Abstract
Objective:
To determine the number of prior concussions associated with increased incidence of persistent post-concussive symptoms (PPCS) in a cohort of acutely concussed pediatric patients.
Design:
Prospective observational cohort study
Setting:
Three university-affiliated concussion clinics
Participants:
270 participants (14.9±1.9 years, 62% male, 54% with prior concussion) were assessed within 14 days of concussion and followed to clinical recovery. Participants with a second head injury before clinical recovery were excluded.
Measures and Main Outcome:
Concussion history, current injury characteristics, recovery time, and risk for prolonged recovery from current concussion.
Results:
There was no statistically significant change in PPCS risk for participants with 0, 1 or 2 prior concussions; however, participants with 3 or more prior concussions had a significantly greater risk of PPCS. Twelve participants sustained a subsequent concussion after clinical recovery from their first injury and were treated as a separate cohort. Our secondary analysis found that these participants took longer to recover and had a greater incidence of PPCS during recovery from their latest concussion.
Conclusion:
Pediatric patients with a history of 3 or more concussions are at greater risk of PPCS than those with fewer than 3 prior concussions.
Keywords: adolescent, pediatric, concussion, Buffalo concussion physical exam, persistent post-concussive symptoms, concussion history
INTRODUCTION
In recent decades concussion has been identified as a major public health concern.1, 2 It has been estimated that the yearly incidence of traumatic brain injury in sport is approximately 3.8 million in the United States, with the majority of these injuries being sport-related concussions (SRC). Due to the high volume of youth who play sports, pediatric and adolescent patients account for the majority of SRCs.1–4 In North America, it has been estimated that 10% of adolescents aged 14–19 years will sustain a concussion each year.5 Concussion results in a variety of physical, cognitive, and mood symptoms.2 The impact of self-reported symptoms on youth’s functioning after concussion is debated,6 but it is usually recognized that symptoms typically improve over the first few weeks after injury in the majority.2,7,8 Nevertheless, 20% to 30% of all concussed youth will not follow the typical recovery profile9–12 and will have prolonged symptoms. Prolonged recovery is defined as symptoms persisting beyond 10–14 days in adults and beyond 4 weeks in children and adolescents.2 Adolescents who take longer than 1 month to recover are considered to have Persistent Post-Concussive Symptoms (PPCS). PPCS has been associated with poor educational, social, and developmental outcomes in all age groups, but especially in adolescents.13,14 Current literature suggests recovery time is related to a variety of intrinsic and extrinsic factors, such as initial symptom burden and a history of psychiatric illness, as well as a history of prior concussion(s) in adults.13,15
The idea that athletes should be removed from sport after having sustained 3 or more concussions was first anecdotally utilized by Quigley in 1945 and reiterated again by Thorndike in 1954.16,17 The definition of concussion has changed significantly since then,18 but recent studies continue to confirm that a history of 3 or more concussions is associated with prolonged recovery in adults.19 However, there is a paucity of research investigating the relationship between concussion history and recovery in the pediatric population. Prior studies in general suffer from small sample sizes and are therefore underpowered and at risk for Type I (false positive) and Type II (false negative) errors.20,21 One reason for these small sample sizes may be that it is relatively uncommon for pediatric patients to report having sustained 3 or more physician-diagnosed concussions.
Decision trees are statistical modeling techniques that can create decision rules. Decision rules are the terminal branches of decision trees where data are partitioned into similar groups around various cut-points to assess population differences with high accuracy.21,22 A cut-point is the dichotomization of a test value in order to assess and create classifications.23 In this study we used cut-point analysis to assess length of recovery as a function of concussion history. We hypothesized that we could develop a decision rule by determining an optimal cut-point that would inform pediatricians and other clinicians as to how many prior concussions increase PPCS risk in children and adolescents. We also describe clinical outcomes in a small cohort of children who were seen for two new concussions during the recruitment period.
METHODS
This prospective cohort study collecting deidentified data from electronic medical records was reviewed and approved by the University at Buffalo Institutional Review Board and an exemption of consent was granted. Male and female pediatric patients seen at any of the three participating community sports medicine concussion centers from September 2015 to March 2019 were selected if they satisfied inclusion criteria: aged 8–18 years old; diagnosed with a concussion within 14 days of injury; documented number of prior concussions; and treated until clinical recovery. Concussions were diagnosed by experienced sports-medicine physicians using recent Concussion in Sports Group (CISG) guidelines,2 including an appropriate history, concussion-like symptoms linked to a concussive head injury or injury to another part of the body with force transmitted to the head, and signs on a concussion-focused physical examination.24
Participants were excluded if they had one of the following: injury involving loss of consciousness for ≥30 minutes; post-traumatic amnesia for ≥ 24 hours; history of moderate to severe brain injury (GCS ≤ 12); gross cervical injury requiring urgent intervention; and focal neurologic signs consistent with an intracerebral lesion, with or without lesion on CT/MRI (if performed). Participants were removed from primary analysis if they had a second concussive head injury before recovery or were lost to follow-up before clearance. Participants who returned to clinic for a new concussion after being cleared by a physician from their prior concussion were analyzed as a separate cohort (secondary analysis).
