Abstract
To investigate the association between the onset, severity, and type of immune‐related adverse events (irAEs) and the efficacy of pembrolizumab in patients with platinum‐pretreated advanced urothelial carcinoma (UC), we retrospectively collected clinical datasets of 755 patients and conducted landmark analysis. Patients who survived for fewer than 3 months were excluded from the evaluation to reduce the immortal time bias. In total, 620 patients were evaluated, of whom 220 patients (35.5%) experienced grade ≥2 irAEs, including 134 patients with grade 2 irAEs and 86 with grade ≥3 irAEs. Propensity score matching extracted 198 patients with and without grade ≥2 irAEs. The onset of grade ≥2 irAEs was associated with longer median progression‐free survival (PFS) (8.3 months vs. 4.5 months, p = 0.003) and overall survival (OS) (20.4 months vs. 14.3 months, p = 0.031) and a higher objective response rate (ORR) (44.8% vs. 30.2%, p = 0.004). Patients with grade 2 irAEs had significantly better oncological outcomes (PFS, OS, and ORR) than grade ≤1 and ≥3 irAEs. Patients with grade ≥3 irAEs had worse outcomes than grade 2 irAEs. Endocrine and skin irAEs were related with better survival outcomes, and the rate of severities was lower in these categories. In conclusion, the occurrence of irAEs, particularly low‐grade irAEs, was predictive of pembrolizumab efficacy in patients with platinum‐pretreated advanced UC.
Keywords: immortal time bias, immune‐related adverse events, pembrolizumab, therapeutic efficacy, urothelial carcinoma
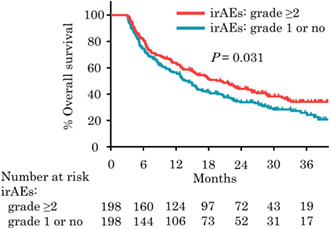
Kaplan‐Meier plots analysis showing OS for 396 patients with grade ≥2 irAEs and with grade 1 or no irAEs who survived longer than 3 months after propensity score matching. The onset of grade ≥2 irAEs was associated with longer median OS (20.4 months vs. 14.3 months, p = 0.031).
1. INTRODUCTION
Urothelial carcinoma (UC) is the fourth leading type of cancer, and more than 10% of cases are diagnosed in advanced stages. 1 Although platinum‐based chemotherapy remains the mainstay of first‐line treatment for metastatic UCs, the prognosis of platinum‐pretreated advanced UC is poor because of the lack of proven later‐line treatments. 2 The establishment of immune checkpoint inhibitors (ICIs) revolutionized the treatment of advanced UC in patients who failed prior platinum chemotherapy, and currently, four different ICIs have been approved by the Food and Drug Administration. 3 , 4 , 5 , 6
Pembrolizumab, a humanized monoclonal anti–programmed death 1 (PD‐1) antibody, exerts antitumor efficacy by inhibiting the negative regulation of immune response, which promotes cytotoxic T cell activity against cancer cells. According to the Keynote‐045 trial, pembrolizumab significantly prolonged overall survival (OS) versus conventional chemotherapy in patients with platinum‐pretreated advanced UCs (10.3 months vs. 7.4 months, p = 0.002). 6 Hyperactivation of the immune system by ICIs could damage normal tissues in various organs, and such events are termed immune‐related adverse events (irAEs). The rate of irAEs with ICIs was reported to be 26.8% for any‐grade events and 6.1% for grade ≥3 events, 7 and 5.6% of patients discontinued treatment because of adverse events (AEs). 6
However, the number of responders to ICIs is limited, and biomarkers for predicting ICI efficacy are lacking. In addition to oncological features such as a microsatellite instability–high status and programmed death ligand 1 (PD‐L1) expression, clinical features including the performance status (PS) and neutrophil‐to‐lymphocyte ratio (NLR) were previously studied as biomarkers. 8 , 9 , 10 Recently, an association between the onset of irAEs and the therapeutic efficacy of ICIs was suggested in melanoma and non–small cell lung cancer (NSCLC). 11 , 12 , 13 Rapid disease progression during ICI treatment would make irAEs clinically undetectable, which is one of the immortal time biases that underlie the evaluation of irAEs. 8 , 14 While there have been reports mentioning the relationship of irAEs with outcome in advanced UC, 15 , 16 , 17 , 18 due to the small sample size, study design, and frequency of irAEs, these previous studies have not accounted for this bias and the results remain controversial. Therefore, we attempted to minimize this immortal time bias by using large‐scale real‐world data, which enabled us to exclude cases who died early and to analyze the real association between irAEs and the efficacy of pembrolizumab in patients with platinum‐pretreated advanced UCs.
2. MATERIALS AND METHODS
2.1. Patients
This multicenter retrospective study was approved by the institutional review boards of 59 medical institutions in Japan (approval number at Kyoto University Graduate School of Medicine was R1783). This study conformed to the provisions of the Declaration of Helsinki. In total, 755 patients with platinum‐pretreated advanced UC who started treatment with pembrolizumab from August 2015 to December 2019 were enrolled. Clinical datasets, including age, gender, smoking history, primary tumor site, variant histology, surgical removal of primary tumor, metastatic sites, number of prior chemotherapy, hemoglobin, albumin, NLR, Eastern Cooperative Oncology Group PS, number of pembrolizumab cycles, and irAEs, were collected until September 2021. In this study, treatment‐related AEs excluding infection and events evidently due to disease progression were regarded as irAEs. Pembrolizumab administered after perioperative chemotherapy was categorized as the first‐line treatment.
