Abstract
Bone‐related events caused by breast cancer bone metastasis substantially compromise the survival and quality of life of patients. Because triple‐negative breast cancer (TNBC) lacks hormone receptors and Her2‐targeted therapeutic options, progress in the treatment of TNBC bone metastasis has been very slow. Intercellular adhesion molecule 1 (ICAM1) is highly expressed in various cancers and plays an important role in tumorigenesis and metastasis. However, the effect and mechanism of ICAM1 in TNBC bone metastasis are still unknown. We found that ICAM1 was highly expressed in TNBC and correlated with prognosis in TNBC patients. Cell lines with high expression of ICAM1 exhibited enhanced bone metastasis in tumor‐bearing mice, and silencing ICAM1 expression significantly inhibited bone metastasis in mice. ICAM1 interacted with integrins to activate the epithelial‐to‐mesenchymal transition program through TGF‐β/SMAD signaling, ultimately enhancing cell invasiveness. Therefore, the findings of the present study provide a strong rationale for the application of ICAM1‐targeted therapy in TNBC patients with bone metastasis.
Keywords: bone metastasis, epithelial‐to‐mesenchymal transition, intercellular adhesion molecule 1, TGF‐β/SMAD signaling, triple‐negative breast cancer
ICAM1 promoted TNBC bone metastasis in TNBC. ICAM1 initialed EMT in TNBC cells. ICAM1 activated TGF‐β/SMAD signaling. " cd_value_code="text
Abbreviations
- BLI
bioluminescence imaging
- BM
highly bone metastatic MDA MB‐231BM cells
- Dor
MDA‐MB‐231 weakly bone metastatic variants
- EMT
epithelial‐to‐mesenchymal transition
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- nTNBC
non‐TNBC
- OS
overall survival
- Par
parental MDA‐MB‐231 cells
- PDor
MDA‐MB‐231 reactivated cells with high bone metastatic activity after dormancy
- PR
progesterone receptor
- RT–qPCR
real‐time quantitative PCR
- TNBC
triple‐negative breast cancer
1. INTRODUCTION
In 2020, for the first time, breast cancer in women surpassed lung cancer to become the most commonly diagnosed cancer. 1 Although only 15%‐20% of breast cancers are triple‐negative breast cancer (TNBC), this subtype is more clinically invasive and has a higher recurrence rate than non‐TNBC (nTNBC) subtypes. 2 , 3 The 5‐year overall survival (OS) rate of female patients with advanced TNBC is only approximately 15%, and more than 90% of deaths are due to metastasis. 4 Bone is the most common site of breast cancer metastasis, and the incidence of bone metastasis in patients with advanced breast cancer is 65%‐75%. 5 Skeletal‐related events, such as pathological fractures, spinal cord compression, and bone surgery, caused by bone metastasis seriously affect quality of life and even cause death. 6 Currently, the goal of these treatments in most cases is not a cure but remission; thus, most treatments are palliative. 7 Radiotherapy, osteoclast suppression, chemotherapy, and supportive interventions are the mainstream recommended regimens. 7 Osteoclast inhibitors, including bisphosphonates, have become the principal therapeutic drugs. 7 , 8 Denosumab can also target receptor activator for NF‐κB ligand (RANKL) to inhibit bone destruction. 9 However, osteoclast inhibitors induce osteonecrotic events in the jaw. 10 Additionally, osteoclast inhibitors did not significantly improve patient OS in premenopausal women without ovarian suppression. 11 , 12
Intercellular adhesion molecule 1 (ICAM1) plays an important role in cell proliferation, cell differentiation, cell motility, apoptosis, and tissue structure. 13 Preliminary studies have shown that higher expression of ICAM1 is associated with tumor migration and invasion in TNBC, unlike in other subtypes. 14, 15 Homophilic interactions of ICAM1 in circulating tumor cell clusters in breast cancer confer increased tumor invasiveness, mediate cell aggregation, enhance tumor endothelial adhesion for endothelial migration, and thus enhance lung metastasis. 16 ICAM1 mediates intercellular adhesion events in immune responses and plays an important role in the tumor immune microenvironment by interacting with its ligands, such as LFA‐1 and MAC‐1. 17 In addition, ICAM1 induces osteoclastogenesis, which creates a microenvironment favorable for bone metastasis of tumor cells. 18 Therefore, ICAM1 may play an important role in breast cancer bone metastasis and may be a new therapeutic target for TNBC bone metastasis. Thus, in this study, the role of ICAM1 in TNBC bone metastasis was investigated, and its molecular mechanism was elucidated for the first time.
2. MATERIALS AND METHODS
2.1. Materials
A hematoxylin‐eosin (HE) staining kit, a tartrate‐resistant acid phosphatase (TRAP) staining kit, phosphate‐buffered saline (PBS), and EDTA antigen retrieval buffer (pH 9.0) were obtained from Servicebio. SYBR Green Master Mix was purchased from MedChemExpress. A diaminobenzidine (DAB) color reagent kit was obtained from DAKO. DMEM, antibiotic‐antimycotic solution, fetal bovine serum (FBS), and bovine serum albumin (BSA) were obtained from Gibco. PCR primers and RNase inhibitors were purchased from Sangon Biotech. Matrigel matrix, 96‐well plates and 24‐well Transwell Millipore chambers were purchased from Corning. Antibody information is provided in Table S1, S2. Horseradish peroxidase (HRP)‐conjugated secondary antibodies were purchased from Abcam or Servicebio. A highly sensitive ECL detection kit (ready‐to‐use) was purchased from Vazyme. All other chemicals were obtained from Sinopharm unless otherwise indicated.
2.2. Animals and xenograft model establishment
Six‐week‐old female BALB/c nu/nu mice were housed in a specific pathogen‐free room with a controlled temperature (24 ± 2°C) and light cycle (12‐hour light/dark cycle). All animals had free access to water and food during the experimental period. All animal experiments were carried out according to the national regulations for animal experimentation and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University (approval number 2020‐KL‐168‐01).
The xenograft experiment was performed as described in our previous work. 19 In brief, firefly luciferase‐labeled MDA‐MB‐231 parental (Par), BM, or shICAM1 BM (KD) cells (0.1 ml/mouse, 1 × 107 or 0.5 × 106 cells/ml) were injected into the left ventricles of mice after anesthetization with sodium pentobarbital (50 mg/kg). BLI was performed to detect tumor cells 2 hours after tumor cell injection. After unsuccessfully injected mice were removed from the study, BLI was performed weekly after the mice were anesthetized with sodium pentobarbital. The BLI signal was normalized using the mean value of the first detected signal intensity. Representative tumor‐bearing mice were selected for X‐ray and PET‐CT imaging on the first 3 days of the experiment and the 2 days before the end of the experiment, respectively. On the last day of the experiment, the anesthetized animals were assessed by BLI, and the suspected bone metastases were then harvested according to the test results. After the bone metastases were fixed with fresh 4% paraformaldehyde solution, they were paraffin embedded and sectioned by routine techniques for HE staining, TRAP staining, and immunohistochemical (IHC) staining.
2.3. Bioinformatic enrichment analyses
Gene over‐representation analysis (ORA) was performed as described in our recent work. 20 In brief, the candidate differentially expressed genes (DEGs) with P value <0.05 and |fold change|>2 were subjected to enrichment analysis with the Reactome database (release version 79, https://reactome.org/). In addition, gene set enrichment analysis (GSEA) was performed using WebGestalt 2019 (http://www.webgestalt.org/). 21 Protein‐protein interaction (PPI) analysis was performed using NetworkAnalyst (version 3.0, https://www.networkanalyst.ca/NetworkAnalyst/faces/home.xhtml). 22 Pathway activity analysis was performed using GSCALite (http://bioinfo.life.hust.edu.cn/web/GSCALite/). 23
2.4. Overall survival analysis of breast cancer patients with different levels of ICAM1 expression
Affymetrix Human Genome U133A Array data for HER2‐negative breast cancer patients were downloaded from Gene Expression Omnibus (GEO; GSE25055, N = 310). Survival differences between the patients with high and low ICAM1 gene expression levels were estimated by plotting Kaplan‐Meier survival curves with the Bioinformatics online resource (http://www.bioinformatics.com.cn/plot_basic_kaplan_meier_survival_curve_plot_040). 24
2.5. Statistical analysis
All results are presented as the mean ± standard deviation values. One‐way analysis of variance (ANOVA) followed by Dunnett's post hoc test was applied to determine statistical significance. For the bioinformatics analyses, statistical analysis was performed according to the default parameters of the corresponding software. A two‐tailed P value <0.05 was considered statistically significant.