Participants were managed according to international recommendations.2, 25 If participants did not recover by 4 weeks after injury, a multidisciplinary approach was implemented that included a physical therapist (for vestibular or vision therapy) and/or a neuropsychologist (for cognitive or mood-related issues).26
Measures and Main Outcome
Demographics, concussion-related history, and physical examination results were recorded by physicians on standardized Buffalo Concussion Physical Exam (BCPE) forms as part of their clinical assessment. Research suggests that self-reported history of concussions may not be accurate;27 therefore, physicians were instructed to include only physician-diagnosed concussions or undiagnosed concussions that were reliable based on history. Recovery time was calculated as the difference in days from injury to when participants were declared recovered. Recovery was defined as: a return to a baseline level of symptoms at rest, a normal physical examination and physician determination of recovery, and the ability to exercise and return-to-school without exacerbation of concussion-like symptoms.28
Statistical Analysis
Univariate statistics were performed. The distribution of the main continuous outcome variable (recovery time) was assessed and an appropriate log transformation was applied. Linear regression for log of recovery time and logistic regression for PPCS were each performed with number of prior concussions as the predictor variable as done in previous research.29 To generate a decision rule, a Fisher’s exact test was used to determine cut-points for the number of prior concussions associated with a statistically significant increase in the risk of PPCS. The misclassification error rate was calculated for each prior concussion cut-point, plotted on a graph, and visually inspected. This partitioning technique used a simple majority classifier that divided participants into two groups based on which side of the cut-point the number of prior concussions fell, and then predicted PPCS for each participant according to the most common classification for group.21,22 Standard errors were calculated using random resampling with replacement.
For the secondary analysis, appropriate non-parametric statistical tests compared participants who were seen for two concussions (Concussion 1 and Concussion 2). The Mann-Whiney U test was used to compare continuous variables and the Fisher’s exact test for categorical variables. Prescribed therapies and persistent impairments were tabulated and described from physicians’ notes.
RESULTS
