Abstract
Study Design
A multicenter cross-sectional analytical retrospective study.
Purpose
To assess functional outcome (FO) after a spinal meningioma (SM) surgery.
Overview of Literature
All studies report functional improvement after SM removal.
Methods
We performed an analytical retrospective cohort study at five different institutions. All patients with a diagnosis of SM were included in this study, including those with recurrent tumors. Meningiomas of the foramen magnum were excluded. Useful histopathological characteristics were separately extracted. Surgical resection was evaluated according to the Simpson grading scale. Patient outcomes and clinical states were assessed with the help of their medical records using four different scales: the modified Ranawat score, the Nurick scale, the Prolo score, the Frankel grade, and the Eastern Cooperative Oncology Group–World Health Organization–Zubrod score.
Results
Between 1991 and 2018, 417 patients were identified, of which 85.8% were female. The median age at surgery was 67.2 years (interquartile range [IQR], 56.7–76.5). The lesion was located in the thoracic region in 77.9% of the patients, cervical region in 16.8%, and lumbar region in 4.1%. Surgical resection was complete in 95.5% of the cases. Only 0.96% of the patients died within the first postoperative month. Neurological status, which improved in 76.9% of the patients, was unchanged in 17.5% and even worsened in 4.4%. Functional status was assessed using the Ranawat score and Nurick scale, with scores of 1 (IQR, 0–2) (i.e., hyperreflexia and asymptomatic; mean, 1.3±1.3) and 1 (IQR, 0–2) (i.e., signs of spinal cord disease, but no difficulty in walking; mean, 1.2±1.4), respectively. Approximately 10.1% of the patients were not ambulant at the last neurosurgical follow-up visit. Older age at surgery was not significantly associated with a chair-bound status (p=0.427).
Conclusions
This large series confirms the favorable FO after spinal meningioma surgery even in the case of seriously impaired preoperative status. A validated scale is needed to assess the factors predicting a worsening of the functional status and guide the management of patients.
Keywords: Spine, Meningioma, Functional outcome
Introduction
Thought to arise from the meningothelial cells of the arachnoid, meningiomas are the most common primary intracranial extracerebral tumors, accounting for 36.8%–37.6% of the tumor cases in the Central Brain Tumor Registry of the United States [1]. Most meningiomas are sporadic, and their incidence is approximately 5/100,000 person-year in France [2,3]. Ionizing radiation, hormonal treatments, and some genetic diseases, such as type 2 neurofibromatosis, are the identified risk factors [4,5].
The 2016 World Health Organization (WHO) classification of tumors affecting the central nervous system recognizes three grades of meningiomas [6]: grade I or benign meningiomas, which are the most common and have usually a good outcome [2,3,7]; grade II, which are atypical or intermediate type [8,9]; and grade III or malignant meningiomas, which are rare and aggressive neoplasms with a poor prognosis [10,11].
The management options include regular monitoring (especially for incidental meningioma), symptom control, surgical excision, irradiation (RT), and chemotherapy (occasionally); however, tailored maximal resection remains the treatment of choice. Further optimal management is difficult to establish, with the role of postoperative RT as standard adjuvant treatment remaining controversial, except for malignant meningiomas [8,9,11].
Meningiomas that developed in the spine are less frequent compared with those that developed intracranially, accounting for approximately 5%–10% of cases [2,12,13]. Meningiomas grow slowly; once they have reached a significant volume, they cause cord and root compression. Depending on their localization, spinal meningiomas (SM) may cause a variety of symptoms, such as pain, sensory and sphincter disturbance, and motor weakness (up to paraplegia). SMs are almost always benign; thus, complete excision should be the goal of the associated surgical therapy, which usually provides a cure for the patients and an improvement in their symptoms.
This study aimed to assess the functional outcome (FO) of patients operated on for a SM.
Materials and Methods
1. Clinical material
We performed a multicenter analytical retrospective cohort study. A retrospective database search was conducted at five different institutions: Queen Elizabeth Hospital (Glasgow, Scotland), Sainte-Anne Hospital (Paris, France), Lariboisière Hospital (Paris, France), Pellegrin Hospital (Bordeaux, France), and Pontchaillou Hospital (Rennes, France). All patients with a diagnosis of SM were included in this study, including those with recurrent tumors. Meningiomas of the foramen magnum were excluded. All pathology reports were carefully examined. Meningioma subtype, mitosis count per 10 high-power fields (mitotic index), Ki-67 index (MIB-1), the presence of necrosis or psammoma, and microscopic pia-mater spinal cord invasion were separately extracted. In cases of recurrence, histology reports were compared with those from previous resections, and histology slides were usually reviewed.