2.2. Objectives
The primary objective of this study was to investigate the relationship between the occurrence of irAEs and the therapeutic efficacy of pembrolizumab in advanced UC. The efficacy of pembrolizumab was assessed using the objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors version 1.1 and by progression‐free survival (PFS) and OS. PFS was defined as the time from the initiation of pembrolizumab to objective disease progression based on radiological assessments, and OS was defined as the time from the initiation of pembrolizumab therapy to death from any cause or the last day of follow‐up. The secondary objective was to assess the impact of the severity and spectrum of irAEs on the efficacy of pembrolizumab. The severity of irAEs was assessed according to Common Terminology Criteria for Adverse Events version 4.0, and grade ≥2 irAEs were regarded as clinically significant irAEs in this study. irAEs were categorized as hematologic, gastrointestinal, general fatigue, hepatic, renal, neurological, pulmonary, skin, endocrine, musculoskeletal, infusion reaction, and other.
2.3. Statistical analysis
Landmark analysis was conducted, and to minimize the immortal time bias, patients who survived more than 3 months were included in the evaluation. Continuous parameters were assessed using the Mann‐Whitney U test or Kruskal‐Wallis test, and categorical data were examined using Pearson's chi‐square test or Fisher's exact test. We used a propensity score matching (PSM) method to achieve the between‐group comparability of the patients with and without clinically significant irAE (Figure 1). PSM was performed using the logistic regression model. Covariates including age, sex, smoking history, primary tumor site (upper urinary tract, bladder and urethra, or both), variant histology, surgical removal of the primary tumor, metastatic sites (lymph nodes, viscera, and liver), number of prior chemotherapies (pembrolizumab in the first, second, third, or fourth line or later), hemoglobin, albumin, NLR, and PS (0, 1, or ≥2) were converted into propensity scores. For the sensitivity analysis, two independent attempts were made to transfer 20% of randomly selected patients with grade ≥2 irAEs to a group of patients with grade 1 or no irAE before PSM was performed. The caliper width was 20% of the standard deviation of the logit of the score. The Kaplan‐Meier method with the log‐rank test was used to estimate OS and PFS. Cox proportional hazard models were used to model the hazard ratios (HRs) and 95% confidence intervals (CIs). All tests were two tailed, and p < 0.05 was defined as statistically significant. All statistical analyses were performed using JMP Pro (version 16.1.0, SAS Institute Inc.).
FIGURE 1.
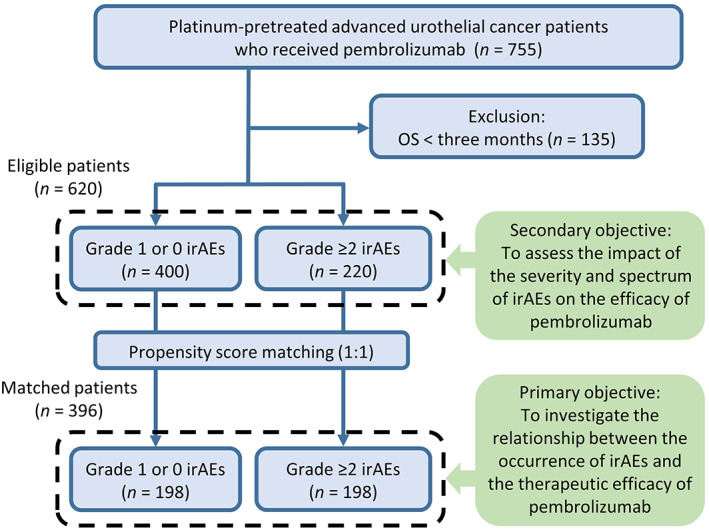
Flowchart of the study
3. RESULTS
3.1. Patients
We identified 220 patients with grade ≥2 irAEs and 400 with grade 1 or no irAEs who survived longer than 3 months. In total, the median patient age was 71 years, 466 patients (75.2%) were male, and 545 patients (87.9%) had a PS of 0 or 1. The primary tumor was located in the upper urinary tract in 272 patients (43.9%) and in the bladder or urethra in 306 patients (49.4%), and 356 patients (57.4%) underwent primary tumor resection. Variant histology appeared in 59 patients (9.5%). Metastasis to the lymph nodes, viscera, and the liver was detected in 415 (66.9%), 363 (58.5%), and 91 patients (14.7%), respectively. Pembrolizumab was mainly initiated as second‐line therapy (60.6%). Baseline patient characteristics are presented in Table 1.
TABLE 1.
