Abstract
Objectives
This study analyzed the clinical outcomes of remdesivir treatment in coronavirus disease 2019 (COVID-19) patients in South Korea.
Methods
This retrospective cohort study involved the secondary analysis of epidemiological data. Among patients diagnosed with COVID-19 from July 2, 2020 to March 23, 2021 (12 AM), 4,868 who received oxygen therapy and were released from isolation after receiving remdesivir treatment were assigned to the treatment group, and 6,068 patients who received oxygen therapy but not remdesivir were assigned to the untreated group. The study subjects included children under the age of 19. The general characteristics and severity were compared between the groups. Differences in the time to death and mortality were also compared.
Results
In the untreated group, the hazard ratio [HR] for mortality was 1.59 (95% confidence interval [CI], 1.40–1.80) among patients aged ≥70 years and 2.32 (95% CI, 2.00–2.69) in patients with severe disease in comparison to the treatment group. In a comparison of survival time among patients with severe disease aged ≥70 years, the HR for mortality before 50 days was 2.09 (95% CI, 1.77–2.46) in the untreated group compared to the treatment group.
Conclusion
Patients with remdesivir treatment showed better clinical outcomes in this study, but these results should be interpreted with caution since this study was not a fully controlled clinical trial.
Keywords: Antiviral, Clinical outcomes, COVID-19 treatment, Remdesivir
Introduction
Remdesivir (Veklury; GILEAD, Foster City, CA, USA), a therapeutic agent for coronavirus disease 2019 (COVID-19), is an antiviral medication that contains nucleoside analogs, which have a similar structure to adenosine triphosphate molecules, and inhibits RNA polymerase. Through this inhibitory activity, remdesivir inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is an RNA virus that causes COVID-19 [1].
Remdesivir was initially developed to treat Ebola virus disease in Western Africa in 2013–2016. After the start of the COVID-19 pandemic in early 2020, Gilead Sciences began conducting clinical trials to assess the effect of remdesivir on SARS-CoV-2 and reported animal experiment results suggesting that remdesivir can be used for Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus [2−4].
In May 2020, the United States Food and Drug Administration (FDA) granted an emergency use authorization for remdesivir based on a clinical trial conducted by the National Institute of Allergy and Infectious Disease (NIAID), in which remdesivir reduced the recovery period of confirmed COVID-19 patients by 31% (from 15 to 11 days) compared to the placebo group. Remdesivir was subsequently approved for emergency use for COVID-19 in South Korea [5].
In early May 2020, in South Korea, the instantaneous reproduction number of COVID-19 increased from 0.2 to 1.8 in about 2 weeks after social distancing was relaxed, and it remained at about 1 until early July. This was due to the effect of cluster outbreaks in Daegu and North Gyeongsang Province [6]. As the number of confirmed cases increased, it tbbecame important to prepare countermeasures for the treatment of confirmed cases.
In October 2020, following the release of an interim clinical study report, the NIAID reported that remdesivir reduced the recovery period by 5 days in a clinical trial involving hospitalized patients with COVID-19. The researchers reported that the remdesivir-treated group had fewer deaths than the placebo group, although the difference in mortality was not statistically significant. Based on other relevant studies, the United States FDA officially approved the use of remdesivir for the treatment of COVID-19 [7,8].
On October 15, 2020, the World Health Organization published the report of a clinical trial assessing the effects of 4 medications, including remdesivir, in 11,266 patients admitted to 500 hospitals in 30 countries from March 2020 to early October 2020. It was reported that remdesivir did not reduce the hospitalization period or mortality, raising controversy regarding the effectiveness of remdesivir against COVID-19 [9].
A study on the effect of remdesivir conducted among 101 COVID-19 patients admitted to 20 medical facilities across South Korea reported that while early remdesivir administration prevented symptoms from worsening in patients with severe COVID-19, remdesivir did not significantly affect fatality, recovery time, and the percentage of recovered patients [10]. In a study of 2,374 soldier patients treated with remdesivir, remdesivir did not affect fatality and hospitalization time [11].