Patients’ demographic and medical data were retrospectively collected. We used radiographic and surgical reports and all available inpatient and outpatient records. Patients’ magnetic resonance imaging (MRI) and/or computed tomography images were studied pre- and postoperatively, if available. Tumor location was divided into four categories: cervical, thoracic, lumbar, and sacral. For statistical convenience, when a tumor was located between two levels, e.g., T10–T11, the lowest one was chosen. Age at diagnosis was defined according to the date of surgery. Surgical resection was evaluated according to the Simpson grading scale using the surgical records [14]. Although Simpson [14] did not include any SM in his original study, the grading system he proposed can also be applied to SM resection; therefore, it was previously used in most of the related studies. We defined total resection (TR) as Simpson grades 1 and 2, and incomplete resection or subtotal resection as Simpson grades 3, 4, and 5. If possible, surgical impression was compared to the postoperative contrast-enhanced images. If radiotherapy was used, data on the technique, overall dose, and time of completion were collected. Radiological relapse was defined as the evidence of tumor regrowth in the case of TR or progression of any residuum in cases of incomplete resections, on the most recent contrast-enhanced MRI scan available. Patient outcome and clinical state were assessed using medical records, patient database of each center, and information obtained from the primary care physicians. As there is no dedicated scale to assess the clinical state of patients with SM, we used four different scales commonly used in spinal pathology evaluation: the modified Ranawat score, the Nurick scale, the Prolo score, the Frankel grade, and the Eastern Cooperative Oncology Group (ECOG)–WHO–Zubrod score [15–18]. Patients were considered to have achieved satisfactory outcomes when they had either no or minimal deficits. We evaluate the functional status before surgery and at the last neurosurgical follow-up.
2. Statistical methods
Continuous variables were reported either as means and standard deviations or as medians and interquartile ranges (IQR); categorical variables are reported as frequencies and proportions. Quantitative variables were categorized according to the median to evaluate their association with the primary outcome. Primary analyses were conducted using available cases (available case analysis). All tests were two-sided, and statistical significance was defined by an alpha level of 0.05 (p<0.05). Analysis was performed using the R programming language and software environment for statistical computing and graphics (R ver. 4.1.1; 2021-08-10; The R Foundation for Statistical Computing, Vienna, Austria) [19]. The statistical program and workflow were written in R Markdown ver. 2.0 with RStudio (RStudio, Boston, MA, USA) for dynamic and reproducible research [20].
3. Compliance with ethical standards
This study was conducted according to the ethical guidelines for epidemiological research of the Helsinki Declaration (2008); French Data Protection Authority (CNIL), an independent national ethical committee (authorization no., 2008538); European General Data Protection Regulation (EU) 2016/679; and French data protection authority. The study was conducted according to the STROBE statement for observational studies in epidemiology and the SAMPL Guidelines [21,22]. The requirement for informed consent was waived due to the retrospective nature of the study.