Baseline characteristics of patients classified by immune‐related adverse events (irAEs) who survived longer than 3 months before and after propensity score matching
| Variable | Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 2 irAEs | Grade ≥3 irAEs | Grade ≥2 irAEs | Grade 1 or no irAEs | P | Grade ≥2 irAEs | Grade 1 or no irAEs | P | SD | |
| n = 134 | n = 86 | n = 220 | n = 400 | n = 198 | n = 198 | ||||
| Age, years, median (range) | 73 (66‐77) | 71 (66‐75) | 72 (66‐77) | 71 (65‐77) | 0.671 | 72 (66‐77) | 73 (67‐77) | 0.352 | 0.041 |
| Gender, n (%) | |||||||||
| Male | 96 (71.6) | 66 (76.7) | 162 (73.6) | 304 (76.0) | 0.560 | 146 (73.7) | 147 (74.2) | 0.909 | 0.011 |
| Female | 38 (28.4) | 20 (23.3) | 58 (26.4) | 96 (24.0) | 52 (26.3) | 51 (25.8) | |||
| Smoking history, n (%) | 72 (53.7) | 50 (58.1) | 122 (55.5) | 228 (57.0) | 0.735 | 111 (56.1) | 117 (59.1) | 0.611 | 0.061 |
| Primary tumor site, n (%) | |||||||||
| Upper urinary tract | 64 (47.8) | 44 (47.8) | 108 (49.1) | 164 (41.0) | 0.124 | 92 (46.5) | 91 (46.0) | 0.954 | 0.010 |
| Bladder/urethra | 58 (43.3) | 37 (43.0) | 95 (43.2) | 211 (52.8) | 91 (46.0) | 89 (45.0) | |||
| Both | 12 (9.0) | 4 (4.7) | 16 (7.3) | 23 (5.8) | 14 (7.1) | 16 (8.1) | |||
| Variant histology, n (%) | 12 (9.0) | 6 (7.0) | 18 (8.2) | 41 (10.3) | 0.475 | 16 (8.1) | 11 (5.6) | 0.426 | 0.099 |
| Surgical removal of primary site, n (%) | 77 (57.5) | 45 (52.3) | 122 (55.5) | 234 (58.5) | 0.497 | 109 (55.1) | 114 (57.6) | 0.685 | 0.403 |
| Metastatic site, n (%) | |||||||||
| Lymph nodes | 90 (67.2) | 56 (65.1) | 146 (66.4) | 269 (67.3) | 0.859 | 133 (67.2) | 133 (67.2) | 1.000 | 0.000 |
| Viscera | 80 (59.7) | 45 (52.3) | 125 (56.8) | 238 (59.5) | 0.551 | 114 (57.6) | 114 (57.6) | 1.000 | 0.000 |
| Liver | 20 (14.9) | 13 (15.1) | 33 (15.0) | 58 (14.5) | 0.906 | 31 (15.7) | 31 (15.7) | 1.000 | 0.000 |
| Line of pembrolizumab, n (%) | |||||||||
| First | 17 (12.7) | 14 (16.3) | 31 (14.1) | 69 (17.3) | 0.232 | 31 (15.7) | 30 (15.2) | 0.888 | 0.072 |
| Second | 82 (61.2) | 48 (55.8) | 130 (59.1) | 246 (61.5) | 119 (60.1) | 126 (63.6) | |||
| Third | 28 (20.9) | 20 (23.3) | 48 (21.8) | 62 (15.5) | 37 (18.7) | 33 (16.7) | |||
| Fourth or more | 7 (5.2) | 4 (4.7) | 11 (5.0) | 23 (5.8) | 11 (5.6) | 9 (4.6) | |||
| Hb, g/dl, median (range) | 11.1 (9.8‐12.1) | 10.9 (9.2‐12.1) | 11.0 (9.6‐12.1) | 10.7 (9.4‐12.1) | 0.270 | 10.9 (9.4‐12.0) | 10.7 (9.6‐12.3) | 0.823 | 0.046 |
| Alb, g/dl, median (range) | 3.8 (3.5‐4.1) | 3.7 (3.2‐4.1) | 3.8 (3.4‐4.1) | 3.8 (3.4‐4.1) | 0.843 | 3.8 (3.4‐4.1) | 3.7 (3.4‐4.1) | 0.610 | 0.002 |
| NLR, median (range) | 3.0 (2.0‐4.3) | 3.4 (2.1‐4.6) | 3.2 (2.0‐4.3) | 3.1 (2.1‐4.7) | 0.515 | 3.2 (2.0‐4.6) | 3.2 (2.2‐4.6) | 0.600 | 0.009 |
| ECOG PS, n (%) | |||||||||
| 0 | 73 (54.5) | 36 (41.9) | 109 (49.6) | 226 (56.5) | 0.168 | 103 (52.0) | 108 (54.6) | 0.878 | 0.052 |
| 1 | 48 (35.8) | 37 (43.0) | 85 (38.6) | 125 (31.3) | 72 (36.4) | 67 (33.8) | |||
| ≥2 | 13 (9.7) | 13 (15.1) | 26 (11.8) | 49 (12.3) | 23 (11.6) | 23 (11.6) | |||
| irAE maximum grade, n (%) | |||||||||
| Grade 2 | 134 (60.9) | 0 | 134 (60.9) | 0 | 121 (61.1) | 0 | |||
| Grade 3 | 0 | 69 (31.4) | 69 (31.4) | 0 | 62 (31.3) | 0 | |||
| Grade 4 | 0 | 17 (7.7) | 17 (7.7) | 0 | 15 (7.6) | 0 | |||
| Grade 5 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Cycles of pembrolizumab, n, median (range) | 9 (5‐20) | 4.5 (3‐10) | 7 (3‐17) | 7 (4‐16) | 0.714 | 7 (4‐17) | 7 (4‐16) | 0.773 | |
Abbreviations: Alb, albumin; ECOG PS, Eastern Cooperative Oncology Group performance status; Hb, hemoglobin; NLR, neutrophil‐to‐lymphocyte ratio; SD, standardized difference.