For other methods, please refer to Appendix S1.
3. RESULTS
3.1. Interleukin‐10 (IL‐10) signaling was the top enriched pathway related to TNBC bone metastasis
The GSE20611 dataset contains data for weakly bone metastatic variants (Dor), reactivated cells with high bone metastatic activity after dormancy in bone metastases (Pdor), and parental MDA‐MB‐231 cells (Par). The intragroup differences in these cells with different levels of bone metastatic activity were small but the intercell differences were significant (Figure 1A). Among these different types of cells, numerous DEGs were found (Figure 1B). Through further expression trend analysis, 312 DEGs associated with high bone metastatic activity were found (Figure 1C,D, Table S3). The expression patterns of these DEGs aptly characterized the predilection of these different types of cells for bone metastasis (Figure 1E). Reactome enrichment analysis with these DEGs indicated that they were enriched in numerous signaling pathways (Figure 1F). Most of the pathways were related to signal transduction, the immune system, etc. (Figure 1G). IL‐10 signaling was the top‐ranked enriched pathway related to TNBC bone metastasis (Figure 1H,L). In addition, the proteins encoded by the IL‐6, VEGFA, CSF2, IL‐8 (CXCL8), and ICAM1 genes were the top‐ranked proteins in the PPI network, and these top‐ranked proteins interacted with other proteins (Figure 1J,K). The pathway activity results of the top 10 genes indicated that these genes were involved in apoptosis, the cell cycle, the DNA damage response, and epithelial‐to‐mesenchymal transition (EMT; Figure 1L).
FIGURE 1.
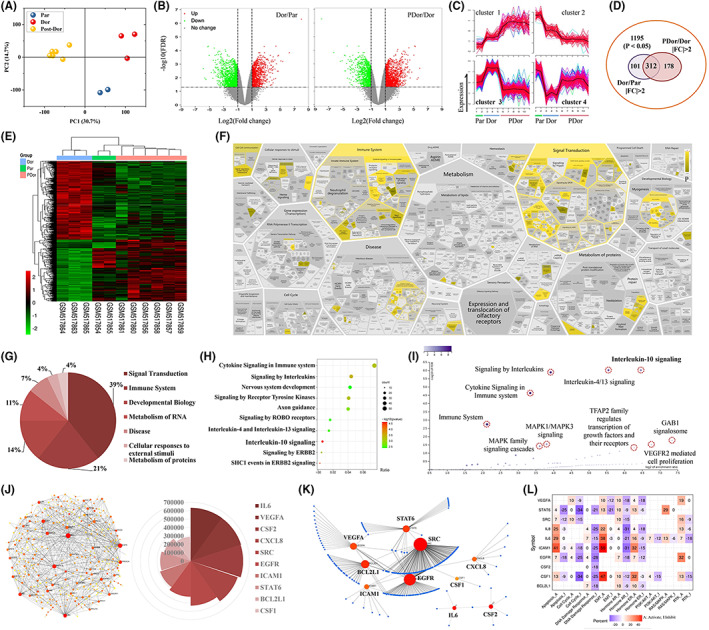
Interleukin‐10 (IL‐10) signaling was the top enriched pathway related to triple‐negative breast cancer (TNBC) bone metastasis according to gene enrichment analysis. A, Principal components analysis (PCA) of the gene set in GSE20611. B, Volcano plot of the DEGs (left: Dor vs. Par; right: PDor vs. Dor). Dor, MDA‐MB‐231 with weakly bone metastatic variants; PDor, MDA‐MB‐231 reactivated cells with high bone metastatic activity after dormancy in bone metastases; and Par, parental MDA‐MB‐231 cells. p < 0.05 and |fold change (FC)| > 2 were set as the criteria for differential expression. C, Cluster analysis of the DEGs. D, Venn diagram showing the overlapping DEGs. E, Heatmap of the 312 candidate DEGs obtained from (D). F, Results of Reactome enrichment analysis with the 312 candidate DEGs. Pathway classification (G), top 10 pathways (H), and pathway distribution (I) results of the Reactome enrichment analysis. J, Protein‐protein interaction (PPI) network of the 312 candidate DEGs and the top 10 proteins according to the MCC score with Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) PPI network functional enrichment analysis. K, PPI network of the top 10 genes in (J). L, Pathway activity analysis of the top 10 genes in (J) using GSCALite. DEGs, differentially expressed genes; MCC, maximal clique centrality
The GSEA results indicated that IL‐10 signaling was inhibited in the weakly bone metastatic breast cancer cell line compared with the parental MDA‐MB‐231 cells (Figure 2A,B). In contrast, IL‐10 signaling was activated in the highly bone metastatic breast cancer cell line compared with the weakly bone metastatic cell line (Figure 2C,D). The expression levels of the genes involved in IL‐10 signaling also indicated the degree of bone metastasis (Figure 2E,F). CSF1, CSF2, IL‐6, IL‐8 (CXCL8), and ICAM1 were the top‐ranked genes in both REACTOME enrichment analysis and GSEA (Figure 2G,H).
FIGURE 2.
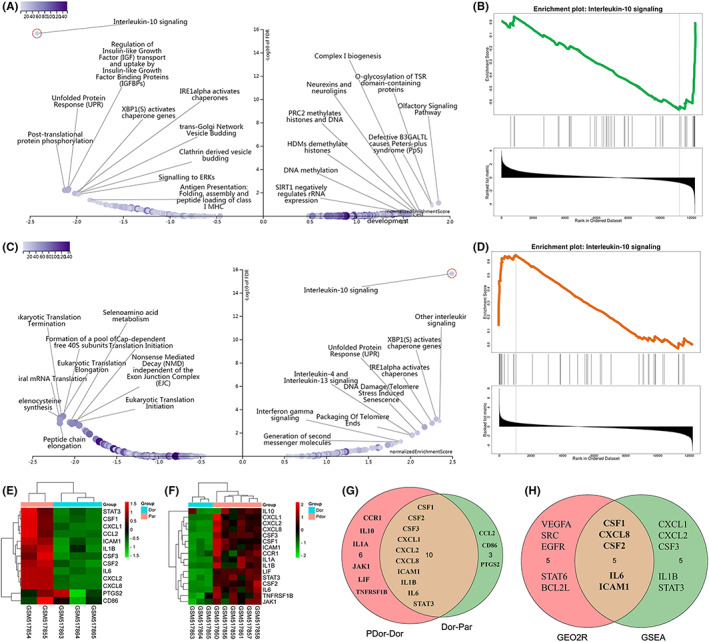
The interleukin‐10 (IL‐10) signaling pathway was the top enriched pathway related to TNBC bone metastasis according to GSEA. Results of GSEA comparing the Dor and Par (A) and PDor and Dor (C) groups in GSE20611 by WebGestalt. B, D, The IL‐10 signaling pathway was the top‐ranked pathway identified using WebGestalt. Heatmap showing the differential expression of genes involved in IL‐10 signaling between the Dor and Par groups (E) and the PDor and Dor groups (F). G, Venn diagram showing the overlapping genes involved in IL‐10 signaling between the Dor and Par and the PDor and Dor groups. H, Venn diagram showing the overlapping genes involved in IL‐10 signaling as identified by gene enrichment analysis and GSEA. GSEA, gene set enrichment analysis; TNBC, triple‐negative breast cancer
3.2. BM cells with high bone metastases have higher levels of ICAM1
In TNBC patients, ICAM1 mRNA and protein levels were significantly increased compared with those in healthy subjects or nTNBC patients in The Cancer Genome Atlas (TCGA) and the Clinical Proteomic Tumor Analysis Consortium (CPTAC) database (Figure 3A,B). In another independent GEO dataset (GSE25055), the ICAM1 mRNA expression level was significantly higher in the TNBC group than in the nTNBC group (Figure 3C). Moreover, patients with higher ICAM1 expression levels had worse survival outcomes (Figure 3D). After multiple cycles of tumor cell injection into the left ventricles of mice combined with primary cell isolation and culture, the highly bone metastatic MDA‐MB‐231BM (BM) TNBC cell line was obtained (Figure 3E). Interestingly, the mRNA and protein expression levels of ICAM1 in BM cells were higher than those in the parental cells (Figure 3F). However, the proliferation of these two cell lines was not significantly different (Figure 3G). The in vitro migration and invasion abilities of BM cells were greatly enhanced compared with those of the parental cells (Figure 3H,I). The BLI results indicated that BM cells, with high expression of ICAM1, had a higher level of bone metastatic activity than the parental cells (Figure 3J). The X‐ray and PET‐CT imaging results also suggested that ICAM1 enhanced the bone‐destroying capability of tumor cells (Figure 3K,L). In addition, the survival time of mice bearing tumors formed from BM cells was significantly shorter than that of mice bearing tumors formed from the parental cells (Figure 3M). The pathological HE and TRAP staining results also confirmed that BM cells indeed exhibited higher levels of bone metastatic activity and bone destruction than the parental cells (Figure 3N,O). Moreover, the expression of ICAM1 in lesions with severe bone metastasis was significantly higher than that in lesions with less severe bone metastasis (Figure 3P).
