Abstract
Background
Several studies have linked various chronic inflammatory skin diseases (CISDs) with inflammatory bowel disease (IBD) in a range of data sources with mixed conclusions.
Objectives
We compared the incidence of IBD – ulcerative colitis (UC) and Crohn disease (CD) – in patients with a CISD vs. similar persons without a CISD.
Methods
In this cohort study using nationwide, longitudinal, commercial insurance claims data from the USA, we identified adults and children who were seen by a dermatologist between 2004 and 2020, and diagnosed with either psoriasis, atopic dermatitis, alopecia areata, vitiligo or hidradenitis suppurativa. Comparator patients were identified through risk-set sampling; they were eligible if they were seen by a dermatologist at least twice and not diagnosed with a CISD. Patient follow-up lasted until either IBD diagnosis, death, disenrolment or end of data stream, whichever came first. IBD events, UC or CD, were identified via validated algorithms: hospitalization or diagnosis with endoscopic confirmation. Incidence rates were computed before and after adjustment via propensity-score decile stratification to account for IBD risk factors. Hazard ratios (HR) and 95% confidence intervals (CIs) were estimated to compare the incidence of IBD in CISD vs. non-CISD.
Results
We identified patients with atopic dermatitis (n = 123 614), psoriasis (n = 83 049), alopecia areata (n = 18 135), vitiligo (n = 9003) or hidradenitis suppurativa (n = 6806), and comparator patients without a CISD (n = 2 376 120). During a median follow-up time of 718 days, and after applying propensity-score adjustment for IBD risk factors, we observed increased risk of both UC (HRUC 2·30, 95% CI 1·61–3·28) and CD (HRCD 2·70, 1·69–4·32) in patients with hidradenitis suppurativa, an increased risk of CD (HRCD 1·23, 1·03–1·46) but not UC (HRUC 1·01, 0·89–1·14) in psoriasis, and no increased risk of IBD in atopic dermatitis (HRUC 1·02, 0·92–1·12; HRCD 1·08, 0·94–1·23), alopecia areata (HRUC 1·18, 0·89–1·56; HRCD 1·26, 0·86–1·86) or vitiligo (HRUC 1·14, 0·77–1·68; HRCD 1·45, 0·87–2·41).
Conclusions
IBD was increased in patients with hidradenitis suppurativa. CD alone was increased in patients with psoriasis. Neither UC nor CD was increased in patients with atopic dermatitis, alopecia areata or vitiligo.
Hidradenitis suppurativa and psoriasis, two chronic inflammatory skin diseases (CISDs), have been linked with an elevated risk of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn disease (CD).1-3 The risk remains unclear for other CISDs, such as atopic dermatitis, alopecia areata and vitiligo.
As immune-mediated diseases often co-occur, a better characterization of IBD across CISDs could lead to earlier identification and treatment of IBD, which has been shown to modify the long-term course of disease.4-6 In addition to immune-mediated disease clustering, treatments such as interleukin (IL)-17A inhibitors used in patients with psoriasis have been associated with causing or unmasking subclinical IBD, particularly CD.7,8 As novel therapies for CISDs come to market it is important to characterize the background rate of IBD in these patients, which will help infer whether an IBD signal rises beyond the background rate inherent to the underlying skin disease.
Previous studies in patients with CISDs have shown variability in IBD rates due to a mix of CISD severity, risk adjustment methodologies, study design, outcome definitions and attention to temporality, namely, whether the IBD occurred after the CISD was already present.1,2,9-12
Given the variable methodologies, it is difficult to compare incidence rates across multiple skin diseases. Currently there is no single population-representative study with a consistent methodology across multiple CISDs that avoids potential recall issues and that limits differential surveillance for the occurrence of IBD. We compared the risks of UC and CD among patients with psoriasis, atopic dermatitis, alopecia areata, vitiligo and hidradenitis suppurativa against those in similar persons without a CISD in a cohort of patients seen by dermatologists in clinical practice between 2004 and 2019.
Patients and methods
Data source
In this cohort study we used a longitudinal insurance claims database, Optum’s deidentified Clinformatics Data Mart Database, from 2004 through 2019. The database is comprised of commercial and Medicare Advantage health plans and contains dated information on plan enrolment, healthcare utilization, demographics and integrated records for inpatient and outpatient encounters, and pharmacy dispensing. All patient information was deidentified. The Brigham and Women’s Hospital’s institutional review board approved this study (#2021P002323), and a signed data licensing agreement was in place.
Study cohort
This study compared patients with CISD to similar patients without CISD identified in dermatology practices. We identified patients of all ages who were diagnosed with a CISD, including psoriasis (excluding psoriatic arthritis), atopic dermatitis, alopecia areata, vitiligo and hidradenitis suppurativa, according to the following criteria: at least two visits to a dermatologist within 1 year, and a diagnosis of the same CISD at each visit (for specific codes, see Table S1 in the Supporting Information).13
In order to have clear conclusions, we further required that the studied skin conditions were mutually exclusive, meaning that a patient who had two encounters with a dermatologist within a year but received a diagnosis of atopic dermatitis at one visit and psoriasis at the next would not qualify. The cohort entry date for the CISD group was the second recorded CISD diagnosis after at least 365 days of continuous enrolment. For the non-CISD group we risk-set sampled 10 patients without CISD from all plan enrolees who had at least two visits to a dermatologist (cohort entry date was the second dermatologist visit) within a year but were not diagnosed with a CISD at any time, ensuring a highly reliable diagnostic separation of the two groups (for more information on the group without CISD, see Tables S2 and S3 in the Supporting Information). Requiring that individuals with and without CISD were seen by a specialist at least twice before cohort entry reduces differential surveillance bias.14 Patients with psoriatic arthritis, defined as a diagnosis of psoriatic arthritis by a dermatologist and by a rheumatologist, were not analysed as an individual group, as it was too small (< 1500).15
The patients without CISD were matched on the cohort entry date of the patients with CISD, ± 60 days, and were required to have at least 365 days of continuous enrolment before the matched cohort entry date. Patients were allowed to contribute only once to the study. Patients both with and without CISD were excluded if they had any of the following conditions at any time since enrolment before their cohort entry: prior IBD, use of an IBD-specific immunomodulatory agent (aminosalicylates, thiopurines or vedolizumab), any abdominal surgery that might be related to IBD [intestinal resection, colectomy (partial and total) or proctectomy], use of an IL-17A inhibitor (ixekizumab, secukinumab or brodalumab), diagnosis of cancer, organ transplant, HIV or HIV medication use, or diagnosis of rheumatoid arthritis, lupus or ankylosing spondylitis (Figure S1; see Supporting Information).16
Outcomes
We identified incident IBD events during all of the available follow-up time. IBD was defined as either a lower-endoscopic procedure – colonoscopy or flexible sigmoidoscopy – followed by a diagnosis of IBD within 3 months, or hospitalization with a discharge diagnosis of IBD (positive predictive value of 81–88%).17,18 Current guidelines report that IBD diagnosis requires endoscopic confirmation, which further informed our choice of outcome definition.19,20 For each IBD outcome we also evaluated the component conditions – UC and CD – separately, using the same criteria of hospitalization or endoscopic confirmation. The event date was the date on which all criteria were fulfilled (see Table S1 for detailed codes).
