Abstract
Apico–basal polarity is a fundamental property of the epithelium that functions as a barrier, holds cells together, and determines the directions of absorption and secretion. Apico–basal polarity is regulated by extracellular matrix‐integrin binding and downstream signaling pathways, including focal adhesion kinase, rouse‐sarcoma oncogene (SRC), and RHO/RHO‐associated kinase (ROCK). Loss of epithelial cell polarity plays a critical role in the progression of cancer cells. However, in differentiated carcinomas, polarity is not completely lost but dysregulated. Recent progress with a three‐dimensional culture of primary cancer cells allowed for studies of the mechanism underlying the abnormality of polarity in differentiated cancers, including flexible switching of polarity status in response to the microenvironment. Invasive micropapillary carcinoma (MPC) is one of the histopathological phenotypes of adenocarcinoma, which is characterized by inverted polarity. Aberrant activation of RHO–ROCK signaling plays a critical role in the MPC phenotype. Establishing in vitro models will contribute to future drug targeting of the abnormal polarity status in cancer.
Keywords: apico–basal polarity, metastasis, micropapillary carcinoma, organoid
The polarity of epithelial cells has been intensively studied. Meanwhile, the relationship between polarity and metastasis in cancer cell clusters has been reported in few studies and remains largely elusive. Here, we review the recent progress in understanding the features of apico‐basal polarity of cancer cell clusters and the role of clusters abnormality plays in the pathophysiology of micropapillary carcinoma.
Abbreviations
- BRAF
v‐raf (rapidly accelerated fibrosarcoma) homolog B
- CRC
colorectal cancer
- CTC
circulating tumor cell
- CTOS
cancer tissue‐originated spheroid
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- FAK
focal adhesion kinase
- HE
Hematoxylin and Eosin
- KRAS
Kirsten rat sarcoma virus
- MDCK
Madin–Darby canine kidney
- MPC
micropapillary carcinoma
- PTC
papillary thyroid carcinoma
- ROCK
RHO‐associated kinase
- SFK
SRC kinase family
- SRC
rouse‐sarcoma oncogene
- TGF β
transforming growth factor β
- TP53
tumor protein p53
1. INTRODUCTION
Recent advances in culturing primary cancer cells in three‐dimensional (3D) conditions have offered a better understanding of the roles of cancer cell clusters. The polarity of epithelial cells has been intensively studied. 1 Meanwhile, the relationship between polarity and metastasis in cancer cell clusters has been reported in few studies and remains largely elusive. Here, we review the recent progress in understanding the features of the apico–basal polarity of cancer cell clusters and the role clusters abnormality plays in the pathophysiology of micropapillary carcinoma.
1.1. Apico–basal polarity of the epithelium
The formation of an epithelial layer with apico–basal polarity is a fundamental process in the development of a multicellular organism. Epithelial cells form sheets, which are essential for barrier function as well as absorption and secretion 2 (Figure 1A). Polarity formation and maintenance require the regulation of tight junctions by proteins, lipids, position sensors (E‐cadherins and integrins), and guanosine triphosphatase switches. 3 The actin and microtubule cytoskeleton are coordinated, and ultimately the apical and basolateral domains are formed. 4 , 5 Par, 6 Scribble, 7 and Crumbs 8 maintain the apical and basolateral membrane domains. These are essential for organizing the intracellular signaling pathways that maintain epithelial homeostasis. Delivery of membrane proteins to the apical surface of epithelial cells is regulated by direct transport from the trans‐Golgi network or transcytosis via endosomes. These mechanisms are differentially utilized depending on the types of epithelial cells, the physiologic requirements of the cells, and the developmental states. 9
FIGURE 1.
