Abstract
The impact of venous thromboembolism in Japanese colorectal cancer patients has not been elucidated. This prespecified subanalysis of the Cancer‐VTE Registry aimed to report venous thromboembolism and event data after 1 year of follow‐up in 2477 patients with colorectal cancer and investigate risk factors of venous thromboembolism. Of 2477 patients, 158 (6.4%) had venous thromboembolism in venous thromboembolism screening at enrollment. Asymptomatic distal deep‐vein thrombosis accounted for 123/158 (77.8%) of venous thromboembolism cases. During the follow‐up period, symptomatic, incidental events requiring treatment and composite venous thromboembolism incidences were 0.3%, 0.8%, and 1.0%, respectively. The incidence of bleeding events, cerebral infarction/transient ischemic attack/systemic embolic event, and all‐cause death were 1.0%, 0.3%, and 4.8%, respectively. These results were consistent with the main study results. In multivariable analysis, venous thromboembolism at baseline was a risk factor of composite venous thromboembolism during the follow‐up period. Japanese patients with colorectal cancer and advancing cancer stage before treatment had more frequent venous thromboembolism complications at baseline, higher incidence of venous thromboembolism events during cancer treatment, and higher mortality.
Keywords: abdominal neoplasms, colon, hemorrhage, rectum, venous thromboembolism
As data on venous thromboembolism (VTE) in Japanese colorectal cancer patients are unclear, this pre‐specified sub‐analysis of the Cancer‐VTE Registry reported VTE and event data after 1‐year follow‐up in 2477 patients with colorectal cancer and investigated risk factors of VTE. Symptomatic, incidental events requiring treatment, and composite VTE incidences were 0.3%, 0.8%, and 1.0%, respectively, and the incidences of bleeding events, cerebral infarction/transient ischemic attack/systemic embolic event, and all‐cause death were1.0%, 0.3%, and 4.8%, respectively. VTE at baseline was a risk factor of composite VTE during the follow‐up period.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CrCL
creatinine clearance
- CT
computed tomography
- DVT
deep‐vein thrombosis
- ECOG PS
Eastern Cooperative Oncology Group performance status
- Hb
hemoglobin
- HR
hazard ratio
- PE
pulmonary embolism
- SEE
systemic embolic event
- TIA
transient ischemic attack
- VTE
venous thromboembolism
- WBC
white blood cells
1. INTRODUCTION
Colorectal cancer is the third most common type of malignancy and the second leading cause of cancer‐related deaths globally. 1 , 2 In 2020, colorectal cancer was the most common malignancy in Japan, affecting >148,500 people and causing >59,000 deaths. 3 For patients with cancer, in addition to coping with the burden of their tumor(s), venous thromboembolism (VTE) may develop as a complication, resulting in a worsened prognosis and increased mortality risk. 4 , 5
The mechanism by which cancer patients are at increased risk of developing VTE is complex and is thought to involve a combination of factors. 6 It is known that in cancer patients, cancer cells can activate hemostasis through multiple pathways and thereby induce systemic hypercoagulability. 7 , 8 Consequently, patients with cancer are prone to developing VTE, a condition that can include both deep‐vein thrombosis (DVT) and pulmonary embolism (PE). 9
Venous thromboembolism is the number one preventable cause of postoperative mortality in patients with intraabdominal malignancies. 10 The incidence rate of VTE has also been shown to be elevated in patients with gastrointestinal cancers. 11 However, there is a lack of data on VTE in colorectal cancer patients. Notably, among patients with colorectal cancer, at least 30% of VTE events following cancer resection occur after hospital discharge. 12 , 13 Few prospective studies have investigated the incidence of and risk factors for VTE in Asian patients, although it is generally accepted that Asian cancer patients have a lower risk of VTE development compared with Western patients. 14 , 15 However, in an analysis of hospitalized Japanese patients receiving chemotherapy for malignancies, the prevalence and incidence of VTE were found to be higher. 16 The incidence of VTE is lower in colorectal cancer than in other types of cancer, such as pancreatic cancer, ovarian cancer, and gastric cancer. 17 , 18 , 19 However, as the incidence of colorectal cancer is high, VTE complications in colorectal cancer patients are likely to be encountered as frequently in clinical practice as those associated with other cancers, including lung cancer. 17 , 19
As the risk of VTE is strongly influenced by study method (prospective or database study, with or without VTE screening) and patient factors (cancer type or stage), and it varies widely among previous studies, it is clearly important to accumulate knowledge from large‐scale studies in Japanese cancer patients. 20 , 21 , 22 , 23 The large‐scale, prospective Cancer‐VTE Registry was initiated to clarify the incidence of VTE and bleeding in Japanese patients with solid tumors and to identify risk factors. 24 , 25 The aims of this prespecified subanalysis, using data from the Cancer‐VTE Registry, were to report VTE and event data after 1 year of follow‐up in the cohort of patients with colorectal cancer by subgroups according to tumor‐related variables, cancer therapy, and patient baseline characteristics.
2. MATERIALS AND METHODS
2.1. Registry design
Full details of the Cancer‐VTE Registry design have been published. 24 , 25 In brief, this was a nationwide, multicenter clinical registry with a prespecified, prospective cohort analysis over 1 year of follow‐up. Patients were enrolled from 170 Japanese medical institutions between March 2017 and February 2019, and the follow‐up period ended in February 2020. This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical Science Studies on Human Subjects by the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor, and Welfare. The ethics committee of each participating institution approved the study protocol and all related documentation.
The study was observational, and the treating physician made all management decisions. Although patients with colorectal, lung, stomach, pancreatic, breast, or gynecologic cancer were enrolled in the main study, this analysis focuses on the cohort of patients with colorectal cancer.