Patients were followed starting on the day after cohort entry until either they were diagnosed with IBD, they disenrolled in their health plan, their data stream ended or they died, whichever came first.
Patient characteristics
Patient characteristics were assessed during the 365 days before cohort entry (Figure S1). We considered age at cohort entry, sex, race, year of cohort entry, history of Clostridioides difficile infection, fecal pathogen test ordered, abdominal or pelvic computed tomography scan or magnetic resonance imaging, prior lower- or upper-gastrointestinal endoscopic procedure, hospitalization related to abdominal diagnoses, systemic glucocorticoid use, cumulative systemic prednisone equivalencies over the prior 365 days, systemic immunomodulatory medication use, number of prior systemic immunomodulatory agents used, number of physician visits and unique prescription medications filled, and a range of other comorbidities, including risk factors for IBD (Table 1).21
Table 1.
Selected baseline patient characteristics comparing patients with vs. without a chronic inflammatory skin disease (CISD), unadjusted
| Patient characteristics, within the 365 days before cohort entry |
Non-CISD (referent) |
CISDs |
|||||
|---|---|---|---|---|---|---|---|
| Psoriasis | Atopic dermatitis | Alopecia areata | Vitiligo | Hidradenitis suppurativa |
Composite CISD | ||
| Number of patients | 2 376 120 | 83 049 | 123 614 | 18 135 | 9003 | 6806 | 242 010 |
| Follow-up (days), median (IQR) | 713 (299–1468) | 657 (285–1274) | 869 (351–1891) | 633 (267–1254) | 665 (292–1287) | 523 (221–992) | 736 (312–1533) |
| Demographics | |||||||
| Age (years) | |||||||
| Mean (SD) | 45·4 (21·7) | 49·1 (17·7) | 41·3 (22·4) | 38·5 (17·4) | 39·7 (21·2) | 36·6 (14·5) | 43·6 (20·7) |
| Median (IQR) | 46 (27–63) | 50 (36 −63) | 43 (23–58) | 37 (27–50) | 40 (22–55) | 35 (25–46) | 45·00 (29–59) |
| 0–17 years | 353 210 (14·9) | 3562 (4·3) | 24 783 (20·0) | 2327 (12·8) | 1957 (21·7) | 577 (8·5) | 33 223 (13·7) |
| 18–25 years | 199 065 (8·4) | 4798 (5·8) | 8518 (6·9) | 1758 (9·7) | 526 (5·8) | 1136 (16·7) | 16 766 (6·9) |
| 26–59 years | 1 106 575 (46·6) | 49 445 (59·5) | 61 714 (49·9) | 11 724 (64·6) | 4707 (52·3) | 4565 (67·1) | 133 074 (55·0) |
| ≥ 60 years | 717 270 (30·2) | 25 244 (30·4) | 28 599 (23·1) | 2326 (12·8) | 1813 (20·1) | 528 (7·8) | 58 947 (24·4) |
| sex | |||||||
| Male | 1 004 600 (42·3) | 40 790 (49·1) | 49 545 (40·1) | 7523 (41·5) | 4177 (46·4) | 1547 (22·7) | 104 232 (43·1) |
| Female | 1 371 520 (57·7) | 42 259 (50·9) | 74 069 (59·9) | 10 612 (58·5) | 4826 (53·6) | 5259 (77·3) | 137 778 (56·9) |
| Region | |||||||
| 1 – Northeast | 293 470 (12·4) | 12 992 (15·6) | 20 412 (16·5) | 3364 (18·5) | 1393 (15·5) | 705 (10·4) | 39 010 (16·1) |
| 2 – North Central | 512 420 (21·6) | 21 293 (25·6) | 27 236 (22·0) | 3728 (20·6) | 1788 (19·9) | 1687 (24·8) | 56 086 (23·2) |
| 3 – South | 1 084 186 (45·6) | 36 527 (44·0) | 55 517 (44·9) | 7899 (43·6) | 4249 (47·2) | 3445 (50·6) | 108 276 (44·7) |
| 4 – West | 483 876 (20·4) | 12 148 (14·6) | 20 385 (16·5) | 3124 (17·2) | 1559 (17·3) | 958 (14·1) | 38 440 (15·9) |
| Racea | |||||||
| White | 1 958 321 (82·4) | 64 892 (78·1) | 89 206 (72·2) | 10 714 (59·1) | 5310 (59·0) | 4369 (64·2) | 175 624 (72·6) |
| Asian | 75 284 (3·2) | 4048 (4·9) | 9468 (7·7) | 1815 (10·0) | 1125 (12·5) | 304 (4·5) | 16 824 (7·0) |
| Black | 150 201 (6·3) | 5935 (7·1) | 13 458 (10·9) | 2233 (12·3) | 901 (10·0) | 1274 (18·7) | 23 889 (9·9) |
| Hispanic | 192 314 (8·1) | 8174 (9·8) | 11 482 (9·3) | 3373 (18·6) | 1667 (18·5) | 859 (12·6) | 25 673 (10·6) |
| GI-related conditions | |||||||
| Abdominal/pelvic CT or MRI | 122 650 (5·2) | 4593 (5·5) | 5603 (4·5) | 726 (4·0) | 354 (3·9) | 534 (7·8) | 11 929 (4·9) |
| Fecal pathogen tests (including Clostridioides difficile) ordered | 22 735 (1·0) | 872 (1·0) | 1387 (1·1) | 167 (0·9) | 89 (1·0) | 108 (1 −6) | 2650 (1·1) |
| GI endoscopy (upper or lower) | 182 942 (7·7) | 6646 (8·0) | 8928 (7·2) | 1045 (5·8) | 614 (6·8) | 458 (6·7) | 17 840 (7·4) |
| Hospitalization related to abdominal issues | 83 338 (3·5) | 4092 (4·9) | 1838 (1·5) | 902 (5·0) | 460 (5·1) | 656 (9·6) | 8062 (3·3) |
| Surgery, any abdominal | 14 703 (0·6) | 556 (0·7) | 670 (0·5) | 76 (0·4) | 39 (0·4) | 89 (1·3) | 1438 (0·6) |
| History of C. difficile infection | 2441 (0·1) | 86 (0·1) | 99 (0·1) | 9 (0·0) | 4 (0·0) | 9 (0·1) | 209 (0·1) |