3.2. irAEs
Among the 620 enrolled patients, 220 patients (35.5%) experienced grade ≥2 irAEs, including 134 patients (60.9%) with grade 2 irAEs and 86 (39.1%) with grade ≥3 irAEs. There were no grade 5 irAEs. The numbers and characteristics of irAEs are presented in Table 2. Among grade ≥2 irAEs, endocrine (n = 60, 9.7%), skin (n = 53, 8.5%), gastrointestinal (n = 43, 6.9%), pulmonary (n = 29, 4.7%), and hepatic events (n = 26, 4.2%) occurred most frequently. The proportion of severe events (grade ≥3/ grade ≥2) for each irAE was higher in the renal (76.9%), pulmonary (65.5%), and musculoskeletal categories (60.0%) and lower in the infusion reaction (0%), skin (13.2%), neurological (18.8%), and endocrine categories (25.0%). Multiple irAEs were observed in 64 patients (29.1%). Treatment discontinuation owing to irAEs occurred in 28 patients (20.9%) with grade 2 irAEs and 28 (32.6%) with grade ≥3 irAEs. There was no predictive factor for the presence of irAEs in our cohort (data not shown).
TABLE 2.
Details of immune‐related adverse events (irAEs)
| Category of irAEs | Grade ≥2 | Grade 2 | Grade ≥3 | ROS |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | % | |
| Endocrine | 60 (9.7) | 45 (7.3) | 15 (2.4) | 25.0 |
| Skin | 53 (8.5) | 46 (7.4) | 7 (1.1) | 13.2 |
| Gastrointestinal | 43 (6.9) | 32 (5.2) | 11 (1.8) | 25.6 |
| Pulmonary | 29 (4.7) | 10 (1.6) | 19 (3.1) | 65.5 |
| Hepatic | 26 (4.2) | 15 (2.4) | 11 (1.8) | 42.3 |
| General fatigue | 23 (3.7) | 16 (2.6) | 7 (1.1) | 30.4 |
| Neurological | 16 (2.6) | 13 (2.1) | 3 (0.5) | 18.8 |
| Hematologic | 14 (2.3) | 10 (1.6) | 4 (0.6) | 28.6 |
| Renal | 13 (2.1) | 3 (0.5) | 10 (1.6) | 76.9 |
| Musculoskeletal | 5 (0.8) | 2 (0.3) | 3 (0.5) | 60.0 |
| Infusion reaction | 4 (0.6) | 4 (0.6) | 0 (0.0) | 0.0 |
| Other | 16 (2.6) | 8 (1.3) | 8 (1.3) | 50.0 |
| Total | 302 | 204 | 98 | 32.5 |
Abbreviations: ROS, rate of severity = grade ≥3 / grade ≥2.
3.3. Occurrence of clinically significant irAEs and efficacy
After stratifying patients on the basis of the occurrence of clinically significant irAEs, 198 patients were extracted from each group by PSM and included in further analyses. Baseline characteristics were well balanced between the groups (Table 1). Median PFS was 8.3 months in patients with grade ≥2 irAEs, versus 4.5 months in patients with grade 1 or no irAEs (p = 0.003; Figure 2A), and median OS was 20.4 months in patients with grade ≥2 irAEs, versus 14.3 months in patients with grade 1 or no irAEs (p = 0.031; Figure 2B). ORR was significantly higher in patients with grade ≥2 irAEs (44.8% vs. 30.2%, p = 0.004). No difference was observed in the median (interquartile range [IQR]) number of cycles of pembrolizumab between patients with grade ≥2 and with grade 1 or no irAEs (7 [4‐17] vs. 7 [4‐16], p = 0.773). Multivariate analysis illustrated that the presence of grade ≥2 irAEs was associated with significantly longer PFS (HR = 0.48, 95% CI = 0.31‐0.73, p < 0.001) and a trend toward favorable OS (HR = 0.71, 95% CI = 0.48‐1.03, p = 0.070; Figure S1). The sensitivity analyses were conducted by transferring 20% of randomly selected patients with grade ≥2 irAEs to patients with grade 1 or no irAEs followed by PSM, and similar favorable prognosis was shown in patients with grade ≥2 irAEs (Figure S2). Additionally, PSM was conducted in patients with OS longer than 6 months, and the occurrence of clinically significant irAEs was also associated with significant improvements in PFS, OS, and ORR (Table S1). In patients with clinically significant irAEs, multivariate analysis illustrated that the nonsmoker was associated with good response (HR = 2.51, 95% CI = 1.25‐5.03, p = 0.010) and PS ≥1 was related with no response (HR = 0.35, 95% CI = 0.17‐0.71, p = 0.004) (Table S2).
FIGURE 2.
