (either rectal or oral). For example, LeCoVax-2, or fractions of Leishmania cells loaded with the desired antigen, could be prepared as tablets for oral administration, and coated for protection against gastric digestion and for a controlled release in the distal part of the small intestine. This would ensure a higher retention time of the antigen in the gut, compared to the time ensured by rectal administration, and the potential translocation of the antigen to lymphoid cells not only in the colon, but also in the ileum and through the M cells and the Peyer’s patches. Therefore, besides the possibility that our study could offer clues for the development of future strategies for the administration of booster doses in anti-COVID-19 vaccination, our results are of more general interest, and provide a strong a case on the potential of L. tarentolae, not only as a platform for vaccine production, but also as a vehicle for antigen delivery through the enteral route.
Funding
C.B. and S.E. received funding from “Fondazione Romeo ed Enrica Invernizzi” (grant agreements no. LIB_VT21SEPIS and LIB_VT22CBAND). S.E. and G.V.Z. received funding from “Erogazione liberale per le attività di ricerca sul Coronavirus” (grant agreements no. LIB_VT20_COVID_19_SEPIS; LIB_VT20_COVID_19_GZUCCOTTI). VisMederi Research provided funding support for reagents and serological assays.
CRediT authorship contribution statement
C.B., S.E., P.F. E.M. and GV.Z.: Conceptualization. I.V.B., S.E., G.C., F.D., M.M., I.R, M.L., L.G., L.B.: Data collection. C.B., S.E., A.M., D.R., P.G., C.R., M.I.: Data analysis and interpretations. C.B., GV.Z.: Supervision. I.V.B., S.E., L.M.: Methodology. C.B., S.E., GV.Z., E.M.: Resources. C.B., S.E., I.V.B.: Writing – original draft. L.G., P.F., A.M., D.R.: Critical revision of the manuscript.
Declaration of Competing Interest
The candidate vaccine Lt-spike and its potential application have been described in the PCT/IB2022/051585 (23 February 2022). The antigen Lt-RBD and its potential application have been described in the patent application no. IT 02021000004160 (23 February 2021). A.M., F.D., M.M., and I.R. are employed by VisMederi Srl; M.L. is employed by VisMederi Research Srl; E.M. is the President and Founder of VisMederi Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Microscopy observations were carried out at The Advanced Microscopy Facility Platform—UNItech NOLIMITS—Università degli Studi di Milano. The networking activity of the UNIMI GSA-IDEA project facilitated the collaboration between the authors of this study. Graphical abstract and figures were created with BioRender.com. The authors thank Dr. Pietro Di Lucia (San Raffaele Scientific Institute) for technical assistance, Prof. Eugenio Scanziani (Università degli Studi di Milano) and Dr. Cesare Resta for their suggestions and useful insights.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2022.106546.
Appendix A. Supplementary material
Supplementary material
.
Data availability
The data that support the findings of this study are included in this published article (and its supplementary information files).
References
- 1.Huang M., Zhang M., Zhu H., Du X., Wang J. Mucosal vaccine delivery: a focus on the breakthrough of specific barriers. Acta Pharm. Sin. B. 2022;12(9):3456–3474. doi: 10.1016/j.apsb.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavelle E.C., Ward R.W. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 2022;22(4):236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh-Mohamed S., Sanders E.C., Gommerman J.L., Tal M.C. Guardians of the oral and nasopharyngeal galaxy: IgA and protection against SARS-CoV-2 infection. Immunol. Rev. 2022;309(1):75–85. doi: 10.1111/imr.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waltz E. How nasal-spray vaccines could change the pandemic. Nature. 2022;609(7926):240–242. doi: 10.1038/d41586-022-02824-3. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 6.Su M., Xu S., Weng J. A bibliometric study of COVID-19 research in Web of Science. Pharmacol. Res. 2021;169 doi: 10.1016/j.phrs.2021.105664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn W.O., Wiley Z. COVID-19 vaccines. Infect. Dis. Clin. North. Am. 2022;36(2):481–494. doi: 10.1016/j.idc.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabitha S., Shobana N., Prakash P., Padmanaban S., Sathiyashree M., Saigeetha S., Chakravarthi S., Uthaman S., Park I.K., Samrot A.V. A review of different vaccines and strategies to combat COVID-19. Vaccin. (Basel) 2022;10(5):737. doi: 10.3390/vaccines10050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björk J., Bonander C., Moghaddassi M., Rasmussen M., Malmqvist U., Inghammar M., Kahn F. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 Omicron BA.1 and BA.2 subvariants - surveillance results from southern Sweden, December 2021 to March 2022. Eur. Surveill. 