| Gagne combined comorbidity score, mean (SD) b | 0·37 (1·33) | 0·38 (1·34) | 0·29 (1·10) | 0·24 (0·87) | 0·27 (0·99) | 0·53 (1·30) | 0·33 (1·18) |
| Comorbid conditions | |||||||
| Smoking | 94 238 (4·0) | 6072 (7·3) | 4870 (3·9) | 702 (3·9) | 211 (2·3) | 989 (14·5) | 12 995 (5·4) |
| Obesity or weight gain | 199 676 (8·4) | 12 016 (14·5) | 8342 (6·7) | 1746 (9·6) | 825 (9·2) | 2055 (30·2) | 25 362 (10·5) |
| Diabetes, severe or complicated | 96 274 (4·1) | 5364 (6·5) | 3316 (2·7) | 428 (2·4) | 381 (4·2) | 463 (6·8) | 10 080 (4·2) |
| COPD | 221 389 (9·3) | 9172 (11·0) | 14 880 (12·0) | 1508 (8·3) | 782 (8·7) | 911 (13·4) | 27 453 (1 1·3) |
| Alcohol abuse | 19 153 (0·8) | 1130 (1·4) | 893 (0·7) | 166 (0·9) | 38 (0·4) | 101 (1·5) | 2346 (1·0) |
| Anaemia | 148 179 (6·2) | 5416 (6·5) | 8508 (6·9) | 1149 (6·3) | 588 (6·5) | 609 (8·9) | 16 394 (6·8) |
| Weight loss | 17 167 (0·7) | 566 (0·7) | 413 (0·3) | 112 (0·6) | 55 (0·6) | 66 (1·0) | 1226 (0·5) |
| Pregnancy | 28 341 (1·2) | 827 (1·0) | 1432 (1·2) | 160 (0·9) | 76 (0·8) | 152 (2·2) | 2655 (1·1) |
| Acne | 571 358 (24·0) | 5084 (6·1) | 16 900 (13·7) | 2324 (12·8) | 983 (10·9) | 1943 (28·5) | 27 296 (11·3) |
| Uveitis | 1789 (0·1) | 75 (0·1) | 117 (0·1) | 9 (0·0) | 7 (0·1) | 6 (0·1) | 216 (0·1) |
| Connective tissue disease | 15 416 (0·6) | 597 (0·7) | 1081 (0·9) | 255 (1·4) | 108 (1 −2) | 78 (1·1) | 2164 (0·9) |
| Other medications | |||||||
| Contraceptive agents | 311 128 (13·1) | 8158 (9·8) | 16 824 (13·6) | 2505 (13·8) | 950 (10·6) | 1673 (24·6) | 30 243 (12·5) |
| Oral retinoids | 38 424 (1·6) | 1680 (2·0) | 1487 (1·2) | 47 (0·3) | 33 (0·4) | 294 (4·3) | 3558 (1·5) |
| NSAIDs and Coxibs | 291 844 (12·3) | 12 514 (15·1) | 15 855 (12·8) | 2259 (12·5) | 1140 (12·7) | 1418 (20·8) | 33 729 (13·9) |
| Systemic glucocorticoid use | |||||||
| Prior use, past 180 days | 295 760 (12·4) | 14 602 (17·6) | 31 566 (25·5) | 2683 (14·8) | 1161 (12·9) | 1360 (20·0) | 51 821 (21·4) |
| Recent use, past 30 days | 46 146 (1·9) | 2178 (2·6) | 7917 (6·4) | 377 (2·1) | 172 (1 −9) | 170 (2·5) | 10 884 (4·5) |
| Sum of daily dose in prednisone mg eq., mean (SD) | 44·5 (298) | 68·8 (311) | 108 (384) | 59·8 (327) | 44·5 (222) | 78·4 (342) | 88·3 (352) |
| DMARD use | |||||||
| Conventional synthetic | 4960 (0·2) | 5278 (6·4) | 874 (0·7) | 136 (0·7) | 26 (0·3) | 41 (0·6) | 6835 (2·8) |
| DMARDsb | |||||||
| Biologic DMARDsc | |||||||
| TNFid | 231 (0·0) | 8384 (10·1) | 79 (0·1) | 13 (0·1) | 7 (0·1) | 527 (7·7) | 9673 (4·0) |
| Non-TNFie | 669 (0·0) | 5019 (6·0) | 270 (0·2) | 27 (0·1) | 2 (0·0) | 9 (0·1) | 5568 (2·3) |
| Targeted synthetic DMARDsf | 1 (0·0) | 1 (0·0) | 1 (0·0) | 20 (0·1) | 1 (0·0) | 1 (0·0) | 29 (0·0) |
| Number of prior unique DMARDs | |||||||
| 0 | 2 369 422 (99·7) | 64 990 (78·3) | 122 290 (98·9) | 17 926 (98·8) | 8958 (99·5) | 6213 (91·3) | 220 678 (91·2) |
| 1 | 5800 (0·2) | 14 756 (17·8) | 1162 (0·9) | 163 (0·9) | 41 (0·5) | 562 (8·3) | 17 275 (7·1) |
| 2 | 846 (0·0) | 2721 (3·3) | 135 (0·1) | 39 (0·2) | 3 (0·0) | 26 (0·4) | 3247 (1·3) |
| ≥ 3 | 52 (0·0) | 582 (0·7) | 27 (0·0) | 7 (0·0) | 1 (0·0) | 5 (0·1) | 810 (0·3) |
| Healthcare utilization | |||||||
| Number of any outpatient visits | |||||||
| Mean (SD) | 5·83 (4·86) | 7·26 (5·73) | 8·00 (6·16) | 6·18 (4·97) | 6·57 (5·79) | 8·46 (6·57) | 7·38 (5·68) |
| Median (IQR) | 4 (3–7) | 6 (4–9) | 6 (4–10) | 5 (3–8) | 5 (3–8) | 7 (4–H) | 6 (4–9) |
| Number of unique medications | |||||||
| Mean (SD) | 5·42 (5·04) | 7·57 (5·87) | 7·42 (5·68) | 5·00 (4·77) | 5·50 (4·97) | 8·63 (6·39) | 7·27 (5·75) |
| Median (IQR) | 4 (2–8) | 6 (3–10) | 6 (3–10) | 4 (2–7) | 4 (2–8) | 7 (4–11) | 6 (3–10) |
| Number of CRP tests ordered, mean (SD) | 0·06 (0·35) | 0·09 (0·42) | 0·07 (0·39) | 0·08 (0·36) | 0·08 (0·36) | 0·11 (0·57) | 0·09 (0·42) |
| Gastroenterologist visit | 228 018 (9·6) | 8677 (10·4) | 12 115 (9·8) | 1479 (8·2) | 856 (9·5) | 647 (9·5) | 23 987 (9·9) |
| Hospitalization | 144 535 (6·1) | 5001 (6·0) | 7255 (5·9) | 582 (3·2) | 323 (3·6) | 543 (8·0) | 13 811 (5·7) |
The data are presented as n (%) unless stated otherwise. This table shows selected patient characteristics, please see Table S2 in the Supporting Information for a full table. COPD, chronic obstructive pulmonary disease; CT, computed tomography scan; DMARD, disease-modifying antirheumatic drug; GI, gastrointestinal; HS, hidradenitis suppurativa; IQR, interquartile range; MRI, magnetic resonance imaging; TNFi, tumour necrosis factor inhibitor.
