Abstract
Objective:
The mechanisms linking obesity to type 2 diabetes (T2D) are not fully understood. Our study aimed to identify obesity-related metabolomic signatures (MESs) and evaluated their relations with incident T2D.
Methods:
In a nested case-control study of 2076 Chinese adults, we measured 140 plasma metabolites at baseline and applied linear regression with LASSO to identify MESs for body mass index (BMI) and waist circumference (WC) and conditional logistic regression to examine their associations with T2D risk.
Results:
We identified and validated 32 metabolites associated with BMI or WC, among which 14 showed positive associations and three showed inverse associations with T2D. Eight and 18 metabolites were selected to build MESs for BMI and WC. Both MESs showed strong linear associations with T2D: OR (95% CI) comparing extreme quartiles was 4.26 (2.00, 9.06) for BMI-MES and 9.60 (4.22, 21.88) for WC-MES (both p-trend<0.001). The MES-T2D associations were particularly evident among individuals with normal WC: OR (95% CI) reached 6.41 (4.11, 9.98) for BMI-MES and 10.38 (6.36, 16.94) for WC-MES. Adding MESs to traditional risk factors and plasma glucose improved C-statistics from 0.79 to 0.83 (p<0.001).
Conclusions:
We identified multiple obesity-related metabolites and metabolomic signatures strongly associated with T2D in Chinese adults.
Keywords: Metabolomics, obesity, type 2 diabetes, risk factors, prediction
Introduction
Type 2 diabetes (T2D) is a leading non-communicable disease worldwide. Globally, 537 million (10.5%) people aged 20-79 years had diabetes in 2019, of whom 90% had T2D, and the number is projected to reach 700 million by 2045 (1). China is home to the largest number of people with diabetes worldwide (1). In a 2015-2017 national survey, the prevalence of diabetes among Chinese adults was 12.8%, corresponding to 130 million adults (2). Understanding the potential mechanisms of T2D and predicting high-risk populations are critical to the prevention and control of T2D.
Obesity is an established risk factor for T2D. Evidence from prospective studies, including our previous work in the Shanghai Women’s Health Study (SWHS), has demonstrated increased risk of incident T2D in Chinese adults with higher levels of general and central obesity measured by body mass index (BMI) and waist circumference (WC), particularly with central obesity (3, 4). Chinese and other East Asian populations are also known to have an elevated T2D risk at a relatively low BMI or WC compared to other races and ethnic groups (5, 6, 7). This may partly be due to higher percentages of body fat at a given BMI and higher amounts of visceral fat at a given WC among Asian descendants, but the mechanisms underlying this increased risk have not been fully elucidated (5, 6). While some measures have been proposed to define “metabolically unhealthy” individuals who have normal weight, their abilities to predict future cardiometabolic events have not been established (8). Thus, how to delineate “metabolically unhealthy” normal weight based on more nuanced metabolic measures in Chinese and other Asian populations remains a major challenge. In addition, since cardiometabolic heterogeneities of obesity (general vs. central obesity) are recognized (9), differential metabolic profiles related to general and central obesity may contribute to the pathophysiology of T2D.
Metabolomics have facilitated high-throughput metabolites profiling and provided novel insights into the etiology of T2D and other diseases (10, 11, 12). While certain metabolites such as branched-chain amino acids (BCAAs)—valine, leucine, and isoleucine—and acylcarnitines such as carnitine (CAR) 3:0 and 5:0 have been implicated in both obesity and T2D (10, 11, 12), to our knowledge, no prior studies have systematically examined the obesity-related metabolomic profiles with incident T2D. To this end, we aimed to identify obesity-related metabolites and metabolomic signatures (MESs) in Chinese adults and examine their associations with incident T2D. In addition, we evaluated the extent to which the identified MESs could mediate the association between BMI/WC and T2D and improve T2D risk prediction beyond established risk factors.
Methods
Study population
We conducted a case-control study nested within two large prospective cohort studies of urban Chinese adults: the SWHS and the Shanghai Men’s Health Study (SMHS). The SWHS enrolled 74,942 women aged 40-70 years in 1996-2000 (response rate: 92.7%) (13), and the SMHS recruited 61,480 men aged 40-74 years in 2002-2006 (response rate: 74.0%) (14). The two cohorts followed similar protocols and administered similar questionnaires. Briefly, information was collected on sociodemographics, lifestyle and dietary factors, medical history, and anthropometrics through in-person interviews at baseline. In addition, a 10 mL blood sample was collected at baseline using an EDTA tube and maintained at 4°C. Within 6 hours, blood samples were processed and aliquoted for long-term storage at −70°C. Cohort participants were followed up via in-person visits every 2-4 years (follow-up rate >91%) and linkage to mortality and cancer registries annually (follow-up rate >99%). The SWHS and SMHS were approved by the Institutional Review Boards of the Shanghai Cancer Institute and Vanderbilt University Medical Center (No. 000340 and 000598), and informed consent was obtained from all study participants.
Nested case-control study design
The current analyses included 1038 incident cases of T2D (see details in “T2D Ascertainment”) and 1038 controls selected using an incidence density sampling method and individually matched by sex, age (±2 years), date of sample collection (±30 days), and the time interval between the last meal and blood draw (i.e., fasting hours, ±2 hours) in both the SWHS and SMHS. The median (inter-quintile range) of fasting hours was 10 (6–15) in our study sample. All cases and controls were free of cancer, cardiovascular disease, and diabetes at baseline. Plasma samples of the matched case-control pairs were placed adjacently in the same batch and sent to the Broad Institute and Beth Israel Deaconess Medical Center for metabolomics profiling (see details in “Metabolomics Profiling”). Our samples were measured in two profiling batches at two different times: Batch 1 (discovery set) included 591 case-control pairs and detected 184 targeted metabolites; Batch 2 (validation set) included 447 case-control pairs and detected 275 targeted metabolites. Our current analyses were focused on the 140 metabolites commonly detected in both batches and with <10% missingness. First, we cross-sectionally screened for obesity-related metabolites using Batch 1 data and validated the findings using Batch 2 data. Second, we prospectively assessed the associations of individual obesity-related metabolites and their combined MESs with T2D risk and evaluated the extent to which the MESs mediated the obesity-T2D association. Third, we evaluated the added predictive value of obesity MESs for T2D compared to established risk factors plus plasma glucose. For T2D-related prospective analyses, we used data of all 1038 case-control pairs. The flowchart of study procedures is shown in Figure 1.