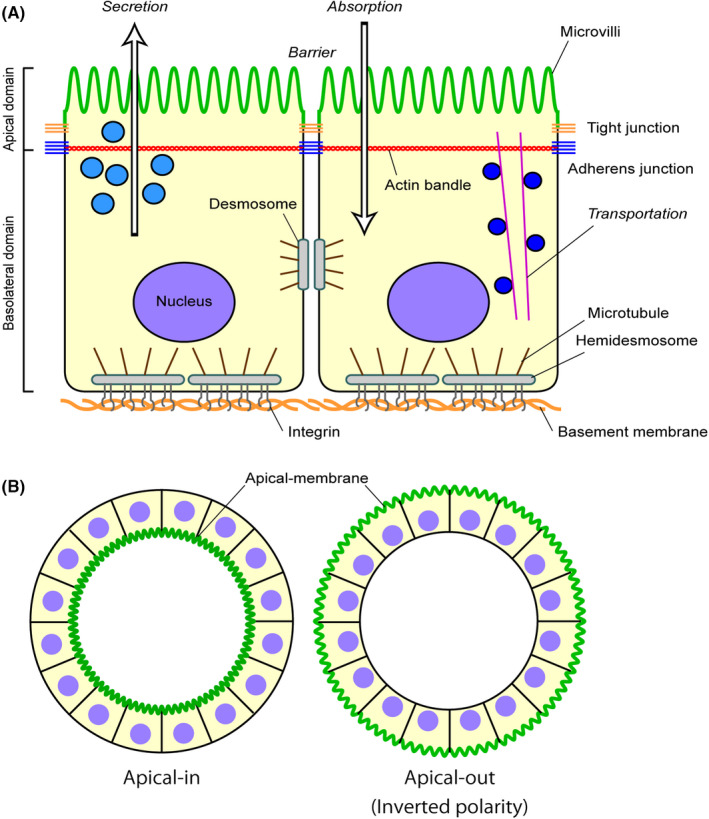
Apico–basal polarity and its inverted form. (A) Apico–basal polarity of the epithelium. A schematic view of the apico–basal polarity. Components and functional roles (italic letters) related to the apico–basal polarity are shown. The apical membrane is indicated in green. (B) Inverted polarity: single cells after proliferation or the aggregates of the MDCK cell line can form multicellular and highly polarized cysts in a collagen gel matrix. When laminin deposition or the integrin/integrin signaling is perturbed, the apical surface is formed outside the cystic structure, showing “inverted polarity.”
1.2. Downstream signaling of ECM‐integrin binding determines the polarity direction
The mechanisms of polarity formation have been studied using multiple models, particularly Madin–Darby canine kidney (MDCK) cells. 10 , 11 In this model system, a suspension of MDCK cells is plated in a collagen gel matrix. These single cells proliferate and differentiate to form multicellular and highly polarized cysts (Figure 1B). When laminin deposition is perturbed, an apical surface forms outside the cystic structure, 12 , 13 which is called “inverted polarity.” Collagen is also important for polarity formation in MDCK cells. Furthermore, inhibition of β1‐integrin can invert the polarity. 14 Thus, extracellular matrix (ECM)‐integrin interactions are involved in establishing epithelial apico–basal polarity and luminal structures.
ECM is a physical scaffold synthesized by cells. The core ECM proteins comprise collagen subunits, proteoglycans, and glycoproteins. 15 Cells interact with ECM molecules via integrins. The extracellular domain of integrins binds to ECM ligands, whereas the intracellular domain binds to cytoskeletal and regulatory proteins. 16
Integrin‐stimulated focal adhesion kinase (FAK) phosphorylation creates a high‐affinity binding site for the SRC‐homology 2 domain of SRC kinase families (SFKs). The binding of SRC to FAK can lead to the activation of SFKs and the formation of a transient FAK–SRC signaling complex that plays a central role in actin cytoskeleton reorganization and migration. 17 FAK also promotes Rac1 activation, specifically at a polarized lamellipodium extension. 18 In MDCK cells, integrin β1 binding to extracellular collagen activates Rac1 14 and leads to basement membrane assembly, 12 which is dependent on RHO–ROCK–myosin signaling. 19 The RHO substrate, RHO‐associated kinase (ROCK), plays a role in laminin deposition and apico–basal polarity formation. 20
1.3. Apico–basal polarity in cancer
Intracellular signaling via SRC, 21 FAK, 22 and RHO 23 is often activated in cancer. In addition to the integrins and their intracellular signaling pathways, polarity determinant proteins are involved in cancer progression. Indeed, the expression or localization of polarity‐related proteins is already altered in the preinvasive stages. 24 Thus, loss of polarity of epithelial cells due to the dysregulation of these proteins plays a key role in the progression of cancer cells. 25 However, in differentiated carcinomas, polarity is somehow retained as one of the 3D characteristics. For example, more than 90% of colorectal cancers are differentiated adenocarcinomas and even the highly differentiated colorectal carcinomas have malignant characteristics. 26 Therefore, polarity in these differentiated carcinomas is disorganized but not lost. Due to the lack of suitable model systems, few studies on the polarity of differentiated adenocarcinomas have been conducted.