From September 2016 to March 2019, 359 children and adolescents (14.94 ±1.86 years old) were diagnosed with a concussion at study centers, and 284 participants met eligibility criteria and were included in the study. Eleven participants were lost to follow-up and 3 had a second head injury before clinical recovery from their initial concussion and were removed from analysis. 12 participants experienced a second concussion after clinical recovery. These participants were a part of the primary analysis and of the secondary analysis. Hence, 270 concussed participants seen within 14 days of their injury (majority sport-related, 84%) were included in the analysis (Figure 1). The 14 participants not included in the analysis did not differ significantly in age or sex compared with the analyzed sample. Sample demographics and concussion history are presented in Table 1.
Figure 1.
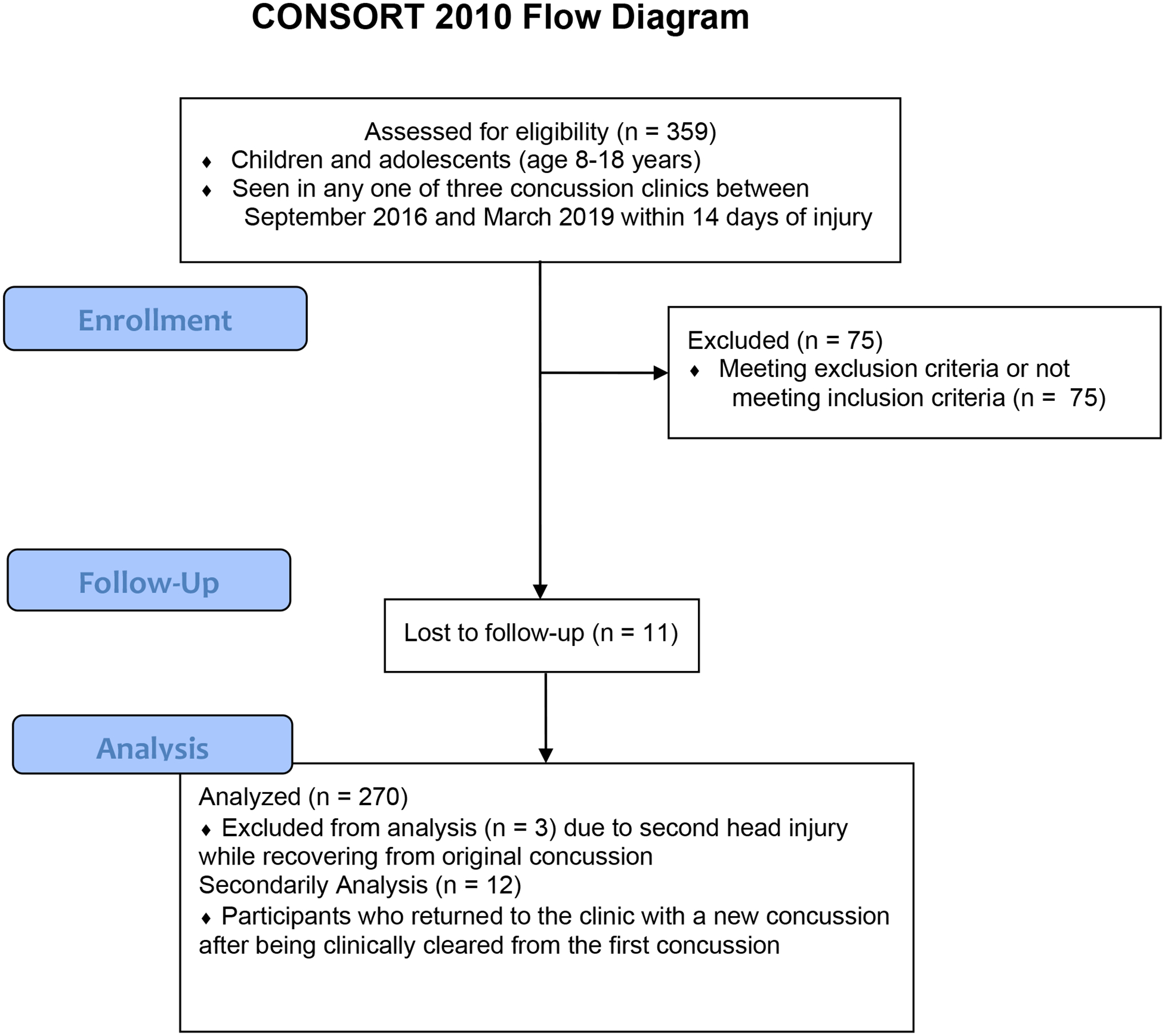
CONSORT Flow Diagram
Table 1.
Participant demographics
| Characteristic | |||
|---|---|---|---|
| n | 270 | ||
| Age, mean (SD) | 14.92 (1.86) years | ||
| Sex, % male (n) | 62% (167) | ||
| Time-since-injury, mean (SD) | 6.00 (3.62) days | ||
| Initial visit symptom severity1, mean (SD) | 33.97 (21.37) | ||
| Recovery time, mean (SD) | 34.50 (36.22) days | ||
| Persistent Post-Concussive Symptoms (PPCS), % (n) | 36.3% (98) | ||
| Loss of consciousness, % (n) | 5.9% (16) | ||
| Sport-related injury, % (n) | 84.1% (227) | ||
| Previous Concussion, % (n) | |||
| 0 | 54.1% (146) | ||
| 1 | 26.7% (72) | ||
| 2 | 13.7% (37) | ||
| 3 | 3.0% (8) | ||
| 4 | 1.5% (4) | ||
| 5 or more | 1.1% (3) | ||
| Concussion 1 | Concussion 2 | p-value | |
| n | 12 | - | |
| Sex | 6 females, 6 males | - | |
| Sport-related injury | 100% | 83.3% | 0.478 |
| Age, in years | 13.75 ± 1.92 | 15.34 ± 2.0 | - |
| Months since recovery from previous concussion | - | 18.75 ± 9.0 | - |
| Injury to first clinic visit, in days | 6.92 ± 4.0 | 7.75 ± 9.6 | 0.914 |
| Symptom severity at diagnosis1 | 28.73 ± 18.7 | 35.09 ± 23.4 | 0.177 |
| LOC2, n(%) | 0 (0%) | 1 (8%) | 0.307 |
| Previous concussions | < 0.001 | ||
| 0 | 6 | 0 | |
| 1 | 6 | 4 | |
| 2 | 0 | 3 | |
| 3 | 0 | 4 | |
| 4 | 0 | 1 | |
| 5 or more | 0 | 0 | |
| Recovery time, in days | 30.75 ± 12.4 | 51.08 ± 57.7 | 0.299 |
| PPCS3, n (%) | 5 (42%) | 5 (42%) | - |
| Symptom severity at clearance | 3.08 ± 4.0 | 5.42 ± 6.7 | 0.287 |
| Directed therapies | 3 cervical PT4 1 vestibular PT | 3 cervical PT, 3 vestibular PT, 1 vision OT5 | - |
| Post-traumatic Diagnosis | None | P1: POTS P2: migraine headache, persistent oculomotor dysfunction |
- |
symptom severity on Post-Concussion Symptom Scale, max = 132;
loss of consciousness;
persistent post-concussive symptoms;
physical therapy;
occupational therapy.