FIGURE 3.
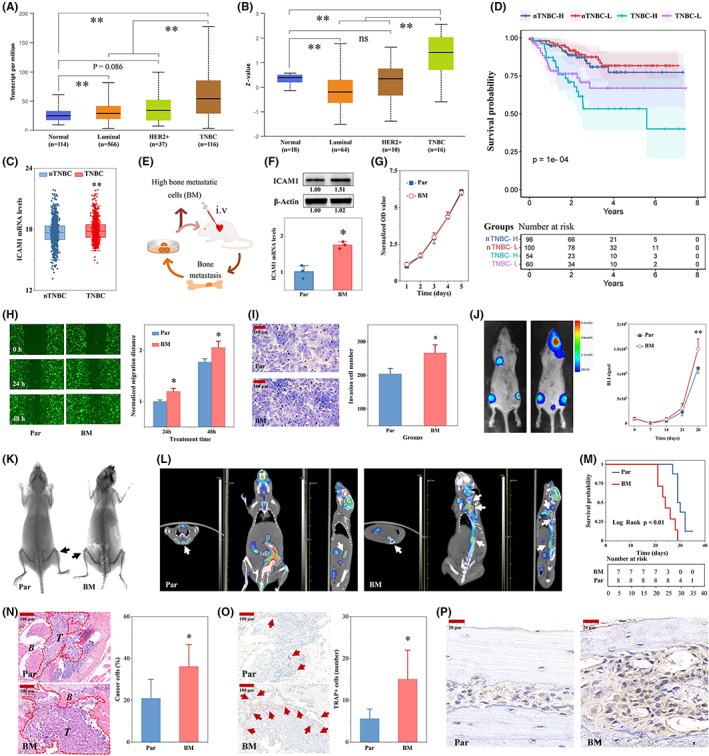
Bone metastatic MDA‐MB‐231BM (BM) cells with high bone metastases have higher levels of ICAM1. A, ICAM1 mRNA expression in the TCGA BRCA cohort was analyzed using UALCAN (http://ualcan.path.uab.edu/index.html; N = 833). B, ICAM1 protein expression in the Clinical Proteomic Tumor Analysis Consortium (CPTAC) BRCA cohort was analyzed using UALCAN (N = 108). C, mRNA expression levels of ICAM1 in GSE25055. D, Differences in the overall survival (OS) of patients with breast cancer in the high and low ICAM1 expression groups in the GSE25055 cohort (N = 310). E, Process for establishing the MDA‐MB‐231BM (BM) TNBC cell line with high bone metastatic activity. F, Protein and mRNA expression levels of ICAM1 in BM cells and the parental cells (N = 3). G, Comparison of cell proliferation between two TNBC cell lines. Representative images and quantitative analysis of migration (H) and invasion (I) in BM TNBC cells and its parental cells (N = 3). J, ICAM1 promoted bone metastasis detected by bioluminescence imaging (BLI) in TNBC cell–bearing mice. Cell suspensions (1 × 107 cells/ml) were prepared, and 1 × 106 cells (0.1 ml/mouse) were injected into the left ventricles of mice. In vivo BLI was used to monitor tumor cell proliferation in mice 2 h after injection and weekly thereafter. The signal intensity was normalized to the signal at 2 h post injection. The MDA‐MB‐231 parental cell group and the BM cell group contained eight and seven mice, respectively. ICAM1 promoted bone metastasis, as detected by X‐ray (K) and PET‐CT (L) imaging, in tumor‐bearing mice 4 weeks after tumor cell injection. M, ICAM1 reduced the survival of tumor‐bearing mice. N, ICAM1 increased tumor cell growth in bone metastases in tumor‐bearing mice (N = 3). O, ICAM1 promoted bone destruction (TRAP+ cells) in tumor‐bearing mice (N = 3). P, Immunohistochemical (IHC) analysis of ICAM1 in bone metastases formed from two different bone metastatic cell lines. *p < 0.05, **p < 0.01 vs. the Par or nTNBC group. ICAM1, intercellular adhesion molecule 1; TNBC, triple‐negative breast cancer
3.3. shICAM1 inhibited TNBC bone metastasis
To further explore the role of ICAM1 in TNBC bone metastasis, we used shRNA to silence ICAM1 gene expression in BM cells. shICAM1 significantly decreased ICAM1 expression at both the mRNA and protein levels (Figure 4A). Although shICAM1 did not noticeably affect cell proliferation, it significantly reduced BM cell migration and invasion (Figure 4B‐D). Interestingly, shICAM1 significantly inhibited cancer cell growth in mice (Figure 4E). Bone destruction was also well controlled by shICAM1 (Figure 4F,G). The proliferation of tumor cells in bone metastases was significantly reduced by shICAM1 (Figure 4H). Additionally, ICAM1 expression in bone metastases was decreased by shICAM1 (Figure 4I).
FIGURE 4.