Results
1. Population description
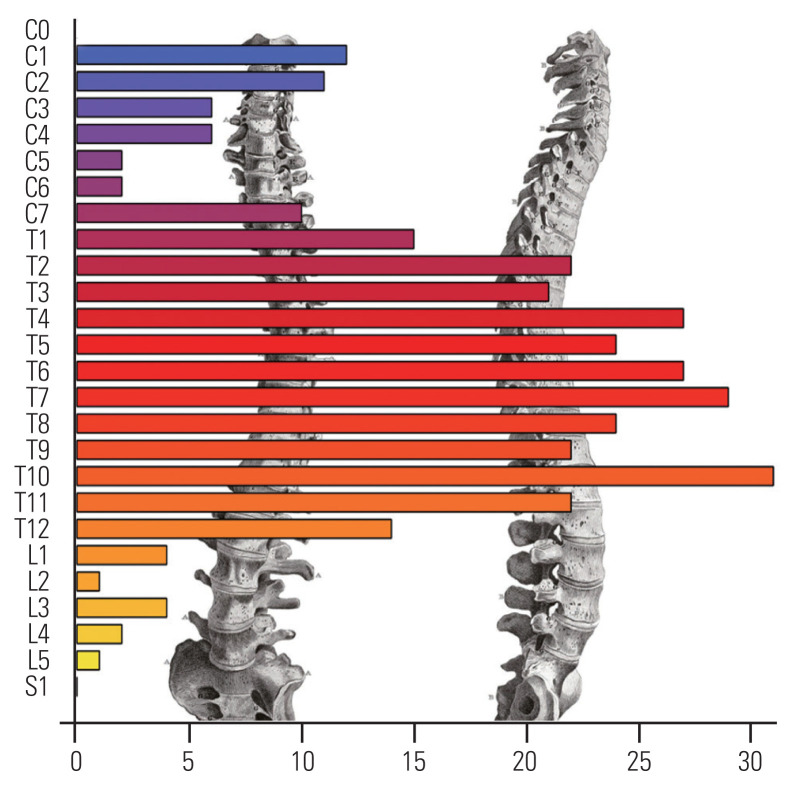
A total of 417 cases of SM that underwent surgery between 1991 and 2018 were included in this study. The majority of the patients were females (85.8%), and the median age at surgery was 67.2 years (IQR, 56.7–76.5). Back and/or limb pain was the most common symptom, occurring in 29.9% of the patients and generally associated with other clinical signs at presentation. Table 1 presents the characteristics of the cohort. The most common location was thoracic in 325 (77.9%) of the cases (Fig. 1).
Table 1.
Characteristics of the cohort (N=417 patients)
| Characteristic | Value |
|---|---|
| Gender male | 59 (14.2) |
| Median age at surgery (yr) | 67.2 (56.7–76.5) |
| Symptoms & clinical signs | |
| Motor and walking impairment | 199 (54.7) |
| Back limb(s) pain | 213 (58.5) |
| Paresthesia, dysesthesia | 115 (31.6) |
| Sphincter disturbance | 48 (13.2) |
| Location | |
| Cervical spine | 70 (16.8) |
| Thoracic spine | 325 (77.9) |
| Lumbar spine | 17 (4.1) |
| Sacrum | 1 (0.2) |
| Side of the meningioma insertion | |
| Right | 140 (38.3) |
| Left | 163 (44.5) |
| Midline | 62 (16.9) |
| Dural insertion | |
| Posterior or posterolateral | 143 (46) |
| Lateral | 71 (22.8) |
| Anterior or anterolateral | 97 (31.2) |
| Tumor volume (cm3) | 1.4 (0.9–2) |
| Resection status | |
| Simpson 1 | 4 (1) |
| Simpson 2 | 382 (94.6) |
| Total resection | 386 (95.5) |
| Simpson 3 | 4 (1) |
| Simpson 4 | 13 (3.2) |
| Simpson 5 | NA |
| Subtotal resection (Simpson 3, 4, & 5) | 17 (4.1) |
| Histological sub-types | |
| Meningothelial meningioma | 128 (32.4) |
| Transitional meningioma | 69 (17.5) |
| Psammomatous meningioma | 138 (34.9) |
| Median mitoses count (/10 HPFs) | 1 (1–1) |
| Median Ki-67 (MIB-1) | 5 (3–6) |
Values are presented as number (%) or median (interquartile range).
NA, not applicable; HPF, high-power field.
Fig. 1.
Count of meningioma by spinal level.
A total of 386 patients (95.5%) underwent a complete resection, i.e., macroscopically complete removal of the tumor and its visible extensions or with coagulation of its dural attachment. The SM repartition did not significantly vary according to gender (p=0.396). There were 24 patients (6.1%) who experienced surgical complications such as cerebrospinal fluid leakage or hematoma. In most cases, a repeat surgery was necessary to solve the problem. Four patients (0.96%) died within the first postoperative month. The median neurosurgical follow-up duration was 1.4 years (IQR, 0.4–4.3).