1.4. Polarity switching
Apico–basal polarity is fundamentally based on the adhesion between cells and is only seen in the multicellular context. The recent development of 3D culture has led to studies of cancer characteristics as cell clusters. The cancer tissue‐originated spheroid (CTOS) method is one cancer organoid method in which cancer cells are prepared and cultured as clusters from patient tumor tissue. 27 Colorectal cancer (CRC) organoids prepared via CTOS methods retain the 3D characteristics of the original patient cancer tissue, especially glandular or cribriform structures of CRC. 27 Notably, CRC organoids can be cultured in floating conditions. This is in contrast to other organoid culture methods 28 in which organoids are cultured in a basement membrane matrix. In floating conditions, the apical membrane of the CRC organoids is formed on the outermost membrane of the organoids (apical‐out status) (Figure 2A). When embedded in Matrigel or collagen, multiple lumens are formed inside the organoids, which are lined by the apical membrane (apical‐in status). 27 , 29 The apical‐in status is a common feature of CRC. Meanwhile, the apical‐out status is also found in patient tumors in lesions with microvessel invasion or micropapillary carcinoma, as described later. These opposite polarity statuses are changeable in both directions. This phenomenon is called polarity switching. 29 Polarity switching in enteroids has been reported by another study. 30
FIGURE 2.
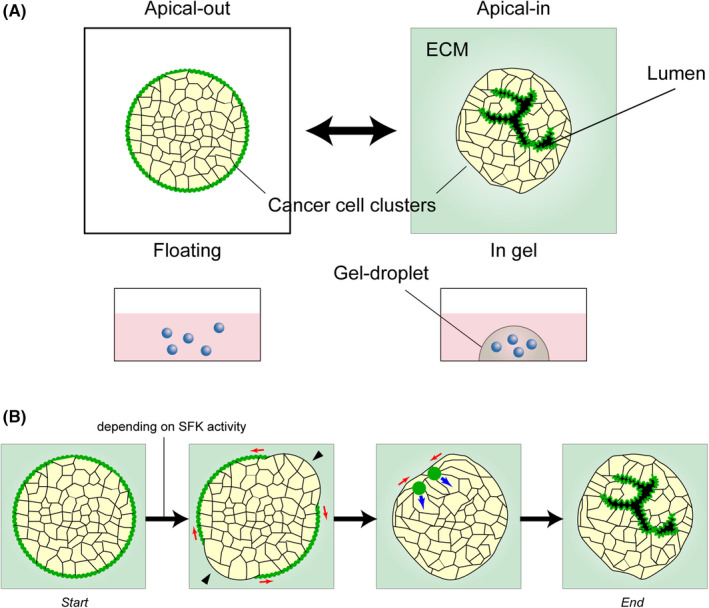
Polarity switching in a cluster of human colorectal cancer. (A) The polarity switching in a cluster of human colorectal cancer. The apical membrane is formed on the outside surfaces of the cancer cell cluster (apical‐out) when cultured in suspension, whereas the apical membrane lines the surface of the lumen inside the cluster (apical‐in) when cultured in extracellular cell‐matrix (ECM). Rapid switching occurs in both directions when the culture conditions are changed. (B) Dynamic process of the polarity switching in a colorectal cancer organoid. The organoid is cultured in the floating condition and embedded in ECM (start), showing apical‐out status. Black arrowheads indicate the regions where the apical membrane is lost. Red arrows indicate the direction of the continuous disappearance of the apical membrane. Green circles indicate the foci of the apical marker. Blue arrows indicate the direction of the movement and expansion of the foci. The organoid eventually adopts the apical‐in status (end). The process depends on SRC kinase family (SFK) activity.
