Abstract
Diagnostic techniques for spinal pathologies have been developed in accordance with advances in technology. Accurate diagnosis of spinal pathology is essential for appropriate management of spinal diseases. Since the development of X-rays in 1895 and computed tomography (CT) in 1967, several diagnostic imaging modalities have been utilized for detecting spinal pathologies, including radiography, CT, magnetic resonance imaging, and radionuclide imaging. In addition to diagnostic imaging technologies, electrodiagnostic tests, including electromyography and nerve conduction studies, play a significant role as diagnostic tools, as spinal diseases are mostly profoundly associated with pathologies of the neural structures, such as the spinal cord and nerve root, and extent of injury at the structure cannot be adequately detected by conventional imaging techniques. In patient-specific treatment strategies, usage of diagnostic modalities is of great importance; thus, we should be aware of the basic details and approaches of the different diagnostic modalities. In this review, the authors discuss the details of the technologies that aid in the diagnosis of spinal pathologies.
Keywords: Spinal diseases, Diagnosis, Images, Electrodiagnostic study
Introduction
The number of patients with spinal disease has gradually increased over time, and the burden related to spinal disease is challenging from economic, social, and others. Similar to other pathologies, the management and prognosis of spinal disease are profoundly linked to accurate diagnosis [1].
With advances in technology, diagnostic techniques have been improved for the accurate diagnosis of spinal pathologies. Since the development of X-rays in 1895, bony structures of the spine have been confirmed, leading to the development of diagnostic approaches using imaging modalities. Although radiography technology has further developed, much of the information about the spine, particularly disc, spinal cord, and root pathologies, cannot be identified due to the inherent limitations of radiography. In 1967, Sir Godfrey Hounsfield invented the first computed tomography (CT) scanner using X-ray technology [2]; in 1977, Raymond Damadian developed the first magnetic resonance imaging (MRI) machine for clinical use in human diseases [3]. The development of these novel technologies had a significant impact on the clinical diagnosis of all human diseases and the establishment of therapeutic guidelines. However, despite these novel developments in diagnostic imaging technologies, there exists paucity in the field of spinal disease diagnosis, as the spinal pathologies are profoundly connected to neural structures, including the spinal cord, cauda equina, and nerve roots, and the extent of injury at the neural structure cannot be detected by conventional imaging techniques. Thus, electrodiagnostic modalities, including electromyography (EMG) and nerve conduction studies (NCSs), have been utilized as spinal diagnostic techniques [4]. In addition to these technologies, modalities, including bone scans, have been developed to better define spinal pathologies.
Owing to these novel techniques, more accurate diagnosis and patient-specific treatment can be offered for spinal diseases. In this review, we provided detailed information about the technologies that aid in the diagnosis of spinal pathologies.
Imaging Modalities
1. Plain radiography
Since the discovery and application of X-rays by Wilhelm Conrad Roentgen in 1895, they have been used as a firstline modality for diseases of human tissues based on osseous anatomy [5]. In spinal pathology, X-rays rapidly became widely used as an invaluable diagnostic tool in spinal imaging. With the development of modern radiographic technologies, advanced imaging modalities have been introduced. Nevertheless, plain radiograph remains the first and most widely used imaging modality for localizing suspected spinal symptoms, including pain, numbness, and weakness [6]. Radiography aids intuitive recognition of the patient’s condition since it provides overall information about each spinal segment and the entire vertebral column in a few images [7]. Notably, imaging is relatively artifact-free compared with other modalities considering that it is achieved in a very short time.
Plain radiography has a comparative advantage over complex modern imaging techniques, as it is possible to acquire images while freely adjusting the patient’s position. Beyond static imaging, it has the advantage of being able to obtain an image wherein the actual movement of the patient is performed while applying a dynamic factor, such as gravity (standing radiograph) and motion (flexion-extension and bending radiographs). Flexionextension lateral radiograph-based dynamic imaging can confirm the presence of instability and determine the dynamics of the spinal lesion [8-13]. In preoperative evaluation and surgical planning, dynamic radiography provides information, such as the effects of gravity, spinal mobility, and instability. Recently, imaging techniques, including bending X-rays [14,15] and prone-traction radiography [16], have been introduced to predict motion in an operative position before surgery. Although dynamic plain radiography is available, including gravity and dynamic elements, it does remain a static image. At the moment of filming, the patient must temporarily stop moving and remain stationary in one position.
As a diagnostic modality, the most serious limitation of plain radiography is the difficulty in identifying soft tissue structures. Occasionally, internal organ structures, such as the bowel gas, overlap bony structures, making it difficult to identify spinal osseous pathology, which can lead to finding false-positive lesions. When skeletal tissues, such as the rib, humeral head, and iliac crest, exist together around the spine, they can overlap and obscure the corresponding spinal column, making it difficult to identify lesions or cause underestimation.
Radiation hazards are an unavoidable risk in X-ray imaging, and although the cumulative amount is not large, it cannot be excluded from cancer risk [17]. Under specific conditions, such as the first trimester period of pregnancy, X-ray imaging is considered an absolute contraindication as it may directly harm the fetus [18]. Efforts are made to minimize radiation exposure when long-term, continuous, and repeated radiation is essential for several torso pathologies, such as in the evaluation of adolescent scoliosis [17].
2. Computed tomography
CT allows good visualization of spinal pathologies, including compression of neural structures and diseases of laterally situated structures (such as foraminal stenosis) [19-21]. Modern spiral CT with multidetector rows allows for fast and continuous data acquisition within a few seconds [22]. In the diagnosis of spinal diseases, CT is particularly valuable for evaluating osseous structures. Volumetric reconstruction of three-dimensional (3D) images and multiplanar post-image acquisition processing provides excellent visualization of the bony structure of the spinal column and intuitive information pre- and postoperatively [23,24]. In particular, CT is appropriate to explore complex anatomical areas (such as occipito-cervical junctions and sacroiliac regions) or lesions wherein normal anatomy has largely been lost due to congenital anomalies, degenerative processes, or previous surgical treatments (Fig. 1) [25].
Fig. 1.
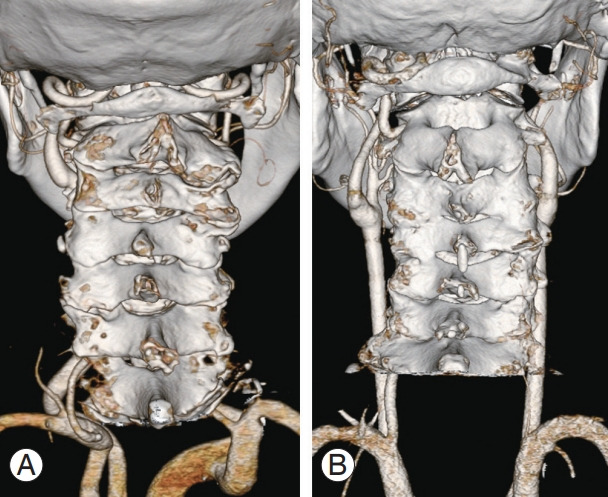
Three-dimensional computed tomography images for preoperative planning. (A) Bilateral vertebral arteries with normal course were observed. (B) Right unilateral vertebral artery without left vertebral artery was detected.