Figure 1. Flowchart of study procedures.

Abbreviations: BMI, body mass index; MES, metabolomic signature; T2D, type 2 diabetes; WC, waist circumference
Anthropometric measurements
Anthropometric measurements were taken by trained interviewers at baseline according to standard protocols. WC was measured at 2.5 cm above the umbilicus in a standing position. Weight, height, and WC were measured in duplicate and recorded to the nearest 0.1 kg and 0.1 cm, respectively, and the average of two measures was used. BMI was calculated as weight in kilograms divided by height in meters squared. Overweight and obesity were defined as BMI of 24.0-27.9 kg/m2 and ≥28.0 kg/m2, respectively, per guidelines for Chinese adults, while central obesity was defined as WC ≥90.0 cm for men and ≥85.0 cm for women (5).
T2D ascertainment
Diabetes was ascertained at baseline and follow-up visits conducted every 2-4 years. T2D was defined as both self-reported physician diagnosis of diabetes and meeting at least one of the following criteria (15): 1) fasting plasma glucose ≥7.0 mmol/L (126 mg/dL) on at least two occasions; 2) 2-h postprandial plasma glucose ≥11.1 mmol/L (200 mg/dL) on at least two occasions; 3) use of antidiabetic drugs; or 4) presence of diabetes symptoms (e.g., frequent urination, increased thirst, increased hunger, or unexplained weight loss) plus fasting plasma glucose ≥7.0 mmol/L or 2-h postprandial plasma glucose ≥11.1 mmol/L. Incident T2D was defined as new-onset diabetes during follow-ups.
Metabolomics profiling
Plasma metabolomics data were measured using blood samples collected at baseline through targeted liquid chromatography-tandem mass spectrometry (LC-MS) platforms. Detailed methods have been described previously (16, 17) and were summarized in the Supplementary Methods. One hundred and eighty-four and 275 targeted LC-MS features were detected in Batch 1 and Batch 2, respectively. The identities of these compounds were confirmed using authentic reference standards or reference samples. Fourteen metabolites were excluded in Batch 2 due to data missingness over 10%, leaving 261 in the remaining dataset, while no metabolites were dropped in Batch 1. The two batches had 140 common metabolites. The median coefficient of variation (CV) for the measured metabolites was 2.8% with a range of 0.7%-74.9%. Over 99% of the metabolites had CVs<30%.
Statistical analyses
Metabolite values were natural log-transformed to improve normality and converted to a z-score with a mean of 0 and a standard deviation (SD) of 1 within each batch of measurements. Missing values of metabolites were replaced by the lowest non-missing ones divided by 2. Missing values of covariates were assigned sex-specific median (continuous variables) or mode (categorical variables) values of the non-missing ones since their missing rates were mostly less than 1%. We estimated associations of BMI and WC with individual metabolites using generalized linear models, adjusting for sex, age, total energy intake (kcal/day), incident T2D case-control status, fasting hours, income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity (measured by metabolic equivalent of task - hours/week), diet quality (measured by a healthy diet score) (15), hypertension status, and menopause status (for women only). False discovery rates (FDRs) were estimated through the Benjamini-Hochberg procedure to account for multiple comparisons (18). Metabolites with FDR-adjusted p<0.05 in the discovery set were considered replicated if they showed FDR-adjusted p<0.05 and the same direction of association in the validation set. Linear correlation coefficients between BMI/WC and validated metabolites were visualized using heat maps.
We estimated odds ratios (ORs) and 95% confidence intervals (95% CIs) for T2D associated with each SD increase in metabolites using conditional logistic regressions with similar adjustments as described above. We applied the adaptive LASSO (least absolute shrinkage and selection operator) regression to select a set of metabolites representing MESs of BMI and WC, respectively. We constructed BMI and WC MESs using the sum of z-scores weighted by regression coefficients for the metabolites remained in the LASSO regression. We examined the two MESs, both continuously and in quartiles (based on the distribution in the controls), in association with risk of T2D overall and in subgroups by age, sex, BMI, and WC.
In addition, we examined how much the obesity MESs mediated the obesity-T2D relationship using the SAS macro “%mediation” (19), by decomposing the total effect of obesity into an indirect effect through MESs and a direct effect. The extent of mediation was estimated as the ratio of the indirect and total effect on a log scale (20).
We further assessed the prediction performance of obesity MESs alone and in addition to traditional T2D risk factors by computing the C-statistics using receiver operating characteristic analyses (21). We compared the model with traditional risk factors (age, sex, smoking, pack-years, alcohol drinking, diet quality, physical activity, family history of diabetes, BMI, and WC), and other models with a combination of traditional risk factors, plasma glucose, and MESs. A similar analysis was conducted for all obesity-related metabolites that individually associated with T2D to predict T2D risk. We also estimated the integrated discrimination improvement (IDI) and net reclassification improvement (NRI) (22) using the SAS macro “%add_predictive”.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). In all T2D association and prediction analyses, tests were two-sided with statistical significance level set to α=0.05.
Results
Baseline characteristics of study participants
Of 2076 participants, 998 (48.1%) were men, and the mean age was 51.7±7.8 years (Table 1). Mean BMI was 24.7±3.3 kg/m2, and 316 (15.2%) had obesity (BMI ≥28.0 kg/m2). Compared to participants with normal weight, those with obesity were older, had higher WC, smoking pack-years (among ever smokers), physical activity level, total energy intake, and prevalence of hypertension and menopause (among women), but were less likely to be current smokers or heavy drinkers, or have a family history of diabetes. Baseline characteristics by incident T2D status are provided in Table S1.
Table 1.