The Central Disease Control Headquarters of South Korea have been supplying remdesivir free of charge to medical facilities to treat severe COVID-19 patients since July 2, 2020 and have been monitoring their clinical outcomes on a daily basis. This study aimed to measure the effect of remdesivir treatment on severe COVID-19 patients by comparing time to death and the hazard ratio (HR) for mortality between the treatment and untreated groups.
Materials and Methods
Subjects
Treatment group
In total, 4,868 confirmed COVID-19 patients from July 2, 2020 to March 23, 2021 who were on oxygen therapy with remdesivir treatment were included. The period of this study was chosen as an interval with minimal effects from vaccination and COVID-19 mutations. The study subjects included 5 children under the age of 19 who received remdesivir treatment.
Untreated (control) group
A total of 6,068 confirmed COVID-19 patients in the same period who were on oxygen therapy without remdesivir treatment were assigned to the untreated group.
Definitions
Severity
In accordance with the domestic eligibility criteria for remdesivir treatment, COVID-19 patients who met the following clinical criteria were selected: (1) evidence of pneumonia on a chest radiograph or chest computed tomography, (2) room air oxygen saturation ≤94%, and (3) patients on oxygen therapy (excluding those on a mechanical ventilator or extracorporeal membrane oxygenation). Severity was divided into severe and mild. Severe cases were defined as patients who received noninvasive ventilation or high-flow nasal oxygenation. Mild cases were defined as patients who received oxygen therapy (less than 15 L of oxygen per minute) with nasal prongs or a facial mask. Severity was classified according to the definitions used in existing papers and the use or non-use of a respiratory machine [12].
Case fatality rate
The case fatality rate was defined as the proportion of deaths among confirmed COVID-19 cases.
Survival time
For deceased patients, survival time was defined as the time from COVID-19 diagnosis to death. For survivors, it was defined as the time from COVID-19 laboratory confirmation to the time of follow-up (March 23, 2021).
Study Period
This study period was from July 2, 2020 (when remdesivir was introduced) to March 23, 2021. This period was selected to minimize the impact of changes in the virus’s properties that could affect clinical outcomes. In other words, this period was not meaningfully affected by the Delta and Omicron variants.
Statistical Analysis
Clinical information, including sex, age, oxygen therapy, and comorbidities (yes or no), was collected from basic epidemiological investigation data from the COVID-19 Information Management System of Korea Disease Control and Prevention Agency (KDCA) and from the COVID-19 Patient Information Management System of the Health Insurance Review and Assessment Service. The chi-square test or Fisher exact test was used to examine the differences in demographic distributions between the treatment and untreated groups. The Kaplan-Meier estimator, the log-rank test, and a Cox proportional-hazard regression model were used to examine the differences in time to death between the 2 groups. All statistical analyses were performed using Microsoft 365 Excel (Microsoft Corp., Redmond, WA, USA), Rex (Rexsoft, Seoul, Korea) and R ver. 3.6.3 (The R Foundation, Vienna, Austria).
Ethics Approval
This study was exempted from review by the Institutional Review Board of the KDCA (IRB No: 2021-11-01-PE-A).
Results
Demographics
In total, 10,936 patients—4,868 in the treatment group and 6,068 in the untreated group—were included in this study. The 2 groups showed overall differences in the general demographics. The percentage of male patients was higher in the treatment group, with 2,666 males (54.8%), than in the untreated group, with 3,033 males (50.0%; p<0.001)
The patients were divided into two 2 groups using the median age of the treatment group (70 years). The percentage of patients aged ≥70 years was higher in the treatment group (48.2%) than in the untreated group (25.4%; p<0.001). Furthermore, 3,358 patients (69.0%) had mild COVID-19 and 1,510 (31.0%) had severe COVID-19 in the treatment group, while 5,501 patients (90.7%) had mild COVID-19 and 567 (9.3%) had severe COVID-19 in the untreated group. The percentage of severe cases was significantly higher in the treatment group than in the untreated group (p<0.001). The percentage of patients with comorbidities was higher in the treatment group (67.6%) than in the untreated group (55.4%, p<0.001). In total, 4,306 patients (88.5%) recovered and 562 (11.5%) died in the treatment group, while 5,518 patients (90.9%) recovered and 550 (9.1%) died in the untreated group. The percentage of deceased patients was significantly higher in the treatment group than in the untreated group (p<0.001; Table 1).