The intrinsic capability of CT in discriminating spinal osseous lesions arises from exceptional sensitivity and specificity to cortical bone abnormalities. Lesions, including cortical discontinuity, thinning, and margin irregularity, can be readily identified on CT. It is useful for identifying skeletal lesions that are very small or unclear on plain radiography [26]. CT provides higher sensitivity than MRI in distinguishing calcified hard disc lesions or ossification of ligamentous structures (such as the posterior longitudinal ligament or ligamentum flavum) [27-29]. Additionally, the evaluator can easily adjust the contrast and window level in the captured image. Subtle soft tissue abnormalities, such as intervertebral disc protrusion, can also be detected through adjustment to a density that can best visualize each soft tissue and bony structure. CT can accurately assess postoperative conditions, including achieving solid spinal fusion, positioning of implanted material, and hardware-related complications [30].
Despite these advantages, CT has inherent limitations in the evaluation of spinal diseases. Since the introduction of CT, cancer risk due to high radiation exposure has been a fundamental challenge [31]. High-dose radiation exposure to patients is required to obtain high-resolution images. Conversely, images of relatively low resolution are derived with low-dose radiation; therefore, there is a possibility that the diagnosis may be missed or misdiagnosed in the case of minute changes in the cortical bone. Distinguishing soft tissues (even with contrast agents) has limitations, particularly between muscles, ligaments, and neural structures around the spine. Spatial artifacts are a challenge, such that the size of the cortical bone appears larger than it is at soft tissue density. When cortical bone diameter/thickness is important, such as in surgical planning for screw placement, a change to bone density setting is recommended. CT images are vulnerable to the formation of metal artifacts from metal foreign bodies in the body, and specific image acquisition and reconstruction are required [32,33]. Particularly, detailed tissue examination may be hindered when previous implantation or foreign bodies containing steel are present around the lesion.
3. Magnetic resonance imaging
MRI is an imaging technology that detects and visualizes changes in the arrangement of hydrogen nuclei (protons) in cells within an artificial magnetic field. Similar to CT, MRI can achieve excellent anatomical and spatial resolution through multiplanar images [34]. Protons are temporarily aligned in one direction when an artificial magnetic field is generated; simultaneously applied radiofrequency pulses disrupt this uniform alignment. The protons attempt to return to their original arrangement when the magnetic field is removed. This shift-back timing varies according to the difference in the number of protons in the tissue, that is, the water content [35,36]. The MRI machine distinguishes shift-back timing (and water content) differences between tissues.
The strength of MRI is that it provides visualization and clear distinction through high-resolution images of individual tissues and organs, particularly soft tissue structures. Spinal diseases affect both the complex bony structure constituting the vertebral column and the diverse soft tissue structures, including ligaments and muscles surrounding the spine, intervertebral discs, and neural structures, including the spinal cord and nerve roots. Therefore, despite its relatively high cost and difficulty in accessibility, MRI, which can visualize both osseous and soft tissues, has become an essential element in the diagnosis of spinal diseases. With the advancement of this technology, the field strength of the superconducting magnets of the MRI machine has gradually increased from 0.15 T initially to 9.4 T at some settings [37]. The standard protocol for spine MRI generally includes axial and sagittal images of T1- and T2-weighted sequences [34]. Depending on the characteristics and phase of the disease, fat suppression T2-weighted images, such as short tau inversion recovery or contrast-enhanced T1 images, are frequently added to sagittal images [34].
T1-weighted images are specialized in providing anatomical details of changes in the bone marrow, osseous structures, discs, and musculotendinous soft tissue [34]. The neural structure is expressed as intermediate signal intensity in the T2-weighted image; however, it provides well-visualized information through perfect contrast with the high signal intensity of the surrounding cerebrospinal fluid [38]. Fat suppression technology provides highly contrasted images for the visualization of pathological structures [39,40].
Similar to CT, intravenous contrast agents can be used in MRI. However, unlike CT, which provides detailed visualization of the vascular structure, gadolinium enhancement used in MRI reduces the relaxation time of molecules around the magnetic field and transmits an image with increased signal intensity of tissue showing hypervascularity. This enhancement appears most clearly at T1 and is used for the diagnosis of epidural fibrous scars, infections, tumors, vascular malformations, or leptomeningeal lesions [41-44].
spine, including the spinal cord, surrounding vessels, and nerve roots [45-47]. Spine MRI is an essential diagnostic tool for evaluating lesions in degenerative diseases, such as spinal stenosis, disc herniation, and myelopathy [47-49]; the vertebrae, ligaments, and spinal cord after trauma [50-52]; and the benign/malignant nature, size, and extent of tumorous lesions [45]. Similar to CT, in the case of acute traumatic osseous lesions, such as fractures, sensitivity, and specificity are high. However, the MRI identification point is different from CT in that it checks for fractures based on signal changes in the bone marrow and cancellous parts rather than direct visualization of the cortical bone. Using signal changes based on bone marrow edema, MRI identifies the presence and location of acute fracture or soft tissue injury and simultaneously distinguishes it from normal tissue without injury. T1-weighted imaging is useful for evaluating the integrity of ligament structures, particularly anterior and posterior longitudinal ligaments and epidural hematomas [53-56]. MRI is also used to diagnose inflammatory conditions, such as multiple sclerosis, sarcoidosis, and transverse myelitis, because it can detect swelling of the spinal cord (acute inflammation) or demyelination (chronic inflammation) [57-59].
Dynamic or axial-loaded MRI was developed to perform MRI in locations that may best reveal pathology. In the diagnosis of cervical myelopathy, dynamic (neck extension) MRI usage is gradually increasing (Fig. 2) [60-63]. Recently, to obtain MRI with gravity added, weightbearing MRI has been introduced in some facilities. It is promising that the pathologic status of the erect spine, including actual weight-bearing, can be reflected in the image [64-67]. However, it is unlikely to replace conventional MRI owing to technically limiting factors, such as lower image resolution [68,69] and longer examination time [67,70]; therefore, it is used as additional or auxiliary imaging technology. Given the cortical bone has sparse free-water content, most signals in MRI are generated by bound water; thus, signal decay is fast, and signal intensity is low [71]. To overcome these shortcomings, technologies, including ultrashort echo time sequences, have been developed to amplify weak cortical bone signals to enhance imaging [72,73].
Fig. 2.