Baseline characteristics of study participants across BMI groups
| Total | Normal weight | Overweight* | Obesity* | |
|---|---|---|---|---|
| N (%) | 2076 | 868 (41.8) | 892 (43.0) | 316 (15.2) |
| Age, years, mean (SD) | 51.7 (7.8) | 51.3 (7.8) | 51.6 (7.6) | 53.2 (8.3) |
| Sex, n (%) | ||||
| Men | 998 (48.1) | 421 (48.5) | 440 (49.3) | 137 (43.4) |
| Women | 1078 (51.9) | 447 (51.5) | 452 (50.7) | 179 (56.7) |
| Income levels, n (%)† | ||||
| Low | 325 (15.7) | 142 (16.4) | 133 (14.9) | 50 (15.8) |
| Lower-middle | 794 (38.3) | 342 (39.4) | 332 (37.2) | 120 (38.0) |
| Upper-middle | 634 (30.5) | 250 (28.8) | 287 (32.2) | 97 (30.7) |
| High | 323 (15.6) | 134 (15.4) | 140 (15.7) | 49 (15.5) |
| BMI, kg/m2 | 24.7 (3.3) | 21.7 (1.7) | 25.8 (1.1) | 30.0 (2.0) |
| WC, cm, mean (SD) | 83.4 (9.4) | 76.8 (7.1) | 86.0 (6.6) | 94.3 (7.3) |
| Smoking, n (%) | ||||
| Never | 1294 (62.3) | 529 (60.9) | 568 (63.7) | 197 (62.3) |
| Former | 85 (4.1) | 24 (2.8) | 36 (4.0) | 25 (7.9) |
| Current | 697 (33.6) | 315 (36.3) | 288 (32.3) | 94 (29.8) |
| Pack-years among ever smokers, mean (SD) | 24.0 (15.3) | 23.5 (14.1) | 24.2 (15.7) | 25.0 (17.6) |
| Drinking, n (%)‡ | ||||
| None | 1690 (81.4) | 689 (79.4) | 740 (83.0) | 261 (82.6) |
| Moderate | 219 (10.6) | 97 (11.2) | 92 (10.3) | 30 (9.5) |
| Heavy | 167 (8.0) | 82 (9.5) | 60 (6.7) | 25 (7.9) |
| Physical activity, MET-hours/week, mean (SD) | 79.0 (47.0) | 78.1 (45.2) | 79.3 (47.8) | 80.9 (49.3) |
| Total energy intake, kcal/day, mean (SD) | 1816.9 (484.3) | 1772.1 (456.6) | 1850.0 (496.0) | 1847.0 (515.8) |
| Healthy diet score, mean (SD) | 24.0 (5.2) | 24.0 (5.0) | 24.0 (5.2) | 23.6 (5.5) |
| Menopause among women, n (%) | 450 (41.7) | 172 (38.5) | 189 (41.8) | 89 (49.7) |
| Hypertension, n (%) | 532 (25.6) | 133 (15.3) | 258 (28.9) | 141 (44.6) |
| Family history of diabetes, n (%) | 483 (23.3) | 188 (21.7) | 232 (26.0) | 63 (19.9) |
Abbreviations: BMI, body mass index; CNY, Chinese Yuan; MET, metabolic equivalent of task; SD, standard deviation; WC, waist circumference.
Overweight and obesity were defined as BMI of 24.0-27.9 kg/m2 and ≥28.0 kg/m2.
Annual income was defined as low, lower-middle, upper-middle, and high: for the Shanghai Women’s Health Study, annual household income <10,000 CNY, ≥10,000 to <20,000 CNY, ≥20,000 to <30,000 CNY, and ≥30,000 CNY; for the Shanghai Men’s Health Study, annual personal income <6,000 CNY, ≥6,000 to <1,2000 CNY, ≥12,000 to <24,000 CNY, and ≥24,000 CNY.
Heavy drinking was defined as >2 drinks per day in men or >1 drink per day in women (1 drink = 14 g ethanol); moderate drinking was defined as >0 to ≤2 drinks per day in men or >0 to ≤1 drink per day in women.
Identification of obesity-related metabolites
Among 1182 participants included in the discovery set, we identified 72 metabolites (out of 184) that correlated with BMI (FDR-adjusted p<0.05). Of the 72 metabolites, 14 were not available in the validation set. Of the remaining 58 metabolites, 24 were confirmed in the validation set of 894 participants (FDR-adjusted p<0.05 and the same direction of association as in the discovery set; Figure 2 and Table S2). Among them, 21 metabolites showed positive associations with BMI with βs ranging from 0.027 to 0.093 for each SD increase, and three showed negative associations with βs ranging from −0.059 to −0.046, all of which were amino acids (asparagine, glycine, and serine).
Figure 2. Change in the metabolite z-score for each 1-kg/m2 increase of body mass index.
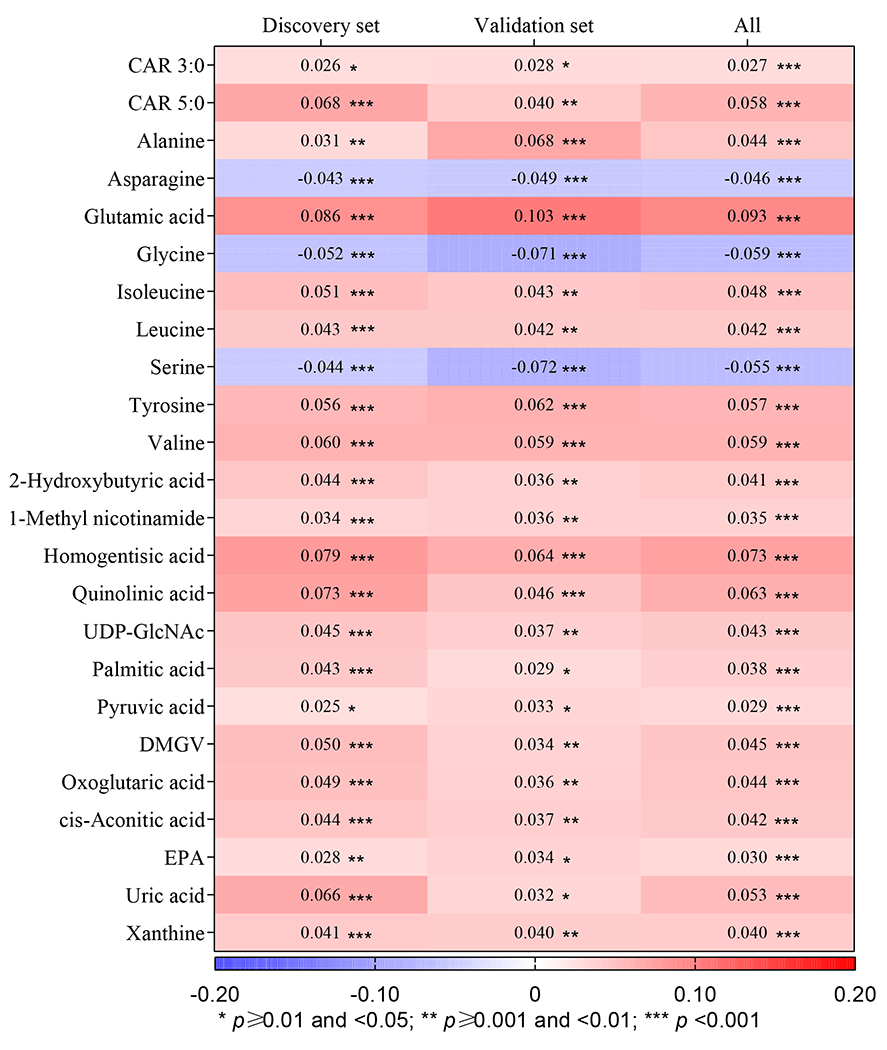
Abbreviations: CAR, carnitine; CNY, Chinese Yuan; DMGV, dimethylguanidino valeric acid; EPA, eicosapentaenoic acid; FDR, false discovery rate; MET, metabolic equivalent of task; T2D, type 2 diabetes; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine. The associations of body mass index with individual plasma metabolites were assessed using generalized linear models, with adjustment for study batch (only in the pooled sample), sex, age (years, continuous), total energy intake (kcal/day, continuous), incident T2D case-control status (yes and no), fasting hours (continuous), income [annual household income <10,000 CNY, ≥10,000 to <20,000 CNY, ≥20,000 to <30,000 CNY, and ≥30,000 CNY in the SWHS; annual personal income <6,000 CNY, ≥6,000 to <12,000 CNY, ≥12,000 to <24,000 CNY, and ≥24,000 CNY in the SMHS], cigarette smoking (never, former, and current), pack-years among smokers (continuous), alcohol drinking [none, moderate (>0 to ≤2 drinks per day in men or >0 to ≤1 drink per day in women), and heavy drinking (>2 drinks per day in men or >1 drink per day in women); 1 drink = 14 g ethanol], physical activity (continuous, MET-hours/week), diet quality (as measured by a healthy diet index), menopause status (yes and no, for women only), and hypertension status (yes and no). βs with FDR-adjusted p values are presented in the figure.