Simple linear and logistic regressions of number of prior concussions and recovery time and PPCS are presented in Figure 2. Number of prior concussions positively correlated with recovery time using simple linear regression (p = 0.024) and with PPCS using logistic regression (p = 0.009). To determine a decision rule, the proportion of participants with PPCS was not significantly different for 0, 1, and 2 prior concussions. Participants were partitioned by number of prior concussions above and below the statistical cut-point for persistent symptoms, shown in Figure 3. In our sample, 15 adolescents out of 270 had 3 or more prior concussions and two-thirds (67%) of those participants developed PPCS. The p-values of the Fisher’s exact test used the values in Figure 3 as 2×2 contingency tables, presented in Figure 4. Only the cut-points for 3, 4, and 5 prior concussions were significantly associated with PPCS. Using a simple majority classifier, we found that the lowest error rate for predicting PPCS was a cut-point of either ≥3 or ≥ 4 prior concussions.
Figure 2:
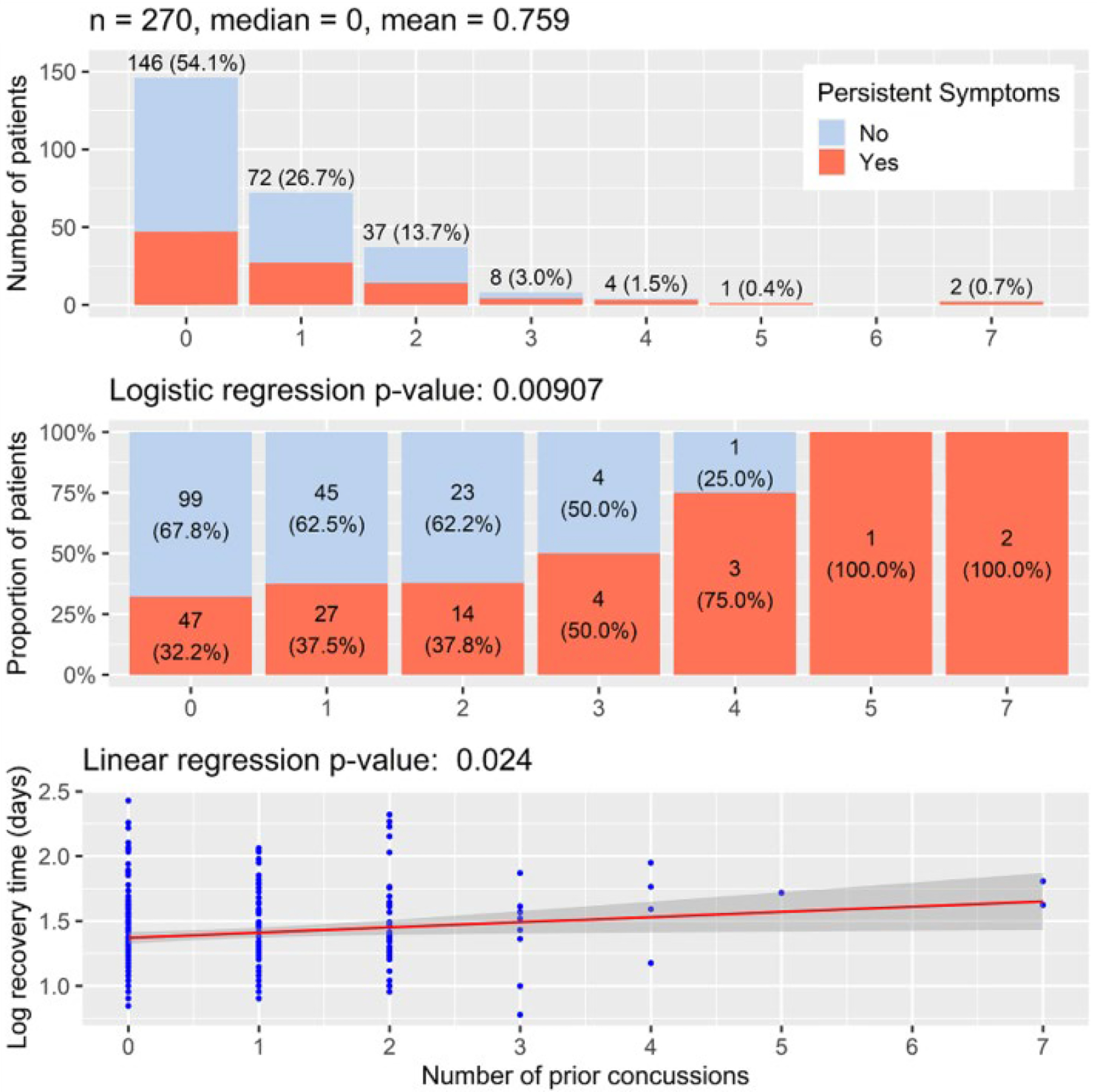
Linear and logistic regression comparing number of prior concussions on recovery time and PPCS (n=270)
Figure 3.