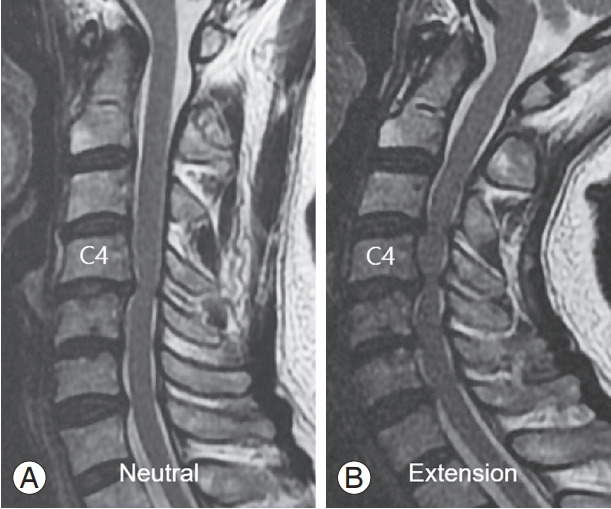
A 63-year-old female patient with degenerative cervical myelopathy. (A) Neutral sagittal magnetic resonance (MR) imaging of the cervical spine showed a C4–5 canal stenosis with signal change of the spinal cord. (B) Extension sagittal MR of the cervical spine showed more aggravated cord compression of C4–5 segment, as well as the cranial and caudal extension of the cervical canal stenosis to C3–7 levels.
Similar to other diagnostic imaging modalities, MRI has drawbacks. Completing an MRI scan takes several minutes, which is longer than the period for completing a CT scan. The increase in magnetic field strength has dramatically improved the MRI resolution; however, this requires more imaging time, thereby causing patient inconvenience and increased possibility of motion artifacts. The possibility of artifact formation based on patient motion is inherent. Moreover, due to interference with magnetic fields, metal artifacts in MRI are formed more extensively than those in CT. In the case of ferrous metal-containing implants, including artificial discs, wires, or screws, extensive artifacts are formed in the area adjacent to the neural structure, thereby obscuring lesions in the adjacent area [74]. There is a possibility that imaging may be impossible or limited due to factors inherent to the patient, such as claustrophobia and the presence of ferromagnetic devices [74,75]. Furthermore, the imaging of tissues with very low water content or thickness, such as the cortical bone and calcified tendon/ligament or cartilage, is not as clearly distinguishable from other soft tissues [76]. For pregnant women, MRI is considered to be a relative contraindication, particularly in the first trimester [77].
Radionuclide Imaging
Bone scans (bone scintigraphy) are one of the primary nuclear medicine tests. Bone scans have high sensitivity and can easily evaluate the bones of the whole body. Generally, this modality scans from the head to the toe using a gamma camera 2–6 hours after intravenous injection with 99mTc-labeled polyphosphonate. However, it includes obtaining a local planar image or a single-photon emission computed tomography (SPECT) image while the gamma camera is fixed. Bone scans are used to evaluate suspicion and extent of metastatic disease, differentiation of fractures and trauma from inflammatory diseases of the skeleton, and evaluation and observation of the prognosis of radiotherapy, benign tumors, arthritis, metabolic bone disease, infection, and other skeletal disorders [78,79].
Although their uptake mechanism is unclear, they are absorbed by chemisorption on the surface of hydroxyapatite by bone metabolism. The degree of accumulation of these drugs depends on biological factors, including bone blood flow and turnover. In the case of a whole-body bone scan, an intravenous injection of a phosphate compound is followed by imaging of the whole-body skeleton or a specific area 2–4 hours later according to the prescribed protocol. The acquired images are 100% and 70% enhanced images; 100% images are used for shallow bone examination, and 70% images are used for thick bones, including the spine. When analyzing the bone condition and vascular distribution of soft tissues is necessary, such as in osteomyelitis, latent fractures, and joint diseases, the radiopharmaceutical is intravenously injected while a simultaneous flow image is obtained for 60 seconds, and a blood pool image is obtained within 10 minutes of injection. After 2–4 hours, a delayed image is obtained by statically capturing the image of the region of interest and evaluated. This image is known as a three-phase bone scan.
Bone scan is superior to X-ray in some circumstances, as it can show abnormal findings with only 3%–5% mineral change, whereas a 30%–50% change is required by other radiological examinations. Since the gamma-ray signal has a linear relationship with lesion severity, it is more useful than MRI in quantitative evaluation.
1. Bone scintigraphy (bone scan)
Radionuclide bone scintigraphy, also called bone scan, is a molecular imaging that is widely used in detecting spinal pathologies. Owing to its high sensitivity, bone scans can detect 3%–5% of bone mineral change; however, its specificity is relatively low. Due to this high sensitivity, it can be used for screening or confirming compression fractures that cannot be detected on plain radiographs. Vertebral compression fractures can be diagnosed using simple radiography, radionuclide bone imaging, and imaging modalities, including MRI. Occult fractures that are not visible on simple radiographs can be diagnosed by MRI or bone scan; however, Zhao et al. [80] found no difference in sensitivity between these two imaging modalities. In the case of MRI, the examination time is long, and the cost is high; therefore, it is considered cost effective to use bone scans for this diagnosis [81,82].
Osteomyelitis and pyogenic arthritis are accompanied by systemic symptoms related to inflammation, which can cause tissue destruction in the bones and joints. However, within 10 days of symptom onset, plain radiographs generally show normal or non-specific findings. Radionuclide imaging is a useful adjunct to MRI, which is the imaging test of choice for spinal infection. Although not useful for detecting soft tissue infections, it is a screening test for spondylodiscitis. Gallium-67 imaging with bone scan increases the specificity of the scan [83,84].
In patients with malignant diseases, prompt diagnosis and appropriate treatment are significant factors for a healthy life. Since most bone metastases are asymptomatic, imaging modalities for early detection have been introduced, with bone scan being the modality of choice [85-88] (Fig. 3). Bone metastasis is often detected late, and serious effects, such as pathologic fracture and cord compression, are highly likely, significantly diminishing patient treatment outcomes and quality of life. Several studies have shown that bone scan sensitivities for bone and vertebral body metastases are high (87% and 84%, respectively) [89,90]. However, when Park et al. [91] considered a bone scan performed on a patient with cervical spinal metastasis, the sensitivity and specificity were 59.1% and 94.6%, respectively. These dissimilar results were attributed to differences in bone metabolism related to the primary cancer type and anatomical characteristics of the cervical spine. Despite some controversial results, the value of a bone scan as a screening test for bone tumors and metastasis is considered high.
Fig. 3.