Similarly, we identified 74 metabolites associated with WC in the discovery set and confirmed 31 metabolites in the validation set (Figure 3 and Table S3). Among them, 26 metabolites showed positive associations with WC with βs ranging from 0.009 to 0.038 for 1-SD increase, while five metabolites showed negative associations with βs ranging from −0.025 to −0.011, including 1,5-anhydrosorbitol, betaine, and the above-mentioned three amino acids. When BMI- and WC-related metabolites were compared, 32 metabolites were associated with either BMI or WC, and 23 metabolites were associated with both (Figure S1). The median intraclass correlation coefficient (a measure of the reliability of the biomarker measurement in relationship to the biological variance of the biomarker) was excellent at 0.998 for these 32 metabolites. Eight metabolites were WC-specific, including six with positive associations (n-acetylalanine, pantothenic acid, kynurenine, choline, ureidopropionic acid, and lactic acid), and two with negative association (betaine and 1,5-anhydrosorbitol). Exploratory pathway analysis of 32 obesity-related metabolites identified six significantly altered pathways, including “alanine, aspartate, and glutamate metabolism”, “glyoxylate and dicarboxylate metabolism”, “glycine, serine, and threonine metabolism”, “pantothenate and CoA biosynthesis”, “D-glutamine and D-glutamate metabolism”, and “citrate cycle (tricarboxylic acid [TCA] cycle)” (Table S4).
Figure 3. Change in the metabolite z-score for each 1-cm increase of waist circumference.

Abbreviations: CAR, carnitine; DMGV, dimethylguanidino valeric acid; EPA, eicosapentaenoic acid; FDR, false discovery rate; T2D, type 2 diabetes; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine. The associations of waist circumference with individual plasma metabolites were assessed using generalized linear models, with adjustment for study batch (only in the pooled sample), sex, age, total energy intake, incident T2D case-control status, fasting hours, income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity, diet quality, menopause status (for women only), and hypertension status. βs with FDR-adjusted p values are presented in the figure.
Associations of individual obesity-related metabolites with incident T2D
Of 32 metabolites related to either BMI or WC, 14 showed significant positive associations with T2D, and three showed inverse associations (all FDR-adjusted p<0.05; Figure 4). Five amino acids (glutamic acid, valine, leucine, alanine, and isoleucine) showed the strongest positive associations with ORs ranging from 1.50 to 1.90 for 1-SD increment. Other nine metabolites showed positive associations with ORs of 1.22-1.43 per SD increment, including uric acid, cis-aconitic acid, lactic acid, 2-hydroxybutyric acid, oxoglutaric acid, palmitic acid, homogentisic acid, dimethylguanidino valeric acid (DMGV), and xanthine. In contrast, 1,5-anhydrosorbitol, glycine, and serine were negatively associated with T2D risk, with ORs of 0.49-0.80 per SD increment.
Figure 4. Association between individual obesity-related metabolites and risk of type 2 diabetes.

Abbreviations: BMI, body mass index; CAR, carnitine; CI, confidence interval; DMGV, dimethylguanidino valeric acid; EPA, eicosapentaenoic acid; FA, fatty acid; FDR, false discovery rate; OR, odds ratio; rNDP, ribonucleotide diphosphate reductase; TCA, tricarboxylic acid; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine. The associations of individual plasma metabolites with type 2 diabetes were assessed using conditional logistic regression, adjusting for study batch, sex, age, total energy intake, fasting hours, income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity, diet quality, BMI, menopause status, hypertension status, and family history of diabetes.
Associations between obesity MESs and incident T2D
The obesity-related metabolites showed close intercorrelations, particularly among those within the same sub-class (Figure S2–3). After accounting for the intercorrelations, the LASSO procedure selected 8 and 18 metabolites to construct the MESs for BMI and WC, respectively (Figure S1).