2.2. Patients
The inclusion/exclusion criteria relating to patients with colorectal cancer were as follows: eligible patients were aged ≥20 years, had stage II‐IV colorectal cancer, 26 had a life expectancy of ≥6 months, had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0‐2, and were enrolled before initiating any planned cancer treatment. Both outpatients and hospitalized patients were eligible. Previous cancer treatment for a primary tumor followed by stable disease for ≥6 months before disease progression/recurrence was allowed. Written informed consent was obtained from all patients before confirming eligibility, and all patient information was anonymized.
All patients were required to undergo VTE screening 2 months before enrollment unless their D‐dimer concentration after cancer diagnosis was ≤1.2 μg/ml regarded as non‐VTE. 24 , 27 VTE screening conformed to current Japanese guidelines, 28 with venous ultrasonography of the lower extremity as the preferred method. Given that this was a registry study conducted under real‐world clinical conditions, no specific provisions for VTE screening during the follow‐up period were made. The diagnosis of symptomatic VTE during the follow‐up period was made by imaging modalities, including compression ultrasonography or contrast CT of the lower extremities, contrast CT of the pulmonary artery, pulmonary angiography, or pulmonary ventilation perfusion scintigraphy.
2.3. Study outcomes
The outcomes of this prespecified subanalysis were to explore baseline VTE prevalence in patients with colorectal cancer and to calculate cumulative incidences of symptomatic VTE, composite VTE (symptomatic VTE events and incidental VTE events requiring treatment), bleeding (major or clinically relevant nonmajor bleeding), cerebral infarction/transient ischemic attack (TIA)/systemic embolic events (SEE), and all‐cause death during the follow‐up period. Incidental VTE events were defined as those in which asymptomatic VTE requiring treatment was detected during imaging or other procedures associated with cancer treatment. Asymptomatic VTE events that did not require treatment were not included as an event. Thrombi that occurred around a central venous catheter were not evaluated and were not counted as VTE events during this study.
We also conducted subgroup analyses to determine the influence of tumor‐related variables (location, recurrence, metastasis, stage, and ECOG PS), cancer therapy (type of treatment, administration as monotherapy or combination therapy, and surgical methodology), and patient baseline characteristics (sex and age) on VTE. All events were adjudicated by independent committees, including neurologists and cardiovascular and VTE specialists.
2.4. Statistical methods
Categorical variables were tabulated using n (%) and continuous variables using mean, SD, and median. Baseline variables were compared by baseline VTE status. For comparisons of continuous variables, a two‐sample t test was used, and for comparisons of categorical variables, a chi‐squared test was used. Time‐to‐event rates were calculated using the cumulative incidence function for each event of interest. Between‐group differences according to baseline VTE status were explored using the Gray test (for VTE, bleeding, and cerebral infarction/TIA/SEE) or the log‐rank test (for all‐cause death). Univariable analyses were conducted to investigate factors correlated with the presence or absence of concurrent VTE at baseline and the occurrence of composite VTE during the follow‐up period. Multivariable analyses were conducted to investigate factors correlated with the occurrence of composite VTE during the follow‐up period using the Fine and Gray models, with all‐cause death as a competing event. In multivariable analysis, the following explanatory variables (adjustment factors) were used: sex, age, location of tumor, cancer stage, ECOG PS, presence or absence of VTE at baseline, and oral anticoagulant treatment. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Patients
Of the patients enrolled in the Cancer‐VTE Registry, 2477 had colorectal cancer. In total, 158 (6.4%) patients had VTE at baseline. Most patients (1947/2477 [78.6%]) had stage II or III disease (Table 1).
TABLE 1.
Baseline demographic and clinical characteristics of patients with colorectal cancer in the Cancer‐VTE Registry
| All colorectal cancer patients (n = 2477 [100.0%]) | Patients with VTE at baseline (n = 158 [6.4%]) | Patients without VTE at baseline (n = 2319 [93.6%]) | p value a | |
|---|---|---|---|---|
| Male sex, n (%) | 1407 (56.8) | 62 (39.2) | 1345 (58.0) | <0.001 |
| Age (years), mean (SD) | 68.1 (11.4) | 73.9 (10.5) | 67.7 (11.4) | <0.001 |
| BMI, kg/m2 | ||||
| Mean (SD) | 22.6 (3.7) | 22.2 (4.3) | 22.6 (3.7) | 0.188 |
| ≥25, n (%) | 577 (23.3) | 36 (22.8) | 541 (23.3) | 0.902 |
| Primary cancer, n (%) | 2385 (96.3) | 149 (94.3) | 2236 (96.4) | 0.173 |
| Cancer type, n (%) | ||||
| Colon | 1587 (64.1) | 114 (72.2) | 1473 (63.5) | 0.029 |
| Rectum | 889 (35.9) | 44 (27.8) | 845 (36.4) | |
| With lymph node metastasis, n (%) | 1473 (59.5) | 105 (66.5) | 1368 (59.0) | 0.064 |
| With distant metastasis, n (%) | 497 (20.1) | 42 (26.6) | 455 (19.6) | 0.035 |
| Cancer stage, n (%) | ||||
| II | 857 (34.6) | 49 (31.0) | 808 (34.8) | 0.050 |
| III | 1090 (44.0) | 63 (39.9) | 1027 (44.3) | |
| IV | 530 (21.4) | 46 (29.1) | 484 (20.9) | |
| ECOG PS, n (%) | ||||
| 0 | 1881 (75.9) | 76 (48.1) | 1805 (77.8) | <0.001 |
| 1 | 488 (19.7) | 64 (40.5) | 424 (18.3) | |
| 2 | 108 (4.4) | 18 (11.4) | 90 (3.9) | |
| DOAC or warfarin use b , n (%) | 130 (5.2) | 45 (28.5) | 85 (3.7) | <0.001 |
| D‐dimer, μg/ml | ||||
| Mean (SD) | 1.2 (2.0) | 3.6 (3.5) | 1.1 (1.8) | <0.001 |
| >1.2, n (%) | 531 (21.4) | 129 (81.6) | 402 (17.3) | <0.001 |
| CrCL, ml/min | ||||
| Mean (SD) | 74 (28) | 63 (26) | 75 (28) | <0.001 |
| ≤50, n (%) | 411 (16.6) | 51 (32.3) | 360 (15.5) | <0.001 |
| Platelet count, ×109/L | ||||
| Mean (SD) | 274 (89) | 295 (104) | 272 (87) | 0.003 |
| ≥350, n (%) | 387 (15.6) | 33 (20.9) | 354 (15.3) | 0.048 |
| Hb, g/dl | ||||
| Mean (SD) | 12.3 (2.2) | 11.0 (2.2) | 12.4 (2.2) | <0.001 |
| <10, n (%) | 379 (15.3) | 54 (34.2) | 325 (14.0) | <0.001 |
| WBC count, ×109/L | ||||
| Mean (SD) | 6.6 (2.2) | 6.7 (2.5) | 6.6 (2.1) | 0.436 |
| ≥11, n (%) | 85 (3.4) | 7 (4.4) | 78 (3.4) | 0.452 |
Abbreviations: BMI, body mass index; CrCL, creatinine clearance; DOAC, direct‐acting oral anticoagulant; ECOG PS, Eastern Cooperative Oncology Group performance status; Hb, hemoglobin; SD, standard deviation; VTE, venous thromboembolism; WBC, white blood cells.