The apical‐out phenotype in floating conditions is a common feature of the CRC organoids, but the ability for polarity switching varied among them in different patients. 29 As for intracellular signaling, polarity switching was strongly suppressed by SFK inhibitors, and partially by an integrin β1 neutralizing antibody and a dynamin inhibitor. 29 Involvement of transforming growth factor β signaling in polarity switching has also been reported. 31 The dynamic process of polarity switching was further studied by Onuma et al. 32 (Figure 2B). They reported that within 1 or 2 h after the apical‐out organoids in the floating condition were embedded in Matrigel, the apical markers were focally lost on the outermost membrane and spread out to fuse. The fused points with the remaining apical marker moved inside of the organoids and formed lumens.
1.5. Polarity switching and metastasis
The contribution of cancer cell clusters to metastasis has been proposed since the 1950s. 33 Circulating tumor cell (CTC) clusters in the blood are associated with significantly worse clinical outcomes compared with single cell CTCs. 34 , 35 , 36 Experimentally, tumor cell clusters generate metastasis more efficiently than single cells. 37 , 38 Additionally, it has been shown, using mouse models, that metastatic foci originate from multiple clones rather than from a single clone, indicating the important role of cancer cell clusters in metastasis. 36 , 39 , 40 Although the apico‐basal polarity status of the CTC clusters has not been well studied, 41 CTC clusters can be apical‐out like the organoids cultured in floating conditions. Cancer cell clusters in the ascites of CRC patients were shown to be in the apical‐out status. 31 Furthermore, the apical‐out status is observed in patient tumor lesions with microvessel invasions. 29 When clusters of cancer cells collectively invade the vascular lumen, they may switch polarity from apical‐in to apical‐out (Figure 3). When apical‐out CTOS organoids in floating conditions were injected into the portal vein of mice, they switched polarity to apical‐in in the liver and eventually formed liver metastasis. 29 When the polarity switching was prevented by inhibitors of SRC or dynamin, the formation of metastasis was suppressed. 29 This suggested that polarity switching is a critical step for metastasis formation by cancer cell clusters.
FIGURE 3.
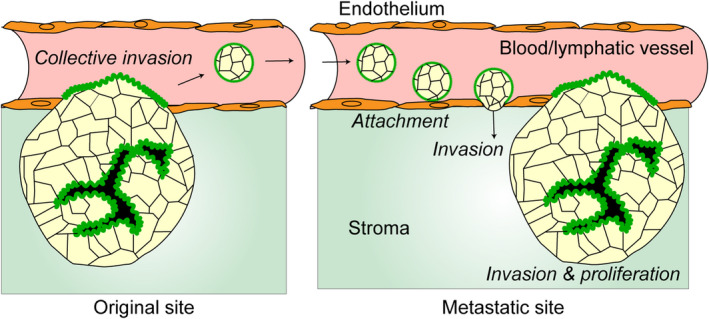
Predictive multisteps in metastasis originated from cancer cell clusters. The cancer cells in the primary lesion show apical‐in status and collectively migrate to the vessels. When they invade the vessels, the polarity switching occurs and the apical membrane faces the bloodstream. The small clusters detach from the mainland due to the shear stress or the budding growth. The survivors from the shear stress and the protective immune system arrive at the metastatic organs and attach to the endothelial cells, clear them, and invade the stroma underneath the vessels, accompanied by the polarity switching. They eventually form metastatic foci after collective migration and proliferation at the metastatic site. Components and functional roles (italic letters) are shown.