Both BMI and WC MESs showed strong linear associations with T2D risk (Table 2). The multivariable-adjusted OR (95% CI) was 10.10 (5.39, 18.91) for the top vs. bottom quartile of the BMI-MES and 12.03 (5.53, 26.20) for the top vs. bottom quartile of the WC-MES (Model 2, both p-trend<0.001). The strong positive associations of obesity MESs with T2D remained after further adjustments for BMI/WC and plasma glucose (the full model: OR [95% CI] = 4.26 [2.00, 9.06] across quartiles of the BMI-MES and 9.60 [4.22, 21.88] across quartiles of the WC-MES).
Table 2.
Association between obesity MESs and risk of type 2 diabetes
| OR (95% CI) |
Per unit increase | p for trend | ||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| BMI MES* | ||||||
| Cases/controls | 55/259 | 135/260 | 233/259 | 615/260 | - | - |
| Model 1† | Reference | 2.46 (1.35, 4.47) | 3.41 (1.87, 6.22) | 10.74 (5.91, 19.52) | 1.76 (1.56, 1.99) | <0.001 |
| Model 2‡ | Reference | 2.44 (1.30, 4.60) | 3.28 (1.74, 6.18) | 10.10 (5.39, 18.91) | 1.72 (1.52, 1.94) | <0.001 |
| Model 2 + BMI | Reference | 1.95 (1.01, 3.77) | 2.30 (1.16, 4.53) | 5.78 (2.83, 11.83) | 1.55 (1.34, 1.79) | <0.001 |
| Model 2 + BMI + plasma glucose§ | Reference | 1.75 (0.87, 3.52) | 1.89 (0.93, 3.85) | 4.26 (2.00, 9.06) | 1.47 (1.26, 1.72) | <0.001 |
| WC MES‖ | ||||||
| Cases/controls | 53/259 | 123/260 | 234/259 | 628/260 | - | - |
| Model 1† | Reference | 3.08 (1.60, 5.93) | 6.68 (3.46, 12.92) | 19.60 (9.89, 38.85) | 1.23 (1.18, 1.28) | <0.001 |
| Model 2¶ | Reference | 2.67 (1.32, 5.38) | 5.00 (2.46, 10.17) | 12.03 (5.53, 26.20) | 1.20 (1.15, 1.26) | <0.001 |
| Model 2 + WC | Reference | 2.67 (1.32, 5.40) | 4.78 (2.34, 9.79) | 11.09 (5.05, 24.38) | 1.20 (1.14, 1.26) | <0.001 |
| Model 2 +WC + plasma glucose§ | Reference | 2.69 (1.28, 5.64) | 4.99 (2.35, 10.57) | 9.60 (4.22, 21.88) | 1.18 (1.13, 1.25) | <0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; MES, metabolomic signature; OR, odds ratio; WC, waist circumference
The BMI MES was based on 15 BMI-related metabolites.
Adjusted for study batch, age, total energy intake, and fasting hours in Model 1.
Adjusted for income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity, diet quality, menopausal status (for women only), hypertension status, and family history of diabetes plus those in Model 1.
Plasma glucose was represented by a composite glucose/fructose/galactose level in the metabolomics data.
The WC MES was based on 21 BMI-related metabolites.
Adjusted for income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity, diet quality, menopausal status (for women only), BMI, hypertension status, and family history of diabetes plus those in Model 1.
The MES-T2D associations were consistent across subgroups by sex and age (Table 3). Strikingly, the associations of obesity MESs with T2D appeared stronger among those with normal WC. Among participants with normal WC, the OR (95% CI) in the full model was 6.41 (4.11, 9.98) across quartiles of the BMI-MES and 10.38 (6.36, 16.94) across quartiles of the WC-MES (p for interaction between MES quartiles and WC categories=0.02 and 0.01, respectively).
Table 3.
Association between obesity MESs and risk of type 2 diabetes in subgroups
| OR (95% CI) |
Per unit increase | p for trend | p for interaction | |||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||||
| BMI-MES* | ||||||||
| Sex | 0.63 | |||||||
| Women | 539/539 | Reference | 1.50 (0.93, 2.43) | 2.48 (1.54, 4.00) | 5.22 (3.18, 8.57) | 1.60 (1.43, 1.80) | <0.001 | |
| Men | 499/499 | Reference | 3.28 (1.58, 6.81) | 4.99 (2.44, 10.20) | 9.33 (4.51, 19.31) | 1.53 (1.35, 1.74) | <0.001 | |
| Age, years | 0.45 | |||||||
| <50 | 541/505 | Reference | 1.78 (1.09, 2.88) | 2.97 (1.83, 4.83) | 6.37 (3.77, 10.76) | 1.62 (1.43, 1.84) | <0.001 | |
| ≥50 | 497/533 | Reference | 2.26 (1.10, 4.63) | 3.24 (1.61, 6.48) | 6.34 (3.17, 12.67) | 1.53 (1.36, 1.72) | <0.001 | |
| BMI, kg/m2 | 0.07 | |||||||
| <24 | 267/601 | Reference | 2.12 (1.28, 3.51) | 2.74 (1.62, 4.64) | 7.96 (4.51, 14.02) | 1.70 (1.48, 1.95) | <0.001 | |
| ≥24 | 771/437 | Reference | 1.57 (0.79, 3.13) | 2.90 (1.50, 5.58) | 4.82 (2.52, 9.23) | 1.48 (1.33, 1.65) | <0.001 | |
| Central obesity | 0.02 | |||||||
| No | 549/818 | Reference | 1.96 (1.29, 2.98) | 2.62 (1.72, 4.00) | 6.41 (4.11, 9.98) | 1.63 (1.47, 1.80) | <0.001 | |
| Yes | 489/220 | Reference | 1.40 (0.30, 6.44) | 3.29 (0.77, 14.14) | 4.48 (1.06, 18.92) | 1.37 (1.18, 1.60) | <0.001 | |
| WC-MES† | ||||||||
| Sex | 0.52 | |||||||
| Women | 539/539 | Reference | 2.08 (1.33, 3.25) | 3.85 (2.43, 6.11) | 8.32 (4.97, 13.93) | 1.19 (1.14, 1.23) | <0.001 | |
| Men | 499/499 | Reference | 4.02 (1.13, 14.33) | 7.38 (2.17, 25.10) | 16.73 (4.90, 57.19) | 1.17 (1.13, 1.22) | <0.001 | |
| Age, years | 0.49 | |||||||
| <50 | 541/505 | Reference | 2.05 (1.25, 3.36) | 3.48 (2.09, 5.81) | 11.62 (6.47, 20.89) | 1.21 (1.16, 1.26) | <0.001 | |
| ≥50 | 497/533 | Reference | 3.24 (1.47, 7.17) | 6.40 (2.98, 13.72) | 11.02 (5.07, 23.95) | 1.17 (1.12, 1.21) | <0.001 | |
| BMI, kg/m2 | 0.06 | |||||||
| <24 | 267/601 | Reference | 2.91 (1.66, 5.10) | 4.75 (2.64, 8.55) | 16.19 (8.30, 31.58) | 1.22 (1.17, 1.28) | <0.001 | |
| ≥24 | 771/437 | Reference | 1.96 (1.05, 3.66) | 3.77 (2.10, 6.78) | 6.85 (3.76, 12.49) | 1.16 (1.12, 1.20) | <0.001 | |
| Central obesity | 0.01 | |||||||
| No | 549/818 | Reference | 2.26 (1.46, 3.51) | 3.91 (2.51, 6.09) | 10.38 (6.36, 16.94) | 1.20 (1.16, 1.24) | <0.001 | |
| Yes | 489/220 | Reference | 2.35 (0.62, 8.91) | 4.34 (1.23, 15.35) | 7.13 (2.01, 25.24) | 1.13 (1.08, 1.19) | <0.001 | |
Abbreviations: BMI, body mass index; CI, confidence interval; MES, metabolomic signature; OR, odds ratio; WC, waist circumference
The BMI MES was based on 15 BMI-related metabolites. Models were adjusted for study batch, sex, age, total energy intake, fasting hours, income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity, diet quality, BMI, menopause status (for women only), hypertension status, family history of diabetes, and plasma glucose (represented by a composite glucose/fructose/galactose level in the metabolomics data).