Difference between patients with VTE and without VTE at baseline. For comparisons of continuous variables, a two‐sample t test was used, and for comparisons of categorical variables, a chi‐squared test was used.
Oral anticoagulant treatment that started before enrollment.
Compared with patients without VTE, patients with VTE at baseline were older, and had a greater proportion of female and stage IV cancer. At baseline, patients with VTE had worsened ECOG PS, lower creatinine clearance (CrCL), and a greater proportion of D‐dimer levels >1.2 μg/ml.
3.2. Venous thromboembolism prevalence at baseline
A breakdown of VTE at baseline is shown in Table 2. Of the 158 (6.4%) patients with colorectal cancer diagnosed with VTE at baseline, 6 (0.2%) had symptomatic VTE, and 13 (0.5%) had PE. Asymptomatic distal DVT accounted for 123/158 (77.8%) of VTE cases. Univariable analysis of factors correlated with the presence of VTE at baseline is shown in Table S1. Female, older (≥65 years of age) patients, with advanced cancer (distant metastasis, stage IV, and ECOG PS 1 and 2), body mass index (BMI) <18.5 kg/m2, history of VTE, bed rest for ≥4 days, platelets ≥350 × 109/L, hemoglobin [Hb]) <10 g/dl, CrCL ≤ 50 ml/min, and D‐dimer >1.2 μg/ml had a greater probability of presenting with VTE at baseline.
TABLE 2.
Summary of VTE prevalence at baseline in patients with colorectal cancer
| All colorectal cancer patients (n = 2477) | Symptomatic VTE | Asymptomatic VTE | |
|---|---|---|---|
| All VTE, n (%) | 158 (6.4) | 6 (0.2) | 152 (6.1) |
| PE (with/without DVT) | 13 (0.5) | 1 (0.0) | 12 (0.5) |
| DVT (with/without PE) | 155 (6.3) | 5 (0.2) | 150 (6.1) |
| Proximal DVT | 30 (1.2) | 3 (0.1) | 27 (1.1) |
| Distal DVT | 125 (5.0) | 2 (0.1) | 123 (5.0) |
Abbreviations: DVT, deep‐vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
3.3. Incidence of events
The mean follow‐up period was 376.2 days. The incidence of each event during the follow‐up period was 0.3% for symptomatic VTE, 0.8% for incidental VTE requiring treatment, 1.0% for composite VTE, 1.0% for bleeding events, 0.3% for cerebral infarction/TIA/SEE, and 4.8% for all‐cause death (Table 3). The incidence of all events except for cerebral infarction/TIA/SEE was higher in patients with VTE at baseline. The components of each event are shown in Table S2.
TABLE 3.
Incidence of events during the follow‐up period
| Event | All colorectal cancer patients (n = 2477 [100%]) | Patients with VTE at baseline (n = 158 [6.4%]) | Patients without VTE at baseline (n = 2319 [93.6%]) | |||
|---|---|---|---|---|---|---|
| Patients with events, n | Incidence (95% CI) | Patients with events, n | Incidence (95% CI) | Patients with events, n | Incidence (95% CI) | |
| Symptomatic VTE | 8 | 0.3 (0.1–0.6) | 2 | 1.3 (0.2–4.5) | 6 | 0.3 (0.1–0.6) |
| Incidental VTE requiring treatment | 19 | 0.8 (0.5–1.2) | 4 | 2.5 (0.7–6.4) | 15 | 0.6 (0.4–1.1) |
| Composite VTE a | 25 | 1.0 (0.7–1.5) | 5 | 3.2 (1.0–7.2) | 20 | 0.9 (0.5–1.3) |
| Bleeding b | 26 | 1.0 (0.7–1.5) | 5 | 3.2 (1.0–7.2) | 21 | 0.9 (0.6–1.4) |
| Cerebral infarction/TIA/SEE | 8 | 0.3 (0.1–0.6) | 0 | 0.0 (0.0–2.3) | 8 | 0.3 (0.1–0.7) |
| All‐cause death | 118 | 4.8 (4.0–5.7) | 22 | 13.9 (8.9–20.3) | 96 | 4.1 (3.4–5.0) |
Abbreviations: CI, confidence interval; SEE, systemic embolic event; TIA, transient ischemic attack; VTE, venous thromboembolism.
A composite of symptomatic VTE events and incidental VTE events requiring treatment.
Included major bleeding and clinically relevant nonmajor bleeding events.