Optum uses proprietary algorithms to define race. Race is a derived ethnicity. The member’s ethnicity is derived using the member’s name and geography. Once the ethnicity is determined, the member is mapped to one of four race categories (A, Asian; B, black; H, Hispanic; w, white).
Nonbiologic immunomodulators were defined as methotrexate, ciclosporin, mycophenolate mofetil, azathioprine, sulfasalazine, leflunomide.
Biologic immunomodulators were defined as adalimumab, etanercept, golimumab, certolizumab, infliximab, dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, rituximab, anakinra, tocilizumab.
TNF inhibitor biologic immunomodulators were defined as adalimumab, etanercept, golimumab, certolizumab, infliximab.
Non-TNF inhibitor biologic immunomodulators were defined as dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, rituximab, anakinra, tocilizumab.
Targeted synthetic immunomodulators were defined as tofacitinib or baricitinib.
Statistical analysis
We tabulated baseline patient characteristics for patients with and without CISD and separately for each CISD component condition: psoriasis, atopic dermatitis, alopecia areata, vitiligo and hidradenitis suppurativa. We recorded the duration of follow-up, including reasons for censoring, and computed incidence rates with 95% confidence intervals (CIs).
We used a propensity-score (PS) decile-stratified analysis to account for potential differences in IBD risk factors, as this is recommended for analyses with few events.22,23 We conducted PS decile stratification both with and without symmetrical trimming of the extremes of the PS distribution by 2·5%.24 We included all pre-exposure patient characteristics, without further variable selection, as independent variables for PS decile stratification.
The proportional hazards model included the CISD indicator variable and nine indicators for the PS deciles using the fifth decile as the reference category. The PS, predicting the presence vs. absence of CISD, was estimated with a multivariable logistic regression for each CISD component condition. We evaluated the success of balancing by computing absolute standardized differences (ASDs) for each characteristic, and recognized characteristics as balanced if the ASD was <10%.25 Kaplan–Meier curves were plotted for CISD and each component condition after stratifying by deciles of PS. Hazard ratios (HRs) comparing the incidence of IBD in patients with vs. without CISD were estimated by fitting Cox proportional hazards regression models.26
We analysed three subgroups of patients: (i) aged 2–17 years, (ii) aged ≥18 years, and (iii) excluding those who had used systemic immunomodulatory agents. While acne has been associated with IBD, it may be the antibiotic treatment rather than the acne itself that is associated with IBD.27-30 As such, we conducted a sensitivity analysis wherein we adjusted for antibiotic use in three categories: (i) anti-anaerobic antibiotics, which have been associated with IBD in children;29 (ii) oral tetracyclines, which have been associated with IBD in women with rosacea;27 and (iii) other antibiotics. Lastly, to demonstrate the robustness of our approach, we included a negative tracer outcome: skull or face fracture. All analyses were conducted using the Aetion Evidence Platform v4·34 (Aetion, New York, NY, USA) (including R 3·4·2; R Foundation, Vienna, Austria), which has been validated in multiple studies.30-33
Results
We identified 242 010 qualifying patients with a diagnosis of a CISD, which comprised patients with psoriasis (n = 83 049), atopic dermatitis (n = 123 614), alopecia areata (n = 18 135), vitiligo (n = 9003) and hidradenitis suppurativa (n = 6806). An additional group of 1403 with psoriatic arthritis was too small to analyse separately. We identified 2 376 120 patients without a CISD. The median follow-up time was 2 years [736 days, interquartile range (IQR) 312–1533] in the CISD group and 2 years (713 days, IQR 299–1468) in the non-CISD group, with similar reasons for censoring in both groups (Table S4; see Supporting Information). We observed some heterogeneity in median follow-up by the CISD group: the longest follow-up was for atopic dermatitis (869 days, IQR 351–1891) and the shortest was for hidradenitis suppurativa (523 days, IQR 221–992).
Before PS balancing, the demographics, comorbidity patterns and systemic immunomodulatory treatments were in line with the known epidemiology and treatment guidelines for each CISD.34-43 Patients with a CISD were overall slightly younger than those without a CISD (mean 43·6 vs. 45·4 years, Table 1); the youngest patients were those with hidradenitis suppurativa (36·6 years) or alopecia (38·5 years). There was a female predominance in hidradenitis suppurativa (77·3%). Across all CISDs, use of systemic immunomodulators was most prevalent in patients with psoriasis (6·4% nonbiologic and 16·3% biologic), compared with those without a CISD (1·3% and 0·04%, respectively). Systemic corticosteroid use was most frequent among those with atopic dermatitis (25·5%) or hidradenitis suppurativa (20·0%). After adjustment with PS stratification and trimming, the distributions of all patient characteristics between individuals with vs. without CISD were well-balanced across all five CISDs and the combined cohort (Table S5; see Supporting Information).