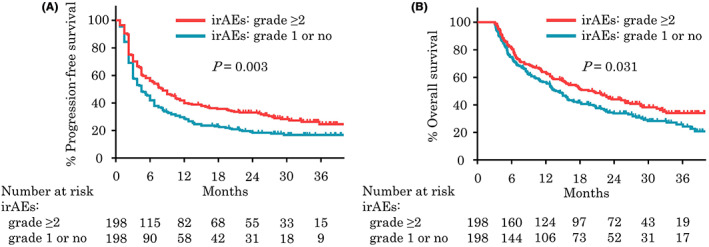
Kaplan‐Meier plots analysis showing progression‐free survival (A) and overall survival (B) for 396 patients with grade ≥2 immune‐related adverse events (irAEs) and with grade 1 or no irAEs who survived longer than 3 months after propensity score matching
3.4. Severity and spectrum of irAEs and efficacy
Comparing patients with grade ≤1 irAEs with those with grade 2 or ≥3 irAEs, median PFS (4.5 months vs. 9.0 months vs. 4.5 months, p < 0.001; Figure 3A), median OS (14.4 months vs. 23.5 months vs. 13.1 months, p < 0.001; Figure 3B), and median ORR (29.6% vs. 45.8% vs. 37.3%, p = 0.003) were significantly better among patients with grade 2 irAEs. No statistical difference in response was observed between patients with grade 1 or no irAEs and those with grade ≥3 irAEs. Interestingly, 56 patients who discontinued pembrolizumab because of irAEs had significantly longer median PFS (19.5 months vs. 5.3 months, p = 0.008) and numerically better median OS (26.9 months vs. 17.8 months, p = 0.112) than patients who continued pembrolizumab despite completing fewer cycles of pembrolizumab (median [IQR]: 5 [3‐8] vs. 8 [4‐20], p < 0.001; Figure 4). This trend was seen not only in grade 2 irAEs but also in grade ≥3 irAEs, and 58 patients who continued treatment in grade ≥3 irAEs had worse outcomes than patients who discontinued treatment (data not shown) (Appendix A).
FIGURE 3.
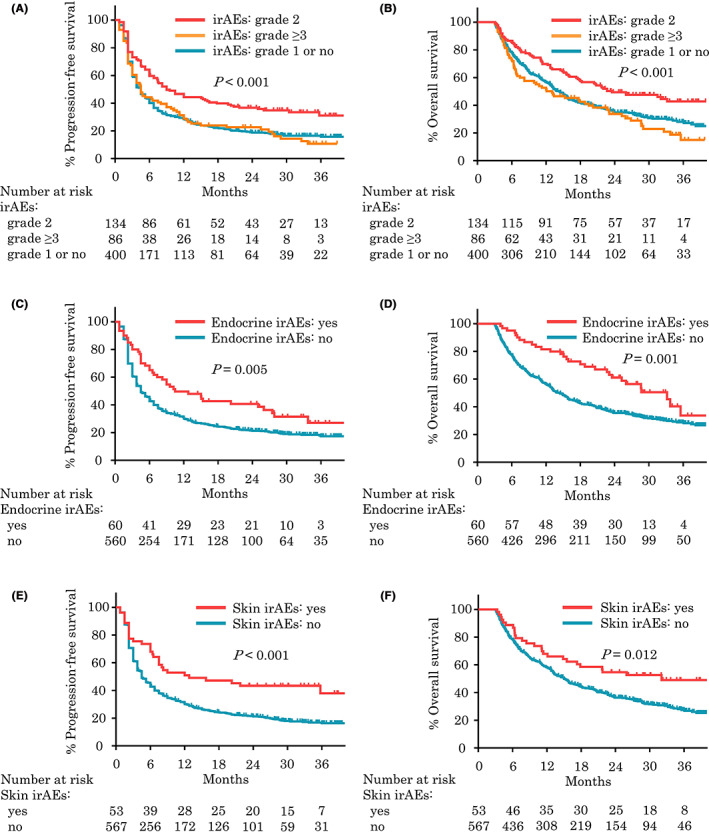
Kaplan‐Meier plots analysis showing progression‐free survival and overall survival for patients with grade 2 or ≥3 immune‐related adverse events (irAEs) (A, B), endocrine irAEs (C, D), and skin irAEs (E, F) who survived longer than 3 months
FIGURE 4.
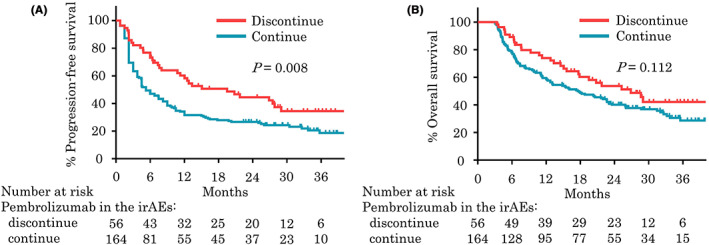
Kaplan‐Meier plots analysis showing progression‐free survival (A) and overall survival (B) for patients with immune‐related adverse events (irAEs) who did and did not discontinue pembrolizumab
When investigating each type of irAEs and the efficacy of pembrolizumab, only endocrine and skin irAEs were related with significantly better survival outcomes. Median PFS was 10.5 months (p = 0.005; Figure 3C) in patients with endocrine irAEs and 12.8 months (p < 0.001; Figure 3E) in those with skin irAEs. Median OS was 33.2 months (p = 0.001; Figure 3D) in patients with endocrine irAEs and 32.1 months (p = 0.012; Figure 3F) in patients with skin irAEs. Patients with single and multiple irAEs had similar objective responses (median PFS: 8.3 months vs. 6.0 months, p = 0.140; median OS: 18.8 months vs. 22.0 months, p = 0.641; Figure S3).