The WC MES was based on 21 BMI-related metabolites. Models were adjusted for study batch, sex, age, total energy intake, fasting hours, income, cigarette smoking, pack-years among ever smokers, alcohol drinking, physical activity, diet quality, BMI, WC, menopause status (for women only), hypertension status, family history of diabetes, and plasma glucose (represented by a composite glucose/fructose/galactose level in the metabolomics data).
The BMI-MES mediated 46.2% of the BMI-T2D association, while the WC-MES mediated 66.0% of the WC-T2D association (Table S5).
Predictive performance of obesity-related metabolites and MESs for incident T2D
BMI-MES or WC-MES alone achieved similar prediction performance compared with a prediction model using traditional T2D risk factors, including sociodemographic and lifestyle factors, family history of diabetes, BMI, and WC (all C-statistics ~0.75; p≥0.12 for pairwise comparisons; Figure 5A). Adding BMI-MES to the model with traditional risk factors increased the C-statistics to 0.78 (95% CI, 0.76, 0.80; p<0.001; Figure 5B), which was comparable to the model with traditional factors plus plasma glucose (C-statistics=0.79, 95% CI, 0.77, 0.81; p=0.22). Moreover, adding BMI-MES to the model with traditional risk factors plus plasma glucose increased the C-statistics to 0.82 (95% CI, 0.80, 0.83; p<0.001), while adding both BMI and WC MESs increased the C-statistics further to 0.83 (95% CI, 0.81, 0.84; p<0.001), which was also statistically higher than the addition of BMI MES alone (p=0.003). Consistently, adding BMI and WC MESs also showed significant NRI and IDI compared with the model with traditional risk factors plus plasma glucose (Table S6). Similar to BMI and WC MESs, seventeen obesity-related metabolites that were individually associated with T2D risk showed favorable prediction performance, and improved the C-statistics when added to the model with traditional risk factors plus glucose (0.84, 95% CI, 0.83, 0.86; p<0.001 Figure S4).
Figure 5. Predictive performance of obesity metabolomic signatures for risk of type 2 diabetes.

Abbreviations: BMI, body mass index; MES, metabolomic signature; ROC, receiver operating characteristic; WC, waist circumference. Traditional risk factors included age, sex, smoking, pack-years among ever smokers, alcohol drinking, diet quality, physical activity, family history of diabetes, BMI, and WC. Plasma glucose was represented by a composite glucose/fructose/galactose level in the metabolomics data. The prediction performance of obesity MESs alone and in addition to traditional T2D risk factors was assessed by computing the C-statistics (i.e., area under the ROC curve). p≥0.12 for pairwise comparisons in Figure 5A, and p=0.22 for comparison between the first two models, and ≤0.003 for other pairwise comparisons in Figure 5B.
Discussion
In a nested sample from two large prospective cohorts of middle-aged, generally normal-weight Chinese adults, we identified and validated 32 circulating metabolites associated with BMI or WC and subsequently generated two obesity MESs. Both BMI and WC MESs showed strong linear relationships with a 4- to 10-fold increased risk of T2D for the comparison between the highest quartile and the lowest, after adjustment for established diabetes risk factors and obesity status. Of note, the MES-T2D associations were even stronger among participants with normal WC, indicating an underlying metabolically unhealthy status that was not captured by usual anthropometrical measurements in these participants, which suggests that metabolomic profiling may be particularly useful for identifying high-risk Asian populations who are more likely to have metabolic disorders and elevated T2D risk without defined obesity. While the MESs could explain large portions of the obesity-T2D association, they could also improve T2D risk prediction beyond traditional risk factors plus plasma glucose.