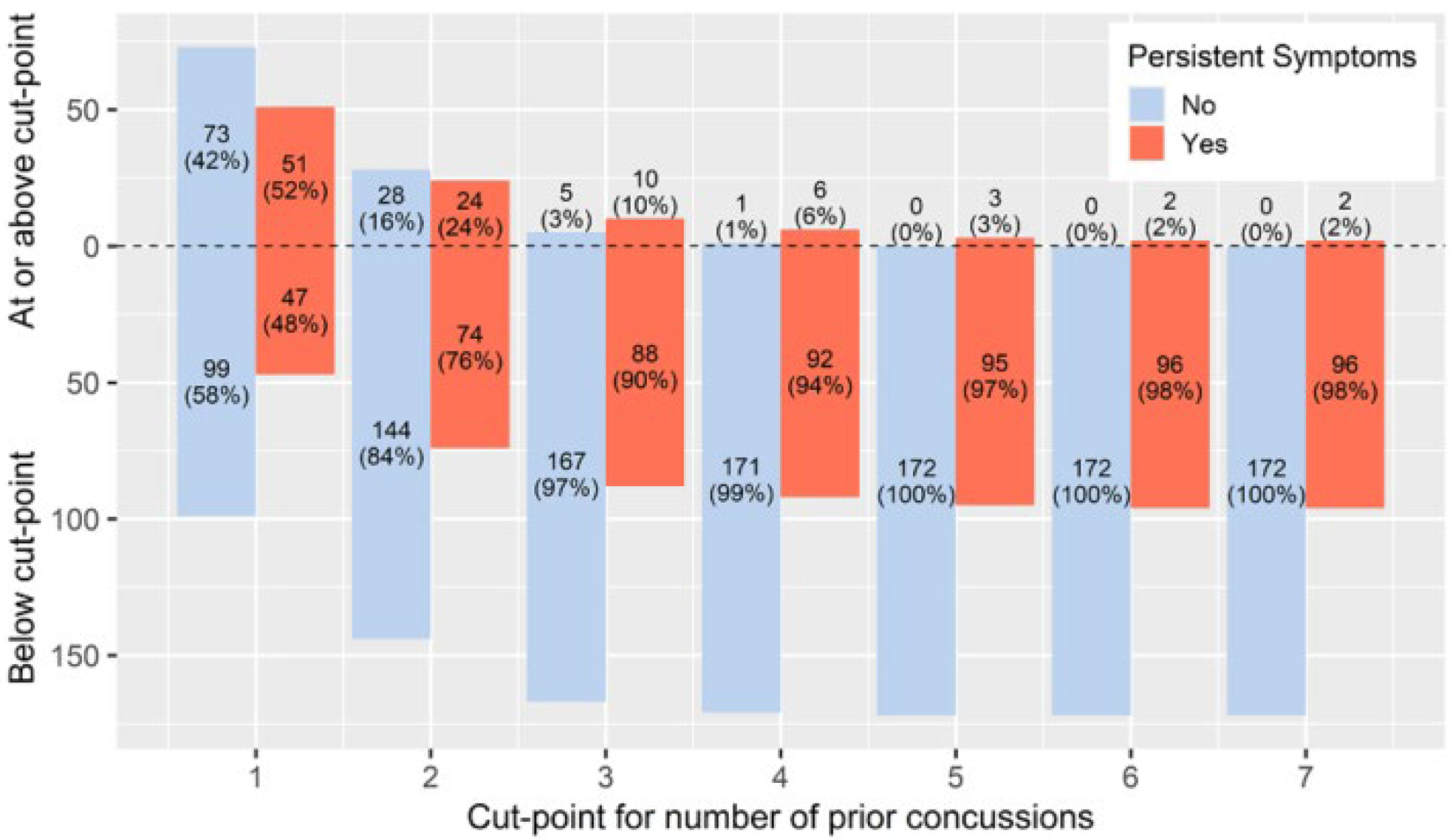
Patients with and without PPCS Above and Below Prior Concussions Cut-point
Figure 4.