4. DISCUSSION
In this retrospective study, the occurrence of irAEs, especially low‐grade irAEs, was strongly associated with higher ORR and longer PFS and OS in patients with platinum‐pretreated advanced UC receiving pembrolizumab. The relationship between the onset of irAEs and ICI efficacy was recently described in some meta‐analyses, and patients with anti‐PD‐1/L1 antibody–related AEs had better PFS, OS, and ORR. 12 , 13 However, most of these findings are from patients with melanoma or NSCLC, with limited reports based on data from patients with UC. 15 , 16 , 17 , 18 , 19 Maher et al. conducted a pooled analysis of 1747 patients with advanced UC from seven clinical trials, and better OS was observed in patients with anti‐PD‐1/L1 antibody–related AEs (HR = 0.45, 95% CI = 0.39‐0.52). 15 Kijima et al. retrospectively evaluated 97 patients with UC receiving pembrolizumab and revealed significant improvements in ORR (52% vs. 16%, p < 0.01) and PFS (11.0 months vs. 3.6 months, p = 0.02) in patients with irAEs. 16
One of the problems in analyzing patients with and without irAEs is the immortal time bias. 8 , 14 Because most irAEs occur within the first few weeks to months of treatment initiation, 20 irAEs in patients with short survival might be clinically undetected, resulting in patients being classified as irAE‐free. In the setting of ICI usage in the second or later lines in particular, survival tends to be short, and this bias could be sufficient to change the results. Unfortunately, most previous reports focusing on patients with UC could not consider this bias because of their small sample sizes or study designs. Recently, Kawai et al. reported that the incidence of irAEs is a predictor of the efficacy of pembrolizumab in UC after minimizing this bias using time‐dependent analysis, whereby the survival time of each patient who experienced irAEs from time of starting pembrolizumab treatment to time of final observation was divided into time from the start of pembrolizumab to the onset of initial irAE and time after the onset of initial irAE. 21 By assigning the former period to the irAEs (−) cohort and the latter period to the irAEs (+) cohort, the survival time of the irAE (+) cohort was underestimated, allowing for a more conservative analysis than landmark analysis. While their method of minimizing the effect of immortal time bias by time‐dependent analysis is commendable, even with that method, this problem has not been completely resolved because the modified irAE (−) cohort still includes many early deaths.
Although our landmark analysis did not fully resolve the immortal bias, by excluding patients with survival periods shorter than 3 months in the present study, this underling bias was reduced, thereby strengthening our findings. Three months seemed to be long enough as the setting of landmark because the median time of onset of overall irAEs is about 2 months, and majority of irAEs appears within 3 months, as previously reported. 22 , 23 The results were similar when the analysis was limited to patients who survived more than 6 months. To further emphasize the significance of irAEs as a predictor of efficacy to pembrolizumab, a sensitivity analysis was also performed. Because only about 20%‐30% of all irAEs occur after 3 months of treatment, 22 , 24 a virtual cohort was created by randomly selecting 20% of patients from a cohort of patients who survived at least 3 months and had grade ≥2 irAEs and transferring them to a grade 1 or no irAE cohort. Despite the attempt narrowing the survival difference between the two cohorts, the presence of irAE was still shown to be a predictive factor.
A representative hypothesis explaining the relationship between ICI efficacy and irAEs is the cross‐reactivity of epitopes common to the tumor and specific organ, and theoretically, hyperactivation of T cells induced by ICIs might result in better tumor responses and higher grades of irAEs. 25 Reports focusing on the association between the severity of irAEs and the efficacy of ICIs are scarce, but several studies including patients with UCs indicated that the development of grade ≥3 irAEs was an independent positive predictor of therapeutic efficacy, which could support the hypothesis. 16 , 26 Conversely, Zhou et al. conducted a meta‐analysis of 30 studies mainly including patients with NSCLC or melanoma and identified favorable outcomes in patients with low‐grade irAEs. 27 Kawai et al. evaluated 176 patients with UCs using time‐dependent analysis, and the incidence of grade 1‐2 irAEs was associated with good prognosis. 21
In the present study, we were able to identify low‐grade irAEs as a positive predictor of the efficacy of pembrolizumab in patients with UC, using different analysis methods and a larger cohort of patients. A prior study mentioned that patients with severe irAEs tended to require stronger immunosuppressants or modification of the treatment schedule to manage these events, and irAEs themselves can be fatal, which could have influenced the outcome. 8 In the present study, no irAE‐related deaths occurred, and patients with severe irAEs were more likely to discontinue pembrolizumab because of these events than patients with low‐grade irAEs. However, discontinuation of pembrolizumab because of irAEs was instead associated with better prognosis despite the short duration of treatment, and this trend was observed irrespective of the severity of irAEs. Our findings indicated that ICI efficacy might be affected more by the onset of irAEs than the duration of treatment, and therefore, modification of the treatment schedule might have less influence than expected. Although whether immunosuppressants reduce the therapeutic efficacy of ICIs is unclear, 8 , 15 the use of these treatments is one possible hypothesis to explain the inferiority of outcomes in patients with severe irAEs than in those with milder irAEs; however, we could not fully evaluate this in this study, representing a study limitation.