1.6. Invasive micropapillary carcinoma
Invasive micropapillary carcinoma (MPC) is a histopathological form of adenocarcinoma that has been reported in a variety of organs, including the colon, breast, bladder, lung, ovary, and salivary glands. 42 , 43 The incidence of MPC is low, but MPC offers a poor prognosis due to high rates of lymphatic invasion and lymph node metastasis, no matter the organ of origin. 42 , 43 MPC is histologically characterized by a lacuna between small papillary carcinoma foci and stroma without a stalk. Notably, the cancer cell foci show inverted polarity. In adenocarcinoma other than MPC, the apical membrane is localized on the luminal side, inside the carcinoma foci, and on the opposite side of the surrounding ECM (apical‐in). Conversely, MPCs have an apical‐out structure, in which the apical membrane is localized outside of the cancer foci despite being surrounded by the ECM. 44 , 45 , 46 Thus, MPCs have an abnormal polarity status.
1.7. Gene mutations in MPC
Several reports have comprehensively analyzed the genetic mutations in MPC. 47 , 48 , 49 , 50 , 51 , 52 Alteration of some genes, such as tumor protein p53 (TP53), Kirsten rat sarcoma virus (KRAS), epidermal growth factor receptor (EGFR), phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit alpha, and v‐raf (rapidly accelerated fibrosarcoma) homolog B (BRAF), is reportedly more frequently detected in MPC than non‐MPC (Table S1), although it is difficult to draw any definitive conclusions because the number of the samples is too low and there are many cases with mixed phenotype both with the MPC and non‐MPC regions within a tumor. Whether they are the “driver” alterations of MPC remains to be functionally elucidated by using in vitro models such as the one reported here. In lung cancer, micropapillary predominant lung adenocarcinoma frequently harbored driver mutations in EGFR. 48 In thyroid cancer, papillary thyroid carcinoma (PTC) is the most common histological type and is less malignant. However, the rare micropapillary/hobnail variant of PTC has been considered an aggressive subtype and has a high incidence of the BRAFV600E mutation. 50 In colorectal cancer, TP53 alterations (mutations and/or accumulation) were detected more frequently than in the micropapillary carcinoma cases. 47 In ovarian cancer, Singer et al. proposed the stepwise progression of low‐grade serous carcinoma from serous borderline tumors to invasive MPC where KRAS mutation is involved. 53 Mutations in tetratricopeptide repeat domain‐7A have been found in patients with multiple intestinal atresias. Furthermore, intestinal organoid cultures from patient biopsies displayed inverted polarity of the epithelial cells. 54 In any case, no single, common mutation responsible for MPC likely exists.
1.8. Experimental model of MPC
Few experimental models of MPC exist. Although not a cancer model, MDCK cells show MPC‐like inverted polarity when cultured in ECM under inhibition of integrin β1. 3 , 10 Apart from polarity dysregulation, 3D cultured HCT116 cells in floating conditions were proposed as an MPC model according to the similarity of glucose metabolism and inactive cell proliferation. 55 Nonetheless, the model should better represent the disease and be established from the disease. Recently, an organoid model of MPC prepared from CRC patient tumors with MPC features was developed. 32 The MPC organoids showed apical‐out status even in the Matrigel embedded condition, recapitulating the MPC phenotype in the tumors (Figure 4). Xenografts generated by the organoids showed the MPC phenotype.
FIGURE 4.
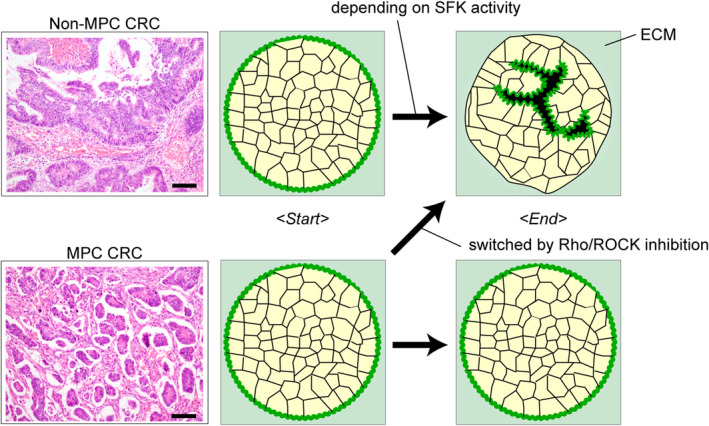
Impaired polarity switching in an micropapillary carcinoma (MPC) colorectal cancer (CRC) organoid. Hematoxylin and Eosin (HE) staining of an MPC (upper left) and a non‐MPC (lower left) CRC tumor is shown, from which the organoids are prepared. Note the apical‐out status of the cancer cells embedded in extracellular matrix (ECM) in the MPC tumor. Polarity switching does not occur in MPC organoids but occurs with RHO/RHO‐associated kinase (ROCK) inhibition.