2. Functional outcome
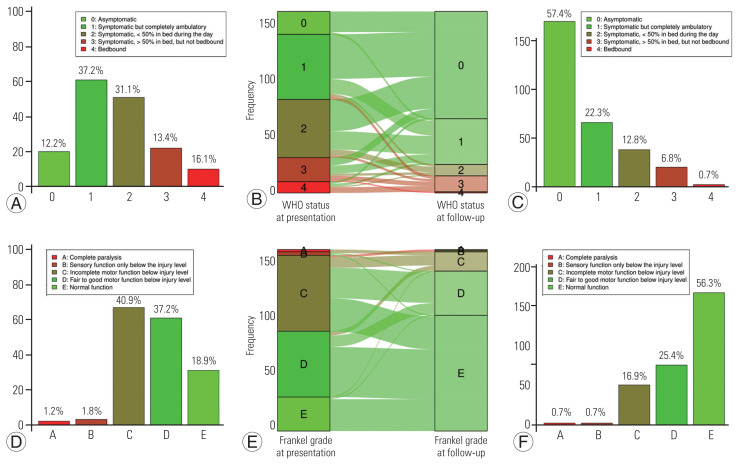
The FO according to each scale used is displayed in Figs. 2 and 3. After surgery, functional status as estimated using the ECOG–WHO–Zubrod score significantly improved from a median score at presentation of 2 (IQR, 1–2; mean±standard deviation [SD], 1.64±1.06) down to 0 (IQR, 0–1; mean±SD, 0.71±0.98) at the last neurosurgical follow-up visit (p<0.001).
Fig. 2.
Functional status at presentation, evolution, and at last neurosurgical follow-up. (A) Functional status at presentation according the Eastern Cooperative Oncology Group (ECOG)–World Health Organization (WHO)–Zubrod score. (B) Alluvial plot of the functional status evolution according the ECOG–WHO–Zubrod score. (C) Functional outcome according the ECOG–WHO–Zubrod score. (D) Functional status at presentation according the Frankel grade. (E) Alluvial plot of the functional status evolution according the Frankel grade. (F) Functional outcome according the Frankel grade.
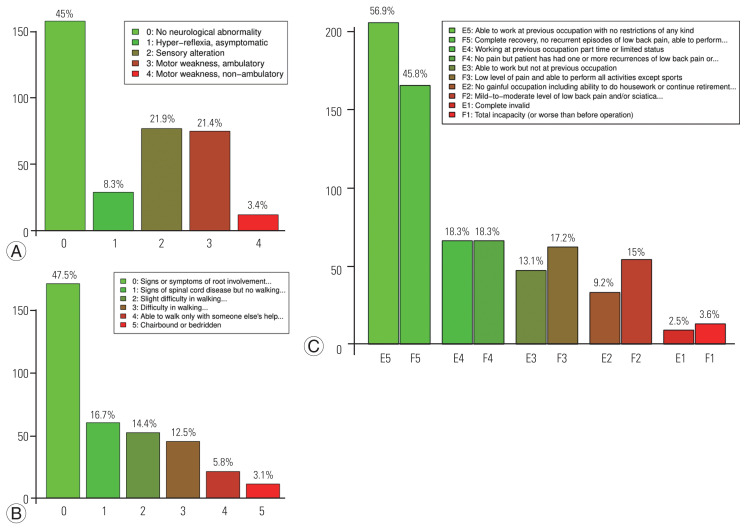
Fig. 3.
Functional status at last neurosurgical follow-up. (A) Functional outcome according the modified Ranawat score. (B) Functional outcome according the Nurick score. (C) Functional outcome according the Prolo scale.
Functional status as assessed using the Frankel grade significantly improved from a median score at presentation of 4 (IQR, 3–4; mean±SD, 3.71±0.84) to 5 (IQR, 4–5; mean±SD, 4.36±0.83) at the last neurosurgical follow-up visit (p<0.001). At the last follow-up visit, the functional status as assessed by the Ranawat score was 1 (IQR, 0–2; i.e., hyperreflexia and asymptomatic; mean±SD, 1.3±1.3); the Nurick score was 1 (IQR, 0–2; i.e., signs of spinal cord disease, but no difficulty in walking; mean±SD, 1.2±1.4) (Fig. 3). Regarding the Prolo score, functional status was 4 at presentation (IQR, 3–5; i.e., one or more episodes of pain recurrences) and 5 after surgery (IQR, 4–5; i.e., the patient can work at previous occupation with no restrictions of any kind). The median total score (ExFx) obtained by adding the scores of each subscale was 9 (IQR, 7–10), which can be rated as excellent (10–9) and/or good (8–7), according to the original study by Prolo et al. [17] (Fig. 3C).