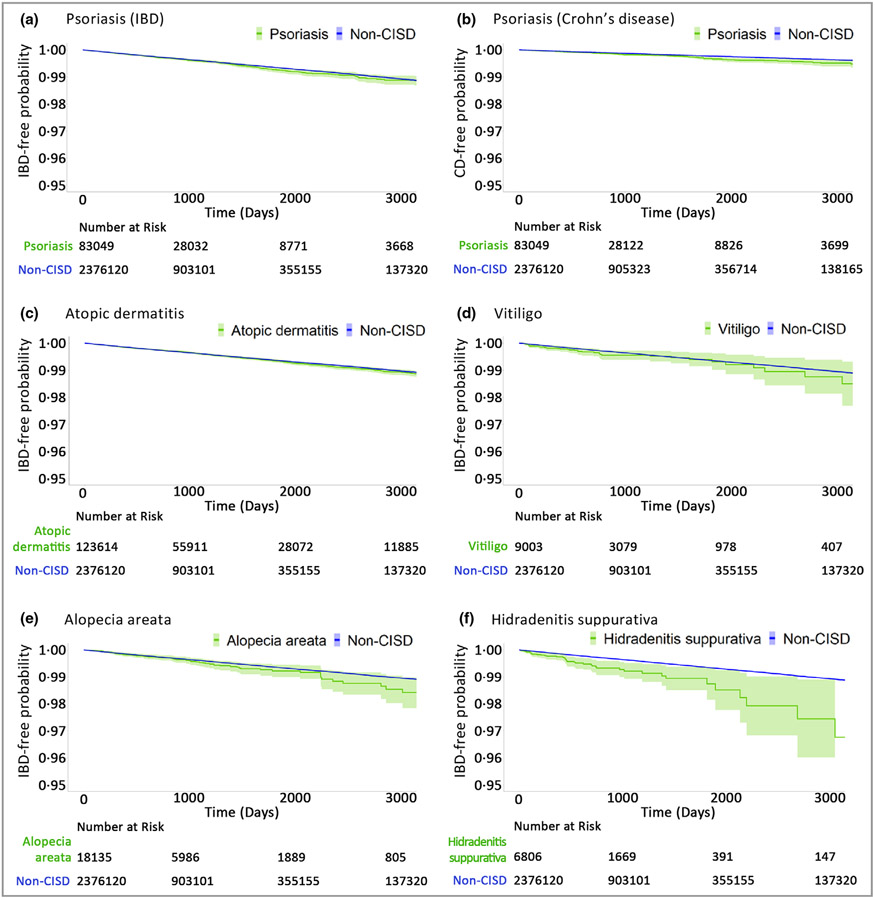
The incidence rate of new IBD was 1·73 per 1000 person-years in patients with CISD. For psoriasis the unadjusted incidence rate (per 1000 person-years) of IBD was 1·91, compared with 1·45 for non-CISD. The PS decile-adjusted HRs (95% CIs) for IBD, UC and CD were 1·06 (0·96–1·18), 1·01 (0·89–1·14) and 1·23 (1·03–1·46), respectively (Table 2). For atopic dermatitis the incidence rate of IBD was 1·62, compared with 1·45 for non-CISD. The PS decile-adjusted HRs for IBD, UC and CD were 1·03 (0·95–1·11), 1·02 (0·92–1·12) and 1·08 (0·94–1·23), respectively. For alopecia areata the incidence rate of IBD was 1·54, vs. 1·45 for non-CISD. The PS decile-adjusted HRs for IBD, UC and CD were 1·18 (0·93–1·50), 1·18 (0·89–1·56) and 1·26 (0·86–1·86), respectively. For vitiligo the incidence rate of IBD was 1·68, vs. 1·45 for non-CISD. The PS decile-adjusted HRs for IBD, UC and CD were 1·25 (0·91–1·71), 1·14 (0·77–1·68) and 1·45 (0·87–2·41), respectively. For hidradenitis suppurativa the incidence rate of IBD was 3·06, vs. 1·45 for non-CISD (Table 2), resulting in an absolute rate difference of 1·62 (95% CI 0·69–2·54) (Table S6; see Supporting Information). The PS decile-adjusted HRs for IBD, UC and CD were 2·21 (1·63–3·00), 2·30 (1·61–28) and 2·70 (1·69–4·32), respectively (Table 2). In patients with hidradenitis suppurativa we observed a numerical reduction in effect size after PS trimming, which removed most patients with prior use of immunomodulating agents from the already small cohort (Table S7; see Supporting Information). Kaplan–Meier plots reflected these results after PS decile adjustment, showing no increase in IBD risk for CISD vs. non-CISD over up to 13 years of follow-up (Figure 1).
Table 2.
Incidence rates and hazard ratios (HRs) of inflammatory bowel disease (IBD), before and after propensity-score decile stratification
| Outcome/CISD | CISD |
Non-CISD |
CISD vs. non-CISD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total patients |
Person-years | Events | Incidence ratea |
Total patients |
Person-years | Events | Incidence ratea |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI)b |
|||
| IBD | ||||||||||||
| Psoriasis | 83 049 | 211 899 | 404 | 1·91 | 2 376 120 | 6 753 361 | 9761 | 1·45 | 1·37 (1·24–1·51) | 1·06 (0·96–1·18) | ||
| Atopic dermatitis | 123 614 | 425 768 | 691 | 1·62 | 2 376 120 | 6 753 361 | 9761 | 1·45 | 1·13 (1·05–1·22) | 1·03 (0·95–1·11) | ||
| Alopecia areata | 18 135 | 45 426 | 70 | 1·54 | 2 376 120 | 6 753 361 | 9761 | 1·45 | 1·06 (0·84–1·35) | 1·18 (0·93–1·50) | ||
| Vitiligo | 9003 | 23 204 | 39 | 1·68 | 2 376 120 | 6 753 361 | 9761 | 1·45 | 1·17 (0·86–1·60) | 1·25 (0·91–1·71) | ||
| HS | 6806 | 13 711 | 42 | 3·06 | 2 376 120 | 6 753 361 | 9761 | 1·45 | 3·00 (2·33–3·87) | 2·21 (1·63–3·00) | ||
| Composite CISD | 242 010c | 723 174 | 1 251 | 1·73 | 2 376 120 | 6 753 361 | 9761 | 1·45 | 1·20 (1·13–1·27) | 1·05 (0·98–1·12) | ||
| Ulcerative colitis | ||||||||||||
| Psoriasis | 83 049 | 212 221 | 287 | 1·35 | 2 376 120 | 6 760 059 | 7178 | 1·06 | 1·31 (1·17–1·47) | 1·01 (0·89–1·14) | ||
| Atopic dermatitis | 123 614 | 426 456 | 479 | 1·12 | 2 376 120 | 6 760 059 | 7178 | 1·06 | 1·06 (0·96–1·16) | 1·02 (0·92–1·12) | ||
| Alopecia areata | 18 135 | 45 483 | 50 | 1·1 | 2 376 120 | 6 760 059 | 7178 | 1·06 | 1·03 (0·78–1·37) | 1·18 (0·89–1·56) | ||
| Vitiligo | 9003 | 23 224 | 26 | 1·12 | 2 376 120 | 6 760 059 | 7178 | 1·06 | 1·05 (0·72–1·55) | 1·14 (0·77–1·68) | ||
| HS | 6806 | 13 735 | 31 | 2·26 | 2 376 120 | 6 760 059 | 7178 | 1·06 | 3·07 (2·29–4·12) | 2·30 (1·61–3·28) | ||
| Composite CISD | 242 010c | 724 286 | 877 | 1·21 | 2 376 120 | 6 760 059 | 7178 | 1·06 | 1·15 (1·07–1·23) | 1·03 (0·95–1·11) | ||
| Crohn disease | ||||||||||||
| Psoriasis | 83 049 | 212 497 | 143 | 0·67 | 2 376 120 | 6 769 184 | 3185 | 0·47 | 1·42 (1·20–1·68) | 1·23 (1·03–1·46) | ||
| Atopic dermatitis | 123 614 | 426 966 | 266 | 0·62 | 2 376 120 | 6 769 184 | 3185 | 0·47 | 1·34 (1·18–1·52) | 1·08 (0·94–1·23) | ||
| Alopecia areata | 18 135 | 45 543 | 26 | 0·57 | 2 376 120 | 6 769 184 | 3185 | 0·47 | 1·23 (0·84–1·80) | 1·26 (0·86–1·86) | ||
| Vitiligo | 9003 | 23 253 | 15 | 0·65 | 2 376 120 | 6 769 184 | 3185 | 0·47 | 1·44 (0·88–2·35) | 1·45 (0·87–2·41) | ||
