Abstract
Study objective
To describe endotracheal intubation practices in emergency departments by staff intubating patients early in the coronavirus disease 2019 (COVID-19) pandemic.
Methods
Multicenter prospective cohort study of endotracheal intubations conducted at 20 US academic emergency departments from May to December 2020, stratified by known or suspected COVID-19 status. We used multivariable regression to measure the association between intubation strategy, COVID-19 known or suspected status, first-pass success, and adverse events.
Results
There were 3,435 unique emergency department endotracheal intubations by 586 participating physicians or advanced practice providers; 565 (18%) patients were known or suspected of having COVID-19 at the time of endotracheal intubation. Compared with patients not known or suspected of COVID-19, endotracheal intubations of patients with known or suspected COVID-19 were more often performed using video laryngoscopy (88% versus 82%, difference 6.3%; 95% confidence interval [CI], 3.0% to 9.6%) and passive nasal oxygenation (44% versus 39%, difference 5.1%; 95% CI, 0.9% to 9.3%). First-pass success was not different between those who were and were not known or suspected of COVID-19 (87% versus 86%, difference 0.6%; 95% CI, –2.4% to 3.6%). Adjusting for patient characteristics and procedure factors in those with low anticipated airway difficulty (n=2,374), adverse events (most commonly hypoxia) occurred more frequently in patients with known or suspected COVID-19 (35% versus 19%, adjusted odds ratio 2.4; 95% CI, 1.7 to 3.3).
Conclusion
Compared with patients not known or suspected of COVID-19, endotracheal intubation of those confirmed or suspected to have COVID-19 was associated with a similar first-pass intubation success rate but higher risk-adjusted adverse events.
Editor’s Capsule Summary.
What is already known on this topic
COVID-19 led many to reassess airway management strategies and to adopt greater use of personal protective equipment.
What question this study addressed
Were there changes in first-pass success or endotracheal intubation techniques during the early phase of the pandemic?
What this study adds to our knowledge
In this multicenter, prospective sample of 3,435 emergency department intubations, first-pass success was not different for the 565 patients known or suspected to have COVID-19 when compared to others. There were only minor differences in intubation devices, medications, and techniques between groups.
How this is relevant to clinical practice
Despite greater personal protective equipment, emergency physicians can maintain quality endotracheal intubation success and practice for patients with known or suspected COVID-19.
Introduction
Background
Front-line health care personnel are at risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because of their contact with diagnosed and undiagnosed patients, time pressures, and frequent performance of aerosol-generating procedures.1 Early in the pandemic, emergency department staff had relatively high SARS-CoV-2 seropositivity rates, even compared with other front-line health care personnel.2, 3, 4, 5 Endotracheal intubation has been associated with an increased risk of transmitting SARS-CoV-2 to health care personnel,6 , 7 and early reports from a multinational registry estimated that more than 10% of health care personnel intubating suspected or confirmed SARS-CoV-2-infected patients developed symptoms or tested positive for the disease within 14 days of intubating a suspected or confirmed SARS-CoV-2-infected patient, despite 88% using recommended personal protective equipment (PPE).8 In response, various approaches were adopted to reduce transmission, including the use of dedicated intubation teams and aerosol hoods.9, 10, 11 Some of these techniques exposed health care personnel and patients to risks of adverse events not encountered using procedures well-established for traditional emergency endotracheal intubation.6 , 12, 13, 14
Importance
Protocols and recommendations were rapidly proposed to mitigate the risks associated with airway management.15, 16, 17 These protocols recommended using enhanced PPE, having the most skilled providers perform intubations, using video laryngoscopy, avoiding aerosol-generating maneuvers, and using supraglottic airways.18 Although previous investigations explored airway management techniques and their associated risk to providers, these studies were predominantly conducted in ICU settings.8 , 19 Understanding the methods by which ED health care personnel manage the airway of patients with known or suspected coronavirus disease 2019 (COVID-19) is essential to our evolving understanding of the ideal way to treat patients with COVID-19 and other contagious respiratory pathogens. In addition, understanding how additional risk mitigation measures (eg, full PPE during intubation) affects success rates and complications of endotracheal intubation is key to improving the care delivered to critically ill patients with COVID-19, as well as critically ill patients in any future pandemic.
Goals of This Investigation
We sought to describe ED airway management practices in patients with known or suspected COVID-19 in the US multicenter network during the early phases of the COVID-19 pandemic and before COVID-19 vaccine availability. The objectives of this analysis were to: (1) compare the ED intubation strategy (eg, approach, medications, and equipment) for those with known or suspected COVID-19 intubations with those not suspected or known to have COVID-19, (2) evaluate the association between ED intubation strategy and intubation outcomes, (3) describe the use of PPE for ED endotracheal intubation, and (4) estimate the accuracy of clinical gestalt in identifying COVID-19 patients undergoing endotracheal intubation.
Materials and Methods
Study Design, Setting, and Participants
The COVID-19 Evaluation of Risks in Emergency Departments (Project COVERED) was a multicenter, prospective cohort study of health care personnel at risk of contracting COVID-19 conducted in 20 US academic medical center EDs with 20 weeks of continuous observation from May 13, 2020 to December 9, 2020. Health care personnel participants recorded detailed information about all intubation and cardiac arrest care. Serial SARS-CoV-2 serology and reverse transcription polymerase chain reaction were used to identify health care personnel infections during the surveillance period. Details of the study design and data collection tools have been reported previously.20, 21, 22, 23 This activity was reviewed and conducted consistent with applicable federal law and Centers for Disease Control and Prevention policy.24 All institutional review boards concurred with this constituting a public health surveillance activity, and health care personnel provided informed consent before participation. This article is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement.25
We recruited ED health care personnel through local advertising to include physicians or advanced practice providers (approximately 40 from each site) who had not previously had SARS-CoV-2 infection and were confirmed seronegative at enrollment. The parent study also enrolled nurses and nonclinical staff, but only physicians/advanced practice providers recorded detailed information about intubations and were included in this analysis. In addition, we replaced participants who left participating sites with other qualifying health care personnel during the observation period. Therefore, this analysis includes only patients who had endotracheal intubation performed by one of the enrolled health care personnel.
Measurements, Definitions, and Data Collection
During 20 weeks of surveillance, health care personnel participants who either performed the endotracheal intubation or were within 3 feet of the procedure completed detailed electronic surveys (Research Electronic Data Capture, REDCap, Vanderbilt University, Nashville, Tennessee) for every endotracheal intubation after the procedure was completed.26 , 27 Local site coordinators verified that ≥95% of endotracheal intubation events were recorded in the data collection tool and monthly auditing ensured ≥95% ongoing capture rates. Local coordinators also abstracted patient-level data after hospital discharge to include SARS-CoV-2 test results and COVID-19 clinical outcomes. When multiple physicians/advanced practice providers reported single endotracheal intubation, the record from the primary health care personnel performing the procedure was retained for this analysis. Health care personnel additionally completed weekly surveys that collected data on workplace and community exposures, PPE use, and new SARS-CoV-2 infections.