3. Improvement of functional status
According to the ECOG–WHO–Zubrod score, 110 patients (68.8%) had improved symptoms after the removal of the SM versus 105 patients (65.6%) according to the Frankel grade and 123 patients (76.9%) if we consider either one classification or the other (Fig. 2C, E).
4. Unchanged functional status
According to the ECOG–WHO–Zubrod score, 40 patients (25%) had unchanged symptoms after the removal of the SM, of which 77.5% were previously asymptomatic or symptomatic but completely ambulant. Regarding the Frankel grade, 47 patients (29.4%) were ambulant, of which 80.9% previously had a normal, fair, or good motor function. If we correlated these results, 28 patients (17.5%) had a correlated unchanged functional status.
5. Worsening of functional status
According to the ECOG–WHO–Zubrod score, 10 patients (6.2%) had a worsening of their symptoms versus eight patients (5%) according to the Frankel grade and seven patients (4.4%) in correlation. For two patients (1.2%), the FO results were discordant. There were 42 patients (10.1%) who were not considered ambulant at the last neurosurgical follow-up visit; all were previously not able to walk independently. Older age was not significantly associated with a chair-bound status after SM surgery (p=0.427).
6. Correlation between the scales used
We explore the correlation between the scales used preoperatively and postoperatively and the relationship between both. There was a strong linear correlation (τ= −0.74, p<0.001) between the Frankel and ECOG–WHO–Zubrod classification for the preoperative assessment of patients before SM removal (Fig. 4A, B). Preoperative status as assessed using the ECOG–WHO–Zubrod classification explained only a fraction of the FO (τ=0.34, p<0.001) defined by the Ranawat at the last follow-up visit with a low τ coefficient correlation (Fig. 4C, D). As suspected, there was a moderate positive linear correlation between ECOG–WHO–Zubrod functional status assessment after SM removal and the modified Ranawat score (τ=0.69, p<0.001) (Fig. 4E, F).
Fig. 4.
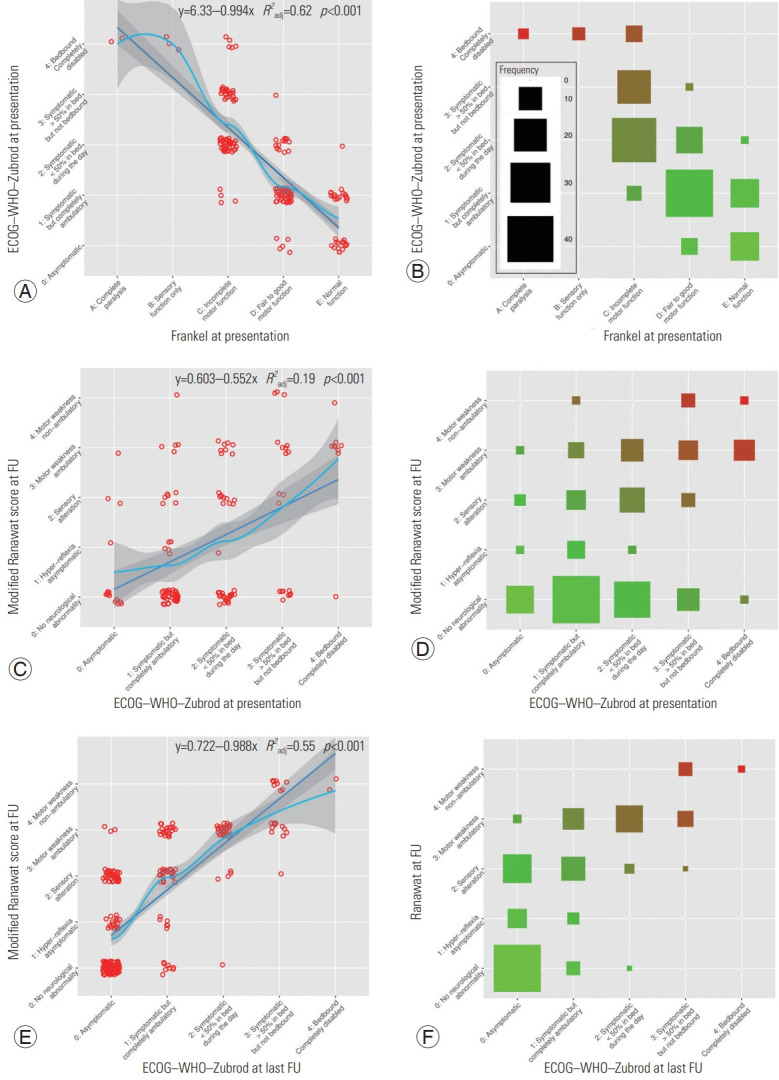
Linear regression plots of the scales used for functional status assessment at presentation and at last neurosurgical follow-up (FU). (A, B) Eastern Cooperative Oncology Group (ECOG)–World Health Organization (WHO)–Zubrod at presentation by Frankel at presentation. (C, D) Modified Ranawat score at last FU by ECOG–WHO–Zubrod at presentation. (E, F) Modified Ranawat score at last FU by ECOG–WHO–Zubrod at last FU.