| HS | 6806 | 13 757 | 16 | 1·16 | 2 376 120 | 6 769 184 | 3185 | 0·47 | 4·13 (2·83–6·03) | 2·70 (1·69–4·32) | ||
| Composite CISD | 242 010c | 725 188 | 468 | 0·65 | 2 376 120 | 6 769 184 | 3185 | 0·47 | 1·38 (1·25–1·52) | 1·12 (1·01–1·24) | ||
CI, confidence interval; CISD, chronic inflammatory skin disease; HS, hidradenitis suppurativa.
Per 1000 person-years.
Adjusted by deciles of propensity score. All patient characteristics were included in the propensity-score model as independent variables.
Patients with psoriatic arthritis, defined as a diagnosis of psoriatic arthritis by a dermatologist and by a rheumatologist, were included in this composite but not analysed as an individual group as it was too small (< 1500).
Figure 1.
The time to inflammatory bowel disease (IBD) comparing patients with vs. without a chronic inflammatory skin disease (CISD), after propensity-score decile stratification. Green represents the exposure group and blue represents the referent (non-CISD) group, with the green and blue shaded regions indicating the corresponding 95% confidence intervals. All Kaplan–Meier curves were not extended beyond the time when <10% of patients remain in the risk set, because they become unreliable. The IBD event is defined as inpatient IBD, or outpatient IBD with endoscopic confirmation.
Among children, aged 2–17 years, we observed an increased incidence of CD in those with atopic dermatitis (IRCD 0·54 per 1000 person-years; 43 events among 22 671 children), compared with those without CISD (IRCD 0·30; 315 events among 349 899 children), resulting in an adjusted HRCD of 1·75 (95% CI 1·22–2·51). We also observed increases in those with psoriasis (IRCD 0·75; seven events among 3538 children) and hidradenitis suppurativa (IR 1·6; two events among 577 children), based on very few events (Figure 2).
Figure 2.
Hazard ratio estimates of developing ulcerative colitis or Crohn disease in patients with vs. without a chronic inflammatory skin disease (CISD), after propensity-score decile stratification. CI, confidence interval; py, person-years. Overall, CISD was defined as a composite of the individual conditions: psoriasis, atopic dermatitis, alopecia areata, vitiligo and hidradenitis suppurativa. Users of immunomodulators patients receiving treatment with nonbiologic immunomodulatory agents (methotrexate, ciclosporin, mycophenolate mofetil, azathioprine, sulfasalazine, leflunomide), biologic immunomodulatory agents (dupilumab, risankizumab-rzaa, tildrakizumab, ixekizumab, secukinumab, guselkumab, ustekinumab, abatacept, adalimumab, etanercept, golimumab, certolizumab, infliximab, rituximab, anakinra, tocilizumab) or targeted synthetic immunomodulatory agents (tofacitinib, baricitinib).
To test the robustness of our study approach we estimated the incidence of face or skull fractures as a negative tracer outcome that is not possibly associated with CISD. We found no association with our negative tracer for CISD (HR 0·95, 95% CI 0·83–1·10) or across all component conditions (Table S7). Additionally, in a sensitivity analysis adjusting for different classes of antibiotic use, we did not see any change in the HRs (Table S8).
Discussion
In this cohort study of a large commercially insured US population, patients with a chronic inflammatory skin disease, diagnosed by a dermatologist and without recorded risk factors for IBD, had an incidence rate of new IBD of 1·7 per 1000 person-years. Of the five CISDs studied here only psoriasis and hidradenitis suppurativa were associated with an increased risk of CD, and hidradenitis suppurativa was additionally associated with UC. Neither atopic dermatitis, alopecia areata nor vitiligo was associated with IBD. These findings indicate continued awareness of co-occurring IBD in patients with hidradenitis suppurativa and CD in those with psoriasis, and support that IBD is not more common among other CISDs than in the general public.
In children aged 2–17 years, IBD was less frequent, with an incidence rate of 0·34 per 1000 for UC and 0·55 per 1000 for CD. In children with atopic dermatitis there was a signal of an increased risk for newly occurring CD compared with children without a CISD. Further investigation will be needed to reliably assess the risk of IBD in children with psoriasis and hidradenitis suppurativa, where fewer than 10 events were observed, resulting in wide CIs.