2022;27(18):2200322. doi: 10.2807/1560-7917.ES.2022.27.18.2200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.León T.M., Dorabawila V., Nelson L., Lutterloh E., Bauer U.E., Backenson B., Bassett M.T., Henry H., Bregman B., Midgley C.M., Myers J.F., Plumb I.D., Reese H.E., Zhao R., Briggs-Hagen M., Hoefer D., Watt J.P., Silk B.J., Jain S., Rosenberg E.S. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis - California and New York, May-November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71(4):125–131. doi: 10.15585/mmwr.mm7104e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chico-Sánchez P., Gras-Valentí P., Algado-Sellés N., Jiménez-Sepúlveda N., Vanaclocha H., Peiró S., Burgos J.S., Berenguer A., Navarro D., Sánchez-Payá J., Valencian vaccine research program (ProVaVac) study group The effectiveness of mRNA vaccines to prevent SARS-CoV-2 infection and hospitalisation for COVID-19 according to the time elapsed since their administration in health professionals in the Valencian Autonomous Community (Spain) Prev. Med. 2022;163 doi: 10.1016/j.ypmed.2022.107237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klatt S., Simpson L., Maslov D.A., Konthur Z. Leishmania tarentolae: taxonomic classification and its application as a promising biotechnological expression host. PLoS Negl. Trop. Dis. 2019;13(7) doi: 10.1371/journal.pntd.0007424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza-Roldan J.A., Votýpka J., Bandi C., Epis S., Modrý D., Tichá L., Volf P., Otranto D. Leishmania tarentolae: a new frontier in the epidemiology and control of the leishmaniases? Transbound. Emerg. Dis. 2022:1–12. doi: 10.1111/tbed.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breton M., Tremblay M.J., Ouellette M., Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect. Immun. 2005;73(10):6372–6382. doi: 10.1128/IAI.73.10.6372-6382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahedifard F., Gholami E., Taheri T., Taslimi Y., Doustdari F., Seyed N., Torkashvand F., Meneses C., Papadopoulou B., Kamhawi S., Valenzuela J.G., Rafati S. Enhanced protective efficacy of nonpathogenic recombinant Leishmania tarentolae expressing cysteine proteinases combined with a sand fly salivary antigen. PLoS Negl. Trop. Dis. 2014;8(3) doi: 10.1371/journal.pntd.0002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdossamadi Z., Taheri T., Seyed N., Montakhab-Yeganeh H., Zahedifard F., Taslimi Y., Habibzadeh S., Gholami E., Gharibzadeh S., Rafati S. Live Leishmania tarentolae secreting HNP1 as an immunotherapeutic tool against Leishmania infection in BALB/c mice. Immunotherapy. 2017;9(13):1089–1102. doi: 10.2217/imt-2017-0076. [DOI] [PubMed] [Google Scholar]
- 17.Ansari N., Rafati S., Taheri T., Roohvand F., Farahmand M., Hajikhezri Z., Keshavarz A., Samimi-Rad K. A non-pathogenic Leishmania tarentolae vector based- HCV polytope DNA vaccine elicits potent and long lasting Th1 and CTL responses in BALB/c mice model. Mol. Immunol. 2019;111:152–161. doi: 10.1016/j.molimm.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Gull K. The cytoskeleton of trypanosomatid parasites. Annu. Rev. Microbiol. 1999;53:629–655. doi: 10.1146/annurev.micro.53.1.629. [DOI] [PubMed] [Google Scholar]
- 19.Delon L., Gibson R.J., Prestidge C.A., Thierry B. Mechanisms of uptake and transport of particulate formulations in the small intestine. J. Control. Release. 2022;343:584–599. doi: 10.1016/j.jconrel.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Yuan I.C., Chu H.J., Ho E.A. Transmission of leishmaniasis to the Chinese hamster (Crycetulus griseus) by feeding of infecting Chinese sand flies Phlebotomus chinensis. Chin. Med. J. 1993;62:204–206. [Google Scholar]
- 21.Reimann M.M., Torres-Santos E.C., Souza C.S.F., Andrade-Neto V.V., Jansen A.M., Brazil R.P., Roque A.L.R. Oral and intragastric: new routes of infection by Leishmania braziliensis and Leishmania infantum? Pathogens. 2022;11(6):688. doi: 10.3390/pathogens11060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geroldinger G., Rezk M., Idris R., Tonner Gruber, M., Moldzio R., Staniek K., Monzote L., Gille L. Techniques to study phagocytosis and uptake of Leishmania tarentolae by J774 Macrophages. Exp. Parasitol. 2019;197:57–64. doi: 10.1016/j.exppara.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-López M., Soto M., Iborra S., Sancho D. Leishmania hijacks myeloid cells for immune escape. Front. Microbiol. 2018;9:883. doi: 10.3389/fmicb.2018.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varotto-Boccazzi I., Epis S., Arnoldi I., Corbett Y., Gabrieli P., Paroni M., Nodari R., Basilico N., Sacchi L., Gramiccia M., Gradoni L., Tranquillo V., Bandi C. Boosting immunity to treat parasitic infections: Asaia bacteria expressing a protein from Wolbachia determine M1 macrophage activation and killing of Leishmania protozoans. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105288. [DOI] [PubMed] [Google Scholar]