Cumulative incidence rates according to VTE at baseline are shown in Figures 1A–C and S1A,B. The cumulative incidence of VTE (either symptomatic [unadjusted HR: 5.06, 95% CI: 1.02‐25.14; Gray test p = 0.027] or composite events [unadjusted HR: 3.82, 95% CI: 1.43‐10.18; Gray test p = 0.004]), bleeding (unadjusted HR: 3.65, 95% CI: 1.38‐9.66; Gray test p = 0.006), and all‐cause death (unadjusted HR: 3.72, 95% CI: 2.34‐5.92; log‐rank test p < 0.001) were higher in patients with VTE at baseline than in those without VTE.
FIGURE 1.
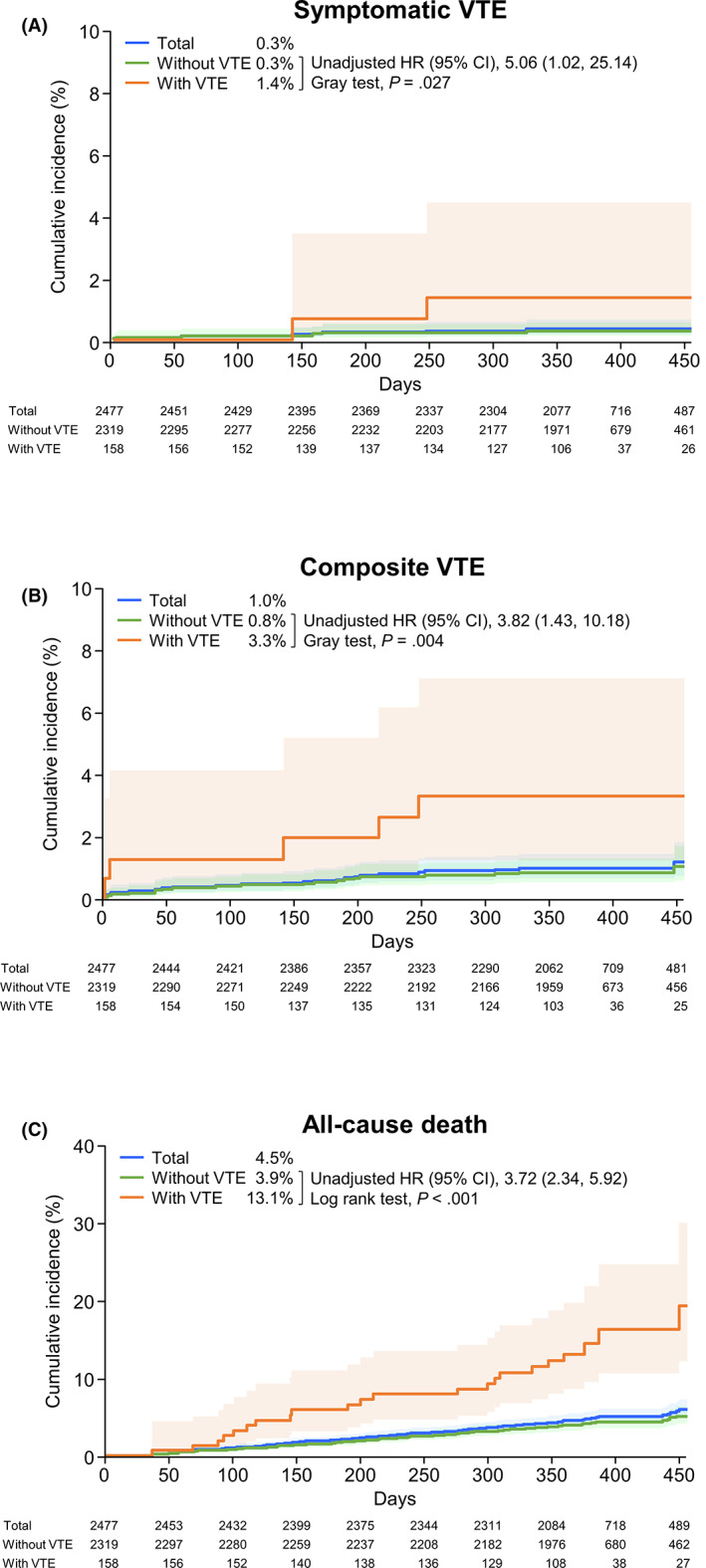
Cumulative incidence of events (time‐to‐event analysis). A, Symptomatic VTE. B, Composite VTE. C, All‐cause death. p values were calculated using either the Gray test (A, B) or the log‐rank test (C). Light shaded areas represent 95% CI. CI, confidence interval; HR, hazard ratio; VTE, venous thromboembolism
3.4. Event occurrence according to baseline variables
The incidence of composite VTE during the follow‐up period according to tumor‐related variables, type of cancer therapy, sex, and age is shown in Table 4. The corresponding data for symptomatic VTE are shown in Table S3. Of the patients with colorectal cancer, 1587/2477 (64.1%) had colon cancer and 889/2477 (35.9%) had rectal cancer. The incidence of events tended to be higher in patients with cancer recurrence (vs primary), lymph node metastasis, distant metastasis, higher cancer stage, and worse ECOG PS. Almost exactly half of the patients with colorectal cancer were treated with surgery alone (1239/2477 [50.0%]), followed by the combination of chemotherapy and surgery (864/2477 [34.9%]), and chemotherapy alone (201/2477 [8.1%]). For all other treatments, the proportion of patients was ≤1%. The incidence of composite VTE was numerically lower in the patients receiving surgery alone (8/1239 [0.6%]) than chemotherapy plus surgery (15/864 [1.7%]).
TABLE 4.