Outcome Measures
Our primary outcome was the description of the characteristics of the initial endotracheal intubation strategy, stratified by patients known or suspected of having COVID-19. We described endotracheal intubation strategies by the following 7 characteristics: (1) preoxygenation strategy, (2) medication strategy (eg, rapid sequence induction, awake intubation, and sedation-only intubation), (3) induction drug, (4) paralytic drug, (5) first-attempt equipment (eg, direct laryngoscopy, video laryngoscopy, and fiberoptic bronchoscopy), (6) use of adjuncts (eg, passive nasal oxygenation, supraglottic devices, intubation boxes, and dedicated intubation teams), and (7) operator experience (eg, resident and faculty physician). Our secondary outcomes included first-pass success, number of attempts, overall success, and adverse events. We defined first-pass success as the successful placement of an endotracheal tube after the first placement of a laryngoscope without removing the laryngoscope from the mouth or changing from the initial technique, as determined by the primary clinician performing the intubation. We defined adverse events within 15 minutes after the endotracheal intubation procedure as hypoxia (pulse oximetry [SpO2]<90%), severe hypoxia (SpO2<80%), hypotension (systolic blood pressure <90 mmHg), esophageal intubation, dental injury, cardiac arrest (after beginning the intubation procedure), and airway failure (ie, death without airway being placed). We also estimated the accuracy of clinical gestalt in predicting which patients undergoing endotracheal intubation had COVID-19.
Exposures
Our primary exposure was confirmed or suspected COVID-19 in the intubated patient as reported by the health care personnel at the time of endotracheal intubation. Confirmed SARS-CoV-2 infection was reported by the health care personnel reporting the endotracheal intubation based on knowledge (from prior testing or patient/family report of a positive test) thought to be accurate at the time of the procedure. For patients without a SARS-CoV-2 test result at the time of endotracheal intubation, health care personnel reported one of the following to indicate their suspicion of COVID-19: “convinced patient had COVID-19” or “pretty sure patient had COVID-19” (suspected); or “convinced patient did not have COVID-19” or “pretty sure patient did not have COVID-19” (not suspected). Each case was classified into mutually exclusive categories of COVID-19 risk as follows: known to have COVID-19 by laboratory result; not known but suspected to have COVID-19; not known but not suspected to have COVID-19, and known not to have COVID-19 by laboratory result. For this analysis, COVID-19 diagnosed after the ED visit did not change the category in which the patient was assigned based on the knowledge available at the time of the procedure. Procedures were also stratified by self-reported severity (eg, emergency, semi-elective, and elective) and self-reported anticipated airway difficulty (the study protocol did not specify the method used for assessing difficulty). For the analysis of the accuracy of prediction of the patient’s COVID-19 status, SARS-CoV-2 testing (usually reverse transcription polymerase chain reaction, per routine institutional clinical protocol) result from hospital admission was used as the reference standard, with confirmed or suspected COVID-19 (defined above) as the primary predictor. Patients could have had multiple intubations, but only single endotracheal intubation on a calendar day was retained. Data were collected from health care personnel within 24 hours of performing the procedure, and the identities of patients and health care personnel were deidentified after linkage.
Primary Data Analysis
Using generalized linear mixed models, intubation strategy characteristics were compared between patients known or suspected of having COVID-19 and those who were not. First-pass success, overall success, and adverse events were compared similarly.
We constructed multivariable models to assess the association between known or suspected COVID-19 status and (1) first-pass success and (2) adverse events. We decided a priori to include anticipated airway difficulty (per report of the intubating clinician), severity, and indication for intubation in our multivariable models because of their known association with both success and adverse events.28 In addition, we added endotracheal intubation strategy (only for intubation strategies that statistically differed between COVID-19 risk groups) to our models to determine whether a change in strategy explained differences in the success or adverse events. Our analysis included screening for interactions and, if evident, stratified analyses. We used generalized linear mixed models with site and person performing the procedure as random effects, using a binomial family and logit link for these models. All table differences were calculated using generalized linear mixed models with a Gaussian distribution and an identity link.
For the evaluation of the test characteristics of clinical gestalt for the diagnosis of COVID-19, we included only those cases with a SARS-CoV-2 test performed on admission. We measured agreement between provider estimation of risk and actual test result, calculating sensitivity, specificity, and likelihood ratios for prediction accuracy.
We used complete case analysis, and missing data were rare (0.9% of cases) because of data validation and error-checking built into the electronic data collection tool. The sample size and power were determined for the parent study, and the analytic results from these analyses represent descriptive outcomes. All 95% confidence intervals (95% CI) were adjusted for clustering using univariate generalized linear mixed models, and all analyses were conducted with R, version 4.1.3 (R Foundation, Vienna, Austria).
Results
Characteristics of Study Subjects
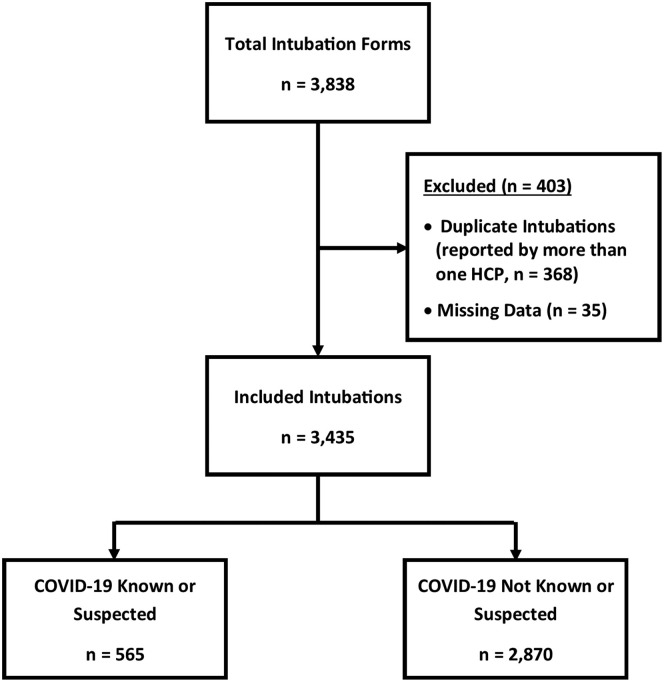
There were 3,435 unique endotracheal intubation events (Figure 1 ) performed by 586 physicians and advanced practice providers. Site-specific endotracheal intubation counts ranged from 65 to 508 (Figure E1, available online at http://www.annemergmed.com), with most intubations occurring from June to September 2020 (Figure E2, available online at http://www.annemergmed.com). A total of 3,043 (89%) endotracheal intubations had unknown patient SARS-CoV-2 results at the time of intubation. Among those with unknown SARS-CoV-2 status, intubating health care personnel suspected COVID-19 (“convinced” or “pretty sure”) in 435 (13%) endotracheal intubations. Two-hundred thirty-seven (7%) patients were ultimately diagnosed with SARS-CoV-2 infection (111 known at the time of intubation and 126 discovered by testing during admission) (Table E1, available online at http://www.annemergmed.com). No SARS-CoV-2 testing was done in 399 (12%) patients, including 264 (66%) who were pronounced dead in the ED. The most common reason for intubation indicated by health care personnel in patients with known or suspected COVID-19 was an acute respiratory failure (58%). For patients without known or suspected COVID-19, the most common reason was poor mental status (61%). Most endotracheal intubations (n=2,784, [81%]) were considered emergency and were predicted not to be difficult ( n=2,374, [69%]) (Table 1 ). After intubation, most endotracheal intubations were judged to be “very easy” or “somewhat easy” (32% and 42%, respectively [not presented in the table]). Hospital mortality in SARS-CoV-2-infected patients was 9.3% (n=22) versus 8.4% (n=237) in those without.