1.9. Aberrant activation of RHO–ROCK signaling in the MPC organoid
Onuma et al. utilized the MPC model to investigate the molecular mechanism underlying the MPC phenotype. 32 RhoA was reported to be a regulator of polarity status as a downstream signaling target of integrin in MDCK cells. 19 Both protein levels and the active form of RhoA were increased in the MPC organoids. Suppression of RhoA signaling enabled the MPC organoids to complete polarity switching in vitro32 (Figure 4). Additionally, when RhoA was suppressed, the xenografts of the MPC organoids showed a non‐MPC phenotype. Notably, the MPC phenotype reverted even when the MPC organoids were pretreated with a ROCK inhibitor before injecting into the mice. This suggested that the etiology of MPC might be a failure of polarity switching due to the disability of sensing ECM.
1.10. Treatment of MPC targeting abnormal polarity
MPC is associated with more malignant phenotypes than other histopathological subtypes. Unfortunately, no specific treatment has been established yet. 56 Clarifying the mechanisms underlying the dysregulated polarity might lead to the development of novel treatments. The interchangeable nature of the inverted polarity status in MPC at least in an experimental setting implies that reversion of the inverted polarity could be an effective therapeutic approach to treat MPC. Indeed, a ROCK inhibitor can prevent the inverted polarity in the MPC CRC organoid model when the inhibitor was added at the polarity switching. 32 However, the delayed addition of the ROCK inhibitor after the formation of the inverted polarity did not show a significant effect. Therefore, activation of ROCK in MPCs might be required only for polarity switching on contact to ECM but not for maintenance of the MPC phenotype.
1.11. Perspectives
The story of dysregulated polarity in differentiated adenocarcinoma is just beginning to unfold. Further research will be necessary. Areas to further investigate include the following: the functional roles of the inverted polarity, the interaction with endothelial cells, peritoneal cells, and the immune system, and the abnormal direction of secretion or shedding of apical proteins. The molecular mechanisms of metastasis in MPC can be investigated via MPC organoids. If the inverted polarity is only seen in pathological states such as cancer, it could provide a crucial target for therapy. Using in vitro models may contribute to future drug discovery.
AUTHOR CONTRIBUTIONS
Conception and design: K.O. and M.I. Administrative support: M.I. Manuscript writing: K.O. and M.I. Final approval of manuscript: K.O. and M.I.
ACKNOWLEDGMENTS
This work was supported by the Japan Society for the Promotion of Science Grant‐in‐Aid for Scientific Research (C) 20K08286 (K.O. and M.I.), Scientific Research (B) 18H02648 (M.I. and K.O.), and a Grant‐in‐Aid from P‐CREATE, a Japan Agency for Medical Research and Development, Japan, JP21cm0106203 (M.I. and K.O.).
FUNDING INFORMATION
This work was supported by the Japan Society for the Promotion of Science Grant‐in‐Aid for Scientific Research (C) 20K08286 (K.O. and M.I.), Scientific Research (B) 18H02648 (M.I. and K.O.), and a Grant‐in‐Aid from P‐CREATE, a Japan Agency for Medical Research and Development, Japan, JP21cm0106203 (M.I. and K.O.).