Incidence of composite VTE during the follow‐up period according to tumor‐related variables, type of cancer therapy, sex, and age
| Variable | All colorectal cancer patients, n (%) | Composite VTE | |
|---|---|---|---|
| n (%) | 95% CI | ||
| All patients with colorectal cancer | 2477 (100) | 25 (1.0) | 0.7–1.5 |
| Sex | |||
| Male | 1407 (56.8) | 12 (0.9) | 0.4–1.5 |
| Female | 1070 (43.2) | 13 (1.2) | 0.6–2.1 |
| Age, years | |||
| <50 | 181 (7.3) | 0 | 0.0–2.0 |
| 50 to <70 | 1068 (43.1) | 9 (0.8) | 0.4–1.6 |
| ≥70 | 1228 (49.6) | 16 (1.3) | 0.7–2.1 |
| Location of tumor | |||
| Colon | 1587 (64.1) | 13 (0.8) | 0.4–1.4 |
| Rectum | 889 (35.9) | 12 (1.3) | 0.7–2.3 |
| Occurrence of tumor | |||
| Primary | 2385 (96.3) | 23 (1.0) | 0.6–1.4 |
| Recurrent | 92 (3.7) | 2 (2.2) | 0.3–7.6 |
| Lymph node metastasis | |||
| No | 1004 (40.5) | 6 (0.6) | 0.2–1.3 |
| Yes | 1473 (59.5) | 19 (1.3) | 0.8–2.0 |
| Presence of distant metastasis | |||
| No | 1980 (79.9) | 16 (0.8) | 0.5–1.3 |
| Yes | 497 (20.1) | 9 (1.8) | 0.8–3.4 |
| Cancer stage | |||
| II | 857 (34.6) | 6 (0.7) | 0.3–1.5 |
| III | 1090 (44.0) | 11 (1.0) | 0.5–1.8 |
| IV | 530 (21.4) | 8 (1.5) | 0.7–3.0 |
| ECOG PS | |||
| 0 | 1881 (75.9) | 19 (1.0) | 0.6–1.6 |
| 1 | 488 (19.7) | 4 (0.8) | 0.2–2.1 |
| 2 | 108 (4.4) | 2 (1.9) | 0.2–6.5 |
| Cancer therapy | |||
| No | 127 (5.1) | 0 | 0.0–2.9 |
| Yes | 2350 (94.9) | 25 (1.1) | 0.7–1.6 |
| Surgery | 2133 (86.1) | 23 (1.1) | 0.7–1.6 |
| Chemotherapy | 1103 (44.5) | 17 (1.5) | 0.9–2.5 |
| Radiation | 43 (1.7) | 0 | 0.0–8.2 |
| Single therapy | |||
| Surgery alone | 1239 (50.0) | 8 (0.6) | 0.3–1.3 |
| Chemotherapy alone | 201 (8.1) | 2 (1.0) | 0.1–3.5 |
| Combination therapy | |||
| Chemotherapy plus surgery | 864 (34.9) | 15 (1.7) | 1.0–2.8 |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; VTE, venous thromboembolism.
3.5. Risk factors for composite VTE during the follow‐up period
Univariable and multivariable analysis of factors correlated with the incidence of composite VTE during the 1‐year follow‐up period is shown in Table 5. Patients with VTE at baseline had a significantly higher risk for presenting a composite VTE event during the follow‐up period (HR: 4.04, 95% CI: 1.46‐11.17; p = 0.007). Female sex, age ≥ 65 years, stage IV, ECOG PS 2, and rectum cancer were factors with a HR >1, but these did not reach statistical significance. Patients receiving oral anticoagulant treatment that started before enrollment was factor with a HR <1 (HR: 0.37, 95% CI: 0.05‐2.83; p = 0.337).
TABLE 5.
Univariable and multivariable analysis of risk factors for composite VTE during the follow‐up period
| Items | Category | N | Events, n (%) | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||||
| Sex | Male | 1407 | 12 (0.9) | 1.00 | – | – | 1.00 | – | – |
| Female | 1070 | 13 (1.2) | 1.42 | 0.65–3.11 | 0.382 | 1.40 | 0.62–3.15 | 0.414 | |
| Age, years | <65 | 791 | 6 (0.8) | 1.00 | – | – | 1.00 | – | – |
| ≥65 | 1686 | 19 (1.1) | 1.49 | 0.60–3.74 | 0.395 | 1.56 | 0.64–3.83 | 0.329 | |
| Location of tumor | Colon | 1587 | 13 (0.8) | 1.00 | – | – | 1.00 | – | – |
| Rectum | 889 | 12 (1.3) | 1.65 | 0.75–3.63 | 0.209 | 1.98 | 0.89–4.42 | 0.094 | |
| Cancer stage | II or III | 1947 | 17 (0.9) | 1.00 | – | – | 1.00 | – | – |
| IV | 530 | 8 (1.5) | 1.76 | 0.76–4.06 | 0.187 | 1.74 | 0.75–4.08 | 0.200 | |
| ECOG PS | 0 or 1 | 2369 | 23 (1.0) | 1.00 | – | – | 1.00 | – | – |
| 2 | 108 | 2 (1.9) | 2.01 | 0.47–8.55 | 0.343 | 1.82 | 0.43–7.64 | 0.415 | |
| VTE at baseline | No | 2319 | 20 (0.9) | 1.00 | – | – | 1.00 | – | – |
| Yes | 158 | 5 (3.2) | 3.82 | 1.43–10.18 | 0.007 | 4.04 | 1.46–11.17 | 0.007 | |
| Oral anticoagulant treatment a | No | 2347 | 24 (1.0) | 1.00 | – | – | 1.00 | – | – |
| Yes | 130 | 1 (0.8) | 0.76 | 0.10–5.63 | 0.787 | 0.37 | 0.05–2.83 | 0.337 | |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; VTE, venous thromboembolism.
Oral anticoagulant treatment that started before enrollment.