Figure 1.
Flow chart of intubations performed by participants at 20 COVERED (COVID-19 Evaluation of Risks in EDs) US academic medical center EDs, May to December 2020. HCP, health care personnel.
Table 1.
Characteristics of US emergency department patients intubated during the COVID-19 pandemic at 20 COVERED (COVID-19 Evaluation of Risks in Emergency Departments) academic medical center emergency departments, May to December 2020.
| Characteristic | Total Sample (n=3,435) |
COVID-19 Confirmed or Suspected(n=565) |
No COVID-19 Confirmed or Suspected(n=2,870) |
Percent Point Difference (95% CI)∗ |
|---|---|---|---|---|
| n (%) | ||||
| Sex | ||||
| Male | 2,279 (66.4) | 331 (58.6) | 1,948 (67.9) | –9.3 (–13.83 to –4.8) |
| Female | 1,148 (33.4) | 234 (41.4) | 914 (31.9) | 9.6 (5.1 to 14.1) |
| Nonbinary | 6 (0.2) | 0 (0.0) | 6 (0.2) | –0.2 (–0.5 to 0.1) |
| Missing | 2 (0.1) | 0 (0.0) | 2 (0.1) | –0.1 (–0.2 to 0.1) |
| Age | ||||
| <18 y | 75 (2.2) | 3 (0.5) | 72 (2.5) | –2.0 (–2.9 to –1.0) |
| 18 to 49 y | 1,345 (39.2) | 133 (23.5) | 1,212 (42.2) | –18.7 (–22.7 to –14.7) |
| 50 to 64 y | 921 (26.8) | 171 (30.3) | 750 (26.1) | 4.1 (–0.1 to 8.4) |
| 65 to 79 y | 731 (21.3) | 179 (31.7) | 552 (19.2) | 12.5 (8.2 to 16.7) |
| ≥80 y | 317 (9.2) | 74 (13.1) | 243 (8.5) | 4.6 (1.6 to 7.7) |
| Missing | 46 (1.3) | 5 (0.9) | 41 (1.4) | –0.5 (–1.5 to 0.5) |
| Race and ethnicity | ||||
| White, non-Hispanic | 1,203 (35.0) | 164 (29.0) | 1,039 (36.2) | –7.2 (–11.4 to –2.9) |
| Black, non-Hispanic | 1,257 (36.6) | 229 (40.5) | 1,028 (35.8) | 4.7 (0.2 to 9.2) |
| Hispanic or Latino | 425 (12.4) | 104 (18.4) | 321 (11.2) | 7.2 (3.7 to 10.7) |
| Other, non-Hispanic | 280 (8.2) | 44 (7.8) | 236 (8.2) | –0.4 (–3.0 to 2.1) |
| Other | 270 (7.9) | 24 (4.3) | 246 (8.6) | –4.3 (–6.4 to –2.3) |
| Severity | ||||
| Emergent | 2,784 (81.0) | 440 (77.9) | 2,344 (81.7) | –3.8 (–7.6 to 0.01) |
| Semiemergent | 595 (17.3) | 113 (20.0) | 482 (16.8) | 3.2 (–0.5 to 6.9) |
| Elective | 56 (1.6) | 12 (2.1) | 44 (1.5) | 0.6 (–0.8 to 2.0) |
| Reason for intubation | ||||
| Acute respiratory failure | 1,035 (30.1) | 329 (58.2) | 706 (24.6) | 33.6 (29.2 to 38.1) |
| Mental status changes | 1,938 (56.4) | 192 (34.0) | 1,746 (60.8) | –26.9 (–31.3 to –22.5) |
| Airway compromise | 152 (4.4) | 3 (0.5) | 149 (5.2) | –4.7 (–5.8 to –3.5) |
| Cardiac arrest | 227 (6.6) | 37 (6.6) | 190 (6.6) | –0.1 (–2.4 to 2.2) |
| Polytrauma | 77 (2.2) | 4 (0.7) | 73 (2.5) | –1.8 (–2.8 to –0.8) |
| Missing | 6 (0.2) | 0 (0.0) | 6 (0.2) | –0.2 (–0.5 to 0.1) |
| Anticipated difficulty | ||||
| Difficult | 1,037 (30.2) | 211 (37.4) | 826 (28.8) | 8.6 (4.1 to 13.0) |
| Not difficult | 2,374 (69.1) | 349 (61.8) | 2,025 (70.6) | –8.8 (–13.2 to –4.3]) |
| Missing | 24 (0.7) | 5 (0.9) | 19 (0.7) | 0.2 (–0.7 to 1.2) |
Percent point differences are calculated using a score test.
Intubation Strategy
The most common initial intubation strategies were rapid sequence induction (n=2,724/3,435, [79%]) with etomidate (n=2,352, [68%]) and rocuronium (n=1,628, [47%]). Video laryngoscopy (n=2,871, [83%]) was the most common technique (Table 2 ). Intubation boxes were used in only 24 (0.7%) endotracheal intubations (16 of which were in known or suspected COVID-19 patients), and dedicated intubation teams were uncommon (0.5% of total intubations) in participating sites. Most endotracheal intubations (n=1,960 [56%]) were performed by residents. Preoxygenation was most frequently performed using a face mask (n=1,374, [40%]). Bag-valve ventilation was used in 2,321 (67%) endotracheal intubations, and a self-inflating bag was used in 2,168 (93%) of these intubations. The patients were monitored with the mechanical ventilators in 3,095 (89%) endotracheal intubations, and most (n=1,690 [55%]) of the ventilators were dual-limb with a bacterial/viral filter (n=1,224, [72%]) placed in-line.
Table 2.
Intubation strategy, stratified by COVID-19 known or suspected status at the time of intubation at 20 COVERED (COVID-19 Evaluation of Risks in Emergency Departments) US academic medical center emergency departments, May to December 2020. Intubation strategy is divided into preoxygenation strategy, medication strategy, induction drug, paralytic drug, first-attempt equipment, adjuncts, and operator experience.