Shared immune pathways play an important role in driving concurrent immune-mediated diseases.44 Inflammatory mediators and cytokines associated with IBD, including tumour necrosis factor-α, IL-17 and IL-23, are also thought to be key drivers in chronic inflammatory diseases such as psoriasis and hidradenitis suppurativa.45-47 Moreover, some of the systemic immunomodulatory agents that treat IBD are also effective treatments for hidradenitis suppurativa and psoriasis, while they are not recommended for treating atopic dermatitis, vitiligo and alopecia areata.48-50 This pathophysiological link may explain our study findings of an increased background risk of IBD for hidradenitis suppurativa and CD for psoriasis but no increased risk for other CISDs.51
Our results help complete and sort the literature and are in line with existing high-validity studies. A meta-analysis of case–control and cross-sectional studies found increased odds of co-occurring IBD in patients with hidradenitis suppurativa, specifically CD (odds ratio 2·12, 95% CI 1·46–3·08) and UC (odds ratio 1~51, 95% CI 1·25–1·82), but there was high statistical heterogeneity in the analysis of CD and inclusion of patients with IBD preceding hidradenitis suppurativa.1 There were mixed results for IBD in patients with atopic dermatitis. A cohort study from Germany found a 20% increased risk of CD (risk ratio 1·20, 95% CI 1·00–1·44) in patients with atopic dermatitis compared with the general population; however, with increasing adjustment for healthcare utilization, the point estimate moved towards the null, indicating presence of surveillance bias.9 In an analysis of the Nurses’ Health Study, there was a threefold increased risk of CD (HR 3·39, 95% CI 1·73–6·65) in patients with self-reported AD; however, this was based on only 10 events of CD in 90 patients with atopic dermatitis, resulting in wide CIs. Our findings are similar to those of other studies that also control for surveillance bias, namely a population-based cohort study from Taiwan, which also required at least two dermatologist-recorded diagnoses of AD and matched on healthcare utilization. In that study the investigators observed no increase of CD (0·05% vs. 0·05%) in patients with AD vs. the general population.11 For psoriasis, we had directionally similar findings, in that we observed an increase in CD, no increase in UC, and no overall increase in IBD, but we did not see the same magnitude of these associations. Existing studies may have had difficulties with differential surveillance for IBD,3,52 incomplete adjustment for medications that treat immune-mediated diseases, few events,2 or less strict outcome definitions for IBD that did not require endoscopic evaluation or hospitalization.3,52
The methodology of our study was applied consistently across all CISDs of interest. For analyses comparing population-based incidence rates across multiple CISDs, several limitations may arise, which we addressed with various approaches. Firstly, to establish a background rate, it is important to make sure that patients in the CISD group did in fact have one of the CISDs and that the comparator patients did not. To address this, we required that patients in both groups were seen by a dermatologist. Assuring that both populations had equal access to specialty care limits differential surveillance, wherein the population with the chronic disease is monitored more closely than the other, raising concern for bias, as closer surveillance increases the chances of capturing an adverse event or comorbidity. The study also separated the five CISDs from each other to avoid contamination by complex patients with multiple conditions and to allow a crisp interpretation of the background rates.
Secondly, we chose a validated outcome that is captured with high accuracy – inpatient IBD diagnosis or outpatient diagnosis with endoscopic confirmation – to avoid biased HR estimates, as is recommended for relative comparisons of incidence rates.53 Thirdly, we used PS methods considering a range of IBD risk factors, comorbidities and treatments to make the groups comparable. We used PS decile stratification as recommended for studies with few events.54 Fourthly, to test that our analytical approach avoided false-positive findings, we used a negative tracer outcome, facial or skull fractures, which are unrelated to skin diseases, and, as expected, found no association.
Some limitations remain. Firstly, claims data can capture strong risk factors for IBD, such as IBD-specific medication dispensing, surgeries, prior IBD diagnoses and healthcare utilization; however, claims data are known to under-record other risk factors, such as smoking and obesity. Risk factors, such as nutritional and environmental, are unavailable in claims. Additionally, the severity and inflammatory burden of the underlying CISD are difficult to assess in claims data. We limited the analysis to patients who sought specialty care by a dermatologist for more than one visit as a proxy for potentially more significant disease burden; however, severity is difficult to capture fully in claims. An analysis excluding patients using systemic treatments focuses on those with less severe CISD. Secondly, statements of no association need to be seen in light of not only the point estimate but also the width of the CI reflecting the actual power in this empirical study. Some of the wide CIs around null findings in our study indicate that there is no major discrepancy, but minor differences cannot be ruled out with this study and require even larger datasets.55 Finally, our study is limited to those patients seen and diagnosed by a dermatologist to optimize the diagnostic accuracy of the dermatological conditions and provide similar surveillance between patients with and without CISD.
This large-scale study expands current knowledge by providing background rates for IBD across multiple CISDs using consistent methods and within a large, single, nationally representative patient population. The methodology allowed us to compare patients with CISDs against those who are similar in all characteristics except they do not have a CISD.
In conclusion, this large-scale analysis of claims data from a representative dermatology patient population found no indication of an increased rate of newly occurring IBD in patients with CISDs, beyond the known increase of IBD in patients with hidradenitis suppurativa and of CD in those with psoriasis, where continued vigilance for IBD is warranted.
Supplementary Material
Figure S1 Cohort study design diagram.
Figure S2 The time to inflammatory bowel disease comparing patients with vs. without a chronic condition.
Table S1 International Classification of Diseases codes used to identify chronic inflammatory skin disease and inflammatory bowel disease.
Table S2 All baseline patient characteristics comparing patients with vs. without a chronic inflammatory skin disease, unadjusted.
Table S3 Exploratory analysis of the top 50 most common dermatological patient characteristics recorded in the group without chronic inflammatory skin disease, on the visit day that defined cohort entry.
Table S4 Follow-up time and reasons for censoring.
Table S5 All baseline characteristics and corresponding absolute standardized differences between patients with vs. without a chronic inflammatory skin disease, before and after propensity-score decile stratification.
Table S6 Incidence rate differences of inflammatory bowel disease, unadjusted.
Table S7 Hazard ratios (95% confidence intervals) of primary outcomes and the negative tracer outcome, with different confounder adjustment models.
Table S8 Hazard ratios for risk of irritable bowel disease in chronic inflammatory skin disease vs. non-chronic inflammatory skin disease, after additional adjustment for antibiotic use.
What is already known about this topic?
Several studies have linked various chronic inflammatory skin diseases (CISDs) with inflammatory bowel disease (IBD) utilizing a range of data sources, with mixed conclusions.
What does this study add?
This large-scale, claims-based cohort study expands current knowledge by providing background rates for IBD across multiple CISDs using consistent methods and within a single, nationally representative patient population.
We observed a relative increased risk of IBD in patients with hidradenitis suppurativa, but the overall incidence rate difference of IBD was generally low.
Crohn disease alone was significantly increased in patients with psoriasis, and neither ulcerative colitis nor Crohn disease was increased in patients with atopic dermatitis, vitiligo or alopecia areata.
Funding sources
This study was funded by a grant (R01 AR080194) from the National Institutes of Health.