4. DISCUSSION
This subanalysis of the Cancer‐VTE Registry is the first large‐scale, prospective study to investigate VTE incidence in Japanese patients with colorectal cancer in a real‐world clinical situation. The results of this study reflect contemporary medical care, in which VTE risk management is widely understood. In this context, VTE occurred in 6.4% of patients screened at the time of cancer diagnosis. During the 1‐year follow‐up period during cancer treatment, symptomatic VTE was 0.3%, asymptomatic VTE requiring treatment was 0.8%, and composite VTE was 1.0%. VTE, bleeding, and all‐cause death occurred more frequently in patients with VTE at baseline than those without baseline VTE, consistent with the results from the overall Cancer‐VTE Registry population. 29
We have previously reported that the overall study results of the Cancer‐VTE Registry show that VTE prevalence at the time of cancer diagnosis was 5.9%, and symptomatic VTE in the 1‐year follow‐up period was 0.5%. Furthermore, VTE frequency in patients with colorectal cancer was lower than that in patients with pancreatic cancer. 25 , 29 Nonetheless, the frequency of VTE in Japanese patients with colorectal cancer in this study (6.4% at the time of cancer diagnosis and 1.0% for composite VTE during the 1‐year follow‐up period) is noticeably higher than that in the general Japanese population. A recent analysis of a medical claims database (n = 5,106,151) found that just 1.1% (n = 55,582) of patients were hospitalized with a diagnosis of VTE over 5 years between 2012 and 2017. 30 Thus, clinicians should be aware that the risk of VTE in patients with colorectal cancer is real, which should be carefully monitored at the time of diagnosis and throughout treatment.
In previous studies of patients with colorectal cancer in Western countries, the rate of VTE development ranged from 2.4% to 10.6%, although it must be noted that study designs and reporting methods varied. 28 , 31 , 32 , 33 In comparison, the 1‐year incidence of composite VTE in our study was lower (1.0%). This finding is consistent with the conventional view that the risk of developing VTE is lower in Asian patients with cancer than Caucasians. 34 , 35 However, it is difficult to accurately compare data from studies with widely heterogeneous methodologies.
Within Japan, there have been few observational studies reporting VTE rates, and of those available, many report only the incidence of VTE after surgery. For example, in patients who underwent laparoscopic surgery for abdominal malignancies, the incidence of VTE varied between 2.8% and approximately 12% with VTE prophylaxis. 36 , 37 , 38 Nonetheless, compared with these previous reports from Japan, the incidence of each event in our study was low. There are many potential reasons for this discrepancy, including the small sample sizes in these prior reports and heterogeneity in patient demographic and clinical background factors. However, we consider that the main difference in VTE frequency was due to the active screening with echocardiography and CT scans, which allowed the diagnostic confirmation of VTE in asymptomatic patients. In this study, such screening tests could not be performed for all patients and may have resulted in a lower frequency of VTE.
Our findings were obtained from a large‐scale, real‐world analysis. Our sample of prospectively evaluated patients was heterogeneous as it included patients aged ≥65 years with various stages of colorectal cancer and advanced disease (presence of distant metastases and ECOG PS of 2). Thus, we consider that our study population better reflected the current clinical situation in Japan. Further, we enrolled patients from both surgical and medical departments at cancer hospitals throughout Japan, and a broad spectrum of patients was included, from those treated only with surgery to those treated only with systemic drugs for advanced cancer. As such, we consider that our data will be used as the foundation for future clinical studies.
The univariable analysis conducted in this study found that possible factors correlating with the presence of VTE before starting cancer treatment were female sex, increased age, advanced cancer progression (ie, cancer stage IV, presence of distant metastasis, higher ECOG PS), BMI <18.5 kg/m2, a history of VTE, bed rest for 4 days or more, higher platelet level, lower Hb level, and CrCL, and higher D‐dimer levels. Based on these findings, caution should be taken in patients with colorectal cancer who present any of these characteristics to ensure that VTE is detected and appropriately managed before initiating cancer treatment.
In this study, the multivariable analysis also examined the factors associated with composite VTE during the follow‐up period and found that the incidence of events was higher in patients with VTE at baseline. In another recent meta‐analysis of VTE after surgery, multiple risk factors for VTE were identified, including advanced or disseminated cancer, chemotherapy, and history of VTE. 39 These items are generally consistent with our findings. In retrospective analyses conducted in the United States and United Kingdom, factors associated with the development of VTE included surgical treatment, 33 , 40 postoperative complications, 40 chemotherapy, 33 , 41 hospital admission, 33 and BMI. 40 In our study, the frequency of VTE did not differ by surgical technique (open/endoscopic), which was consistent with the previous study for surgical patients. 36
Venous thromboembolism is known to increase mortality in patients with cancer 35 and, in this study, a 3.7‐fold increased risk of all‐cause death was observed in patients with baseline VTE versus those without baseline VTE. This is consistent with previous studies that report VTE to be a risk factor for mortality in a range of patients of different ethnicities with colorectal cancer. 42 , 43 , 44 , 45 , 46 In this subanalysis, we did not confirm whether the presence of VTE is an independent predictor of all‐cause death. However, in the main analysis of this registry, VTE at baseline was shown to be a significant independent predictor of all‐cause death (adjusted HR: 1.26; 95% CI: 1.04‐1.53; p = 0.019) in patients with solid tumors, highlighting the importance of VTE screening before initiating cancer treatment. 29
The study limitations are similar to those of the main publication and are related to the patient population (potential selection bias due to eligibility restriction by cancer type and stage), the observational design (whereby no procedures or visits were mandated, and follow‐up testing may have differed among centers), and the relatively short follow‐up duration (1 year). Patients on palliative therapy were also excluded, so generalization to such patients is not possible. Finally, no data were collected on pharmacologic or physical (eg, intermittent pneumatic compression or elastic stocking use) prophylaxis of VTE during perioperative periods, and these impacts are not known.
In conclusion, in Japanese patients with colorectal cancer undergoing cancer treatment, the incidence of VTE was 1.0% during the 1‐year follow‐up period. The incidence of VTE tended to increase with advancing cancer stage. The presence of VTE at the time of cancer diagnosis was found to increase not only VTE events during cancer treatment but also death. It is important to evaluate the presence or absence of VTE at the time of cancer diagnosis before proceeding with treatment for colorectal cancer.
Funding information
This study was supported by Daiichi Sankyo Co., Ltd., which was involved in the study design, planning of the data analysis, data interpretation, and development of the manuscript, but was not involved in data management and statistical analysis.