| Characteristic | COVID-19 Confirmed or Suspected (n=565) |
No COVID-19 Confirmed or Suspected (n=2,870) |
Difference (95% CI)∗ |
|---|---|---|---|
| n (%) | |||
| Preoxygenation | |||
| Nasal cannula | 50 (8.8) | 393 (13.7) | –6.2 (–9.2 to –3.2) |
| Face mask | 194 (34.3) | 1,167 (40.7) | –6.0 (–10.3 to –1.7) |
| High-flow nasal cannula | 57 (10.1) | 55 (1.9) | 7.9 (6.3 to 9.5) |
| CPAP/BiPap | 84 (14.9) | 139 (4.8) | 10.9 (8.6 to 13.1) |
| Bag-valve-mask | 160 (28.3) | 871 (30.3) | –2.2 (–6.2 to 1.9) |
| None | 20 (3.5) | 245 (8.5) | –4.9 (–7.3 to –2.5) |
| Medication strategy | |||
| Rapid sequence induction | 454 (80.4) | 2,244 (78.2) | 1.9 (–1.9 to 5.6) |
| Awake | 1 (0.2) | 32 (1.1) | –0.9 (–1.6 to –0.3)† |
| Sedation only | 6 (1.1) | 31 (1.1) | –0.1 (–1.0 to 0.8) |
| No sedation | 104 (18.4) | 563 (19.6) | –0.8 (–4.4 to 2.8) |
| Induction drug‡ | |||
| Propofol | 12 (2.1) | 84 (2.9) | –0.8 (–2.2 to 0.6)† |
| Etomidate | 394 (69.7) | 1,935 (67.4) | 1.1 (–3.0 to 5.3) |
| Ketamine | 55 (9.7) | 262 (9.1) | 1.8 (–0.8 to 4.4) |
| Midazolam | 5 (0.9) | 35 (1.2) | –0.4 (–1.4 to 0.6) |
| Fentanyl | 10 (1.8) | 37 (1.3) | 0.4 (–0.6 to 1.5) |
| Other medication | 0 (0) | 11 (0.4) | –0.4 (–0.7 to –0.1)† |
| No induction medication | 107 (18.9) | 584 (20.3) | –1.1 (–4.7 to 2.6) |
| Paralytic drug‡ | |||
| Succinylcholine | 190 (33.6) | 981 (34.2) | –3.6 (–7.7 to 0.5) |
| Rocuronium | 271 (48.0) | 1,343 (46.8) | 4.4 (0.1 to 8.7) |
| Vecuronium | 0 (0) | 5 (0.2) | –0.2 (–0.4 to 0.1)† |
| Cisatracurium | 0 (0) | 1 (0) | –0.0 (–0.1 to 0.1) |
| No paralysis medication | 108 (19.1) | 564 (19.7) | –0.7 (–4.2 to 2.9) |
| First-attempt equipment | |||
| Direct laryngoscopy | 55 (9.7) | 491 (17.1) | –6.5 (–9.7 to –3.3) |
| Video laryngoscopy | 501 (88.7) | 2,338 (81.5) | 6.3 (3.0 to 9.6) |
| Supraglottic airway | 2 (0.4) | 5 (0.2) | 0.2 (–0.4 to 0.8)† |
| Retrograde intubation | 0 (0) | 1 (0) | –0 (–0.1 to 0.1)† |
| Fiberoptic intubation | 3 (0.5) | 31 (1.1) | –0.5 (–1.4 to 0.4) |
| Cricothyroidotomy | 4 (0.7) | 4 (0.1) | 0.6 (–0.2 to 1.4)† |
| Adjuncts‡ | |||
| Passive nasal oxygenation | 245 (43.4) | 1,039 (36.2) | 5.1 (0.9 to 9.3) |
| Intubation boxes | 16 (2.8) | 8 (0.3) | 2.6 (1.8 to 3.6) |
| Dedicated intubation teams | 5 (0.9) | 13 (0.5) | –0.1 (–0.7 to 0.6) |
| Supraglottic devices | 3 (0.5) | 6 (0.2) | 0.3 (–0.4 to 1.0)† |
| Operator experience | |||
| First-year resident | 76 (13.5) | 373 (13.0) | 1.7 (–1.2 to 4.6) |
| Second-year resident | 148 (26.2) | 779 (27.1) | 2.1 (–1.8- to 5.9) |
| Third- or fourth-year resident | 82 (14.5) | 470 (16.4) | –0.1 (–3.3 to 3.1) |
| Fellow | 3 (0.5) | 15 (0.5) | 0.1 (–0.6 to 0.8) |
| Attending/advanced practice provider | 256 (45.3) | 1,233 (43.0) | –3.9 (–8.2 to 0.4) |
BiPap, bilevel positive airway pressure ventilation; CPAP, continuous positive airway pressure ventilation.
Difference is calculated using univariate linear mixed models with random effects for site and person performing the procedure. Because of that, some values may have a difference calculated that differs from the arithmetic difference because of cluster effects and rounding.
Low counts affected estimability. Unadjusted differences are calculated instead using a score test.
Percentages will not add up to 100% within a column since many elements of strategy could have more than 1 response (ie, multiple medications could be given).
Video laryngoscopy was used more frequently in patients with known or suspected COVID-19 versus those not known or suspected (88% versus 82%, difference 6.3%, 95% CI 3.0% to 9.6%). Between-group proportions of initial medication strategy, induction drug, or paralytic drug selection were not different. Passive nasal oxygenation was used more frequently in COVID-19 known or suspected endotracheal intubations (44% versus 39%, difference 5.1%, 95% CI 0.9% to 9.3%). Preoxygenation was performed more frequently in COVID-19 known or suspected endotracheal intubations with high-flow nasal cannula or noninvasive positive pressure ventilation (26% versus 7%, difference 18.9%, 95% CI 16.2% to 21.5%). Table 2 shows the intubation strategy stratified by COVID-19 known or suspected status at the time of intubation; other elements were similarly stratified by COVID-19 known or suspected status.
Use of PPE
Full barrier airborne PPE (eg, eye protection, N95 respirator or higher, gloves, and gown) were used for most intubations (n=2,055, [59%]) during the surveillance period. Most health care personnel (92%) caring for patients known or suspected of COVID-19 used N95 respirators or powered air-purifying respirators. Powered air-purifying respirator use was more common in those with known or suspected COVID-19 (17% versus 11%, difference 5.4%, 95% CI 3.4% to 7.4%) than in those without. N95 respirator use (82% versus 80%, difference 1.8%, 95% CI –1.0% to 4.6%) and eye protection (82% versus 80%, difference 1.9%, 95% CI –0.9% to 4.7%) were not different. Table 3 shows PPE use stratified by COVID-19 known or suspected status.
Table 3.
Personal protective equipment used during intubation, stratified by known or suspected COVID-19 status at the time of intubation, at 20 COVERED (COVID-19 Evaluation of Risks in EDs) US academic medical center Emergency Departments, May to December 2020. “Full Barrier Centers for Disease Control and Prevention-Adherence” was defined as eye protection, gown, gloves, and respiratory protection, which included N95 or powered air-purifying respirator.
| Characteristic | COVID-19 Confirmed or Suspected (n=565)† |
No COVID-19 Confirmed or Suspected (n=2,870)† |
Proportion Difference (95% CI)∗ |
|---|---|---|---|
| n (%) | |||
| Full barrier CDC-adherence | 374 (66.2) | 1,663 (57.9) | 7.5 (3.8 to 11.3) |
| Eye protection | 474 (83.9) | 2,324 (81.0) | 1.9 (–0.9 to 4.7) |
| Gowns | 457 (80.9) | 2,074 (72.3) | 8.6 (5.1 to 12.2) |
| Gloves | 545 (96.5) | 2,760 (96.2) | 0.9 (–0.8 to 2.6) |
| Surgical mask | 254 (45.0) | 1,214 (42.3) | -3.2 (–6.2 to –0.3) |
| N95 or higher/PAPR | 546 (96.6) | 2,618 (91.2) | 4.3 (2.2 to 6.5) |
CDC, Centers for Disease Control and Prevention; PAPR, powered air-purifying respirator.
Difference is calculated using univariate linear mixed models with random effects for site and person performing the procedure. Because of that, some values may have a difference calculated that differs from the arithmetic difference because of rounding.