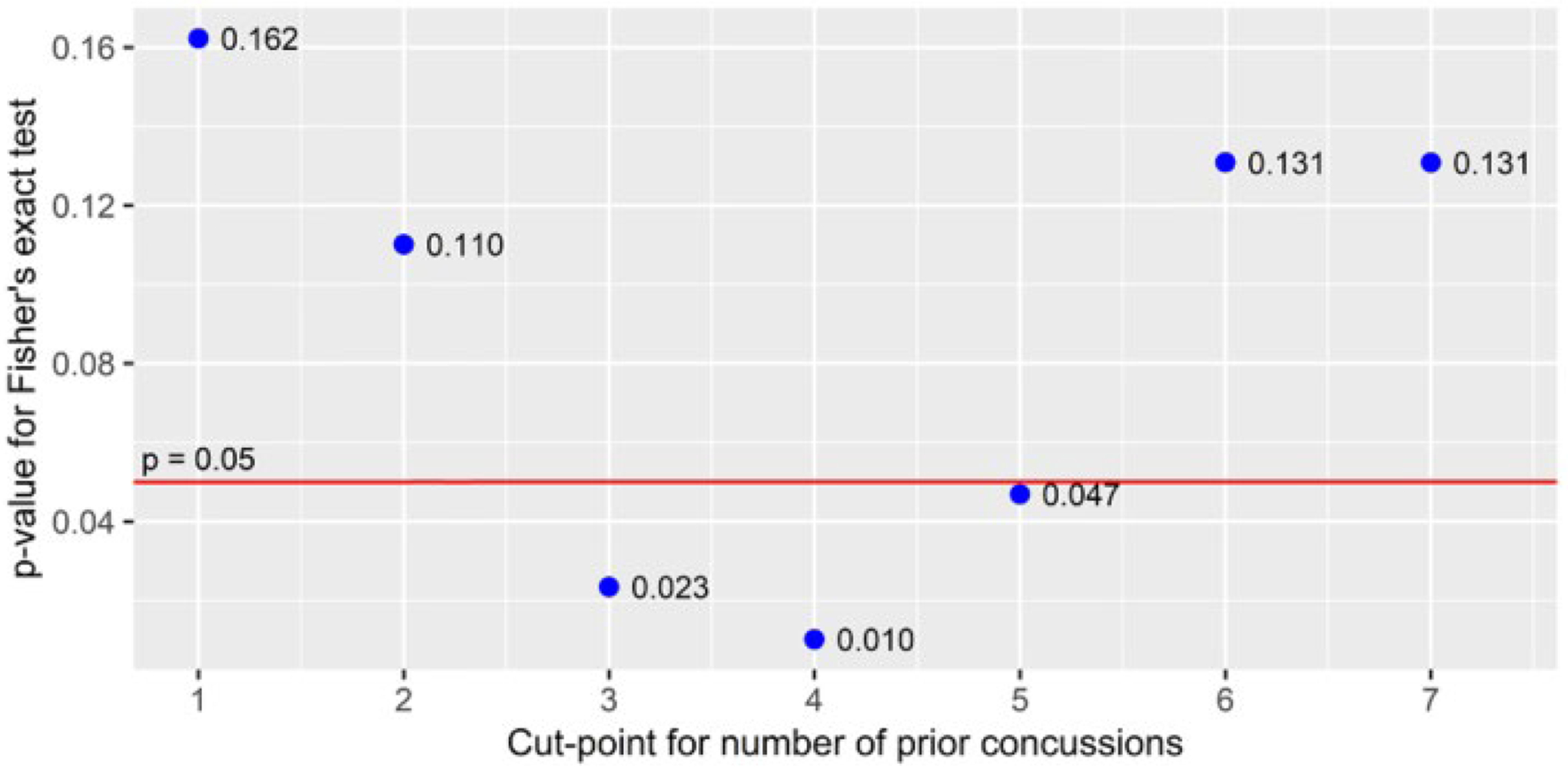
Fisher’s Exact Test for Independence of Proportions of PPCS (n=270)
In the secondary analysis, 12 participants were diagnosed with a new concussion after clearance from the first concussion. Injury characteristics and outcomes of Concussion 1 and Concussion 2 are also presented in Table 1. It is important to note that the labels “Concussion 1” and “Concussion 2” refer only to concussions seen by study clinicians during the enrollment period; 50% of participants had a history of prior concussion when Concussion 1 occurred. Participants recovered in a mean of 30.75 ± 12.4 days (median = 28 days) from Concussion 1 and in a mean of 51.08 ± 57.7 days (median = 24 days) from Concussion 2 (non-significant, p = 0.299). The proportion of participants who developed PPCS was the same for Concussion 1 and Concussion 2 (42%). The maximum duration of recovery after Concussion 1 was 60 days. After Concussion 2, recovery times for 2 participants were considered to be outliers (114 and 208 days). No participants in the small sample of subsequent concussions were diagnosed with persistent impairments after clearance from Concussion 1. However, 2 out of 12 participants after Concussion 2 were diagnosed with persistent impairments after clinical recovery. One patient had postural orthostatic tachycardia syndrome (POTS) and the other patient had both post-traumatic migraine headaches and persistent oculomotor impairments requiring directed vision therapy.