DISCLOSURE
Masataka Ikeda received lecture fees and honoraria from Daiichi Sankyo Co., Ltd.; Bayer Yakuhin Ltd.; and Bristol Myers Squibb K.K. and received research funds from Daiichi Sankyo Co., Ltd. Hiroyuki Uetake had no conflicts of interest to declare. Takayuki Yoshino received lecture fees and honoraria from Taiho Pharmaceutical Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; Eli Lilly Japan K.K.; Merck Biopharma Co., Ltd.; Bayer Yakuhin Ltd.; Ono Pharmaceutical Co., Ltd.; and MSD K.K. and received research funds from Ono Pharmaceutical Co., Ltd.; Sanofi K.K.; Daiichi Sankyo Co., Ltd.; PAREXEL International Inc.; Pfizer Japan Inc.; Taiho Pharmaceutical Co., Ltd.; MSD K.K.; Amgen K.K.; Genomedia Inc.; Sysmex Corp.; Chugai Pharmaceutical Co., Ltd.; and Nippon Boehringer Ingelheim Co., Ltd. Taishi Hata received lecture fees and honoraria from Daiichi Sankyo Co., Ltd. Mari S. Oba had no conflicts of interest to declare. Atsushi Takita and Tetsuya Kimura were employees of Daiichi Sankyo Co., Ltd.
ETHICAL APPROVAL
The Cancer‐VTE Registry was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical Science Studies on Human Subjects of the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
The ethics committee of each participating institution approved the study protocol and all related documentation.
INFORMED CONSENT
Patients provided written informed consent for participation and all patient data were anonymized.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY
UMIN Clinical Trials Registry: UMIN000024942.
ANIMAL STUDIES
N/A.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors wish to express their condolences in memory of the principal investigator of this study, Professor Yasuo Ohashi, who passed away on March 11, 2021. The authors thank EP‐CRSU Co., Ltd. and Mediscience Planning Inc. for their partial support in the conduct of this Registry and Edanz (www.edanz.com) for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The authors thank Jun Hosokawa of Daiichi Sankyo Co., Ltd. for supporting the preparation of the manuscript.
Ikeda M, Uetake H, Yoshino T, et al. Incidence and risk factors for venous thromboembolism, bleeding, and death in colorectal cancer (Cancer‐VTE Registry). Cancer Sci. 2022;113:3901‐3911. doi: 10.1111/cas.15527
DATA AVAILABILITY STATEMENT
The anonymized data underlying the results presented in this manuscript may be made available to researchers upon submission of a reasonable request to the corresponding author. The decision to disclose the data will be made by the corresponding author and the funder, Daiichi Sankyo Co., Ltd. The data disclosure can be requested for 36 months from the article publication.
REFERENCES
- 1. IARC/WHO cancer factsheet. Globocan 2020: colorectal cancer. World Health Organization; 2020. https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9‐Colorectum‐fact‐sheet.pdf. Accessed October 12, 2020. [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 3. IARC/WHO cancer factsheet. Globocan 2020: Japan. World Health Organization; 2020. https://gco.iarc.fr/today/data/factsheets/populations/392‐japan‐fact‐sheets.pdf. Accessed October 12, 2020. [Google Scholar]
- 4. Fernandes CJ, Morinaga LTK, Alves JLJ, et al. Cancer‐associated thrombosis: the when, how and why. Eur Respir Rev. 2019;28:180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi Y, Hu X, Chen J, Ying X, Shi Y. The risk factors of VTE and survival prognosis of patients with malignant cancer: implication for nursing and treatment. Clin Appl Thromb Hemost. 2020;26:1076029620971053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhami SPS, Patmore S, O'Sullivan JM. Advances in the management of cancer‐associated thrombosis. Semin Thromb Hemost. 2021;47:139‐149. [DOI] [PubMed] [Google Scholar]
- 7. Falanga A, Marchetti M. Hemostatic biomarkers in cancer progression. Thromb Res. 2018;164(Suppl 1):S54‐S61. [DOI] [PubMed] [Google Scholar]
- 8. Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902‐4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ay C, Pabinger I, Cohen AT. Cancer‐associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117:219‐230. [DOI] [PubMed] [Google Scholar]
- 10. Sandén P, Svensson PJ, Själander A. Venous thromboembolism and cancer risk. J Thromb Thrombolysis. 2017;43:68‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riess H, Habbel P, Jühling A, Sinn M, Pelzer U. Primary prevention and treatment of venous thromboembolic events in patients with gastrointestinal cancers – Review. World J Gastrointest Oncol. 2016;8:258‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davenport DL, Vargas HD, Kasten MW, Xenos ES. Timing and perioperative risk factors for in‐hospital and post‐discharge venous thromboembolism after colorectal cancer resection. Clin Appl Thromb Hemost. 2012;18:569‐575. [DOI] [PubMed] [Google Scholar]
- 13. Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Nguyen NT, Stamos MJ. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg. 2014;18:2169‐2177. [DOI] [PubMed] [Google Scholar]
- 14. Yeo DX, Junnarkar S, Balasubramaniam S, et al. Incidence of venous thromboembolism and its pharmacological prophylaxis in Asian general surgery patients: a systematic review. World J Surg. 2015;39:150‐157. [DOI] [PubMed] [Google Scholar]
- 15. Yhim HY, Jang MJ, Bang SM, et al. Incidence of venous thromboembolism following major surgery in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2014;12:1035‐1043. [DOI] [PubMed] [Google Scholar]
- 16. Kitayama H, Kondo T, Sugiyama J, et al. Venous thromboembolism in hospitalized patients receiving chemotherapy for malignancies at Japanese community hospital: prospective observational study. BMC Cancer. 2017;17:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer‐associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers. 2018;10:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wun T, White RH. Epidemiology of cancer‐related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22:9‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iorga RA, Bratu OG, Marcu RD, et al. Venous thromboembolism in cancer patients: still looking for answers. Exp Ther Med. 2019;18:5026‐5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adelborg K, Corraini P, Darvalics B, et al. Risk of thromboembolic and bleeding outcomes following hematological cancers: a Danish population‐based cohort study. J Thromb Haemost. 2019;17:1305‐1318. [DOI] [PubMed] [Google Scholar]