Percentages will not add up to 100% within a column since more than one element of protection may have been used.
Among 237 endotracheal intubations of confirmed SARS-CoV-2-positive patients, 88 (37%) were intubated without Centers for Disease Control and Prevention guideline-adherent full barrier airborne precautions. The most frequent PPE element responsible for lack of adherence was not wearing gowns (n=52, [59%]), followed by lack of eye protection (n=38, [43%], Figure E3, available online at http://www.annemergmed.com). Most providers were in the patient room for less than 30 minutes for the intubation procedure, and the duration in the room was not different between those with and without known or suspected COVID-19 (Figure E4, available online at http://www.annemergmed.com). Providers rated confidence in their PPE as very high or moderately high in 95% of endotracheal intubations (n=3,295 [not presented in figures]).
Intubation Outcomes
First-pass success was reported in 3,027 (87%) endotracheal intubation attempts, and there was no difference in first-pass success between known or suspected COVID-19 intubations versus those not confirmed or suspected (87% versus 86%, difference 0.6%, 95% CI –2.4% to 3.6%). Endotracheal intubations of patients ultimately confirmed to be SARS-CoV-2-infected the first-pass success of 84%. In cases with failure of the first attempt, the most frequently used rescue device was a bougie (n=95, [21%]). Only 108 (3%) endotracheal intubations required more than 2 attempts, and the median number of attempts (unadjusted) was not different between the 2 groups (Table E2, available online at http://www.annemergmed.com); 14 (0.4%) patients required cricothyroidotomy as a rescue procedure. Successful endotracheal intubation was performed in 3,455 (99.6%) patients. Two patients died without being successfully intubated, and both had a cardiac arrest that occurred prior to ED arrival and during the endotracheal intubation attempt (Table E3, available online at http://www.annemergmed.com).
Peri-intubation adverse events occurred in 35% (n=200) of confirmed or suspected COVID-19 patients compared with 19% (n=544) of nonconfirmed or suspected COVID-19-patients (difference 16.2%, 95% CI 12.5% to 19.9%). The most common adverse event was hypoxia, which was significantly more likely in patients who were later SARS-CoV-2 confirmed (32% versus 16%, difference 16%, 95% CI 11% to 21%); 57% of patients with hypoxia had severe hypoxia (SpO2<80%). Adverse events were not more likely when a technique other than rapid sequence induction was used (22% in rapid sequence induction versus 24% in all other techniques, difference –2%, 95% CI –6% to 1%). Adverse events were not different for those who used full barrier precautions vs. those who did not (22% versus 23%, difference –0.6%, 95% CI –3.7% to 2.5%, not presented in figures). Adverse events are detailed in Table 4 .
Table 4.
Intubation adverse events, stratified by COVID-19 known or suspected status at the time of intubation at 20 COVERED COVID-19 (Evaluation of Risks in Emergency Departments) US academic medical center emergency departments, May to December 2020. Hypoxia was defined as minimum pulse oxygenation (SpO2) less than 90%, severe hypoxia was defined as SpO2 less than 80%, and hypotension were defined as minimum systolic blood pressure less than 90 mmHg. All adverse events were observed within 15 minutes of the intubation procedure.
| Characteristic | COVID-19 Confirmed or Suspected (n=565)† |
No COVID-19 Confirmed or Suspected (n=2,870)† |
Proportion Difference (95% CI)∗ |
|---|---|---|---|
| n (%) | |||
| Hypoxia | 157 (27.8) | 381 (13.3) | 14.2 (10.9 to 17.4) |
| Severe hypoxia | 85 (15.0) | 174 (6.1) | 8.5 (6.1 to 10.9) |
| Hypotension | 40 (7.1) | 119 (4.1) | 2.9 (1.0 to 4.8) |
| Esophageal intubation | 4 (0.7) | 33 (1.1) | –0.3 (-1.3 to 0.6) |
| Dental injury | 1 (0.2) | 9 (0.3) | –0.1 (-0.6 to 0.4) |
| Cardiac arrest | 35 (6.2) | 102 (3.6) | 2.3 (0.5 to 4.1) |
| Failed airway | 1 (0.2) | 3 (0.1) | 0.1 (-0.2 to 0.4) |
| No adverse events | 365 (64.6) | 2,326 (81.0) | –16.2 (–19.9 to –12.5) |
Difference is calculated using univariate linear mixed models with random effects for site and person performing the procedure. Because of that, some values may have a difference calculated that differs from the arithmetic difference because of rounding.
Percentages will not add up to 100% within a column since more than one adverse event may have been recorded.
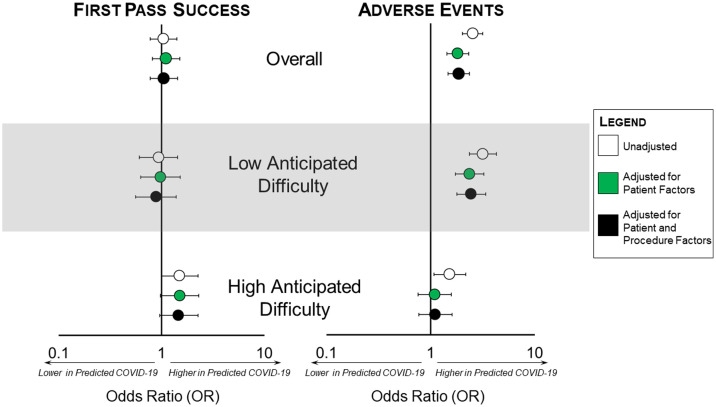
Adjusting for anticipated difficulty, severity, and reason for endotracheal intubation (patient factors), known or suspected COVID-19 was not associated with different first-pass success (adjusted odds ratio [aOR] 1.0, 95% CI 0.6 to 1.5 for low anticipated difficulty; aOR 1.5, 95% CI 1.0 to 2.3 for great anticipated difficulty), but higher odds of adverse events in intubations not anticipated to be difficult (aOR 2.4, 95% CI 1.7 to 3.7). An interaction term was present for adverse events between COVID-19 known or suspected status and expected difficulty (P =.004), so results are presented stratified by anticipated difficulty.
To measure the contribution of endotracheal intubation strategy to adverse events, we additionally included procedure-level variables video laryngoscopy and passive nasal oxygenation in the subsequent multivariable model. Including both patient and procedural factors as covariates in the model, the effect size of the association of known or suspected COVID-19 with adverse events did not change for patients with predicted low airway difficulty (aOR 2.46 [95% CI 1.79 to 3.39] versus 2.37 [95% CI 1.73 to 3.26]) or high airway difficulty (aOR 1.11 [95% CI 0.77 to 1.61] versus 1.10 [95% CI 0.76 to 1.59]), so we concluded that intubation strategy was unlikely to confound the relationship between COVID-19 known or suspected status and increased adverse events (Figure 2 and Table E4, available online at http://www.annemergmed.com).
Figure 2.