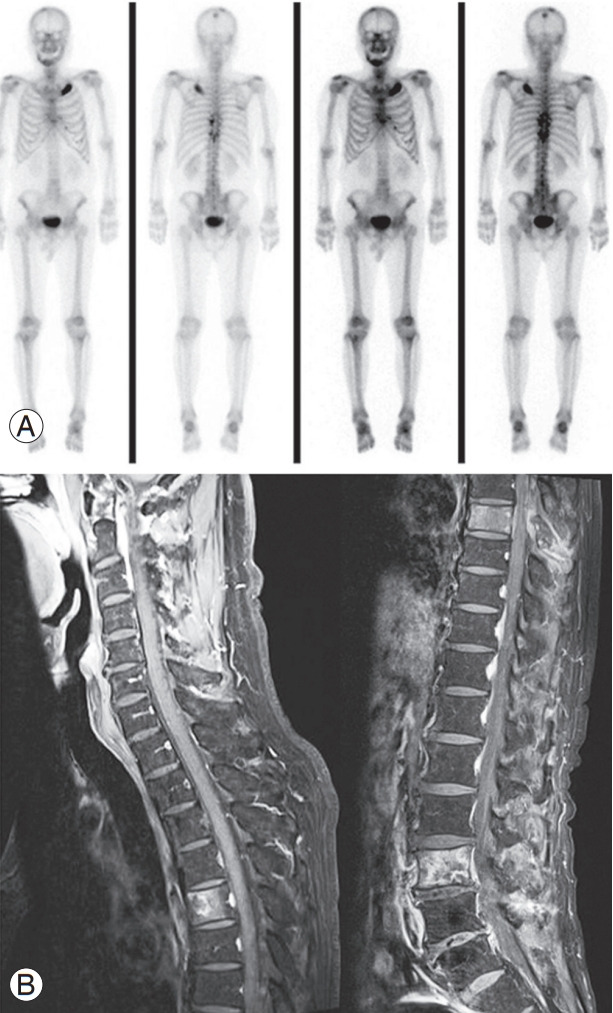
A 75-year-old male patient was admitted to the emergency room with lower motor weakness and sensory change that gradually developed and was diagnosed with T7–9 epidural space mass lesion and prostate cancer multiple metastasis. (A) A preoperative whole-body bone scan showed hot-uptake of the skull, mandible, multiple ribs, T7–11, and L4–5, suggesting metastasis. (B) In the preoperative enhanced magnetic resonance image, metastasis was confirmed in the T5, T7, and L3 vertebral bodies, and T7–9 cord compression was seen.
Imaging tests are significant for diagnosing rheumatoid arthritis and confirming the course of disease. Although simple imaging is widely used, plain radiographs have limitations in early diagnosis, whereas bone scans examine the whole body to identify early disease. Diagnosis of inflammatory diseases, such as rheumatoid arthritis, ankylosing spondylitis, and Reiter syndrome, is important as they accompany sacroiliac arthritis. To quantitatively evaluate sacroiliac joint inflammation, the sacroiliac joint/scrum ratio is computed based on the central portion of the sacrum in a bone scan. Sacroiliac arthritis is suspected if the value is 1.15 or more, and diagnosis is positive if it is 1.2 or more [78]. Gheita et al. [92] suggested that bone scans are useful for detecting disease activity and peripheral arthritis in patients with subclinical axial seronegative spondyloarthritis. Currently, bone scintigraphy of the spine is widely used.
2. Single-photon emission computed tomography/computed tomography
SPECT/CT is a fusion of CT imaging and SPECT, which acquires an image by rotating a gamma camera around the patient using radioactive isotopes to reconstruct a cross-sectional image. SPECT/CT complements SPECT, which has poor spatial resolution, to increase diagnostic specificity, and enable quantitative measurements [93,94]. Horger et al. [95] found that SPECT/CT was 85% more accurate than bone scan alone. Accuracy increases in areas with complex anatomical structures, such as axial skeletal structures, where planar evaluation is difficult. Arthritis causes back pain when it occurs in the facet joint of the spine; however, it is difficult to distinguish early due to slight changes using plain radiography. Using SPECT, osteoarthritis is diagnosed by confirming an increase in intake in the facet joint with a diagnostic sensitivity and specificity of 85%–100% and 71%, respectively; SPECT/CT can be used to localize the uptake site, which results in more accurate diagnostic results [96-99].
SPECT/CT is a highly recommended diagnostic tool for specific spinal pathologies, including spondylolysis, occult fracture, infection, and malignancy. While bone scans have high diagnostic sensitivity, SPECT/CT can evaluate the spine as a 3D tomography image; therefore, it can have a higher diagnostic accuracy than bone scans [100,101]. Studies have shown that SPECT/CT can be a useful diagnostic method for detecting spinal diseases, including malignant tumors, active phases of arthritis, minor trauma, infections, pseudoarthrosis, and postoperative pain caused by minor factors [102,103].
Electrodiagnostic Studies
1. Central motor conduction time study
Central motor conduction time (CMCT) studies evaluate the state of the corticospinal tract in the brain or spinal cord based on motor evoked potentials (MEPs) (Fig. 4A). Generally, the values for CMCTs are recorded from the bilateral biceps brachii (BB), abductor pollicis brevis (APB), and tibialis anterior (TA) muscles. Electrodes are attached to the selected muscles to induce an MEP. Magnetic stimulation is initiated over the primary motor cortex and delivered to the muscles on the contralateral side of the stimulated cortex by inducing muscle contraction. Muscle contractions are recorded as MEPs, and the latency is calculated; CMCT is estimated by subtracting the latency of nerve conduction between the spinal nerve root around the intervertebral foramen and the muscle, where an electrode was attached, from the latency of nerve conduction from the cerebral cortex to the muscle via the corticospinal tract. The cut-off values are 8.5, 9.2, and 18.1 ms for BB-CMCT, APB-CMCT, and TA-CMCT, respectively [104].
Fig. 4.
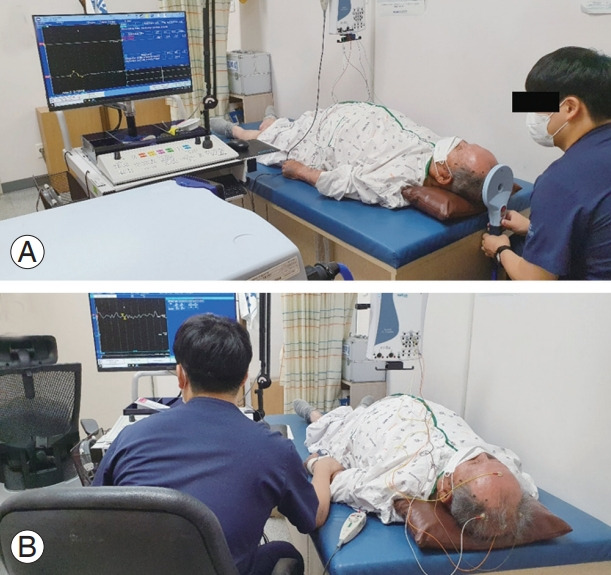
Assessment of central motor conduction time (A) and sensory evoked potential (B).
2. Sensory evoked potential study
Sensory evoked potential (SEP) studies evaluate the state of the sensory tract in the brain or spinal cord. The SEPs of the bilateral median and posterior tibial (PT) nerves are generally assessed (Fig. 4B); SEPs of median nerve stimulation at the wrist (N19, P23) and PT nerve at the ankle (N37, N45) are recorded on the scalp overlying the primary sensory area (median nerve: C3’ and C4,’ PT nerve:Cz’) in the parietal lobe contralateral to the stimulated side. The cut-off values are 21.0 (N19) and 24.6 ms (P23) for median-SEP and 43.4 (P37) and 51.1 ms (N45) for PTSEP [104].