- 21. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta‐analysis. PLoS Med. 2012;9:e1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer ‐ a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404‐1413. [DOI] [PubMed] [Google Scholar]
- 24. Ohashi Y, Ikeda M, Kunitoh H, et al. Venous thromboembolism in patients with cancer: design and rationale of a multicentre, prospective registry (Cancer‐VTE Registry). BMJ Open. 2018;8:e018910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohashi Y, Ikeda M, Kunitoh H, et al. Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer‐VTE Registry. Jpn J Clin Oncol. 2020;50:1246‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 8th ed (Japanese ver.). KANEHARA & Co., LTD; 2017. [Google Scholar]
- 27. Nomura H, Wada H, Mizuno T, et al. Negative predictive value of D‐dimer for diagnosis of venous thromboembolism. Int J Hematol. 2008;87:250‐255. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka S, Nishigami K, Taniguchi N, et al. Criteria for ultrasound diagnosis of deep venous thrombosis of lower extremities. J Med Ultrason. 2008;35:33‐36. [DOI] [PubMed] [Google Scholar]
- 29. Ohashi Y, Ikeda M, Kunitoh H, et al. One‐year incidence of venous thromboembolism, bleeding, and death in patients with solid tumors newly initiating cancer treatment: Results from the Cancer‐VTE Registry. Thromb Res. 2022;213:203‐213. 10.1016/j.thromres.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 30. Yamashita Y, Morimoto T, Yoshikawa Y, et al. Temporal trends in the practice pattern for venous thromboembolism in Japan: insight from JROAD‐DPC. J Am Heart Assoc. 2020;9:e014582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahern TP, Horváth‐Puhó E, Spindler KL, Sørensen HT, Ording AG, Erichsen R. Colorectal cancer, comorbidity, and risk of venous thromboembolism: assessment of biological interactions in a Danish nationwide cohort. Br J Cancer. 2016;114:96‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high‐risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648‐655. [DOI] [PubMed] [Google Scholar]
- 33. Metcalf RL, Al‐Hadithi E, Hopley N, et al. Characterisation and risk assessment of venous thromboembolism in gastrointestinal cancers. World J Gastrointest Oncol. 2017;9:363‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Angchaisuksiri P. Cancer‐associated thrombosis in Asia. Thromb J. 2016;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee LH, Nagarajan C, Tan CW, Ng HJ. Epidemiology of cancer‐associated thrombosis in Asia: a systematic review. Front Cardiovasc Med. 2021;8:669288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hata T, Yasui M, Ikeda M, et al. Efficacy and safety of anticoagulant prophylaxis for prevention of postoperative venous thromboembolism in Japanese patients undergoing laparoscopic colorectal cancer surgery. Ann Gastroenterol Surg. 2019;3:568‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakagawa K, Watanabe J, Ota M, et al. Efficacy and safety of enoxaparin for preventing venous thromboembolic events after laparoscopic colorectal cancer surgery: a randomized‐controlled trial (YCOG 1404). Surg Today. 2020;50:68‐75. [DOI] [PubMed] [Google Scholar]
- 38. Obitsu T, Tanaka N, Oyama A, et al. Efficacy and safety of low‐molecular‐weight heparin on prevention of venous thromboembolism after laparoscopic operation for gastrointestinal malignancy in Japanese patients: a multicenter, open‐label, prospective, randomized controlled trial. J Am Coll Surg. 2020;231:501‐509.e502. [DOI] [PubMed] [Google Scholar]
- 39. Li YD, Li HD, Zhang SX. Effect of thromboprophylaxis on the incidence of venous thromboembolism in surgical patients with colorectal cancer: a meta‐analysis. Int Angiol. 2020;39:353‐360. [DOI] [PubMed] [Google Scholar]
- 40. Schlick CJR, Liu JY, Yang AD, Bentrem DJ, Bilimoria KY, Merkow RP. Pre‐operative, intra‐operative, and postoperative factors associated with post‐discharge venous thromboembolism following colorectal cancer resection. J Gastrointest Surg. 2020;24:144‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walker AJ, West J, Card TR, Humes DJ, Grainge MJ. Variation in the risk of venous thromboembolism in people with colorectal cancer: a population‐based cohort study from England. J Thromb Haemost. 2014;12:641‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bozkaya Y, Özdemir N, Erdem GU, et al. Mortality risk analysis of asymptomatic and symptomatic venous thromboembolism in patients with metastatic colorectal cancer. J Cancer Res Ther. 2018;14:1330‐1335. [DOI] [PubMed] [Google Scholar]
- 43. Hanna N, Bikov KA, McNally D, Onwudiwe NC, Dalal M, Mullins DC. Impact of venous thromboembolism on mortality of elderly Medicare patients with stage III colon cancer. Oncologist. 2012;17:1191‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ades S, Pulluri B, Holmes CE, Lal I, Kumar S, Littenberg B. Risk factors for venous thromboembolism in metastatic colorectal cancer with contemporary treatment: a SEER‐medicare analysis. Cancer Med. 2022;11:1817‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112‐1118. [DOI] [PubMed] [Google Scholar]
- 46. Choi S, Lee K‐W, Bang S‐M, et al. Different characteristics and prognostic impact of deep‐vein thrombosis/pulmonary embolism and intraabdominal venous thrombosis in colorectal cancer patients. Thromb Haemost. 2011;106:1084‐1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The anonymized data underlying the results presented in this manuscript may be made available to researchers upon submission of a reasonable request to the corresponding author. The decision to disclose the data will be made by the corresponding author and the funder, Daiichi Sankyo Co., Ltd. The data disclosure can be requested for 36 months from the article publication.