Table 1.
General demographics of the treatment and untreated groups
| Characteristic | Treatment group | Untreated group | p-value |
|---|---|---|---|
| Count | 4,868 (100) | 6,068 (100) | |
| Sex | <0.001 | ||
| Male | 2,666 (54.8) | 3,033 (50.0) | |
| Female | 2,202 (45.2) | 3,035 (50.0) | |
| Age (y) | <0.001 | ||
| <70 | 2,523 (51.8) | 4,524 (74.6) | |
| ≥70 | 2,345 (48.2) | 1,544 (25.4) | |
| Severity | <0.001 | ||
| Mild | 3,358 (69.0) | 5,501 (90.7) | |
| Severe | 1,510 (31.0) | 567 (9.3) | |
| Comorbidities | <0.001 | ||
| Yes | 3,289 (67.6) | 3,364 (55.4) | |
| No | 1,579 (32.4) | 2,704 (44.6) | |
| Outcome status | <0.001 | ||
| Recovery | 4,306 (88.5) | 5,518 (90.9) | |
| Death | 562 (11.5) | 550 (9.1) |
Data are presented as n (%).
Survival Analysis
The survival analysis was adjusted for age and severity to minimize impact of underlying differences in general demographics between the treatment and untreated groups. Age was divided into <70 years and ≥70 years, and severity was categorized as mild and severe. Severe cases aged ≥70 years were analyzed separately.
Patients aged <70 years
The differences in survival time among 7,047 patients aged <70 years were estimated. Approximately 77.0% and 84.0% of treated and untreated patients survived after 50 days, respectively. The treatment group had a higher survival rate than the untreated group for patients aged <70 years (p<0.001; Figure 1). The HR for mortality was 0.46 in the untreated group (95% confidence interval [CI], 0.34–0.62) compared to the treatment group for patients aged <70 years; reflecting a significantly lower mortality risk than in the treatment group (Table 2).
Figure 1.
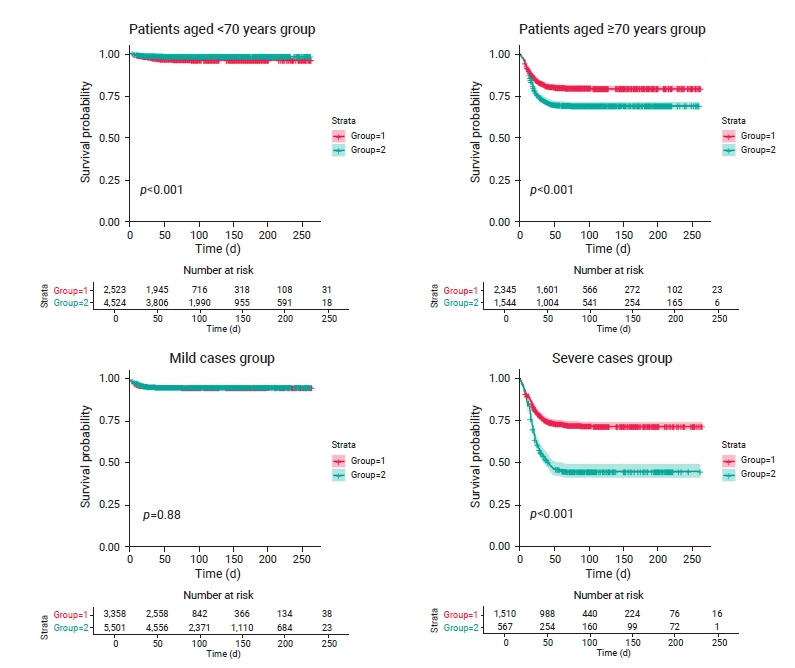
The treatment group had a higher survival rate than the untreated group for patients aged <70 years (p<0.001). The treatment group had a higher survival rate than the untreated group for patients aged ≥70 years (p<0.001). In mild cases, the difference in survival time between the 2 groups was not statistically significant (p=0.88). In severe cases, the treatment group had a higher survival rate than the untreated group (p<0.001). Group1 is a treated group and Group 2 is a untreated group.