CONFLICT OF INTEREST
K.O. and M.I. belong to the Department of Clinical Bio‐resource Research and Development at Kyoto University, which is sponsored by KBBM, Inc. M.I. is an inventor of the patents related to the CTOS method. The corresponding author is a current associate editor of Cancer Science.
ETHICS STATEMENT
Approval of the research protocol by an institutional reviewer board: N/A. Informed consent: N/A. Registry and the registration no. of the study/trial: N/A. Animal studies: N/A.
Supporting information
Table S1
Onuma K, Inoue M. Abnormality of apico–basal polarity in adenocarcinoma. Cancer Sci. 2022;113:3657‐3663. doi: 10.1111/cas.15549
REFERENCES
- 1. Buckley CE, St JD. Apical‐basal polarity and the control of epithelial form and function. Nat Rev Mol Cell Biol. 2022;23:559‐577. [DOI] [PubMed] [Google Scholar]
- 2. Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bryant DM, Roignot J, Datta A, et al. A molecular switch for the orientation of epithelial cell polarization. Dev Cell. 2014;31:171‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez‐Boulan E, Musch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim Biophys Acta. 2005;1744:455‐464. [DOI] [PubMed] [Google Scholar]
- 6. Goehring NW. PAR polarity: from complexity to design principles. Exp Cell Res. 2014;328:258‐266. [DOI] [PubMed] [Google Scholar]
- 7. Bonello TT, Peifer M. Scribble: a master scaffold in polarity, adhesion, synaptogenesis, and proliferation. J Cell Biol. 2019;218:742‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan B, Yatim S, Peng S, Gunaratne J, Hunziker W, Ludwig A. The mammalian crumbs complex defines a distinct polarity domain apical of epithelial tight junctions. Curr Biol. 2020;30(2791–804):e6. [DOI] [PubMed] [Google Scholar]
- 9. Stoops EH, Caplan MJ. Trafficking to the apical and basolateral membranes in polarized epithelial cells. J Am Soc Nephrol. 2014;25:1375‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell‐cell and cell‐substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):137‐151. [DOI] [PubMed] [Google Scholar]
- 11. Bryant DM, Datta A, Rodriguez‐Fraticelli AE, Peranen J, Martin‐Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Brien LE, Jou TS, Pollack AL, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831‐838. [DOI] [PubMed] [Google Scholar]
- 13. Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu W, Datta A, Leroy P, et al. Beta1‐integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the "omics" era. Matrix Biol. 2016;49:10‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928‐7946. [DOI] [PubMed] [Google Scholar]
- 18. Choma DP, Milano V, Pumiglia KM, DiPersio CM. Integrin alpha3beta1‐dependent activation of FAK/Src regulates Rac1‐mediated keratinocyte polarization on laminin‐5. J Invest Dermatol. 2007;127:31‐40. [DOI] [PubMed] [Google Scholar]
- 19. Yu W, Shewan AM, Brakeman P, et al. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 2008;9:923‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M. ROCK1‐directed basement membrane positioning coordinates epithelial tissue polarity. Development. 2012;139:411‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470‐480. [DOI] [PubMed] [Google Scholar]
- 22. Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahai E, Marshall CJ. RHO‐GTPases and cancer. Nat Rev Cancer. 2002;2:133‐142. [DOI] [PubMed] [Google Scholar]
- 24. Bostwick DG, Cheng L. Precursors of prostate cancer. Histopathology. 2012;60:4‐27. [DOI] [PubMed] [Google Scholar]
- 25. Fomicheva M, Tross EM, Macara IG. Polarity proteins in oncogenesis. Curr Opin Cell Biol. 2020;62:26‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376‐388. [DOI] [PubMed] [Google Scholar]
- 27. Kondo J, Endo H, Okuyama H, et al. Retaining cell‐cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci USA. 2011;108:6235‐6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sato T, Stange DE, Ferrante M, et al. Long‐term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762‐1772. [DOI] [PubMed] [Google Scholar]
- 29. Okuyama H, Kondo J, Sato Y, et al. Dynamic change of polarity in primary cultured spheroids of human colorectal adenocarcinoma and its role in metastasis. Am J Pathol. 2016;186:899‐911. [DOI] [PubMed] [Google Scholar]