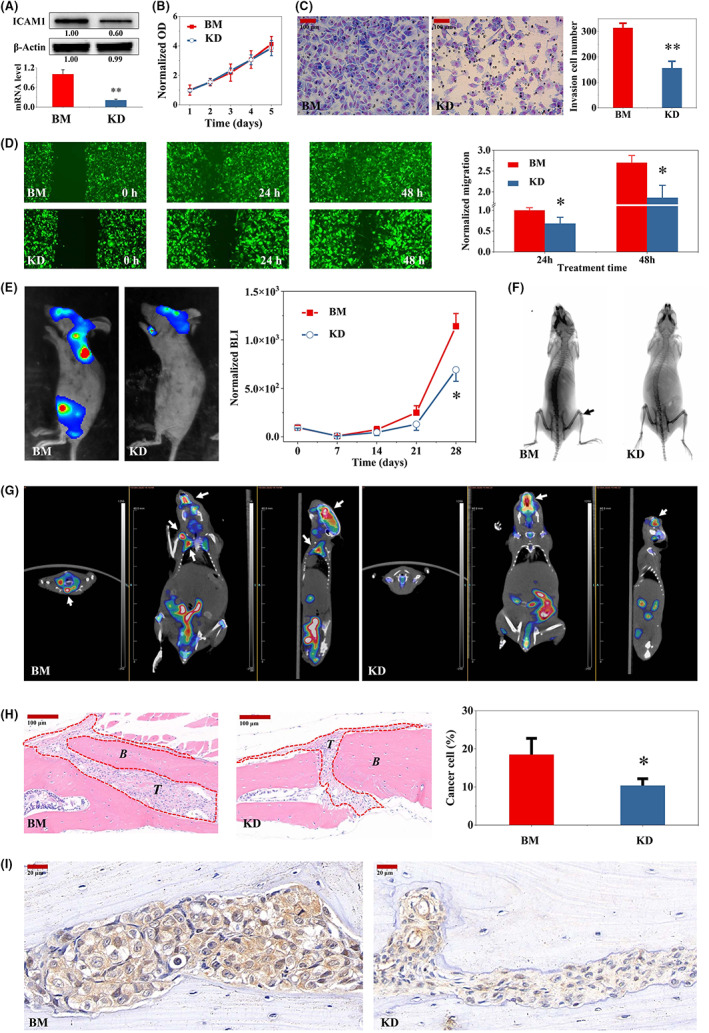
shRNA‐mediated ICAM1 gene silencing inhibited bone metastasis in mice. A, shICAM1 inhibited ICAM1 gene expression in BM cells. B, Effects of shICAM1 on the proliferation of BM cells. Representative images and quantification of migration (C) and invasion (D) in shICAM1 cells and the parental BM TNBC cell line (N = 3). E, shICAM1 inhibited bone metastasis in mice. Except for the cell density of 0.5 × 106 cells/mouse for BM and shICAM1 BM cells, the rest of the procedure was conducted as described in Figure 3J (N = 3). shICAM1 decreased bone metastasis, as detected by X‐ray (F) and PET‐CT (G) imaging, in tumor‐bearing mice 4 weeks after tumor cell injection. The X‐ray images were finally processed with a color inversion algorithm. H, shICAM1 reduced tumor cell growth in bone metastases in tumor‐bearing mice (N = 3). I, IHC analysis of ICAM1 expression in bone metastases formed from BM and shICAM1 BM cells. *p < 0.05, **p < 0.01 vs. the shCtrl group. ICAM1, intercellular adhesion molecule 1; IHC, immunohistochemical; TNBC, triple‐negative breast cancer
3.4. ICAM1 was correlated with EMT‐, apoptosis‐, and cell cycle–related genes
To explore the signaling pathways regulated by ICAM1, we performed Reactome enrichment analysis with the genes significantly differentially regulated by siICAM1 (Table S4) based on previous work. 16 Cytoskeleton‐ or cell movement‐, apoptosis‐, and TGF‐β–related signaling pathways were the main enriched pathways (Figure 5A). Therefore, correlation analysis between the main genes in the above pathways and ICAM1 was performed, and interesting results were obtained. Integrins, especially αL, αM, and β2, were positively correlated with ICAM1 (p < 0.05; Figure 5B). EMT‐related transcription factors (TFs), such as SNAIL and ZEB, were positively correlated with ICAM1 (Figure 5B). EMT marker tight‐junction genes were negatively correlated with ICAM1, while gap junction genes were positively correlated with ICAM1 (Figure 5B). MMPs were positively correlated with ICAM1 (Figure 5B). TGF‐β1 was positively correlated with ICAM1, and TGFBR3 was negatively correlated with ICAM1 (Figure 5B). Antiapoptotic genes, such as BCL‐xL, MCL1, and BAG6, were positively correlated with ICAM1, while proapoptotic genes, such as BAD and RASA1, were negatively correlated with ICAM1 (Figure 5B). Cell cycle regulatory genes, such as CCNB1, CCNG1, and CDKs, were negatively correlated with ICAM1, while CDKN1A and CCND1 were positively correlated with ICAM1 (Figure 5B). Migration‐related genes were also positively correlated with ICAM1, as were the integrin downstream genes GSK3A and ILK (Figure 5B). Although most NOTCH, BMP, and WNT pathway genes were positively correlated with ICAM1, the correlations were not significant (Figure 5B). In addition, PI3K/AKT pathway genes, such as PIK3C2A, PIK3C2G, and AKT1, were negatively correlated with ICAM1 (Figure 5B). Validation analysis using BM cells and the parental cells was then performed. The results were consistent with the results of the correlation analysis (Figure 5C). Flow cytometric analysis also showed G1/S arrest in BM cells, which have high expression of ICAM1, and indicated a significant decrease in their apoptosis rate (Figure 5D,E). Finally, we selected typical representatives from the above genes for Western blot verification and found similar results (Figure 5F). In particular, phospho‐SMAD2/3 were elevated in highly bone metastatic BM cells compared with MDA‐MB‐231 cells, and ICAM1 silencing significantly reduced phospho‐SMAD2/3 levels in BM cells (Figure 5F). We then compared the expression of ICAM1, TGF‐β1, integrin β2, and EMT markers in bone metastases formed in tumor‐bearing mice from cancer cells with different levels of bone metastatic activity. BM cells resulted in more severe bone damage, accompanied by high expression levels of ICAM1, TGF‐β1, integrin β2, and N‐cadherin (Figure 6). After shRNA‐mediated ICAM1 gene silencing, the expression levels of ICAM1, TGF‐β1, integrin β2, and N‐cadherin were decreased, and bone metastasis was significantly alleviated (Figure 6).
FIGURE 5.
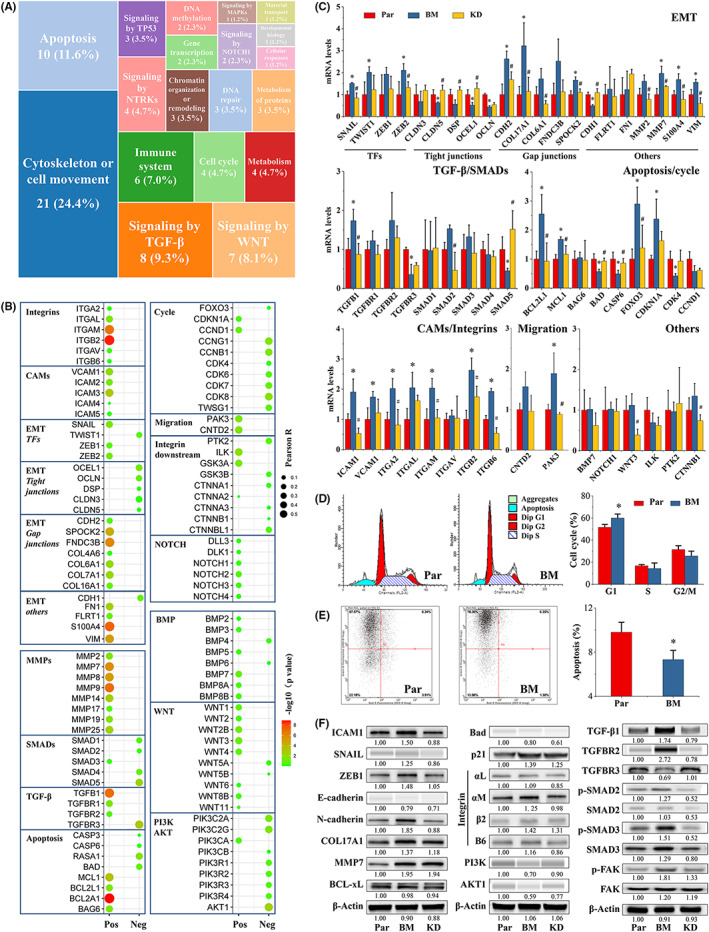
ICAM1 regulated the expression of EMT‐, cell cycle–, and apoptosis‐related genes. A, Heatmap of the signaling pathways significantly differentially regulated by siICAM1 in MDA‐MB‐231 cells, as determined using Reactome enrichment analysis. The candidate genes used for Reactome enrichment analysis are shown in Table S4. B, Pearson correlation analysis of ICAM1 gene expression with the expression of the indicated genes in GSE25055. Pos, positive; Neg, negative. C, mRNA levels in parental, BM, and shICAM1 cells were determined by RT‐qPCR as described in Section 2 (N = 3). D, shICAM1 induced G1/S arrest in BM cells. The flow cytometry method is described in Section 2 (N = 3). E, shICAM1 induced apoptosis in BM cells. The flow cytometry method is described in Section 2 (N = 3). F, Protein levels in Par, BM, and shICAM1 cells. Western blotting was carried out with a Mini‐PROTEAN Tetra cell system or a Wes Automated System as described in Section 2. The amount of total protein loaded was 10 μg. *p < 0.05 vs. the Par group. # p < 0.05 vs. the BM group. EMT, epithelial‐to‐mesenchymal transition; ICAM1, intercellular adhesion molecule 1
FIGURE 6.