Metabolomic profiling has the potential to uncover a wide spectrum of metabolic alterations in obesity (23), which are otherwise poorly captured by the current definitions based on BMI and WC. To our knowledge, the present study is the largest metabolomic study (n>2,000) that examined both BMI- and WC-related metabolites in an East Asian population. Compared with adults of European descendants, East Asian adults are more likely to have elevated visceral fat and T2D risk at the same BMI level (5, 6), so lower cut-offs of BMI and WC have been used to define general and central obesity for Asian adults. To date, a handful of smaller studies have assessed obesity-related metabolomic profiles among Chinese adults (24, 25, 26, 27). However, most of them treated obesity as a dichotomized outcome based on cut-offs not specific to Chinese populations (24, 25, 27), and none of them examined central obesity or its indicators such as WC. In the present study, we identified 32 plasma metabolites, of which 23 were related to both BMI and WC. Glutamic acid, homogentistic acid, quinolinic acid, valine, CAR 5:0, tyrosine, and uric acid showed the strongest positive associations, while glycine, serine, and asparagine showed the strongest inverse associations. In addition, eight metabolites were only related to WC (see the Results section). The mostly converging but somewhat differential metabolomic profiles of BMI and WC support the use of WC to complement BMI for defining obesity in clinical and research settings (9, 28). The majority of the obesity-related metabolites we identified have been reported previously (e.g., BCAAs and their catabolites) (11, 23, 26), yet some were relatively novel (e.g., ureidopropionic acid, 1-methyl nicotinamide, homogentisic acid, quinolinic acid, uridine diphosphate N-acetylglucosamine, DMGV, and cis-aconitic acid). Of these novel metabolites, ureidopropionic acid was only related to WC but not BMI, and three metabolites, including cis-aconitic acid (a TCA cycle metabolite), homogentisic acid (a tyrosine metabolite), and DMGV, were further associated with increased T2D risk in our prospective analysis. Interestingly, DMGV has recently been reported to be a liver fat biomarker and correlated with consumption of sugar sweetened beverages (29, 30). Liver fat is one of the major forms of ectopic fat, which is difficult to capture with BMI or WC and frequently necessitates imaging to confirm (9). Thus, our findings further support the potential utility of metabolomic profiling as a high-throughput method to identify metabolic aberrations of adiposity, particularly among individuals with normal BMI or WC.
Among the 32 obesity-related metabolites, 17 were significantly associated with incident T2D after controlling for multiple comparisons and a series of T2D risk factors, including glucose level. In particular, glutamic acid, BCAAs (valine, leucine, and isoleucine), and alanine showed the strongest positive associations among all obesity-related metabolites in our work. While the potential role of these amino acids in obesity, insulin resistance, and T2D has long been recognized (10, 31, 32, 33), their associations with incident T2D were only reported among Asian populations in prospective studies in recent years (34, 35, 36, 37, 38). Meanwhile, 1,5-anhydrosorbitol, glycine, and serine showed the strongest associations with reduced risks of T2D in our current study, as consistently reported in our previous analysis in the SWHS/SMHS (35) as well as other populations (10). It is worth noting that 1,5-anhydrosorbitol is a validated biomarker for monitoring glycemic control among patients with diabetes in different populations, including Asians (39, 40). Furthermore, our study demonstrated that TCA cycle-related metabolites (e.g., oxoglutaric acid, cis-aconitic acid, and lactic acid) and lipid-related metabolites (e.g., palmitic acid and 2-hydroxybutyric acid) are involved in the obesity-T2D relationship in Chinese, which is supported by reports from other populations (11, 12). Finally, our study confirmed for the first time in a generally lean Asian population that DMGV could be a marker of obesity and can be used to predict T2D risk, as first indicated in a US population (29).
When the identified BMI- or WC-related metabolites were constructed into two MESs, we found strong and robust associations of these two MESs with incident T2D, even among participants with normal BMI or WC (based on Chinese population-specific criteria), which was similarly observed in European populations (41). While the molecular mechanisms for stronger MES-T2D associations among participants with normal WC cannot be examined in our study, this observation suggests that unhealthy metabolic status reflected by circulating metabolomic may be particularly useful for identifying individuals without central obesity but at high risk for T2D. We also found that the MESs mediated ~46-66% of the BMI/WC-T2D associations and predicted T2D risk with similar discrimination as traditional T2D risk factors (C-statistics=~0.75). Furthermore, they improved risk prediction when added to traditional risk factors and plasma glucose (C-statistics=0.83). These findings provided compelling evidence that obesity MESs may help to differentiate “metabolically unhealthy” from “metabolically healthy” adults, especially among those with normal BMIs or WCs. Accumulating evidence suggests that currently established metabolic biomarkers (e.g., fasting glucose, blood lipids, blood pressure, and C-reactive protein) may lead to considerable misclassifications of these two phenotypes among adults with normal weight, and thus have limited utility to predict future disease risk (8). Whether plasma metabolites could be incorporated to define metabolic health and improve cardiometabolic disease risk prediction has attracted increasing attention. Several prospective studies have shown that circulating metabolites significantly improve T2D prediction beyond traditional clinical risk factors alone, but their predictive utility in addition to glucose is less clear (31, 37, 38, 42, 43). Our findings indicate that obesity-related metabolites improve T2D prediction beyond traditional risk factors and plasma glucose, which will need to be validated in more prospective studies. Additionally, we found that those identified metabolites mediated up to 66% of the obesity-T2D association, nominating potential metabolic pathways for further research to elucidate disease pathophysiology. On the other hand, 35%−55% of this association remains unexplained, and further explorations—potentially with nontargeted LC-MS methods—are warranted. Collectively, our findings indicate that a panel of obesity-related metabolites may complement BMI and WC in identifying individuals at high risk for T2D, particularly among participants with normal WC.
Our study has substantial strengths, including a prospective study design, a large sample size of individually matched cases and controls, standardized in-person assessments of BMI, WC, and T2D, and detailed information for major covariates. However, we acknowledge several limitations. First, as our analyses only assessed certain plasma metabolites of known identity, constrained by the metabolomics platforms, other metabolites with essential physiological functions such as lipids and bile acids were not fully captured in our present analyses. Second, the relations of BMI and WC with metabolites could be bidirectional. The assumption underlying the mediation analysis for BMI/WC-T2D association could thus be potentially undermined. Third, BMI and WC were proxy measures for general and central obesity. Future studies on body fat measured by imaging techniques could improve the precision of our work. Finally, our sample included middle-aged, generally lean, urban Chinese adults, so the generalizability of our findings to other Chinese or Asian populations will need to be confirmed.
Conclusion
We identified 32 plasma metabolites related to BMI or WC and constructed two obesity MESs that showed strong associations with incident T2D, especially among participants with normal WC. The obesity MESs could explain a large portion of the obesity-T2D association and improve the prediction of T2D beyond traditional risk factors and plasma glucose. More large prospective studies are needed to validate our findings and evaluate the clinical utility of metabolomic profiling for cardiometabolic disease prevention.
Supplementary Material
Study importance.