Table 2.
Hazard ratios for mortality in untreated and treated patients by group
| Type | HR (95% CI) |
|---|---|
| Patients aged <70 y | |
| Treated (n=2,523) | 1 |
| Untreated (n=4,524) | 0.46 (0.34−0.62) |
| Patients aged ≥70 y | |
| Treated (n=2,345) | 1 |
| Untreated (n=1,544) | 1.59 (1.40−1.80) |
| Mild casea) | |
| Treated (n=3,358) | 1 |
| Untreated (n=5,501) | 0.98 (0.80−1.21) |
| Severe caseb) | |
| Treated (n=1,510) | 1 |
| Untreated (n=567) | 2.32 (2.00−2.69) |
| Severe cases and aged ≥70 y | |
| Treated (n=874) | 1 |
| Untreated (n=387) | 2.09 (1.77−2.46) |
HR, hazard ratio; CI, confidence interval.
Mild case: patient requiring oxygen therapy (nasal cannula or mask).
Severe case: patient requiring high-flow oxygen therapy, mechanical ventilator, or extracorporeal membrane oxygenation/continuous renal replacement therapy.
Patients aged ≥70 years
Survival time was compared among 3,889 patients aged ≥70 years. Approximately 68.0% and 65.0% of the treated and untreated patients survived after 50 days, respectively. The treatment group had a higher survival rate than the untreated group (p<0.001; Figure 1). The HR was 1.59 (95% CI, 1.40–1.80) in the untreated group compared to the treatment group for patients aged ≥70 years, and this difference was statistically significant (Table 2).
Mild cases
The differences in survival time among 8,859 mild cases were estimated. Approximately 76.0% and 82.0% of treated and untreated patients survived after 50 days, respectively; the difference in survival time between the 2 groups was not statistically significant (p=0.88; Figure 1), with an HR of 0.98 (95% CI, 0.80–1.21) in the untreated group compared to the treatment group (Table 2).
Severe cases
Survival time among 2,077 severe cases was compared between the treatment and untreated groups. Based on survival curves, 25.0% of treated survivors did not survive after 40 days, and 25.0% and 50.0% of untreated patients did not survive after 18 and 43 days, respectively. About 65.0% and 45.0% of the treated and untreated patients survived after 50 days, respectively. The treatment group had a higher survival rate than the untreated group (p<0.001; Figure 1). The HR was 2.32 (95% CI, 2.00–2.69) in the untreated group compared to the treatment group (Table 2).
Severe cases aged ≥70 years
Differences in survival time among 1,261 severe cases aged ≥70 years were estimated. A quarter (25.0%) of treated patients did not survive after 21 days, and 25.0% and 50.0% of untreated survivors did not survive after 16 and 26 days, respectively. About 57.0% of treated and 33.0% of untreated patients survived after 50 days. The treatment group had a significantly higher survival rate (p<0.001, Figure S1), as reflected by an HR for mortality of 2.09 (95% CI, 1.77–2.46) in the untreated group compared to the treatment group (Table 2).
Cox Proportional-Hazard Regression Model
The Cox proportional-hazard regression model is a method developed by David Roxbee Cox. To identify significant differences in COVID-19 mortality based on remdesivir treatment, each factor was serially introduced to a regression model. No noticeable difference in mortality risk was found between the treatment and untreated groups after controlling only for age (HR, 1.11; 95% CI, 0.99–1.26). However, the HR for mortality was 1.74 (95% CI, 1.54–1.97) in the untreated group compared to the treatment group after controlling for age and severity, and the HR was 1.76 (95% CI, 1.56–1.98) in the untreated group after controlling for age, severity, and comorbidities, similar to the results observed from the previous model (Table 3).