Discussion
SMs are the most common intradural spinal tumors, with a prevalence of 30.7%, with the main differential diagnosis being schwannoma of the spinal nerve root [13]. The primary goal of surgery is to achieve complete tumor removal while avoiding additional neurological damage. SM resection is usually a relatively simple neurosurgical intervention with reported rates of complete resection (Simpson grade I or II) often above 90%. Such procedures are characterized by low associated rates of morbidity and mortality. Compared with intracranial meningioma, aggressive SMs are infrequent. Therefore, once removed, recurrence is uncommon. Therefore, focusing on the FO rather than on the oncologic outcome is of great importance. SMs are usually slowly growing tumors originating from arachnoid membranes; thus, they produce symptoms only after reaching a distinct size and significant spinal cord compression. Their clinical presentation is rather nonspecific, with back pain often being the leading symptom, whereas radiating pain, motor deficits, impaired sensibility, and sphincter disturbance only progress very slowly. Local pain is often misinterpreted until the diagnosis is suspected on MRI requested when significant spinal cord compression occurs at an advanced stage of the disease. In most patients, the diagnosis is thus not confirmed until motor deficits or gait disturbances arise, witnessing a long-lasting spinal cord compression. Data on FO after SM surgery are scarce. Moreover, until recently, no dedicated scale to assess the functional status before and after SM removal was available. Several scores developed for pathologies or surgical procedures involving the osteoligamentous structure of the spine were used by different authors who reported on the subject.
1. Epidemiology of spinal meningioma
Overall, across all studies, there is an agreement of reported figures on demographic, surgical, and histopathological data. Our population is similar to those described in previous reports, with 85.8% of female participants and a median age at surgery of 67.2 years (IQR, 56.7–76.5 years). All studies on meningiomas, regardless of their insertion, have shown a female prevalence of approximately ¾, which suggests the influence of sexual hormones, as meningiomas are known to be hormone-sensitive and usually express progesterone receptors (PRs) [2]. Despite the abundant expression of PR, which is found in 88% of meningioma cases, how their expression is regulated remains unknown, especially since estrogen receptors are virtually absent in these tumors [23,24]. SMs occur even more frequently in women compared with their intracranial counterparts, yet no satisfactory explanation for this has been provided [13]. As expected, SM frequency is higher in the elderly and maximum for the 60–80-year category, with the 75–79-year-old group having the highest incidence of SM according to Westwick and Shamji [13]. SM was present at an older age (65.4 years in our study), compared with intracranial meningiomas that had a mean age at surgery of approximately 58 years [2,3,13]. These two features of the overrepresentation of females and people of older age groups at the time of surgery make likely SM a different entity compared with intracranial meningioma.
As previously reported, SMs occur all along the spine but are preferentially found at the thoracic level in 77.9% of the cases [25]. It seems obvious as the thoracic spine comprises the most vertebrae of all levels of the spine (approximately 67%). However, Fig. 1 clearly shows that the distribution of meningiomas along the spine is inhomogeneous. The factors that account for this irregular distribution remain unknown.