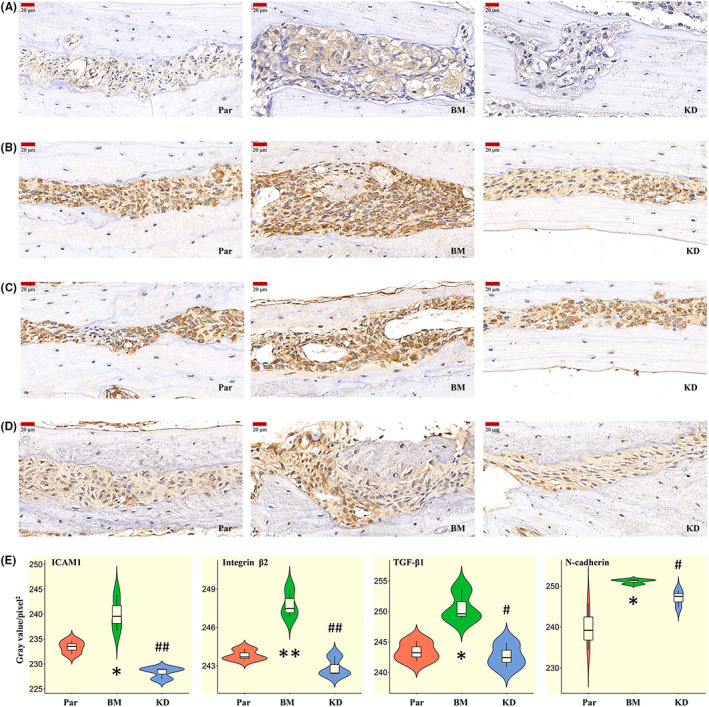
ICAM1‐mediated promotion of TNBC bone metastasis was associated with TGF‐β1 and EMT. Representative images of IHC staining of ICAM1 (A), integrin β2 (B), TGF‐β1 (C), and N‐cadherin (D) in bone metastases in TNBC tumor‐bearing mice in the three groups. E, Violin plot of the mean gray values in the bone metastases, as calculated with ImageJ (N = 3). *p < 0.05, **p < 0.01 vs. the Par group; # p < 0.05, ## p < 0.01 vs. the BM group. EMT, epithelial‐to‐mesenchymal transition; IHC, immunohistochemical; ICAM1, intercellular adhesion molecule 1; TNBC, triple‐negative breast cancer
3.5. ICAM1 regulated cell migration via integrin‐mediated TGF‐β/EMT signaling
The above results suggested that interactions between ICAM 1 and its integrin ligands may regulate the EMT process in TNBC in a TGF‐β dependent manner. Therefore, BM cells were treated with or without anti‐ICAM1, anti‐integrin, or anti‐TGF‐β1 neutralizing antibodies to compare the effects of these antibodies on cell migration and EMT. The anti‐ICAM1, anti‐integrin, and anti‐TGF‐β1 neutralizing antibodies significantly inhibited BM cell migration (Figure 7A,B). Moreover, combined treatment with the anti‐ICAM1 and anti‐TGF‐β1 neutralizing antibodies further significantly enhanced this inhibition (Figure 7A,B). These neutralizing antibodies significantly decreased the levels of EMT markers, such as N‐cadherin, SNAIL, and ZEB1 (Figure 7C). Additionally, both anti‐ICAM1 antibody and anti‐TGF‐β1 antibody, as well as their combination, significantly decreased phospho‐SMAD3 levels (Figure 7C).
FIGURE 7.
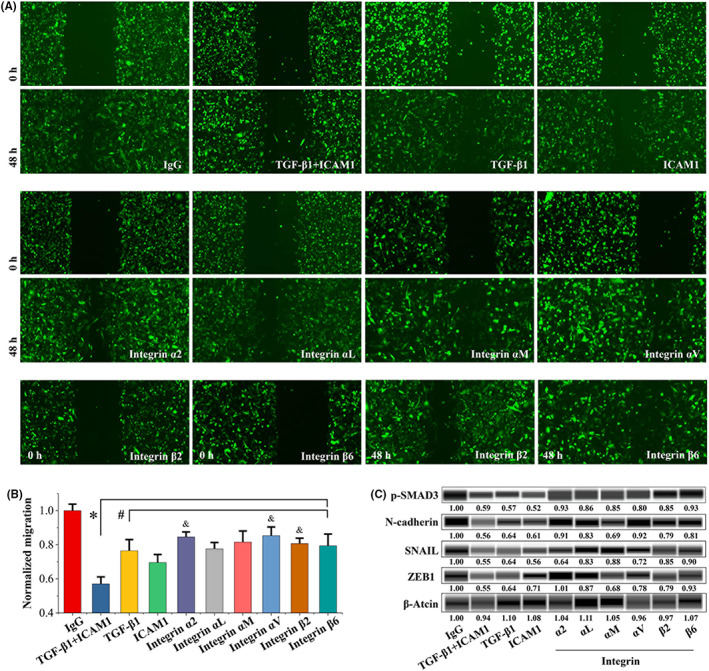
ICAM1 promoted TNBC cell migration in an integrin‐mediated TGF‐β/EMT–dependent manner. A, Representative images of BM cells treated with control IgG or anti‐ICAM1, anti‐integrin, or anti‐TGF‐β1 neutralizing antibodies (10 μg/ml). The wound‐healing migration assay protocol is described in Section 2 (N = 3). B, Quantitative analysis of the migration assay results in (A). *p < 0.05 vs. the control IgG group. # p < 0.05 vs. the anti‐ICAM1 plus anti‐TGF‐β1 antibody group. & p < 0.05 vs. the ICAM1 antibody group. C, Effects of control IgG or anti‐ICAM1, anti‐integrin, or anti‐TGF‐β1 antibody (10 μg/ml) treatment on protein expression in BM cells. Western blotting was performed with a Wes Automated System as described in Section 2. The amount of total protein loaded was 30 μg. ICAM1, intercellular adhesion molecule 1; TNBC, triple‐negative breast cancer
4. DISCUSSION
A previous study showed that ICAM1 was highly expressed in TNBC samples compared with nTNBC and normal samples, suggesting that ICAM1 is a therapeutic target for TNBC. 14 Another recent study showed that ICAM1 expression was higher in lung metastatic TNBC cell lines than in the parental cell lines and that siRNA‐mediated silencing of ICAM1 inhibited TNBC lung metastasis. 16 In this study, ICAM1 expression was significantly higher in highly bone metastatic TNBC BM cells than in the parental cells, and shICAM1 reduced bone metastasis in tumor‐bearing mice. Mechanistically, ICAM1 promoted TNBC bone metastasis through integrin‐mediated TGF‐β/EMT signaling.