- 30. Co JY, Margalef‐Catala M, Li X, et al. Controlling epithelial polarity: a human Enteroid model for host‐pathogen interactions. Cell Rep. 2019;26(2509–20):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zajac O, Raingeaud J, Libanje F, et al. Tumour spheres with inverted polarity drive the formation of peritoneal metastases in patients with hypermethylated colorectal carcinomas. Nat Cell Biol. 2018;20:296‐306. [DOI] [PubMed] [Google Scholar]
- 32. Onuma K, Sato Y, Okuyama H, et al. Aberrant activation of rho/ROCK signaling in impaired polarity switching of colorectal micropapillary carcinoma. J Pathol. 2021;255:84‐94. [DOI] [PubMed] [Google Scholar]
- 33. Watanabe S. The metastasizability of tumor cells. Cancer. 1954;7:215‐223. [DOI] [PubMed] [Google Scholar]
- 34. Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small‐cell lung cancer. J Clin Oncol. 2012;30:525‐532. [DOI] [PubMed] [Google Scholar]
- 35. Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889‐894. [PubMed] [Google Scholar]
- 38. Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649‐5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maddipati R, Stanger BZ. Pancreatic cancer Metastases Harbor evidence of Polyclonality. Cancer Discov. 2015;5:1086‐1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wrenn ED, Yamamoto A, Moore BM, et al. Regulation of collective metastasis by Nanolumenal signaling. Cell. 2020;183:395.e19‐410.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heikenwalder M, Lorentzen A. The role of polarisation of circulating tumour cells in cancer metastasis. Cell Mol Life Sci. 2019;76:3765‐3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11:297‐303. [DOI] [PubMed] [Google Scholar]
- 43. Yang YL, Liu BB, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: an update. Arch Pathol Lab Med. 2016;140:799‐805. [DOI] [PubMed] [Google Scholar]
- 44. Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660‐662. [PubMed] [Google Scholar]
- 45. Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004;17:1045‐1050. [DOI] [PubMed] [Google Scholar]
- 46. Li YS, Kaneko M, Sakamoto DG, Takeshima Y, Inai K. The reversed apical pattern of MUC1 expression is characteristics of invasive micropapillary carcinoma of the breast. Breast Cancer. 2006;13:58‐63. [DOI] [PubMed] [Google Scholar]
- 47. Verdu M, Roman R, Calvo M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol. 2011;24:729‐738. [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Wang R, Cai D, et al. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol. 2014;9:1772‐1778. [DOI] [PubMed] [Google Scholar]
- 49. Gruel N, Benhamo V, Bhalshankar J, et al. Polarity gene alterations in pure invasive micropapillary carcinomas of the breast. Breast Cancer Res. 2014;16:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morandi L, Righi A, Maletta F, et al. Somatic mutation profiling of hobnail variant of papillary thyroid carcinoma. Endocr Relat Cancer. 2017;24:107‐117. [DOI] [PubMed] [Google Scholar]
- 51. Zhang S, Xu Y, Zhao P, et al. Integrated analysis of genomic and immunological features in lung adenocarcinoma with micropapillary component. Front Oncol. 2021;11:652193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi Q, Shao K, Jia H, et al. Genomic alterations and evolution of cell clusters in metastatic invasive micropapillary carcinoma of the breast. Nat Commun. 2022;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singer G, Kurman RJ, Chang HW, Cho SK, Shih IM. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160:1223‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bigorgne AE, Farin HF, Lemoine R, et al. TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J Clin Invest. 2014;124:328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vyas M, Patel N, Celli R, Wajapeyee N, Jain D, Zhang X. Glucose metabolic reprogramming and cell proliferation arrest in colorectal micropapillary carcinoma. Gastroenterology Res. 2019;12:128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li W, Han Y, Wang C, et al. Precise pathologic diagnosis and individualized treatment improve the outcomes of invasive micropapillary carcinoma of the breast: a 12‐year prospective clinical study. Mod Pathol. 2018;31:956‐964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