Adjusted odds of first-pass success and adverse events related to COVID-19 known or suspected status in emergency department intubations during the COVID-19 pandemic at 20 COVERED (COVID-19 Evaluation of Risks in Emergency Departments) academic medical center emergency departments, May to December 2020. Because an interaction term between expected airway difficulty and known or suspected COVID-19 status was present, models are presented for the entire data set and analysis stratified by predicted airway difficulty. White dots indicate unadjusted estimates, green dots represent estimates adjusted for patient-level factors (acuity, reason for intubation), and black dots represent estimates adjusted for both patient-level and procedure-level factors (acuity, reason for intubation, passive nasal oxygenation, video laryngoscopy). Error bars represent 95% CIs. The forest plot on the left of the figure represents the outcome of first-pass success, and the forest plot on the right side of the figure represents adverse events. Adverse events included hypoxia, hypotension, esophageal intubation, dental injury, cardiac arrest, and failed airway.
COVID-19 Prediction
Among 237 patients who ultimately tested positive for SARS-CoV-2, 111 (47%) were known to be infected at the time of endotracheal intubation, and 3 (1%) had a prior negative test known at the time of endotracheal intubation. Among the 123 (52%) with unknown status at the time of endotracheal intubation who ultimately tested positive, 66 (54%) were thought likely to have COVID-19. Among 2,830 patients ultimately found to be SARS-CoV-2 negative, 255 (9%) were known to have a negative test at the time of endotracheal intubation, and 2,528 (90%) had unknown status, of which 2,189 (87%) were thought unlikely to have COVID-19 (16 had a previous positive test, but a negative test at hospital admission). Among 2,651 intubated with uncertain COVID-19 status, the sensitivity of clinical gestalt for a positive COVID-19 test was 54% (95% CI 37% to 77%), and the specificity was 87% (95% CI 84% to 90%), yielding a positive likelihood ratio of 4.0 (95% CI 2.6 to 6.1) and a negative likelihood ratio of 0.5 (95% CI 0.4 to 0.8, Table E1, available online at http://www.annemergmed.com).
Limitations
This study has several limitations. First, although our study was conducted in multiple academic centers, our sample was a prospectively identified convenience sample of physicians and advanced practice providers at participating sites with variation in procedural experience, so it may not be representative of endotracheal intubations at these or other EDs. Second, our intubation data were self-reported by the individual who performed the procedure (including determining first-pass success), creating the potential for misclassification of strategy or recall of success. Furthermore, we included adverse events only very early after the completion of intubation. Nevertheless, our very high capture proportion of procedures performed by participants provides confidence that our sample was internally representative. Third, not all patients were tested for COVID-19 because some sites had not implemented uniform testing during the surveillance period. Finally, with current high health care personnel and patient vaccination rates, COVID-19 therapeutics, and new variants, provider behavior may have evolved either during the study period or because our data collection ended. However, with increasing breakthrough infections due to new SARS-CoV-2 variants demonstrating immune evasion and ongoing trepidation about occupational COVID-19 exposure, our results remain relevant to our understanding of how COVID-19 influences provider decisionmaking and patient outcomes.
Discussion
In this study of endotracheal intubation early in the COVID-19 pandemic, we found that patients known or suspected of having COVID-19 were intubated using similar initial techniques as those not known or suspected of having COVID-19, except for slightly increased use of video laryngoscopy, passive nasal oxygenation, and preoxygenation with either high-flow nasal cannula or noninvasive positive pressure ventilation. With the advent of the pandemic, a number of reports of novel intubation devices and techniques have been proposed, but the use of these techniques in our cohort of academic medical center EDs was rare.9, 10, 11 Despite more aggressive use of empiric hypoxia-prevention strategies, we observed a higher proportion of hypoxia and severe hypoxia in intubations of patients with COVID-19 than among those without, which is likely related to the severity of lung injury. First-pass success in both COVID-19 and non-COVID-19 endotracheal intubations was not different, and the rates observed were similar to those reported in other pre-COVID-19 reports of ED intubation success.29 , 30
Largely, these findings are likely reassuring to ED practitioners and patients. Despite very high adherence to full barrier PPE guidelines, ED providers maintained good intubation quality metrics. Some have hypothesized that increased use of PPE would decrease intubation success and could be associated with increased adverse events, which we did not observe.31 The high endotracheal intubation success rate in the face of enhanced PPE use is especially relevant because of clinicians' imperfect accuracy in predicting COVID-19 status among patients with unknown test results, even during the height of the COVID-19 surge early in the pandemic. In addition, we previously reported that physicians who performed endotracheal intubation for SARS-CoV-2-infected patients had only a modest risk of acquiring COVID-19, especially when appropriate PPE was used.23 These observations support widespread universal PPE use during periods of high COVID-19 transmission, which was noted in our study.
Several prior studies from early in the pandemic have described outcomes of COVID-19 endotracheal intubations. For example, a retrospective case series of 117 patients from 2 Canadian hospitals using electronic medical record documentation reported similar first-pass success, but hypoxia was only documented in 24% of patients. These centers used specialized intubation teams that may have had a lower adverse event rate, but our higher hypoxemia rate from real-time endotracheal intubation reports may have captured more adverse events ultimately not documented in medical records.32 Another report based on electronic medical record data also showed increased use of video laryngoscopy and frequency of hypoxic events, similar to our findings.33 A study in an Australian ED showed that hypoxia was more common (18% versus 10%) in the COVID-19 period (after a new protocol was implemented in March 2020), and the authors attributed that increased prevalence to changes in the procedures and team for ED endotracheal intubation.34 Our data, with robust measurement of COVID-19 status and risk adjustment, suggests that intubation strategy is not associated with worsened clinical outcomes, but the changes in intubation practices observed in our study were much more modest and may have represented increasing procedural comfort as case counts surged in the United States. In our study, the increased frequency of hypoxia observed in COVID-19 patients seems more attributable to the disease than to the airway management strategy. These studies are all limited by reporting bias from medical records data. However, they also reinforce that in our much larger multicenter cohort with robust real-time reporting, risk assessment, and COVID-19 testing, the techniques used in patients with known or suspected COVID-19 were not different from those observed among patients without this infection. Any increase in the risk of adverse events is likely due to the disease rather than changing the strategy of performing the procedure.
The increased risk of adverse events among patients with SARS-CoV-2 infection continues to deserve attention. COVID-19-suspected patients were usually intubated for acute hypoxemic respiratory failure and anticipated to have greater airway difficulty—perhaps because of their tenuous physiologic status. Despite potential concerns with infection control, these patients were managed with more aggressive use of passive nasal preoxygenation and preoxygenation with a high-flow nasal cannula or noninvasive positive pressure ventilation. Even when we adjusted for intubation strategy that differed in the COVID-19 known or suspected group (ie, passive nasal oxygenation and video laryngoscopy), adverse events were still more common in those with low anticipated airway difficulty, suggesting that they were driven by lung injury from SARS-CoV-2 status itself rather than changes in intubation decisions. In the parent prospective surveillance study, we found that only 6 intubating health care personnel (2.5%) tested positive for SARS-CoV-2 in the period after intubation.23 These findings support the use of standard strategies to maintain safe intubating conditions, even in those suspected of having a highly transmissible novel respiratory disease.
The other notable finding from the present study was the prevalence of PPE lapses. Although overall PPE use was very high, 37% of COVID-19 intubations were performed without full Centers for Disease Control and Prevention-adherent protection. Most of these lapses were related to lack of gown use. These likely unintended lapses, coupled with the modest ability of intubating clinicians to predict who had COVID-19, reinforce the need for universal PPE, engineering controls, and standardization to circumvent the barriers to PPE use—especially during early surges in case volumes.