3. Nerve conduction study and electromyography
NCS and EMG evaluate the state of peripheral nerves and muscles [105,106]; the information on the degree and site of nerve injury can be obtained. Using EMG, the presence of muscle pathology can be investigated. In NCS, electrical stimulation is delivered to the nerves by the stimulating electrode, while the recording electrode attached to specific sites on the nerves receives electrical activity [105]. Subsequently, the latency for the muscles to contract in response to the nerve electrical stimulation, conduction velocity, and amplitude are assessed. EMG evaluates the electro-physiological condition of a muscle by the insertion of a thin needle electrode directly into the muscle tissue [107]. Electrophysiological alterations occur in a muscle if nerve or muscle damage is present. In spinal disorders, NCS/EMG can be used to evaluate the presence and level of radiculopathy. Before spinal surgery, NCS/EMG is used for the differentiation of other possible unexpected disorders, including motor neuron disease, Guillain–Barré syndrome, and peripheral demyelinating disease [108,109]. Therefore, NCS/EMG can prevent unnecessary spinal surgery by accurately diagnosing the lesions causing neurological symptoms.
Conclusions
Accurate diagnosis and patient-specific treatment strategies for spinal diseases are of paramount importance although remain challenging despite the advancement of diagnostic technologies. In recent years, these technologies have significantly improved, allowing for greater accuracy and reliability to be achieved in diagnosing spinal pathologies. Diagnostic technologies, such as X-rays, CT, MRI, and electrodiagnostic tests, can aid in appropriate patient management and accurately determine prognosis. Therefore, to determine the best approach for each patient, clinicians should be aware of the essential diagnostic modalities for the spine.
Footnotes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: GWL; data curation: GUK, GWL; formal analysis: GWL; funding acquisition: GWL; methodology: GUK, GWL; project administration: GWL; visualization: WTP, MCC; writing–original draft: GUK, WTP, MCC, GWL; and writing–review & editing: GWL.
References
- 1.Kim GU, Chang MC, Kim TU, Lee GW. Diagnostic modality in spine disease: a review. Asian Spine J. 2020;14:910–20. doi: 10.31616/asj.2020.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya KB. Godfrey Newbold Hounsfield (1919-2004): the man who revolutionized neuroimaging. Ann Indian Acad Neurol. 2016;19:448–50. doi: 10.4103/0972-2327.194414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young IR. Significant events in the development of MRI. J Magn Reson Imaging. 2004;20:183–6. doi: 10.1002/jmri.20123. [DOI] [PubMed] [Google Scholar]
- 4.Kazamel M, Warren PP. History of electromyography and nerve conduction studies: a tribute to the founding fathers. J Clin Neurosci. 2017;43:54–60. doi: 10.1016/j.jocn.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Dewing SB. Modern radiology in historical perspective. Springfield (IL): Charles C. Thomas;; 1962. [Google Scholar]
- 6.Choi BW, Choi MS, Chang H. Radiological assessment of the effects of anterior cervical discectomy and fusion on distraction of the posterior ligamentum flavum in patients with degenerative cervical spines. Clin Orthop Surg. 2021;13:499–504. doi: 10.4055/cios20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon MS, Choi WR, Lim HG, Lee SY, Wi SM. Pavlov’s ratio of the cervical spine in a Korean population: a comparative study by age in patients with minor trauma without neurologic symptoms. Clin Orthop Surg. 2021;13:71–5. doi: 10.4055/cios19174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leone A, Guglielmi G, Cassar-Pullicino VN, Bonomo L. Lumbar intervertebral instability: a review. Radiology. 2007;245:62–77. doi: 10.1148/radiol.2451051359. [DOI] [PubMed] [Google Scholar]
- 9.Yao G, Cheung JP, Shigematsu H, et al. Characterization and predictive value of segmental curve flexibility in adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2017;42:1622–8. doi: 10.1097/BRS.0000000000002046. [DOI] [PubMed] [Google Scholar]
- 10.Wood KB, Popp CA, Transfeldt EE, Geissele AE. Radiographic evaluation of instability in spondylolisthesis. Spine (Phila Pa 1976) 1994;19:1697–703. doi: 10.1097/00007632-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Matsuyama Y. Surgical treatment for adult spinal deformity: conceptual approach and surgical strategy. Spine Surg Relat Res. 2017;1:56–60. doi: 10.22603/ssrr.1.2016-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J. 2010;19:1824–36. doi: 10.1007/s00586-010-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon MS, Choi WR, Lim HG, Jeon SM, Yu CG. Effect of congenital C4-5 synostosis on adjacent mobile segments: radiographic assessment. Asian Spine J. 2021;15:139–42. doi: 10.31616/asj.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki S, Shigematsu H, Tanaka M, et al. Segmental flexibility in adolescent idiopathic scoliosis assessed using the fulcrum-bending radiography method. Clin Spine Surg. 2020;33:E376–80. doi: 10.1097/BSD.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 15.Mishra N, Ramlan A, Tang KH, et al. A novel technique to achieve maximal bending in flexibility assessment by slot-scanning digital radiography in scoliosis: the new gold standard? Eur J Radiol. 2021;141:109805. doi: 10.1016/j.ejrad.2021.109805. [DOI] [PubMed] [Google Scholar]
- 16.Cheung JP, Fong HK, Cheung PW. Predicting spondylolisthesis correction with prone traction radiographs. Bone Joint J. 2020;102-B:1062–71. doi: 10.1302/0301-620X.102B8.BJJ-2020-0528.R1. [DOI] [PubMed] [Google Scholar]
- 17.Luan FJ, Zhang J, Mak KC, Liu ZH, Wang HQ. Low radiation X-rays: benefiting people globally by reducing cancer risks. Int J Med Sci. 2021;18:73–80. doi: 10.7150/ijms.48050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matzon JL, Lutsky KF, Ricci EK, Beredjiklian PK. Considerations in the radiologic evaluation of the pregnant orthopaedic patient. J Am Acad Orthop Surg. 2015;23:485–91. doi: 10.5435/JAAOS-D-14-00274. [DOI] [PubMed] [Google Scholar]
- 19.Jahnke RW, Hart BL. Cervical stenosis, spondylosis, and herniated disc disease. Radiol Clin North Am. 1991;29:777–91. [PubMed] [Google Scholar]
- 20.Landman JA, Hoffman JC, Jr, Braun IF, Barrow DL. Value of computed tomographic myelography in the recognition of cervical herniated disk. AJNR Am J Neuroradiol. 1984;5:391–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Simon JE, Lukin RR. Diskogenic disease of the cervical spine. Semin Roentgenol. 1988;23:118–24. doi: 10.1016/s0037-198x(88)80008-7. [DOI] [PubMed] [Google Scholar]