The important cell membrane protein ICAM not only mediates intercellular adhesion but also interacts with its ligands via either heterophilic or homophilic interactions, resulting in the transduction of extracellular signals into a cell. 13 , 25 , 26 A previous study showed that the higher the expression of ICAM1 is, the worse the prognosis is, especially in patients with ER‐negative breast cancer. 27 Neutrophils promote TNBC cell migration through ICAM1 clustering–mediated focal adhesion rearrangement and cytoskeletal rearrangement. 15 In addition, Taftaf et al. showed that circulating tumor cells confer higher tumor invasiveness by inducing homophilic ICAM1‐ICAM1 interactions for tumor‐endothelial adhesion to achieve endothelial migration, ultimately leading to lung metastasis in breast cancer. 16 Disruption of these homophilic interactions inhibits TNBC cell migration. Our study is consistent with previous studies suggesting that ICAM1 clustering–mediated homophilic interactions are critical for the invasive and metastatic capacities of TNBC cells. 16 , 28
In addition, ICAM1 participates in heterophilic interactions with its ligands LFA‐1 (αL/β2 integrin) and MAC‐1 (αM/β2 integrin), and this interaction plays an important role in promoting the migration of leukocytes and endothelial cells. 17 , 29 The affinity between ICAM1 with LFA‐1 and Mac‐1 is different, and the differential affinities may regulate leukocyte adhesion and movement. 30, 31, 32, 33 However, in TNBC cells cultured in vitro, the expression of the integrin β2 subunit is very low. 34 Similarly, we found that the β2 integrin protein level was very low in MDA‐MB‐231 cells cultured in vitro. However, β2 integrin protein in bone metastases was unexpectedly increased. Additionally, disruption of the heterophilic interaction between ICAM1 and integrins with anti‐αL, anti‐αM, or anti‐β2 integrin neutralizing antibody inhibited cell migration. This study demonstrates that heterophilic interactions between ICAM1 and its ligands play an important role in the migration of TNBC cells. The different levels of α6/β1 integrin lead to gene expression differences in cell cycle and proliferation can determine whether they can initiate TGF‐β and integrin/FAK signaling. 35 Additionally, integrin β1 activates FAK, which phosphorylates TGFBR1, thereby enhancing the binding of TGFBR1 to TGFBR2 and activating the TGF‐β/SMAD pathway in breast cancer bone metastasis. 36 According to the “seed and soil” hypothesis, it is relatively difficult for most cancer cells to metastasize to other organs, and only a few cancer cells that acquire mutations can metastasize via alterations in the expression of some genes and can better adapt to a new environment to survive and eventually metastasize. 37 Therefore, abnormal changes in the expression of ICAM1 or its ligands may be an important factor in bone metastasis of TNBC cells. This observation provides a new therapeutic target for the treatment of TNBC bone metastases.
Epithelial‐to‐mesenchymal transition is associated with the formation of cancer stem cells. 38 , 39 , 40 EMT is induced by paracrine signaling factors to which cells are exposed, especially TGF‐β. 41 In addition, various combinations of other signaling mediators, such as WNT proteins, cytokines, growth factors, and extracellular matrix (ECM)‐integrin interactions, induce EMT. 42 Ultimately, these signals lead to the inducible expression and functional activation of various regulators, especially EMT‐related transcription factors (EMT‐TFs), during EMT. 43 We found that ICAM1 was highly correlated with TFs and markers of EMT in TNBC cells. Moreover, in mice bearing tumors formed by TNBC cells with different levels of bone metastatic activity, the degree of bone damage was not only highly correlated with the expression of ICAM1 but also correlated with the expression of EMT‐TFs and markers, such as N‐cadherin, SNAIL, and ZEB. Thus, we believe that ICAM1 promotes bone metastasis through EMT signaling in TNBC. In addition, TGF‐β signaling is a key pathway in EMT. 41 , 44 , 45 In fact, TGF‐β stimulates EMT in epithelial cells and enhances their motility. 46 Moreover, the interaction of TGF‐β with integrins (αV/β6 or αV/β8) results in release of active TGF‐β, suggesting that TGF‐β activity is regulated by integrins. 47 , 48 Although the mechanism by which ICAM1 interacts with integrins to affect TGF‐β signaling remains unknown, ICAM1 expression is highly positively correlated with TGF‐β1 expression and subsequently activates the SMAD pathway, ultimately initiating the EMT program. In addition, heterophilic interactions between ICAM1 and integrins regulate β‐catenin and thus activate EMT. 49 Cell movement–related signaling pathways, such as microtubule organization, actomyosin structure organization, and endomembrane system organization pathways, are regulated by ICAM1. 16 Activation of these pathways related to cell movement is critical for cancer cell migration through the EMT program. 50 , 51 Therefore, due to the important influence of ICAM1 on cell migration and movement through EMT, targeting ICAM1 is important for controlling cancer metastasis. In fact, our results were consistent with previous work suggesting that targeting ICAM1 and inhibiting ICAM1 expression can inhibit breast cancer metastasis. 16
Furthermore, cell cycle and apoptosis‐related pathways were regulated upon silencing of ICAM1. G1/S arrest and apoptotic inhibition was found in BM cells compared with the parental cells. We speculate that this seeming contradiction may be a compromise or an adaptive adjustment made by the highly bone metastatic TNBC cell line to undergo metastasis. To metastasize, BM cells use as much energy as possible for metastasis‐related signaling instead of proliferation. 52 This may also explain why the proliferation of the three cell lines with different levels of bone metastatic activity did not differ significantly although their ICAM1 expression level differed. In other words, the cell cycle arrest induced by ICAM1 may be counteracted by the inhibition of apoptosis, ultimately reflected in the lack of an apparent change in cell proliferation. However, a previous study suggested that ICAM1 promoted cancer cell cluster formation and siICAM1 inhibited cluster formation in a mammosphere assay using poly (2‐hydroxyethyl methacrylate) (poly‐HEMA) in PRIME‐XV tumorsphere serum‐free medium. 16 Unfortunately, the effects of ICAM1 on cell proliferation were not directly compared in that study. 16 Studies in this paper and other papers indicate that ICAM1 barely promotes cell proliferation and that silencing ICAM1 expression does not noticeably affect cell proliferation. 53 However, these studies, including ours, are based on conventional 2D cell culture, not 3D culture conditions. These observations seem to suggest that under 3D culture conditions, ICAM1 can promote the clustering of cancer cells, which may affect cell proliferation. However, Taftaf et al. did not directly compare the effects of ICAM1 on cell proliferation under 2D and 3D culture conditions, and these effects need to be confirmed by further comparative studies. Additionally, previous studies showed that the occurrence of EMT is accompanied not only by cell cycle arrest but also by apoptosis induction. 52 , 54 Although the present study and most previous studies confirmed that EMT is accompanied by cell cycle arrest, the fate of apoptosis differed, possibly because of the differences in cancers or metastatic sites.
Herein, we show that ICAM1 affects TGF activity by mediating interactions with its ligands and ultimately activating the TGF‐β/SMAD pathway. Combined with findings from previous studies, our findings suggest that active TGF‐β1 can be released by the release of integrins (αV/β6 or αV/β8) from an inactive state. 47 , 48 However, the main ligands of ICAM1 are LFA‐1 and MAC‐1, namely, αL/β2 and αM/β2 integrin, respectively. 55 Although we found that ICAM1 expression was highly positively correlated with αV/β6 integrin expression and that the presence of neutralizing antibodies against αV or β6 integrins inhibited cell migration, whether ICAM1 can directly bind to αV/β6 integrins and thereby release active TGF‐β1 remains unknown. Alternatively, the binding of ICAM1 to LFA‐1 and MAC‐1 may affect the activity of αV/β6 integrins. Confirmation of these phenomena requires further direct evidence. Additionally, TGFBR3, a transmembrane receptor protein for TGF‐β, undergoes ectodomain shedding, releasing soluble TGFBR3, which binds and sequesters ligand to inhibit downstream TGF‐β/SMAD signaling, and ultimately reduces breast cancer cell invasion and tumor‐induced angiogenesis. 56 , 57 , 58 The significantly reduced soluble TGFBR3 in TNBC is not sufficient to neutralize TGF‐β and ultimately activate the TGF‐β pathway. Moreover, TNBC cells release ICAM1 through exosomes or extracellular vesicles, inducing not only tumor immune responses but also osteoclast differentiation and thereby creating a tumor microenvironment favorable for tumor cells. 18 , 59 Therefore, the role of this secreted ICAM1 in breast cancer bone metastasis also needs further investigation.