- 25.Breton M., Zhao C., Ouellette M., Tremblay M.J., Papadopoulou B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) Gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1. Infect., J. Gen. Virol. 2007;88(1):217–225. doi: 10.1099/vir.0.81995-0. [DOI] [PubMed] [Google Scholar]
- 26.Varotto-Boccazzi I., Garziano M., Cattaneo G.M., Bisaglia B., Gabrieli P., Biasin M., Manenti A., Rubolini D., Clerici M., Montomoli E., Zuccotti G.V., Trabattoni D., Epis S., Bandi C. Leishmania tarentolae as an antigen delivery platform: dendritic cell maturation after Infection with a clone engineered to express the SARS-CoV-2 Spike protein. Vaccines. 2022;10:803. doi: 10.3390/vaccines10050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varotto-Boccazzi I., Manenti A., Dapporto F., Gourlay L.J., Bisaglia B., Gabrieli P., Forneris F., Faravelli S., Bollati V., Rubolini D., Zuccotti G., Montomoli E., Epis S., Bandi C. Epidemic preparedness-Leishmania tarentolae as an easy-to-handle tool to produce antigens for viral diagnosis: application to COVID-19. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.736530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milani G.P., Montomoli E., UNICORN Consortium investigators, Bollati V., Albetti B., Bandi C., Bellini T., Bonzini M., Buscaglia M., Cantarella C., Cantone L., Carugno M., Casartelli S., Cavaletti G., D'Alessandro S., De Chiara F., Delbue S., Dioni L., Eberini I., Favero C., Ferrari L., Ferraroni M., Galastri L., Galli C., Hoxha M., Iodice S., La Vecchia C., Macchi C., Manini I., Marchi S., Mariani J., Pariani E., Pesatori A.C., Rota F., Ruscica M., Schioppo T., Tarantini L., Trombetta C.M., Valsecchi M.G., Vicenzi M., Zanchetta G. SARS-CoV-2 infection among asymptomatic homebound subjects in Milan, Italy. Eur. J. Intern. Med. 2020;78:161–163. doi: 10.1016/j.ejim.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonti E., Jiménez de Oya N., Galliverti G., Moseman E.A., Di Lucia P., Amabile A., Sammicheli S., De Giovanni M., Sironi L., Chevrier N., Sitia G., Gennari L., Guidotti L.G., von Andrian U.H., Iannacone M. Bisphosphonates target B cells to enhance humoral immune responses. Cell. Rep. 2013;5(2):323–330. doi: 10.1016/j.celrep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manenti A., Maggetti M., Casa E., Martinuzzi D., Torelli A., Trombetta C.M., Marchi S., Montomoli E. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J. Med. Virol. 2020;92(10):2096–2104. doi: 10.1002/jmv.25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manenti A., Molesti E., Maggetti M., Torelli A., Lapini G., Montomoli E. The theory and practice of the viral dose in neutralization assay: Insights on SARS-CoV-2 "doublethink" effect. J. Virol. Methods. 2021;297 doi: 10.1016/j.jviromet.2021.114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Mächler M., Bolker B.M. GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 2017;9(2):378–400. [Google Scholar]
- 33.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 34.R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL 〈https://www.R-project.org/〉.
- 35.Rothen D.A., Krenger P.S., Nonic A., Balke I., Vogt A.S., Chang X., Manenti A., Vedovi F., Resevica G., Walton S.M., Zeltins A., Montomoli E., Vogel M., Bachmann M.F., Mohsen M.O. Intranasal administration of a VLP-based vaccine induces neutralizing antibodies against SARS-CoV-2 and variants of concern. Allergy. 2022;77(8):2446–2458. doi: 10.1111/all.15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czerkinsky C., Holmgren J. Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. Curr. Top. Microbiol. Immunol. 2012;354:1–18. doi: 10.1007/82_2010_112. [DOI] [PubMed] [Google Scholar]
- 37.van Splunter M., van Hoffen E., Floris-Vollenbroek E.G., Timmerman H., de Bos E.L., Meijer B., Ulfman L.H., Witteman B., Wells J.M., Brugman S., Savelkoul H.F.J., van Neerven R.J.J. Oral cholera vaccination promotes homing of IgA+ memory B cells to the large intestine and the respiratory tract. Mucosal Immunol. 2018;11(4):1254–1264. doi: 10.1038/s41385-018-0006-7. [DOI] [PubMed] [Google Scholar]
- 38.Pishesha N., Harmand T.J., Ploegh H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022 doi: 10.1038/s41577-022-00707-2. [DOI] [PubMed] [Google Scholar]
- 39.Song Y., Wang Y., Thakur R., Meidan V.M., Michniak B. Mucosal drug delivery: membranes, methodologies, and applications. Crit. Rev. Ther. Drug Carr. Syst. 2004;21(3):195–256. doi: 10.1615/critrevtherdrugcarriersyst.v21.i3.20. [DOI] [PubMed] [Google Scholar]
- 40.Hua S. Physiological and pharmaceutical considerations for rectal drug formulations. Front. Pharmacol. 2019;10:1196. doi: 10.3389/fphar.2019.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that support the findings of this study are included in this published article (and its supplementary information files).