- 22.Chawla S. Multidetector computed tomography imaging of the spine. J Comput Assist Tomogr. 2004;28 Suppl 1:S28–31. doi: 10.1097/01.rct.0000120853.80935.a8. [DOI] [PubMed] [Google Scholar]
- 23.Blackmore CC, Mann FA, Wilson AJ. Helical CT in the primary trauma evaluation of the cervical spine: an evidence-based approach. Skeletal Radiol. 2000;29:632–9. doi: 10.1007/s002560000270. [DOI] [PubMed] [Google Scholar]
- 24.Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58:902–5. doi: 10.1097/01.ta.0000162138.36519.2a. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Choi DY, Shin MH, Kim JT, Kim IS, Hong JT. Anatomical feasibility for safe occipital condyle screw fixation. Eur Spine J. 2016;25:1674–82. doi: 10.1007/s00586-016-4399-2. [DOI] [PubMed] [Google Scholar]
- 26.Newton PO, Hahn GW, Fricka KB, Wenger DR. Utility of three-dimensional and multiplanar reformatted computed tomography for evaluation of pediatric congenital spine abnormalities. Spine (Phila Pa 1976) 2002;27:844–50. doi: 10.1097/00007632-200204150-00012. [DOI] [PubMed] [Google Scholar]
- 27.Firooznia H, Rafii M, Golimbu C, Tyler I, Benjamin VM, Pinto RS. Computed tomography of calcification and ossification of posterior longitudinal ligament of the spine. J Comput Tomogr. 1984;8:317–24. doi: 10.1016/0149-936x(84)90082-1. [DOI] [PubMed] [Google Scholar]
- 28.Izumi T, Hirano T, Watanabe K, Sano A, Ito T, Endo N. Three-dimensional evaluation of volume change in ossification of the posterior longitudinal ligament of the cervical spine using computed tomography. Eur Spine J. 2013;22:2569–74. doi: 10.1007/s00586-013-2989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura S, Nagoshi N, Iwanami A, et al. Prevalence and distribution of diffuse idiopathic skeletal hyperostosis on whole-spine computed tomography in patients with cervical ossification of the posterior longitudinal ligament: a multicenter study. Clin Spine Surg. 2018;31:E460–5. doi: 10.1097/BSD.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 30.Ghodasara N, Yi PH, Clark K, Fishman EK, Farshad M, Fritz J. Postoperative spinal CT: what the radiologist needs to know. Radiographics. 2019;39:1840–61. doi: 10.1148/rg.2019190050. [DOI] [PubMed] [Google Scholar]
- 31.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–7. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeom JS, Chung MS, Lee CK, Kim Y, Kim N, Lee JB. Evaluation of pedicle screw position on computerized tomography scans: technical note. J Neurosurg. 2003;98(1 Suppl):104–9. doi: 10.3171/spi.2003.98.1.0104. [DOI] [PubMed] [Google Scholar]
- 33.Wellenberg RH, Hakvoort ET, Slump CH, Boomsma MF, Maas M, Streekstra GJ. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur J Radiol. 2018;107:60–9. doi: 10.1016/j.ejrad.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Hartley KG, Damon BM, Patterson GT, Long JH, Holt GE. MRI techniques: a review and update for the orthopaedic surgeon. J Am Acad Orthop Surg. 2012;20:775–87. doi: 10.5435/JAAOS-20-12-775. [DOI] [PubMed] [Google Scholar]
- 35.Hansen BB. Introducing standing weight-bearing MRI in the diagnostics of low back pain and degenerative spinal disorders. Dan Med J. 2017;64:B5416. [PubMed] [Google Scholar]
- 36.Berger A. Magnetic resonance imaging. BMJ. 2002;324:35. doi: 10.1136/bmj.324.7328.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edelman RR. The history of MR imaging as seen through the pages of radiology. Radiology. 2014;273(2 Suppl):S181–200. doi: 10.1148/radiol.14140706. [DOI] [PubMed] [Google Scholar]
- 38.Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–49. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delfaut EM, Beltran J, Johnson G, Rousseau J, Marchandise X, Cotten A. Fat suppression in MR imaging: techniques and pitfalls. Radiographics. 1999;19:373–82. doi: 10.1148/radiographics.19.2.g99mr03373. [DOI] [PubMed] [Google Scholar]
- 40.Alyas F, Saifuddin A, Connell D. MR imaging evaluation of the bone marrow and marrow infiltrative disorders of the lumbar spine. Magn Reson Imaging Clin N Am. 2007;15:199–219. doi: 10.1016/j.mric.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Rubin JB, Enzmann DR, Wright A. CSF-gated MR imaging of the spine: theory and clinical implementation. Radiology. 1987;163:784–92. doi: 10.1148/radiology.163.3.3575733. [DOI] [PubMed] [Google Scholar]
- 42.Taber KH, Herrick RC, Weathers SW, Kumar AJ, Schomer DF, Hayman LA. Pitfalls and artifacts encountered in clinical MR imaging of the spine. Radiographics. 1998;18:1499–521. doi: 10.1148/radiographics.18.6.9821197. [DOI] [PubMed] [Google Scholar]
- 43.Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–66. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 44.Haughton V, Schreibman K, De Smet A. Contrast between scar and recurrent herniated disk on contrastenhanced MR images. AJNR Am J Neuroradiol. 2002;23:1652–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley WG, Jr, Waluch V, Yadley RA, Wycoff RR. Comparison of CT and MR in 400 patients with suspected disease of the brain and cervical spinal cord. Radiology. 1984;152:695–702. doi: 10.1148/radiology.152.3.6463251. [DOI] [PubMed] [Google Scholar]
- 46.Pfirrmann CW, Dora C, Schmid MR, Zanetti M, Hodler J, Boos N. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004;230:583–8. doi: 10.1148/radiol.2302021289. [DOI] [PubMed] [Google Scholar]
- 47.Lee GY, Lee JW, Choi HS, Oh KJ, Kang HS. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40:1033–9. doi: 10.1007/s00256-011-1102-x. [DOI] [PubMed] [Google Scholar]
- 48.Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194:1095–8. doi: 10.2214/AJR.09.2772. [DOI] [PubMed] [Google Scholar]
- 49.Park HJ, Kim JH, Lee JW, et al. Clinical correlation of a new and practical magnetic resonance grading system for cervical foraminal stenosis assessment. Acta Radiol. 2015;56:727–32. doi: 10.1177/0284185114537929. [DOI] [PubMed] [Google Scholar]
- 50.Kaniewska M, de Beus JM, Ahlhelm F, et al. Whole spine localizers of magnetic resonance imaging detect unexpected vertebral fractures. Acta Radiol. 2019;60:742–8. doi: 10.1177/0284185118796673. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Fintelmann FJ, Kamath RS, Kattapuram SV, Rosenthal DI. Limited magnetic resonance imaging of the lumbar spine has high sensitivity for detection of acute fractures, infection, and malignancy. Skeletal Radiol. 2016;45:1687–93. doi: 10.1007/s00256-016-2493-5. [DOI] [PubMed] [Google Scholar]