The relationship between the sites of irAEs and efficacy of ICIs was also previously reported in various types of cancer. In a meta‐analysis evaluating different categories of irAEs, endocrine and skin irAEs were linked to significantly longer PFS, whereas gastrointestinal, pulmonary, and hepatic irAEs had no effect on survival. 27 Recently, the development of cutaneous irAEs and their subtypes were suggested to be protective against mortality in patients treated with PD‐1 or PD‐L1 therapy. 28 In patients with UC, endocrine irAEs were associated with higher ORR and longer PFS. 16 Although why specific irAEs positively affect therapeutic efficacy remained unknown, the prior report mentioned that the manageability of events differs among the types of irAEs. 11 Our results suggested the endocrine and skin irAEs are positive prognostic factors for the outcomes of pembrolizumab in patients with UC. The proportion of severe irAEs was lower for endocrine and skin irAEs, supporting the idea that less severe irAEs contribute to better prognosis. Although some studies suggested that the number of irAEs (single or multiple) was associated with the therapeutic efficacy of ICIs, 11 , 26 we detected no difference in the present study.
This study had multiple limitations. First, selection bias attributable to the retrospective setting could exist, and comparisons between patients with and without irAEs are inevitably associated with the immortal time bias, as we previously mentioned. The time to irAE onset represents useful data, but we could not collect this information in this study. Second, we did not include grade 1 irAEs as low grade irAEs in our analysis, although it does not fit the general definition. However, regarding AEs as truly immune related or not is often challenging especially in lower grades, and it was difficult to collect all grade 1 irAEs retrospectively and accurately separate them from no irAEs, which may lead to bias. In addition, regarding grade 1 and grade 2 irAEs as same group may also lead to overestimation of the prognosis of grade 2 irAEs, because management of irAEs is usually different between two groups. To reduce these biases for analyzing clinically significant irAEs, patients were divided into three groups (grade ≤1, 2, and ≥3) in this study. Third, this study lacked a central review of imaging and pathology, as well as toxicity, and prognostic oncological biomarkers such as PD‐L1 were not assessed. Fourth, the management of irAEs, including the usage of immunosuppressants or corticosteroids and the decision to discontinue ICIs, was not standardized in our study. We could not evaluate the impact of immunosuppressants on ICI efficacy owing to heterogeneity. Additionally, promptly treated irAEs might be considered lower‐grade events, and vice versa. Nevertheless, this is the largest real‐world analysis to investigate the association between irAEs and the efficacy of pembrolizumab in advanced UC.
In conclusion, the occurrence of irAEs, particularly low‐grade irAEs, was a positive predictor of therapeutic efficacy in patients with platinum‐pretreated advanced UC receiving pembrolizumab. The discontinuation of pembrolizumab because of irAEs, which frequently occurred in patients with severe irAEs, was associated with favorable objective responses. The severity of endocrine and skin irAEs was low, and these events were numerically related with good clinical outcomes.
DISCLOSURE
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The research protocol was approved by the Institutional Review Board (IRB) of Kyoto University Graduate School of Medicine (approval number R1783) and the local IRB at each participating institute.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Fig. S1
Fig. S2
Fig. S3
Table S1
Table S2
ACKNOWLEDGMENT
We thank Joe Barber Jr., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
APPENDIX A.
Study institutes: Osaka City University, Akita University, Hirosaki University, National Cancer Center Hospital, Hamamatsu University School of Medicine, Yamagata University, Kyoto University, University of Tsukuba, Nara Medical University, Shizuoka General Hospital, University of the Ryukyus, Iwate Medical University, Oita University, Hiroshima University, Shimane University, Kansai Medical University, Osaka University, Kagawa University, University of Yamanashi, Japanese Red Cross Wakayama Medical Center, Kyoto Prefectural University of Medicine, Kobe City Nishi‐Kobe Medical Center, Japanese Red Cross Osaka Hospital, Nagoya University, Harasanshin Hospital, Hokkaido University, Japanese Red Cross Otsu Hospital, Kagoshima University, Kyushu University, Shikoku Cancer Center, Tenri Hospital, Hakodate Goryoukaku Hospital, Kitasato University, Kyoto Katsura Hospital, National Hospital Organization Kyoto Medical Center, Kumamoto University, National Hospital Organization Himeji Medical Center, Tazuke Kofukai Medical Research Institute Kitano Hospital, Toyooka Hospital, Hokkaido Cancer Center, University of Miyazaki, Hitachi General Hospital, The Jikei University Kashiwa Hospital, Shimada Municipal Hospital, Mie University, Yamaguchi University, Ibaraki Prefectural Central Hospital, Kyoto City Hospital, Kochi University, Ijinkai Takeda General Hospital, University of Toyama, Otsu City Hospital, Sapporo Medical University, Kansai Electric Power Hospital, Kurume University, Hyogo College of Medicine, Hirakata Kohsai Hospital, Rakuwakai Otowa Memorial Hospital.