Table 3.
Factors affecting mortality among coronavirus disease 2,019 patients treated or not treated with remdesivir
| Variable | Model 1a) | Model 2b) | Model 3c) |
|---|---|---|---|
| Remdesivir administered (n=10,936) | |||
| Yes | 1 | 1 | 1 |
| No | 1.11 (0.99−1.26) | 1.74 (1.54−1.97) | 1.76 (1.56−1.98) |
| Age (n=10,936) | |||
| <70 y | 1 | 1 | 1 |
| ≥70 y | 3.95 (3.47−4.49) | 2.67 (2.35−3.04) | 2.27 (2.00−2.58) |
| Severity (n=10,936) | |||
| Mild | 1 | 1 | |
| Severe | 8.51 (7.46−9.72) | 7.61 (6.68−8.67) | |
| Comorbidities (n=10,936) | |||
| No | 1 | ||
| Yes | 2.75 (2.33−3.24) |
Data are presented as hazard ratio (95% confidence interval).
Controlled for age.
Controlled for age and severity.
Controlled for age, severity, and comorbidities.
Discussion
Significant differences were found in all demographic characteristics, including sex, age, severity, and isolation status, between the treatment and untreated groups. These differences were inevitable because this study was not designed as a clinical trial and the 2 groups could not be fully controlled. Confirmed COVID-19 patients during the study period could receive remdesivir treatment if they were eligible, but the decision was still made by the treating physician and it could have been affected by many other uncontrollable factors.
A limitation is that KDCA’s COVID-19 clinical information collection systems do not provide details regarding comorbidities and only show the presence of comorbidities. For this reason, differences in comorbidities in the study population could not be further estimated. However, no noticeable differences in the distribution of comorbidities were found among deceased patients aside from neurological disorders, which were more frequent in the untreated group than in the treatment group.
In the survival analysis considering different age groups, the untreated group showed a lower mortality risk (HR, 0.46) than the treatment group among patients aged <70 years. The lower risk in the untreated group may be attributed to the higher proportion of mild cases and the lower proportion of patients with comorbidities aged <70 years in the untreated group.
For patients aged ≥70 years, the HR for mortality in the untreated group was 1.59 compared to the treatment group, showing better outcomes in the treatment group. A high proportion of patients aged ≥70 years had severe COVID-19. A recent study on patients admitted to a hospital for COVID-19 in South Korea reported that early remdesivir administration prevented symptoms from worsening in patients with severe COVID-19 [10], and this study also showed that the early use of remdesivir reduced the risk of COVID-19 mortality by reducing symptom exacerbation in severe cases.
In the survival analysis considering different severity groups, no differences in the time to death and HR for mortality were found between the treatment and untreated groups in mild cases, possibly because mild cases often recover regardless of remdesivir treatment. However, the HR for mortality was 2.32 times among severe cases in the untreated group, and the time to death (in 25.0% of patients) was 40 days in the treatment group and 18 days in the untreated group. Considering that the untreated group had a higher HR and shorter time to death than the treatment group despite the treated group having a higher proportion of severe cases, remdesivir showed positive results in preventing death. Although the results of the study are similar in that they showed a therapeutic effect in severely ill patients, there are some differences from other studies conducted by the National Institutes of Health of the United States on the effectiveness of remdesivir (ACTT-1). That study found that patients requiring low-flow nasal oxygen therapy (that is, a nasal cannula or face mask oxygenation, 15 L per minute or less) had the greatest benefit from remdesivir. This benefit was attenuated in patients requiring high-flow nasal oxygenation and noninvasive ventilation and largely absent in those requiring invasive ventilation or extracorporeal membrane oxygenation [7].