- 52.Williams RL, Hardman JA, Lyons K. MR imaging of suspected acute spinal instability. Injury. 1998;29:109–13. doi: 10.1016/s0020-1383(97)00148-4. [DOI] [PubMed] [Google Scholar]
- 53.Ricart PA, Verma R, Fineberg SJ, et al. Post-traumatic cervical spine epidural hematoma: incidence and risk factors. Injury. 2017;48:2529–33. doi: 10.1016/j.injury.2017.08.060. [DOI] [PubMed] [Google Scholar]
- 54.Kumar Y, Hayashi D. Role of magnetic resonance imaging in acute spinal trauma: a pictorial review. BMC Musculoskelet Disord. 2016;17:310. doi: 10.1186/s12891-016-1169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan J, Shen L, Fang L, et al. Correlations between posterior longitudinal injury and parameters of vertebral body damage. J Surg Res. 2015;199:552–6. doi: 10.1016/j.jss.2015.04.068. [DOI] [PubMed] [Google Scholar]
- 56.Henninger B, Kaser V, Ostermann S, et al. Cervical disc and ligamentous injury in hyperextension trauma: MRI and intraoperative correlation. J Neuroimaging. 2020;30:104–9. doi: 10.1111/jon.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeSanto J, Ross JS. Spine infection/inflammation. Radiol Clin North Am. 2011;49:105–27. doi: 10.1016/j.rcl.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Cadiou S, Robin F, Guillin R, et al. Spondyloarthritis and sarcoidosis: related or fake friends?: a systematic literature review. Joint Bone Spine. 2020;87:579–87. doi: 10.1016/j.jbspin.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Philpott C, Brotchie P. Comparison of MRI sequences for evaluation of multiple sclerosis of the cervical spinal cord at 3 T. Eur J Radiol. 2011;80:780–5. doi: 10.1016/j.ejrad.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 60.Chen CJ, Hsu HL, Niu CC, et al. Cervical degenerative disease at flexion-extension MR imaging: prediction criteria. Radiology. 2003;227:136–42. doi: 10.1148/radiol.2271020116. [DOI] [PubMed] [Google Scholar]
- 61.Guppy KH, Hawk M, Chakrabarti I, Banerjee A. The use of flexion-extension magnetic resonance imaging for evaluating signal intensity changes of the cervical spinal cord. J Neurosurg Spine. 2009;10:366–73. doi: 10.3171/2009.1.SPINE08567. [DOI] [PubMed] [Google Scholar]
- 62.Harada T, Tsuji Y, Mikami Y, et al. The clinical usefulness of preoperative dynamic MRI to select decompression levels for cervical spondylotic myelopathy. Magn Reson Imaging. 2010;28:820–5. doi: 10.1016/j.mri.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Zeitoun D, Rangel A, Lazennec JY, Catonne Y, Pascal-Moussellard H. Preoperative evaluation of the cervical spondylotic myelopathy with flexionextension magnetic resonance imaging: about a prospective study of fifty patients. Spine (Phila Pa 1976) 2011;36:E1134–9. doi: 10.1097/BRS.0b013e3181f822c7. [DOI] [PubMed] [Google Scholar]
- 64.Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679–86. doi: 10.1007/s00586-009-0919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weishaupt D, Schmid MR, Zanetti M, et al. Positional MR imaging of the lumbar spine: does it demonstrate nerve root compromise not visible at conventional MR imaging? Radiology. 2000;215:247–53. doi: 10.1148/radiology.215.1.r00ap06247. [DOI] [PubMed] [Google Scholar]
- 66.Mataki K, Koda M, Shibao Y, et al. Successful visualization of dynamic change of lumbar nerve root compression with the patient in both upright and prone positions using dynamic digital tomosynthesis-radiculography in patients with lumbar foraminal stenosis: an initial report of three cases. J Clin Neurosci. 2019;62:256–9. doi: 10.1016/j.jocn.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 67.Lee RK, Griffith JF, Lau YY, et al. Diagnostic capability of low- versus high-field magnetic resonance imaging for lumbar degenerative disease. Spine (Phila Pa 1976) 2015;40:382–91. doi: 10.1097/BRS.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 68.Tarantino U, Fanucci E, Iundusi R, et al. Lumbar spine MRI in upright position for diagnosing acute and chronic low back pain: statistical analysis of morphological changes. J Orthop Traumatol. 2013;14:15–22. doi: 10.1007/s10195-012-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alyas F, Connell D, Saifuddin A. Upright positional MRI of the lumbar spine. Clin Radiol. 2008;63:1035–48. doi: 10.1016/j.crad.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 70.Gilbert JW, Wheeler GR, Lingreen RA, Johnson RR. Open stand-up MRI: a new instrument for positional neuroimaging. J Spinal Disord Tech. 2006;19:151–4. doi: 10.1097/01.bsd.0000188665.54014.8d. [DOI] [PubMed] [Google Scholar]
- 71.Florkow MC, Willemsen K, Mascarenhas VV, Oei EH, van Stralen M, Seevinck PR. Magnetic resonance imaging versus computed tomography for three-dimensional bone imaging of musculoskeletal pathologies: a review. J Magn Reson Imaging. 2022;56:11–34. doi: 10.1002/jmri.28067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010;207:304–11. doi: 10.1016/j.jmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Bae WC, Chen PC, Chung CB, Masuda K, D’Lima D, Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27:848–57. doi: 10.1002/jbmr.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudisch A, Kremser C, Peer S, Kathrein A, Judmaier W, Daniaux H. Metallic artifacts in magnetic resonance imaging of patients with spinal fusion: a comparison of implant materials and imaging sequences. Spine (Phila Pa 1976) 1998;23:692–9. doi: 10.1097/00007632-199803150-00009. [DOI] [PubMed] [Google Scholar]
- 75.Raj V, O’Dwyer R, Pathmanathan R, Vaidhyanath R. MRI and cardiac pacing devices: beware the rules are changing. Br J Radiol. 2011;84:857–9. doi: 10.1259/bjr/22160941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong L, Zeng QY, Jinkins JR. CT and MRI characteristics of ossification of the ligamenta flava in the thoracic spine. Eur Radiol. 2001;11:1798–802. doi: 10.1007/s003300000788. [DOI] [PubMed] [Google Scholar]
- 77.Haldorsen IS, Lura N, Blaakær J, Fischerova D, Werner HM. What is the role of imaging at primary diagnostic work-up in uterine cervical cancer? Curr Oncol Rep. 2019;21:77. doi: 10.1007/s11912-019-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang KW, Kim SE, Lee DS, Jung JK. Koh’s nuclear medicine. 4th ed. Seoul: Korea Medicine;; 2019. [Google Scholar]
- 79.O’Malley JP, Ziessman HA, Thrall JH. Nuclear medicine and molecular imaging: the requisites. 5th ed. Philadelphia (PA): Elsevier Health Sciences; 2020. [Google Scholar]
- 80.Zhao QM, Gu XF, Liu ZT, Cheng L. The value of radionuclide bone imaging in defining fresh fractures among osteoporotic vertebral compression fractures. J Craniofac Surg. 2016;27:745–8. doi: 10.1097/SCS.0000000000002594. [DOI] [PubMed] [Google Scholar]