Otsuka H, Kita Y, Ito K, et al. Immune‐related adverse events in urothelial cancer patients: Adjustment for immortal time bias. Cancer Sci. 2022;113:3912‐3921. doi: 10.1111/cas.15539
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7‐33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2. Cathomas R, Lorch A, Bruins HM, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81(1):95‐103. doi: 10.1016/j.eururo.2021.09.026 [DOI] [PubMed] [Google Scholar]
- 3. Powels T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum‐treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open‐label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748‐757. doi: 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 4. Sharma P, Retz M, Siefker‐Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2017;18(3):312‐322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 5. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti‐programmed death‐ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117‐2124. doi: 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015‐1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grimm MO, Bex A, De Santis M, et al. Safe use of immune checkpoint inhibitors in the multidisciplinary management of urological cancer: the European Association of Urology position in 2019. Eur Urol. 2019;76(3):368‐380. doi: 10.1016/j.eururo.2019.05.041 [DOI] [PubMed] [Google Scholar]
- 8. Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi T, Ito K, Kojima T, et al. Pre‐pembrolizumab neutrophil‐to‐lymphocyte ratio (NLR) predicts the efficacy of second‐line pembrolizumab treatment in urothelial cancer regardless of the pre‐chemo NLR. Cancer Immunol Immunother. 2022;71(2):461‐471. doi: 10.1007/s00262-021-03000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito K, Kobayashi T, Kojima T, et al. Pembrolizumab for treating advanced urothelial carcinoma in patients with impaired performance status: analysis of a Japanese nationwide cohort. Cancer Med. 2021;10(10):3188‐3196. doi: 10.1002/cam4.3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune‐related adverse events spectrum and efficacy of anti‐PD‐1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237‐247. doi: 10.1016/j.cllc.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 12. Park R, Lopes L, Saeed A. Anti‐PD‐1/L1‐associated immune‐related adverse events as harbinger of favorable clinical outcome: systemic review and meta‐analysis. Clin Transl Oncol. 2021;23(1):100‐109. doi: 10.1007/s12094-020-02397-5 [DOI] [PubMed] [Google Scholar]
- 13. Hussaini S, Chehade R, Boldt RG, et al. Association between immune‐related side effects and efficacy and benefit of immune checkpoint inhibitors‐a systematic review and meta‐analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 14. Giobbie‐Hurder A, Gelber RD, Regan MM. Challenges of guarantee‐time bias. J Clin Oncol. 2013;31(23):2963‐2969. doi: 10.1200/JCO.2013.49.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730‐2737. doi: 10.1200/JCO.19.00318 [DOI] [PubMed] [Google Scholar]
- 16. Kijima T, Fukushima H, Kusuhara S, et al. Association between the occurrence and spectrum of immune‐related adverse events and efficacy of pembrolizumab in Asian patients with advanced urothelial cancer: multicenter retrospective analyses and systematic literature review. Clin Genitourin Cancer. 2021;19(3):208‐216. doi: 10.1016/j.clgc.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi K, Suzuki K, Hiraide M, et al. Association of immune‐related adverse events with pembrolizumab efficacy in the treatment of advanced urothelial carcinoma. Oncology. 2020;98(4):237‐242. doi: 10.1159/000505340 [DOI] [PubMed] [Google Scholar]
- 18. Kawai T, Sato Y, Makino K, et al. Immune‐related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur J Cancer. 2019;116:114‐115. doi: 10.1016/j.ejca.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 19. Narita T, Hatakeyama S, Numakura K, et al. Comparison of pembrolizumab with conventional chemotherapy after first‐line platinum‐based chemotherapy for advanced urothelial carcinoma in real‐world practice: a multicenter retrospective study. Int J Urol. 2021;28(9):899‐905. doi: 10.1111/iju.14601 [DOI] [PubMed] [Google Scholar]
- 20. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158‐168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 21. Kawai T, Taguchi S, Nakagawa T, et al. Impact of immune‐related adverse events on the therapeutic efficacy of pembrolizumab in urothelial carcinoma: a multicenter retrospective study using time‐dependent analysis. J Immunother Cancer. 2022;10(2):e003965. doi: 10.1136/jitc-2021-003965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS, for the MDX010‐20 Investigators . Patterns of onset and resolution of immune‐related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675‐1682. doi: 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 23. Ngamphaiboon N, Ithimakin S, Siripoon T, Sintawichiai N, Sriuranpong V. Patterns and outcomes of immune‐related adverse events in solid tumor patients treated with immune checkpoint inhibitors in Thailand: a multicenter analysis. BMC Cancer. 2021;21(1):1275. doi: 10.1186/s12885-021-09003-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nuzzo PV, Pond GR, Abou Alaiwi S, et al. Conditional immune toxicity rate in patients with metastatic renal and urothelial cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8(1):e000371. doi: 10.1136/jitc-2019-000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immne‐related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73‐82. doi: 10.2147/ITT.S126227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma VT, Su CT, Hu M, et al. Characterization of outcomes in patients with advanced genitourinary malignancies treated with immune checkpoint inhibitors. Urol Oncol. 2021;39(7):437.e1‐437.e9. doi: 10.1016/j.urolonc.2021.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune‐related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta‐analysis. BMC Med. 2020;18(1):87. doi: 10.1186/s12916-020-01549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang K, Seo J, Tiu BC, et al. Association of cutaneous immune‐related adverse events with increased survival in patients treated with anti‐programmed cell death 1 and anti‐programmed cell death ligand 1 therapy. JAMA Dermatol. 2022;158(2):189‐193. doi: 10.1001/jamadermatol.2021.5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1
Fig. S2
Fig. S3
Table S1
Table S2