Obesity is an established risk factor for type 2 diabetes (T2D), but the metabolic mechanisms linking obesity with T2D risk are not fully understood.
We identified 32 plasma metabolites related to body mass index or waist circumference in a Chinese population. Two metabolomic signatures were derived from those metabolites and showed strong associations with incident T2D, even among participants with normal weight or waist circumference.
Our work highlights that obesity related circulating metabolites could explain the obesity-T2D link to a large extent and potentially improve T2D risk prediction.
Acknowledgments
We thank the study participants of the SWHS and SMHS for their participation in the original cohort studies. The work was presented as a research poster in the American Diabetes Association’s 81st Scientific Sessions on June 25-29, 2021. The data described in the manuscript will be made available upon reasonable request and approval of the corresponding author of the manuscript and the Data Use Committees of the SWHS and SMHS.
Funding
This study was funded by R01 DK108159 to TJW, XOS, and REG from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH). The Shanghai Women’s Health Study was funded by UM1 CA182910 to WZ; the Shanghai Men’s Health Study was funded by UM1 CA173640 to XOS from the National Cancer Institute at the NIH. DY is supported by R01 HL149779 from the National Heart, Lung, and Blood Institute and R01 DK126721 from the NIDDK at the NIH. Plasma sample preparation was performed by the Survey and Biospecimen Shared Resource which is supported in part by the Vanderbilt-Ingram Cancer Center (P30CA68485). The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 10th Edition 2021. [cited 2022 May 25]. Available from: https://www.diabetesatlas.org/en/.
- 2.Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ 2020;369: m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal AD, Jin F, Shu XO, Yang G, Elasy TA, Chow WH, et al. Body fat distribution and risk of diabetes among Chinese women. Int J Obes Relat Metab Disord 2004;28: 594–599. [DOI] [PubMed] [Google Scholar]
- 4.Bragg F, Tang K, Guo Y, Iona A, Du H, Holmes MV, et al. Associations of general and central adiposity with incident diabetes in Chinese men and women. Diabetes Care 2018;41: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol 2021;9: 373–392. [DOI] [PubMed] [Google Scholar]
- 6.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teufel F, Seiglie JA, Geldsetzer P, Theilmann M, Marcus ME, Ebert C, et al. Body-mass index and diabetes risk in 57 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 685 616 adults. Lancet 2021;398: 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefan N, Schick F, Häring H-U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab 2017;26: 292–300. [DOI] [PubMed] [Google Scholar]
- 9.Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7: 715–725. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZZ, Gerszten RE. Metabolomics and proteomics in type 2 diabetes. Circ Res 2020;126: 1613–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Sadanala KC, Kim EK. A metabolomic approach to understanding the metabolic link between obesity and diabetes. Mol Cells 2015;38: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Q, Li Y, Wang M, Tang Z, Wang T, Liu C, et al. Progress in metabonomics of type 2 diabetes mellitus. Molecules 2018;23: 1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol 2005;162: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 14.Shu XO, Li H, Yang G, Gao J, Cai H, Takata Y, et al. Cohort Profile: The Shanghai Men’s Health Study. Int J Epidemiol 2015;44: 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu D, Zheng W, Cai H, Xiang YB, Li H, Gao YT, et al. Long-term diet quality and risk of type 2 diabetes among urban Chinese adults. Diabetes Care 2018;41: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018;137: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimberly WT, O’Sullivan JF, Nath AK, Keyes M, Shi X, Larson MG, et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2017;2: e92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57: 289–300. [Google Scholar]
- 19.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 2010;172: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44: 837–845. [PubMed] [Google Scholar]
- 22.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019;15: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie G, Ma X, Zhao A, Wang C, Zhang Y, Nieman D, et al. The metabolite profiles of the obese population are gender-dependent. J Proteome Res 2014;13: 4062–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SM, Yang RY, Wang M, Ji FS, Li HX, Tang YM, et al. Identification of serum metabolites associated with obesity and traditional risk factors for metabolic disease in Chinese adults. Nutr Metab Cardiovasc Dis 2018;28: 112–118. [DOI] [PubMed] [Google Scholar]
- 26.Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics 2014;10: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu HT, Fu XY, Xu B, Zuo LL, Ma HB, Wang SR. Untargeted metabolomics approach (UPLC-Q-TOF-MS) explores the biomarkers of serum and urine in overweight/obese young men. Asia Pac J Clin Nutr 2018;27: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 28.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020;16: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest 2017;127: 4394–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottosson F, Ericson U, Almgren P, Smith E, Brunkwall L, Hellstrand S, et al. Dimethylguanidino valerate: a lifestyle-related metabolite associated with future coronary artery disease and cardiovascular mortality. J Am Heart Assoc 2019;8: e012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9: 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125: 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Wang Y, Ong CN, Subramaniam T, Choi HW, Yuan JM, et al. Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016;59: 2349–2359. [DOI] [PubMed] [Google Scholar]
- 35.Yu D, Moore SC, Matthews CE, Xiang YB, Zhang X, Gao YT, et al. Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang SJ, Kwak SY, Jo G, Song TJ, Shin MJ. Serum metabolite profile associated with incident type 2 diabetes in Koreans: findings from the Korean Genome and Epidemiology Study. Sci Rep 2018;8: 8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Akter S, Kuwahara K, Matsushita Y, Nakagawa T, Konishi M, et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci Rep 2019;9: 7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu G, Zheng Y, Wang H, Sun J, Ma H, Xiao Y, et al. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int J Epidemiol 2016;45: 1507–1516. [DOI] [PubMed] [Google Scholar]
- 39.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet 1996;347: 1514–1518. [DOI] [PubMed] [Google Scholar]
- 40.McGill JB, Cole TG, Nowatzke W, Houghton S, Ammirati EB, Gautille T, et al. Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycemia: a U.S. trial of the GlycoMark assay. Diabetes Care 2004;27: 1859–1865. [DOI] [PubMed] [Google Scholar]
- 41.Ottosson F, Smith E, Ericson U, Brunkwall L, Orho-Melander M, Di Somma S, et al. Metabolome-defined obesity and the risk of future type 2 diabetes and mortality. Diabetes Care 2022;45: 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebholz CM, Yu B, Zheng Z, Chang P, Tin A, Kottgen A, et al. Serum metabolomic profile of incident diabetes. Diabetologia 2018;61: 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