In conclusion, confirmed or suspected COVID-19 status was associated with only minor changes in COVID-19 endotracheal intubation strategy but higher risk-adjusted adverse events, most commonly hypoxia, during intubation. First-pass success among patients with confirmed or suspected COVID-19 was not different from those who were not suspected of infection. Future work should focus on how hypoxia-prevention strategies can be preemptively applied to reduce the rate of complications in these patients while maintaining health care personnel safety and preventing occupational COVID-19 acquisition.
Acknowledgments
The authors thank the COVERED participants, the participating institutions/emergency departments, and individuals. In addition, the authors acknowledge the following participating Project COVERED emergency departments: Allegheny General Hospital, Pittsburgh, PA; Baystate Medical Center, Springfield, MA; Denver Health Medical Center, Denver, CO; Detroit Receiving Hospital/Sinai-Grace Hospital, Detroit, MI; Hennepin County Medical Center, Minneapolis, MN; Jackson Memorial Hospital, Miami, FL; Johns Hopkins Medical Institute, Baltimore, MD; University Medical Center, New Orleans, LA; Mount Sinai Hospital East/Elmhurst Hospital Center, New York, NY; Orlando Regional Medical Center, Orlando, FL; University of Alabama at Birmingham Hospital, Birmingham, AL; Ronald Reagan–UCLA Medical Center/Olive View–UCLA Medical Center, Los Angeles, CA; University of Iowa, Iowa City, IA; University of Massachusetts Memorial Medical Center, Worcester, MA; University of Mississippi Medical Center, Jackson, MS; UCSF Zuckerberg San Francisco General, San Francisco, CA; UT Southwestern Medical Center/Parkland Memorial Hospital, Dallas, TX; Truman Medical Center, Kansas City, MO; Thomas Jefferson University, Philadelphia, PA; and Washington University Barnes-Jewish Hospital, St. Louis, MO. Aishat Adeyemi, BA, Lisa Allen, MPH, MPA, Gregory Almonte, Otuwe Anya, MS, Paula Arellano-Cruz, BS, Ruzana Aronov, Danielle Beckham, RN, MSN, CRC, Lauren Buck, BS, Samuel Ceckowski, BS, Maxime Centeno, BS, Virginia, Chan, BS, Anna Marie Chang, MD, MSCE, Melissa Connor, RN, Gabriella Dashler, BS, Jenna Davis, MSN, Cynthia Delgado, BS, Veronica Delgado PA, MS, Brianna DiFronzo, Radhika L. Edpuganti, BS, Alyssa Espinera, MD, Fresa Estevez, BA, Shelly Ann Evans, MBA, RN, Cathy Fairfield, BSN, Phillip Fairweather, MD, Theodore Falcon, BS, David Gallegos, BBA, Samuel Ganier, MD, Stephanie Gravitz, MPH, Jeffrey Harrison, RN, Kyle Herbert MD, Judy Hermans, MPH, Emily Hopkins, MSPH, Alan Jones, MD, Kia M. Jones, DrPh, Momina Khan, BS, Karin Hoth, PhD, Laura Iavicoli, MD, Robin Kemball, MPH, Laurie Kemble, BHSRT, CCRC, Stuart Kessler, MD, Catherine Lind RN, NP, Karina Loayza, LCSW, Carol Lynn Lyle, PA, MPH, Virginia B. Mangolds, MS, RN, Hannah Makarevich-Manilla, MPH, CCRC, Thomas Mazzocco, RN, Sarah Meram, MS, Valerie H. Mika, MS, Reynaldo Padilla, BA, Giacomo Passaglia, BS, Rebekah Peacock, BSN, Danielle Perez, BS, Kye E. Poronsky, MA, Eric Raines, EMT-P, Monica N. Ramage, MSN, RN, Kavita Rampertaap MSN, RN, Sarah Reineck, BS, Nicole Renzi, RN, Erin P. Ricketts, MSPH, Stephanie Rodriguez, MA, Justin Sabol, BS, Valeria Samame, BA, Katie Schneider, MSN, Robert Sellman PA, Kristine Sernulka BSN, CCRP, Edward Sheriff, PhD, MPH, Jennifer Siller, DNP, RN, Colleen Smith, MD, Timothy Smith, BS, Kelly Szabo, MPH, CCRC, Meghan Tinetti BSN, CCRP, Denise Tritt, CIM, CRCP, Julia Vargas, BS, Samuel Vargas, BS, Kavey Vidal, BS, Lori Wilkerson, RN, CRC, Darleen Williams, DNP, APRN-CNS, Sallie Anne Wright, MPH, BSMT, and Isaias Yin, LVN. The authors acknowledge Paul Casella, MFA for his editorial assistance.
Footnotes
Please see page 146 for the Editor’s Capsule Summary of this article.
Supervising editor: Steven M. Green, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Author contributions: NMM and DAT were responsible for conceptualization, study design, data interpretation, drafting the manuscript, project administration, funding acquisition. ESL, KKH, and PT were responsible for data management, data analysis, interpretation, and revising the manuscript for important intellectual content. JNC and BD were responsible for conceptualization, data interpretation, and revising the manuscript for important intellectual content. AK was responsible for data management, study coordination, and revising the manuscript for important intellectual content. WRM was responsible for conceptualization, study design, interpretation of the data analysis, and revising the manuscript for important intellectual content. TMF was responsible for data interpretation and drafting a portion of the manuscript. KW was responsible for data management, study coordination, and revising the manuscript for important intellectual content. CM, PKK, and SS were responsible for conceptualization, acquisition of funding, data interpretation, and revising the manuscript for important intellectual content. NMM takes ultimate responsibility for the integrity of the data, and all authors approved the final manuscript prior to publication.
Authorship: All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fundingandsupport: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. This project was funded by a cooperative agreement from the Centers for Disease Control and Prevention (U01CK000480) and the Institute for Clinical and Translational Science at the University of Iowa through a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health (UL1TR002537). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Readers: click on the link to go directly to a survey in which you can provide feedback to Annals on this particular article.
A podcast for this article is available at www.annemergmed.com.
Appendix
†The Project COVERED Emergency Department Network includes Monica Bahamon, MPH, Makini Chisolm-Straker, MD, MPH ; Brett Faine, Pharm D, MS ; Brian M. Fuller, MD; James Galbraith, MD; Philip A. Giordano, MD; John P. Haran, MD, PhD; Elisabeth Hesse, MD, MTM&H; Amanda Higgins, MS; Jeremiah Hinson, MD; Karin Hoth, PhD ; Stacey House, MD, PhD ; Ahamed H. Idris, MD ; Efrat Kean, MD; Elizabeth Krebs, MD, MSc; Michael C. Kurz, MD; MS, Lilly Lee SM, MD; Stephen Y. Liang, MD, MPHS ; Stephen C. Lim, MD; Juan Carlos C. Montoy, MD, PhD; Gregory Moran, MD; Utsav Nandi, MD, MSCI; Kavitha Pathmarajah, MPH; James H. Paxton, MD; Yesenia Perez, BA;Lynne D. Richardson, MD; Robert M. Rodriguez MD; Richard Rothman, MD, PhD; Walter A. Schrading, MD; Jessica Shuck, BA; Patricia Slev, MD; Howard A. Smithline, MD; Michelle St. Romain, MD; Kimberly Souffront, PhD, FNP-BC, RN; Mark T. Steele, MD; Amy Stubbs, MD, Morgan B. Swanson, BS, Josh Tiao, MD, Jesus R. Torres, MD, MPH; Stacy A. Trent, MD, MPH; Lisandra Uribe, BS; Arvind Venkat, MD; Gregory Volturo, MD; Kurt D. Weber, MD; and James Willey, MD.