2. Functional outcome
All studies report functional improvement after SM removal; however, considering the variety of classifications used to assess pre- and postoperative functional status, it is not easy to summarize and compare these data [12]. According to the series reported by Sandalcioglu et al. [12], the outcome was either improved or unchanged in 96.2% of the patients at the last follow-up visit, and 12% of their patients were unable to independently walk as compared with 94.4% and 10.1%, respectively, in our study. The two results are similar [12]. In a literature review by Maiti et al. [26], improved or unchanged FO was reported in at least 90% of the patients across all 14 studies assessed. Even patients with severe preoperative states may experience an excellent neurological recovery after careful surgery and appropriate rehabilitation, as found in our study. As different FO scales have been described in the literature, a direct comparison is not feasible. The Nurick grade, Frankel grade, (modified) Japanese Orthopaedic Association score, or (modified) McCormick grade have been used by different authors [16,18]. We decided to use five different scales to assess our population because there is no dedicated one to evaluate FO for SM and compare the results among them using correlations. We postulated that meningioma may occur everywhere along the spinal canal and thus produce a large variety of symptoms similar to those of arthritis-related degenerative cervical or thoracic myelopathy for which the Nurick or modified Ranawat score can be used [15,16]. Searching for the correlation between the classifications we used, we usually found a moderate (τ=0.65 for Frankel versus Prolo functional) to high association (τ=0.86 for Ranawat versus Nurick) between those scales. The classifications we used are not strictly superposable despite the fact that they all intend to grade the functional status of patients in five or six categories. The difference between our study and the others is that we used several different scales to assess the FO along with a pre- and postoperative assessment. We also established that FO improvement is invariably present, regardless of the scales used. This is corroborated by the fact that there is a correlation between the five scales used. Moreover, we found a correlation between the preoperative and postoperative functional status. There is no ideal scale, but the more details it comprises, the less likely used it is due to its complexity and time-consuming utilization. Moreover, usually, the more items there are, the less reliable the classification is due to inter- and intraindividual assessment variations.
The (modified) McCormick grade, although originally used to classify intramedullary tumors, has been used by many authors for SM FO assessment as it is easy to use, with only four categories (despite their somewhat imprecise definitions) [27]. We think that functional evaluation could be further simplified in only three grades: those who are paucisymptomatic or asymptomatic, fully ambulant, and back to a normal life; those who are not ambulant and dependent; and those in-between, especially as most patients with SM are elderly people.
Frati et al. [28] proposed the “Spinal Meningiomas Prognostic Evaluation Scale” system, a novel and simple evaluation scale based on preoperatively known features, to predict and assess the risk of functional postoperative worsening. They identified four items, namely, anterior/anterolateral location, surgery for SM recurrence, sphincter involvement, and patient not ambulant, as being fairly predictive of the long-term outcome of SM surgery [28].
3. Limitations
The retrospective nature of the study and the lack of clarity regarding treatment rationales and nonhomogeneous management strategies without random assignment need to be considered when interpreting the results. A central neuropathology review was not possible due to limited resources. However, a study of histopathologic assessments between “parent institutions” and central review found a high concordance of 93.0% for WHO grade I meningioma for grading [29]. There was a lack of precision in gathering information on the patient using electronic medical records software, especially regarding the preoperative clinical state and patient outcome. Further data collection and missing data recovery are needed to improve the quality and statistical power of this study. Follow-up details found in patients’ medical records are most of the time too scarce to properly assess the patient’s real state. Nonetheless, the inclusion of more patients and extended follow-up would be helpful to validate our findings.