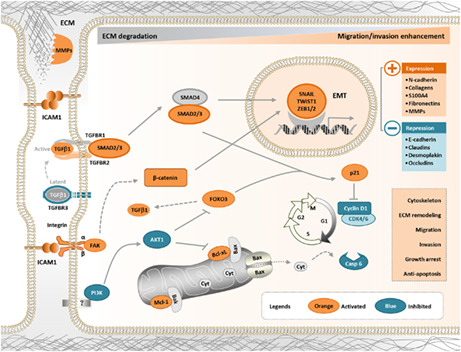
Therefore, the heterophilic interactions between ICAM1 and its integrin ligands regulate integrin activity, which in turn affects the activity of extracellular TGF‐β and activates the TGF‐β/SMAD pathway. The activated TGF‐β/SMAD pathway ultimately promotes EMT by regulating the expression of EMT‐related genes, such as CDHs, SNAIL, ZEB, and MMPs. In contrast, ICAM1 regulates cell cycle– and apoptosis‐related genes by inhibiting the PI3K/AKT pathway and ultimately exerts antiapoptotic effects and cell cycle arrest. In addition, ICAM1 may activate other pathways to promote metastasis through homophilic interactions. 16 In summary, ICAM1 promotes the EMT program, induces cell cycle progression, promotes apoptotic resistance through integrin‐mediated TGF‐β/SMAD signaling, and finally results in bone metastasis in TNBC (Figure 8). Therefore, the findings of the present study provide a strong rationale for the application of ICAM1‐targeted therapy in breast cancer with bone metastasis.
FIGURE 8.
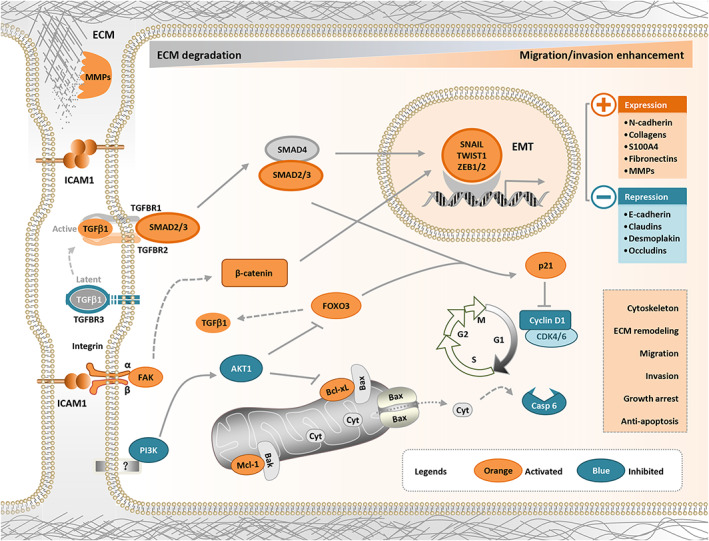
ICAM1 promotes bone metastasis of TNBC cells by regulating EMT through interactions with integrins in a TGF‐β–dependent manner. ICAM1 promotes the EMT program, induces cell cycle progression, promotes apoptotic resistance through integrin‐mediated TGF‐β/SMAD signaling, and finally results in bone metastasis in TNBC. EMT, epithelial‐to‐mesenchymal transition; ICAM1, intercellular adhesion molecule 1; TNBC, triple‐negative breast cancer
AUTHOR CONTRIBUTIONS
Fu Z and Liu S conceived and designed the experiments. Chen M performed the animal experiments, bioinformatics analysis, RT–qPCR, western blotting, and statistical analysis. Wu C carried out the wound healing, Transwell, HE staining, TRAP staining, IHC, and cell proliferation assays. Chen M wrote the manuscript. All authors reviewed and approved the final manuscript.
DISCLOSURE
The authors declare no conflict of interest.
ANIMAL STUDIES
All animal experiments were carried out according to the national regulations for animal experimentation and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University (approval number 2020‐KL‐168‐01).
Supporting information
Appendix S1
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We appreciate the assistance with the animal experiments from Dr. Xiufei Gao in the First Affiliated Hospital of Zhejiang Chinese Medical University. This study was supported by grants from the National Natural Science Foundation of China (No. 81704075), the Shanghai Municipal Health Commission (No. ZY3‐LCPT‐2‐1002), the Science and Technology Commission of Shanghai Municipality (No. 20Z21900300), and the Shanghai Hospital Development Center (No. SHDC2020CR1050B).
Chen M, Wu C, Fu Z, Liu S. ICAM1 promotes bone metastasis via integrin‐mediated TGF‐β/EMT signaling in triple‐negative breast cancer. Cancer Sci. 2022;113:3751‐3765. doi: 10.1111/cas.15532
Mingcang Chen and Chunyu Wu contributed equally to this work.
Contributor Information
Zhengwei Fu, Email: azwfu@zjut.edu.cn.
Sheng Liu, Email: sliu_tcm@163.com.
REFERENCES
- 1. Sung H, Ferlay J, Siege RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005‐2015). Breast. 2018;39:131‐138. doi: 10.1016/j.breast.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 3. Lyons TG. Targeted therapies for triple‐negative breast cancer. Curr Treat Options Oncol. 2019;20(11):82. doi: 10.1007/s11864-019-0682-x [DOI] [PubMed] [Google Scholar]
- 4. Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14‐27. doi: 10.1016/j.semcancer.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 5. Zarrer J, Haider MT, Smit DJ. Pathological crosstalk between metastatic breast cancer cells and the bone microenvironment. Biomolecules. 2020;10(2):337. doi: 10.3390/biom10020337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102(7):456‐463. doi: 10.1093/jnci/djq029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang Z, Wang H, Wang S, et al. Chinese expert consensus statement on the clinical diagnosis and treatment of breast cancer bone metastasis and bone related disease. Transl Breast Cancer Res. 2021;2:2. doi: 10.21037/tbcr-20-65 [DOI] [Google Scholar]
- 8. Xing L, Ebetino FH, Boeckman RK Jr, et al. Targeting anti‐cancer agents to bone using bisphosphonates. Bone. 2020;138:115492. doi: 10.1016/j.bone.2020.115492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McClung MR. Denosumab for the treatment of osteoporosis. In: Dempste DW, Cauley AJ, Bouxsein ML, Cosman F, eds. Marcus and feldman's Osteoporosis (5th Edition): Denosumab for the Treatment of Osteoporosis. Academic Press; 2021:1737‐1755. [Google Scholar]
- 10. Loyson T, Cann TV, Schöffski P, et al. Incidence of osteonecrosis of the jaw in patients with bone metastases treated sequentially with bisphosphonates and denosumab. Acta Clin Belg. 2018;73(2):100‐109. doi: 10.1080/17843286.2017.1348001 [DOI] [PubMed] [Google Scholar]
- 11. Coleman RE, Gnant M, Paterson A, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta‐analysis of individual patient data from randomized trials. Lancet. 2015;386:1353‐1361. doi: 10.1016/S0140-6736(15)60908-4 [DOI] [PubMed] [Google Scholar]
- 12. Steinman RA, Brufsky AM, Oesterreich S. Zoledronic acid effectiveness against breast cancer metastases ‐ a role for estrogen in the microenvironment? Breast Cancer Res. 2012;14(5):213. doi: 10.1186/bcr3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bui TM, Wiesolek HL, Sumagin R. ICAM‐1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108(3):787‐799. doi: 10.1002/JLB.2MR0220-549R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo P, Huang J, Wang L, et al. ICAM‐1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci USA. 2014;111(41):14710‐14715. doi: 10.1073/pnas.1408556111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA‐MB‐468 human breast carcinoma cells via ICAM‐1. Exp Cell Res. 2010;316(1):138‐148. doi: 10.1016/j.yexcr.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 16. Taftaf R, Liu X, Singh S, et al. ICAM1 initiates CTC cluster formation and transendothelial migration in lung metastasis of breast cancer. Nat Commun. 2021;12(1):4867. doi: 10.1038/s41467-021-25189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manuel R, Enric E. Role of LFA‐1 and ICAM‐1 in cancer. Cancer. 2017;9(11):153. doi: 10.3390/cancers9110153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alečković M. The Role of Secreted Proteins and Exosomes in Cancer Metastasis. Princeton University; 2016:111‐116. [Google Scholar]
- 19. Wu C, Yang S, Sun Z, Han X, Ye Y, Liu S. Characterization of the attenuation of breast cancer bone metastasis in mice by zoledronic acid using 99mTc bone scintigraphy. Pathol Oncol Res. 2014;20(3):747‐754. doi: 10.1007/s12253-014-9756-z [DOI] [PubMed] [Google Scholar]
- 20. Li H, Chen M, Yang Z, et al. Amorphophalli Rhizoma inhibits breast cancer growth, proliferation, migration, and invasion via the PI3K/AKT pathway. J Ethnopharmacol. 2022;286:114926. doi: 10.1016/j.jep.2021.114926 [DOI] [PubMed] [Google Scholar]
- 21. Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47(W1):W199‐W205. doi: 10.1093/nar/gkz401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta‐analysis. Nucleic Acids Res. 2019;47(W1):W234‐W241. doi: 10.1093/nar/gkz240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771‐3772. doi: 10.1093/bioinformatics/bty411 [DOI] [PubMed] [Google Scholar]