Differences in survival rate were identified in severe cases aged ≥70 years. The time to death (in 25.0% of patients) was 21 days in the treatment group and 16 days in the untreated group, and the HR for mortality was 2.09 in the untreated group. These results show that the therapeutic effect of remdesivir was consistently observed after considering patients’ age and severity.
In the Cox proportional-hazard regression analysis, the HR for mortality was 1.76 in the untreated group. This result is consistent with the final report of the NIAID, in which remdesivir lowered mortality compared to the placebo group [7]. However, in the definition of mild and severe cases, the NIAID in the United States defined a condition that did not require hospitalization as mild, and all COVID-19 patients with pneumonia and hypoxemia were defined as severe cases. This difference may be relevant for the interpretation of the results.
Conclusion
This study showed similar results as previous studies on the therapeutic effect of remdesivir. However, the results of this study should be interpreted with caution as this study was not designed as a clinical trial. Nevertheless, this study has meaningful findings, as it included all patients with remdesivir treatment since the approval and supply of the medication in South Korea. A further analysis of the effect of comorbidities on remdesivir treatment outcomes and the effects of other COVID-19 variants must be conducted to achieve a better understanding of the effect of remdesivir on COVID-19.
Footnotes
Ethics Approval
This study was exempted from review by the Institutional Review Board of the Korea Disease Control and Prevention Agency (IRB No: 2021-11-01-PE-A). The requirement for informed consent was waived because of the retrospective nature of this study.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
None.
Availability of Data
Detailed data used in this study cannot be disclosed to the outside because it contains personal information in accordance with Article 2 Paragraph 1 of the Personal Information Protection Act. If you have additional questions about the study, please contact the corresponding author, Dr. Jin Gwack, gwackjin@korea.kr.
Authors’ Contributions
Conceptualization: BIK; Formal analysis: MY; Project administration: JK; Supervision: JK, BIK, JG; Writing–original draft: MY; Writing–review & editing: all authors.
Supplementary Material
Figure S1. Survival curves of severe cases aged ≥70 years with or without remdesivir treatment. The treatment group had a higher survival rate (p<0.001). Supplementary data are available at https://doi.org/10.24171/j.phrp.2022.0138.
References
- 1.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo MK, Jordan R, Arvey A, Sudhamsu J, et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joseph SS, Samuel M. Gilead working with China to test Ebola drug as new coronavirus treatment [Internet]. Reuters; 2020 [cited 2022 Apr 27]. Available from: https://www.reuters.com/article/us-health-china-gilead-sciences-idUSKBN1ZU2RX.
- 5.Gillenwater S, Rahaghi F, Hadeh A. Remdesivir for the treatment of Covid-19: preliminary report. N Engl J Med. 2020;383:992. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 6.Ryu S, Ali ST, Noh E, et al. Transmission dynamics and control of two epidemic waves of SARS-CoV-2 in South Korea. BMC Infect Dis. 2021;21:485. doi: 10.1186/s12879-021-06204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19: final report. 2020.11.5. N Engl J Med. 2020;383:1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Food and Drug Administration (FDA). FDA news release. FDA approves first treatment for COVID-19 [Internet]. Silver Spring, MD: FDA; 2020 [cited 2022 Apr 27]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19.
- 9.WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid-19: interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joo EJ, Ko JH, Kim SE, et al. Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study. J Korean Med Sci. 2021;36:e83. doi: 10.3346/jkms.2021.36.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohl ME, Miller DR, Lund BC, et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw Open. 2021;4:e2114741. doi: 10.1001/jamanetworkopen.2021.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung HK, Kim JY, Heo J, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January-May 2020. J Korean Med Sci. 2020;35:e280. doi: 10.3346/jkms.2020.35.e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Survival curves of severe cases aged ≥70 years with or without remdesivir treatment. The treatment group had a higher survival rate (p<0.001). Supplementary data are available at https://doi.org/10.24171/j.phrp.2022.0138.