Conclusions
This large series confirms the existence of a favorable FO after SM surgery even in a case of a seriously impaired preoperative status. A validated scale is needed to assess the factors predicting a worsening of the functional status and guide the management of patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champeaux C, Weller J, Katsahian S. Epidemiology of meningiomas: a nationwide study of surgically treated tumours on French medico-administrative data. Cancer Epidemiol. 2019;58:63–70. doi: 10.1016/j.canep.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Zouaoui S, Darlix A, Rigau V, et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie. 2018;64:15–21. doi: 10.1016/j.neuchi.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Champeaux-Depond C, Weller J, Resche-Rigon M. Neurofibromatosis type 2: a nationwide population-based study focused on survival after meningioma surgery. Clin Neurol Neurosurg. 2020;198:106236. doi: 10.1016/j.clineuro.2020.106236. [DOI] [PubMed] [Google Scholar]
- 5.Champeaux-Depond C, Weller J, Froelich S, Sartor A. Cyproterone acetate and meningioma: a nationwide-wide population based study. J Neurooncol. 2021;151:331–8. doi: 10.1007/s11060-020-03672-9. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 7.Champeaux C, Houston D, Dunn L, Resche-Rigon M. Intracranial WHO grade I meningioma: a competing risk analysis of progression and disease-specific survival. Acta Neurochir (Wien) 2019;161:2541–9. doi: 10.1007/s00701-019-04096-9. [DOI] [PubMed] [Google Scholar]
- 8.Champeaux C, Houston D, Dunn L. Atypical meningioma: a study on recurrence and disease-specific survival. Neurochirurgie. 2017;63:273–81. doi: 10.1016/j.neuchi.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Champeaux C, Dunn L. World Health Organization grade II meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. 2016;89:180–6. doi: 10.1016/j.wneu.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Champeaux C, Jecko V, Houston D, et al. Malignant meningioma: an international multicentre retrospective study. Neurosurgery. 2019;85:E461–9. doi: 10.1093/neuros/nyy610. [DOI] [PubMed] [Google Scholar]
- 11.Champeaux C, Wilson E, Brandner S, Shieff C, Thorne L. World Health Organization grade III meningiomas: a retrospective study for outcome and prognostic factors assessment. Br J Neurosurg. 2015;29:693–8. doi: 10.3109/02688697.2015.1054350. [DOI] [PubMed] [Google Scholar]
- 12.Sandalcioglu IE, Hunold A, Muller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035–41. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westwick HJ, Shamji MF. Effects of sex on the incidence and prognosis of spinal meningiomas: a surveillance, epidemiology, and end results study. J Neurosurg Spine. 2015;23:368–73. doi: 10.3171/2014.12.SPINE14974. [DOI] [PubMed] [Google Scholar]
- 14.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyres KS, Gray DH, Robertson P. Posterior surgical treatment for the rheumatoid cervical spine. Br J Rheumatol. 1998;37:756–9. doi: 10.1093/rheumatology/37.7.756. [DOI] [PubMed] [Google Scholar]
- 16.Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. doi: 10.1093/brain/95.1.87. [DOI] [PubMed] [Google Scholar]
- 17.Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations: a paradigm applied to posterior lumbar interbody fusions. Spine (Phila Pa 1976) 1986;11:601–6. doi: 10.1097/00007632-198607000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–92. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team . R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; 2014. [Google Scholar]
- 20.RStudio Team . RStudio: integrated development environment for R. Boston (MA): RStudio Inc; 2015. [Google Scholar]
- 21.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Lang TA, Altman DG. Basic statistical reporting for articles published in biomedical journals: the “Statistical Analyses and Methods in the Published Literature” or the SAMPL guidelines. Int J Nurs Stud. 2015;52:5–9. doi: 10.1016/j.ijnurstu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Blankenstein MA, Verheijen FM, Jacobs JM, Donker TH, van Duijnhoven MW, Thijssen JH. Occurrence, regulation, and significance of progesterone receptors in human meningioma. Steroids. 2000;65:795–800. doi: 10.1016/s0039-128x(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 24.Goffin J. Estrogen- and progesterone-receptors in meningiomas: review article. Clin Neurol Neurosurg. 1986;88:169–75. doi: 10.1016/s0303-8467(86)80024-5. [DOI] [PubMed] [Google Scholar]
- 25.Hohenberger C, Gugg C, Schmidt NO, Zeman F, Schebesch KM. Functional outcome after surgical treatment of spinal meningioma. J Clin Neurosci. 2020;77:62–6. doi: 10.1016/j.jocn.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41:E6. doi: 10.3171/2016.5.FOCUS16163. [DOI] [PubMed] [Google Scholar]
- 27.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–32. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 28.Frati A, Pesce A, Toccaceli G, Fraschetti F, Caruso R, Raco A. Spinal Meningiomas Prognostic Evaluation Score (SPES): predicting the neurological outcomes in spinal meningioma surgery. Neurosurg Rev. 2019;42:115–25. doi: 10.1007/s10143-018-0961-1. [DOI] [PubMed] [Google Scholar]
- 29.Rogers CL, Perry A, Pugh S, et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro Oncol. 2016;18:565–74. doi: 10.1093/neuonc/nov247. [DOI] [PMC free article] [PubMed] [Google Scholar]