DISCUSSION
We found that a history of 3 or more prior concussions was associated with developing PPCS in pediatric patients, which is the same number of prior concussions associated with greater PPCS risk in adults.11 Understanding the potential for increased risk of prolonged recovery is vital to making informed clinical decisions regarding concussion management in youth. In our study, both the Fisher’s exact test and error rate analysis supported the decision rule that a history of 3 or 4 concussions significantly increases the likelihood of having PPCS. We did not find a significant increase in PPCS for a history of one or two prior concussions, consistent with previous literature.19
Our secondary analysis suggests that subsequent concussions increase the risk of persistent impairments and the need for adjunct therapies. Therefore, adolescents who experience multiple concussions may not only delay their return to sport and daily life but also increase healthcare costs due to the need for additional treatment.30 The insignificant difference in recovery time between Concussion 1 and Concussion 2 likely reflects the small sample size within our secondary analysis. Even though the secondary analysis sample size was small, the main analysis population represented the typical pediatric concussion population: most concussions were sport-related, the proportion of sexes was evenly split, and the incidence of PPCS was approximately 30%, which is consistent with the risk for adolescents reported in the prospective concussion literature.9–12
Due to the fact that decision trees and decision rules can create distinct classifications within populations, these statistical models can provide useful data to help clinicians make evidence-based decisions. The partitioning technique used in this study, a Fisher’s exact test, is valid for small sample sizes and protects against Type I error. We have shown statistical significance within our model (Figure 4), supporting our hypothesis that 3 or more concussions increases the risk of PPCS. Nevertheless, the data do not suggest that a child should be removed from sport or daily activity simply for having sustained 3 or more concussions. The decision whether and when to return to activity after concussion is a choice that a patient, their guardian(s) and physician should make together, taking into consideration factors beyond a potential increased risk for PPCS, including prior health history, psychological history, and financial situation.31 Each concussion is unique, and each patient is unique with a different threshold for injury risk. It is plausible to have a protracted recovery from a single concussion that warrants a recommendation to limit participation in certain recreational or sport activities. It is equally possible to recommend no limitations for patients with a history of rapid and complete recovery from multiple concussions. Our finding that 3 or more concussions appears to significantly increase the risk of prolonged recovery in children is one piece of useful information that can help clinicians and student athletes make more informed decisions about concussion management.
Our study has limitations. There was only 1 participant with 5 prior concussions, none with 6 prior concussions and 2 participants with 7 prior concussions. However, the Fisher’s exact test is valid for smaller samples sizes, reducing the chance of a Type I error. We also recognize that many variables affect concussion recovery such as sex, symptom burden, and time to initial assessment. We believe our data reflect the need for future research using similar statistical approaches, as participants with multiple concussions may be difficult to enroll in randomized trials. Another limitation is potential loss of power from unequal sample sizes; the primary analysis had 284 participants while the secondary analysis included only 12 participants.
CONCLUSION
Our findings suggest that, similar to adults, pediatric patients who sustain a concussion have an increased likelihood of suffering PPCS and persistent impairments after sustaining three or more prior concussions. Moreover, pediatric patients who sustain subsequent concussions often need adjunct therapies that may increase health care costs. Additional academic accommodations in school may be required for a longer period of time for pediatric patients with a history of 3 or more concussions, so it may be useful to alert school-based clinicians (school nurses, athletic trainers, etc.) and school administrative personnel to this possibility in the early stages of the patient’s recovery. When making decisions regarding return to school, return to sport and to other activities, school-based providers, clinic-based providers, patients and their families may want to consider the implications of prior concussion history, especially in pediatric patients who report three or more prior concussions.