- 24. Heatmap was plotted by. Accessed July 30, 2022. https://www.bioinformatics.com.cn
- 25. Haddon L, Hugh J. MUC1‐mediated motility in breast cancer: a review highlighting the role of the MUC1/ICAM‐1/Src signaling triad. Clin Exp Metastasis. 2015;32(4):393‐403. doi: 10.1007/s10585-015-9711-8 [DOI] [PubMed] [Google Scholar]
- 26. Ghazy AA, Elsheredy HG, Abouelella AM, Rashwan EK, Khaled BEA, Elsheredy AG. Significance of intracellular adhesion Molecule‐1 polymorphism and IP‐10 among breast cancer patients. Egypt J Immunol. 2020;27(1):187‐195. PMID: 33236621. [PubMed] [Google Scholar]
- 27. Schröder C, Witzel I, Müller V, et al. Prognostic value of intercellular adhesion molecule (ICAM)‐1 expression in breast cancer. J Cancer Res Clin Oncol. 2011;137(8):1193‐1201. doi: 10.1007/s00432-011-0984-2 [DOI] [PubMed] [Google Scholar]
- 28. Zhu L, Mu Q, Yu J, Griffin JI, Xu X, Ho RJY. ICAM‐1 targeted drug combination nanoparticles enhanced gemcitabine‐paclitaxel exposure and breast cancer suppression in mouse models. Pharmaceutics. 2021;14(1):89. doi: 10.3390/pharmaceutics14010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yusuf‐Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA‐1/ICAM‐1 and VLA‐4/VCAM‐1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22(2):146‐167. doi: 10.1002/med.10001 [DOI] [PubMed] [Google Scholar]
- 30. Mao D, Lü S, Li N, Zhang Y, Long M. Conformational stability analyses of alpha subunit I domain of LFA‐1 and Mac‐1. PLoS One. 2011;6(8):e24188. doi: 10.1371/journal.pone.0024188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569‐2575. doi: 10.1084/jem.20060925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E‐ or P‐selectin induces the extended but not high‐affinity conformation of LFA‐1 in neutrophils. Blood. 2010;116(4):617‐624. doi: 10.1182/blood-2010-01-266122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith C. Cooperative interactions of LFA‐1 and Mac‐1 with intercellular adhesion molecule‐1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83(6):2008‐2017. doi: 10.1172/JCI114111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.. Taftaf R. ICAM1 Initiates CTC Cluster Formation and Lung Metastasis of Triple Negative Breast Cancer. Northwestern University. 28497317; 2021. [DOI] [PMC free article] [PubMed]
- 35. Schober M, Fuchs E. Tumor‐initiating stem cells of squamous cell carcinomas and their control by TGF‐β and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USA. 2011;108(26):10544‐10549. doi: 10.1073/pnas.1107807108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang L, Qu J, Qi Y, et al. EZH2 engages TGFβ signaling to promote breast cancer bone metastasis via integrin β1‐FAK activation. Nat Commun. 2022;13(1):2543. doi: 10.1038/s41467-022-30105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fidler I. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453‐458. doi: 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]
- 38. Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21(5):325‐338. doi: 10.1038/s41568-021-00332-6 [DOI] [PubMed] [Google Scholar]
- 39. Mani SA, Guo W, Liao MJ, et al. The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704‐715. doi: 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell. 2016;166:21‐45. doi: 10.1016/j.cell.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 41. Moustakas A, Kowanetz M, Thuault S. Smad signal transduction. In: Dijke PT, Heldin CH, eds. TGF‐β/SMAD signaling in epithelial to mesenchymal transition. Springer; 2006; 5:131‐150. [Google Scholar]
- 42. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial‐mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178‐196. doi: 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611‐629. doi: 10.1038/nrclinonc.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scimeca M, Trivigno D, Bonfiglio R, et al. Breast cancer metastasis to bone: from epithelial to mesenchymal transition to breast osteoblast‐like cells. Semin Cancer Biol. 2021;72:155‐164. doi: 10.1016/j.semcancer.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 45. Xu X, Zhang L, He X, et al. TGF‐β plays a vital role in triple‐negative breast cancer (TNBC) drug‐resistance through regulating stemness, EMT and apoptosis. Biochem Biophys Res Commun. 2018;502(1):160‐165. doi: 10.1016/j.bbrc.2018.05.139 [DOI] [PubMed] [Google Scholar]
- 46. Xu J, Lamouille S, Derynck R. TGF‐beta‐induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156‐172. doi: 10.1038/cr.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. GARP regulates the bioavailability and activation of TGFβ. Mol Biol Cell. 2012;23(6):1129‐1139. doi: 10.1091/mbc.E11-12-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dong X, Zhao B, Iacob RE, et al. Force interacts with macromolecular structure in activation of TGF‐β. Nature. 2017;542(7639):55‐59. doi: 10.1038/nature21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mamuya FA, Duncan MK. aV integrins and TGF‐β‐induced EMT: a circle of regulation. J Cell Mol Med. 2012;16(3):445‐455. doi: 10.1111/j.1582-4934.2011.01419.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Campbell K, Casanova J. A common framework for EMT and collective cell migration. Development. 2016;143(23):4291‐4300. doi: 10.1242/dev.139071 [DOI] [PubMed] [Google Scholar]
- 51. Aiello NM, Kang Y. Context‐dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016‐1026. doi: 10.1084/jem.20181827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hao Y, Baker D, Ten Dijke P. TGF‐β‐mediated epithelial‐mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11):2767. doi: 10.3390/ijms20112767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosette C, Roth RB, Oeth P, et al. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26(5):943‐950. doi: 10.1093/carcin/bgi070 [DOI] [PubMed] [Google Scholar]
- 54. Song J, Shi W. The concomitant apoptosis and EMT underlie the fundamental functions of TGF‐β. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):91‐97. doi: 10.1093/abbs/gmx117 [DOI] [PubMed] [Google Scholar]
- 55. Li N, Yang H, Wang M, Lü S, Zhang Y, Long M. Ligand‐specific binding forces of LFA‐1 and mac‐1 in neutrophil adhesion and crawling. Mol Biol Cell. 2018;29(4):408‐418. doi: 10.1091/mbc.E16-12-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee JD, Hempel N, Lee NY, Blobe GC. The type III TGF‐beta receptor suppresses breast cancer progression through GIPC‐mediated inhibition of TGF‐beta signaling. Carcinogenesis. 2010;31(2):175‐183. doi: 10.1093/carcin/bgp271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dong M, How T, Kirkbride KC, et al. The type III TGF‐beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117(1):206‐217. doi: 10.1172/JCI29293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meyer AE, Gatza CE, How T, Starr M, Nixon AB, Blobe GC. Role of TGF‐β receptor III localization in polarity and breast cancer progression. Mol Biol Cell. 2014;25(15):2291‐2304. doi: 10.1091/mbc.E14-03-0825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang W, Zhong W, Wang B, et al. ICAM‐1‐mediated adhesion is a prerequisite for exosome‐induced T cell suppression. Dev Cell. 2022;57(3):329‐343. doi: 10.1016/j.devcel.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1
Table S2
Table S3
Table S4