Supplementary Data
References
- 1.Hughes M.M., Groenewold M.R., Lessem S.E., et al. Update: characteristics of health care personnel with COVID-19 – United States, February 12-July 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1364–1368. doi: 10.15585/mmwr.mm6938a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker J.M., Nelson K.N., Overton E., et al. Quantification of occupational and community risk factors for SARS-CoV-2 seropositivity among health care workers in a Large U.S. Health care system. Ann Intern Med. 2021;174:649–654. doi: 10.7326/M20-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iversen K., Bundgaard H., Hasselbalch R.B., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen T., Levin N., Niehus K., et al. Prevalence of IgG antibodies to SARS-CoV-2 among emergency department employees. Am J Emerg Med. 2020;38:2752–2753. doi: 10.1016/j.ajem.2020.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jespersen S., Mikkelsen S., Greve T., et al. SARS-CoV-2 seroprevalence survey among 17,971 healthcare and administrative personnel at hospitals, prehospital services, and specialist practitioners in the Central Denmark Region. Clin Infect Dis. 2021;73:e2853–e2860. doi: 10.1093/cid/ciaa1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran K., Cimon K., Severn M., et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLOS ONE. 2012;7:e35797–e35798. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan V.W., Ng H.H., Rahman L., et al. Transmission of severe acute respiratory syndrome coronavirus 1 and severe acute respiratory syndrome coronavirus 2 during aerosol-generating procedures in critical care: A systematic review and meta-analysis of observational studies. Crit Care Med. 2021;49:1159–1168. doi: 10.1097/CCM.0000000000004965. [DOI] [PubMed] [Google Scholar]
- 8.El-Boghdadly K., Wong D.J.N., Owen R., et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia. 2020;75:1437–1447. doi: 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad I., Jeyarajah J., Nair G., et al. A prospective, observational, cohort study of airway management of patients with COVID-19 by specialist tracheal intubation teams. Can J Anaesth. 2021;68:196–203. doi: 10.1007/s12630-020-01804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor Azhar M.N., Bustam A., Poh K., et al. COVID-19 aerosol box as protection from droplet and aerosol contaminations in healthcare workers performing airway intubation: A randomised cross-over simulation study. Emerg Med J. 2021;38:111–117. doi: 10.1136/emermed-2020-210514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayya D., O’Neill O.J., Feiertag T.D., et al. The use of oxygen hoods in patients failing on conventional high-flow oxygen delivery systems, the effects on oxygenation, mechanical ventilation and mortality rates in hypoxic patients with COVID-19. A Prospective Controlled Cohort Study. Respir Med. 2021;179:106312–106313. doi: 10.1016/j.rmed.2021.106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman D.N., de Perio M.A., Radonovich L.J., Jr. COVID-19 and risks posed to personnel during endotracheal intubation. JAMA. 2020;323:2027–2028. doi: 10.1001/jama.2020.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canelli R., Connor C.W., Gonzalez M., et al. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:1957–1958. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung J.C., Ho L.T., Cheng J.V., et al. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020;8:e19–e20. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao W., Wang T., Jiang B., et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125:e28–e37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook T.M., El-Boghdadly K., McGuire B., et al. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan E.H., Gibson L.E., Berra L., et al. In-hospital airway management of COVID-19 patients. Crit Care. 2020;24(1):292. doi: 10.1186/s13054-020-03018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown C.A., III, Mosier J.M., Carlson J.N., et al. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1:80–84. doi: 10.1002/emp2.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong D.J.N., El-Boghdadly K., Owen R., et al. Emergency airway management in patients with COVID-19: A prospective international multicenter cohort study. Anesthesiology. 2021;135:292–303. doi: 10.1097/ALN.0000000000003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohr N.M., Harland K.K., Krishnadasan A., et al. Diagnosed and undiagnosed COVID-19 in US Emergency Department health care personnel: a cross-sectional analysis. Ann Emerg Med. 2021;78:27–34. doi: 10.1016/j.annemergmed.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez R.M., Montoy J.C.C., Hoth K.F., et al. Symptoms of anxiety, burnout, and PTSD and the mitigation effect of serologic testing in emergency department personnel during the COVID-19 pandemic. Ann Emerg Med. 2021;78:35–43.e2. doi: 10.1016/j.annemergmed.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrading W.A., Trent S.A., Paxton J.H., et al. Vaccination rates and acceptance of SARS-CoV-2 vaccination among U.S. Emergency Department health care personnel. Acad Emerg Med. 2021;28:455–458. doi: 10.1111/acem.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr N.M., Krishnadasan A., Harland K.K., et al. Emergency department personnel patient care-related COVID-19 risk. PLOS ONE. 2022;17:e0271597–e0271598. doi: 10.1371/journal.pone.0271597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.45 Code of Federal Regulations part 46:102(l)(2), 21 Code of Federal Regulations part 56; 42 United States Code §241(d); 5 §552a; 44 United States Code §3501 et seq.
- 25.von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Harris P.A., Taylor R., Thielke R., et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208–103209. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown C.A., III, Bair A.E., Pallin D.J., et al. Techniques, success, and adverse events of emergency department adult intubations. Ann Emerg Med. 2015;65:363–370.e1. doi: 10.1016/j.annemergmed.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Trent S.A., Kaji A.H., Carlson J.N., et al. Video laryngoscopy is associated with first-pass success in emergency department intubations for trauma patients: a propensity score matched analysis of the national emergency airway registry. Ann Emerg Med. 2021;78:708–719. doi: 10.1016/j.annemergmed.2021.07.115. [DOI] [PubMed] [Google Scholar]
- 30.Mohr N.M., Pape S.G., Runde D., et al. Etomidate use is associated with less hypotension than ketamine for emergency department sepsis intubations: a NEAR cohort study. Acad Emerg Med. 2020;27:1140–1149. doi: 10.1111/acem.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Çağlar A., Kaçer İ., Hacımustafaoğlu M., et al. Impact of personal protective equipment on prehospital endotracheal intubation performance in simulated manikin. Australas Emerg Care. 2021;24:235–239. doi: 10.1016/j.auec.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvis N., Schiavo S., Bartoszko J., et al. A specialized airway management team for COVID-19 patients: a retrospective study of the experience of two Canadian hospitals in Toronto. Can J Anaesth. 2022;69:333–342. doi: 10.1007/s12630-021-02169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins A., Stapleton S., Rodriguez G., et al. Emergency tracheal intubation in patients with COVID-19: A single-center, retrospective cohort study. West J Emerg Med. 2021;22:678–686. doi: 10.5811/westjem.2020.2.49665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groombridge C.J., Maini A., Olaussen A., et al. Unintended consequences: the impact of airway management modifications introduced in response to COVID-19 on intubations in a tertiary centre emergency department. Emerg Med Australas. 2021;33:728–733. doi: 10.1111/1742-6723.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.