Funding and Support
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R01NS094444 and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 to the University at Buffalo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Conder RL, Conder AA. Sports-related concussions. N C Med J. 2015;76(2):89–95. [DOI] [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–47. [DOI] [PubMed] [Google Scholar]
- 3.Halstead ME, Walter KD Moffatt K, et al. Sport-related concussion in children and adolescents. Pediatrics 2018;142(6):2018–3074. [DOI] [PubMed] [Google Scholar]
- 4.Guskiewicz KM, McLeod TCV. (2011). Pediatric sports-related concussion. J Phys Med Rehabil. 2011;3(4), 353–364.2. [DOI] [PubMed] [Google Scholar]
- 5.Black AM, Meeuwisse DW, Eliason PH, et al. Sport participation and injury rates in high school: a Canadian survey of 2029 high school students. J Safety Res 2021;78:314–21. [DOI] [PubMed] [Google Scholar]
- 6.Alves WM, Macciocchi SN, Barth J. Postconcussive symptoms after uncomplicated mild head injury. J Head Trauma Rehabil. 1993;8(3):48–59. [Google Scholar]
- 7.Barlow KM, Crawford S, Stevenson A, et al. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374–81. [DOI] [PubMed] [Google Scholar]
- 8.Rutherford WH, Merrett JD, McDonald JR. Symptoms at one year following concussion from minor head injuries. Injury. 1979;10(3):225–230. [DOI] [PubMed] [Google Scholar]
- 9.Ponsford J, Willmott C, Rothwell A, et al. Cognitive and behavioral outcome following mild traumatic head injury in children. J Head Trauma Rehabil. 1999;14(4):360–372. [DOI] [PubMed] [Google Scholar]
- 10.Mittenberg W, Wittner M, Miller L. Postconcussion syndrome occurs in children. Neuropsychology. 1997;11(3):447–452. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon I, Swaine B, Friedman D, et al. Mild traumatic brain injury affects children’s self-efficacy related to their physical activity performance. J Head Trauma Rehabil. 2005;20(5):436–449. [DOI] [PubMed] [Google Scholar]
- 12.Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014–25. [DOI] [PubMed] [Google Scholar]
- 13.Kerr ZY, Zuckerman SL, Wasserman EB, et al. Concussion symptoms and return to play time in youth, high school, and college American football athletes. JAMA Pediatr 2016;170(7): 647–653. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman EB, Bazarian JJ, Mapstone M, Block R, van Wijngaarden E. Academic dysfunction after a concussion among US high school and college students. Am J Public Health. 2016;106(7):1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scopaz KA, Hatzenbuehler JR. Risk modifiers for concussion and prolonged recovery. Sports Health. 2013;5(6):537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrory P When to retire after concussion? Br J Sports Med. 2001;35(6):380–382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Thorndike A Serious recurrent injuries of athletes: contraindications to further competitive participation. N Engl J Med. 1952;247(15) 554–6. [DOI] [PubMed] [Google Scholar]
- 18.Aubry M, Cantu R, Dvorak J, et al. Concussion in sport group. Summary and agreement statement of the First International Conference on Concussion in Sport, Vienna 2001. Recommendations for the improvement of safety and health of athletes who may suffer concussive injuries. Br J Sports Med. 2001;36(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guskiewicz KM, McCrea Z, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549–5510. [DOI] [PubMed] [Google Scholar]
- 20.Akobeng AK. Understanding type I and type II errors, statistical power and sample size. Acta Paediatr Esp. 2016;105(6):605–9. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Li J, Li Y, Wong WK. A model‐based multithreshold method for subgroup identification. Stat Med. 2019;38(14):2605–31. [DOI] [PubMed] [Google Scholar]
- 22.Vogenberg FR. Predictive and prognostic models: implications for healthcare decision-making in a modern recession. Am Health Drug Benefits. 2009;2(6):218–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Unal I Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haider MN, Leddy JJ, Du W, et al. Practical management: brief physical examination for sport-related concussion in the outpatient setting. Clin J Sport Med. 2018;30(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon KG, Clugston JR, Dec K, et al. American Medical Society for sports medicine position statement on concussion in sport. Br J Sports Med. 2019;53(4):213–25. [DOI] [PubMed] [Google Scholar]
- 26.Zasler N, Haider MN, Grzibowski NR, Leddy JJ. Physician medical assessment in a multidisciplinary concussion clinic. J Head Trauma Rehabil. 2019;34(6):409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beakey M, Roe M, Tiernan S, Keenan B, Collins K. Cross-sectional investigation of self-reported concussions and reporting behaviors in 866 adolescent rugby union players: implications for educational strategies. Clin J Sport Med. 2020;30 Suppl 1:S75–S81. [DOI] [PubMed] [Google Scholar]
- 28.Haider MN, Leddy JJ, Pavlesen S, et al. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med. 2018;52(18):1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haider MN, Cunningham A, Darling S, et al. Derivation of the Buffalo Concussion Physical Examination risk of delayed recovery (RDR) score to identify children at risk for persistent postconcussive symptoms. Br J Sports Med. 2021;55(24):1427–1433. [DOI] [PubMed] [Google Scholar]
- 30.Yengo-Kahn AM, Kelly PD, Liles DC, et al. The cost of a single concussion in American high school football: a retrospective cohort study. Concussion. 2020;5(4):CNC81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