- 81.Jordan E, Choe D, Miller T, Chamarthy M, Brook A, Freeman LM. Utility of bone scintigraphy to determine the appropriate vertebral augmentation levels. Clin Nucl Med. 2010;35:687–91. doi: 10.1097/RLU.0b013e3181e9fb07. [DOI] [PubMed] [Google Scholar]
- 82.Jun DS, An BK, Yu CH, Hwang KH, Paik JW. Practical use of bone scan in patients with an osteoporotic vertebral compression fracture. J Korean Med Sci. 2015;30:194–8. doi: 10.3346/jkms.2015.30.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minoves Font M. Clinical applications of nuclear medicine in the diagnosis and assessment of musculoskeletal sports injuries. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2020;39:112–34. doi: 10.1016/j.remn.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Love C, Palestro CJ. Nuclear medicine imaging of bone infections. Clin Radiol. 2016;71:632–46. doi: 10.1016/j.crad.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Gosfield E, 3rd, Alavi A, Kneeland B. Comparison of radionuclide bone scans and magnetic resonance imaging in detecting spinal metastases. J Nucl Med. 1993;34:2191–8. [PubMed] [Google Scholar]
- 86.Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–14. doi: 10.1016/S1470-2045(09)70088-9. [DOI] [PubMed] [Google Scholar]
- 87.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 88.Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med. 2001;31:28–49. doi: 10.1053/snuc.2001.18742. [DOI] [PubMed] [Google Scholar]
- 89.Uchida K, Nakajima H, Miyazaki T, et al. (18)F-FDG PET/CT for diagnosis of osteosclerotic and osteolytic vertebral metastatic lesions: comparison with bone scintigraphy. Asian Spine J. 2013;7:96–103. doi: 10.4184/asj.2013.7.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu T, Cheng T, Xu W, Yan WL, Liu J, Yang HL. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40:523–31. doi: 10.1007/s00256-010-0963-8. [DOI] [PubMed] [Google Scholar]
- 91.Park SM, Park JW, Lee HJ, et al. Diagnostic value of Technetium-99m bone scintigraphy in the detection of cervical spine metastases in oncological patients. Spine (Phila Pa 1976) 2017;42:1699–705. doi: 10.1097/BRS.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 92.Gheita TA, Azkalany GS, Kenawy SA, Kandeel AA. Bone scintigraphy in axial seronegative spondyloarthritis patients: role in detection of subclinical peripheral arthritis and disease activity. Int J Rheum Dis. 2015;18:553–9. doi: 10.1111/1756-185X.12527. [DOI] [PubMed] [Google Scholar]
- 93.Horger M, Bares R. The role of single-photon emission computed tomography/computed tomography in benign and malignant bone disease. Semin Nucl Med. 2006;36:286–94. doi: 10.1053/j.semnuclmed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Linke R, Kuwert T, Uder M, Forst R, Wuest W. Skeletal SPECT/CT of the peripheral extremities. AJR Am J Roentgenol. 2010;194:W329–35. doi: 10.2214/AJR.09.3288. [DOI] [PubMed] [Google Scholar]
- 95.Horger M, Eschmann SM, Pfannenberg C, et al. Evaluation of combined transmission and emission tomography for classification of skeletal lesions. AJR Am J Roentgenol. 2004;183:655–61. doi: 10.2214/ajr.183.3.1830655. [DOI] [PubMed] [Google Scholar]
- 96.Gnanasegaran G, Paycha F, Strobel K, et al. Bone SPECT/CT in postoperative spine. Semin Nucl Med. 2018;48:410–24. doi: 10.1053/j.semnuclmed.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Al-Riyami K, Voo S, Gnanasegaran G, et al. The role of bone SPECT/CT in patients with persistent or recurrent lumbar pain following lumbar spine stabilization surgery. Eur J Nucl Med Mol Imaging. 2019;46:989–98. doi: 10.1007/s00259-018-4141-x. [DOI] [PubMed] [Google Scholar]
- 98.Brusko GD, Perez-Roman RJ, Tapamo H, Burks SS, Serafini AN, Wang MY. Preoperative SPECT imaging as a tool for surgical planning in patients with axial neck and back pain. Neurosurg Focus. 2019;47:E19. doi: 10.3171/2019.9.FOCUS19648. [DOI] [PubMed] [Google Scholar]
- 99.Tender GC, Davidson C, Shields J, et al. Primary pain generator identification by CT-SPECT in patients with degenerative spinal disease. Neurosurg Focus. 2019;47:E18. doi: 10.3171/2019.9.FOCUS19608. [DOI] [PubMed] [Google Scholar]
- 100.Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MF. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20:28–33. [PubMed] [Google Scholar]
- 101.Perez-Roman RJ, Brusko GD, Burks SS, Serafini AN, Wang MY. Use of single-photon emission computed tomography imaging for hypermetabolic facet identification in diagnosis of cervical and axial back pain. World Neurosurg. 2020;137:e487–92. doi: 10.1016/j.wneu.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 102.Ryan RJ, Gibson T, Fogelman I. The identification of spinal pathology in chronic low back pain using single photon emission computed tomography. Nucl Med Commun. 1992;13:497–502. doi: 10.1097/00006231-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 103.McDonald M, Cooper R, Wang MY. Use of computed tomography-single-photon emission computed tomography fusion for diagnosing painful facet arthropathy: technical note. Neurosurg Focus. 2007;22:E2. doi: 10.3171/foc.2007.22.1.2. [DOI] [PubMed] [Google Scholar]
- 104.Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic medicine. 2nd ed. Philadelphia (PA): Hanley & Belfus; 2002. [Google Scholar]
- 105.Lee DG, Chang MC. Dorsal scapular nerve injury after trigger point injection into the rhomboid major muscle: a case report. J Back Musculoskelet Rehabil. 2018;31:211–4. doi: 10.3233/BMR-169740. [DOI] [PubMed] [Google Scholar]
- 106.Kwak SY, Boudier-Reveret M, Chang MC. Watch out for slowly progressive weakness of the distal upper limb: it could be chronic acquired demyelinating neuropathy! Ann Palliat Med. 2020;9:1285–7. doi: 10.21037/apm.2020.04.15. [DOI] [PubMed] [Google Scholar]
- 107.Rubin DI. Needle electromyography: basic concepts. Handb Clin Neurol. 2019;160:243–56. doi: 10.1016/B978-0-444-64032-1.00016-3. [DOI] [PubMed] [Google Scholar]
- 108.Chang MC. Missed diagnosis of chronic inflammatory demyelinating polyneuropathy in a patient with cervical myelopathy due to ossification of posterior longitudinal ligament. Neurol Int. 2018;10:7690. doi: 10.4081/ni.2018.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kwak S, Boudier-Reveret M, Cho HK, Chang MC. Multifocal acquired demyelinating sensory and motor neuropathy misdiagnosed as carpal tunnel syndrome: a case report. J Int Med Res. 2021;49:300060521998896. doi: 10.1177/0300060521998896. [DOI] [PMC free article] [PubMed] [Google Scholar]