Footnotes
Conflicts of interest
Y.J. and C.Y. declare they have no conflicts of interest. M.C.S. has received research grants to the Brigham and Women’s Hospital from UCB Pharma and AbbVie for work unrelated to the topic of this study. J.K. is a consultant for Roche and Pfizer. R.W. has received a research grant to the Brigham and Women’s Hospital from UCB Pharma for work unrelated to the topic of this study and has worked as a consultant to Aetion. J.F.M. is a consultant and/or investigator for Merck, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Celgene, Sanofi, Regeneron, Arena, Sun Pharma, Biogen, Pfizer, EMD Sorono, Avotres and LEO Pharma. A.M. reports personal fees from Pfizer, Digital Diagnostics, 3Derm, AbbVie, Bioniz, Concert, Lilly and Hims; and participation in clinical trials with Incyte, Aclaris, Concert and Lilly outside the submitted work. J.I.S. is an advisor, speaker and/or consultant for AbbVie, Afyx, Arena, Asana, BioMX, Bluefin, Bodewell, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO, Luna, Menlo, Novartis, Pfizer, RAPT, Regeneron and Sanofi; and a speaker for Eli Lilly, Incyte, LEO, Pfizer, Regeneron and Sanofi; and his institution has received grants from Galderma. S.S. is the principal investigator of multiple investigator-initiated grants to the Brigham and Women’s Hospital from the FDA, NIH, PCORI and Boehringer Ingelheim unrelated to the topic of this study. He is a consultant to Aetion, a software manufacturer of which he owns equity. His interests were declared, reviewed and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies. R.J.G. has received grants to the Brigham and Women’s Hospital from Amgen, AstraZeneca, Kowa, Novartis and Pfizer for research unrelated to the current investigation.
Ethics statement
The Brigham and Women’s Hospital’s institutional review board approved this study (IRB# 2021P002323), and a signed data licensing agreement was in place.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Chen WT, Chi CC. Association of hidradenitis suppurativa with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol 2019; 155:1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo CH, Khalili H, Lochhead P et al. Immune-mediated diseases and risk of Crohn’s disease or ulcerative colitis: a prospective cohort study. Aliment Pharmacol Ther 2021; 53:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeberg A, Mallbris L, Warren RB et al. Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol 2016; 175:487–92. [DOI] [PubMed] [Google Scholar]
- 4.Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr Drug Targets 2014; 15:1056–63. [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Fiorino G, Peyrin-Biroulet L. Early intervention in Crohn’s disease: towards disease modification trials. Gut 2017; 66:2179–87. [DOI] [PubMed] [Google Scholar]
- 6.Solitano V, D’Amico F, Zacharopoulou E et al. Early intervention in ulcerative colitis: ready for prime time? J Clin Med 2020; 9:2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hueber W, Sands BE, Lewitzky S et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emond B, Ellis LA, Chakravarty SD et al. Real-world incidence of inflammatory bowel disease among patients with other chronic inflammatory diseases treated with interleukin-17a or phosphodiesterase 4 inhibitors. Curr Med Res Opin 2019; 35:1751–9. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt J, Schwarz K, Baurecht H et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol 2016; 137:130–6. [DOI] [PubMed] [Google Scholar]
- 10.Wu LC, Hwang CY, Chung PI et al. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case–control study in Taiwan. Pediatr Allergy Immunol 2014; 25:586–92. [DOI] [PubMed] [Google Scholar]
- 11.Weng YC, Juan CK, Ho HJ et al. Atopic dermatitis does not increase the risk of inflammatory bowel disease: a nationwide cohort study. J Dermatol 2021; 48:168–74. [DOI] [PubMed] [Google Scholar]
- 12.Charlton R, Green A, Shaddick G et al. Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis:a population-based cohort study. Ann Rheum Dis 2018; 77:277–80. [DOI] [PubMed] [Google Scholar]
- 13.Schneeweiss MC, Kim SC, Wyss R et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol 2021; 157:805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneeweiss S, Seeger JD, Maclure M et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001; 154:854–64. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Ford JA, Jin Y et al. Validation of claims-based algorithms for psoriatic arthritis. Pharmacoepidemiol Drug Saf 2020; 29:404–8. [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss S, Rassen JA, Brown JS et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med 2019; 170:398–406. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Allison JE, Herrinton LJ. Validity of computerized diagnoses, procedures, and drugs for inflammatory bowel disease in a northern California managed care organization. Pharmacoepidemiol Drug Saf 2009; 18:1086–93. [DOI] [PubMed] [Google Scholar]
- 18.Singla M, Hutfless S, Al Kazzi E et al. Clinical codes combined with procedure codes increase diagnostic accuracy of Crohn’s disease in a US Military health record. BMJ Open Gastroenterol 2020; 7:e000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lichtenstein GR, Loftus EV, Isaacs KL et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113:481–517. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DT, Ananthakrishnan AN, Siegel CA et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114:384–413. [DOI] [PubMed] [Google Scholar]
- 21.Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (23 Suppl. 1):I9–16. [DOI] [PubMed] [Google Scholar]
- 22.Brookhart MA, Schneeweiss S, Rothman KJ et al. Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 24.Yoshida K, Solomon DH, Haneuse S et al. Multinomial extension of propensity score trimming methods: a simulation study. Am J Epidemiol 2019; 188:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin JM, Rassen JA, Ackermann D et al. Metrics for covariate balance in cohort studies of causal effects. Stat Med 2014; 33:1685–99. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life tables. J R Stat Soc B 1972; 20:187–220. [Google Scholar]
- 27.Li WQ, Cho E, Khalili H et al. Rosacea, use of tetracycline, and risk of incident inflammatory bowel disease in women. Clin Gastroenterol Hepatol 2016; 14:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ungaro R, Bernstein CN, Gearry R et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol 2014; 109:1728–38. [DOI] [PubMed] [Google Scholar]
- 29.Kronman MP, Zaoutis TE, Haynes K et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012; 130:e794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolis DJ, Fanelli M, Hoffstad O, Lewis JD. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol 2010; 105:2610–16. [DOI] [PubMed] [Google Scholar]
- 31.Wang SV, Verpillat P, Rassen JA et al. Transparency and reproducibility of observational cohort studies using large healthcare databases. Clin Pharmacol Ther 2016; 99:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SC, Solomon DH, Rogers JR et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol 2017; 69:1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patorno E, Schneeweiss S, Gopalakrishnan C et al. Using real-world data to predict findings of an ongoing phase IV cardiovascular outcome trial: cardiovascular safety of linagliptin versus glimepiride. Diabetes Care 2019; 42:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidbury R, Davis DM, Cohen DE et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71:327–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menter A, Strober BE, Kaplan DH et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019; 80:1029–72. [DOI] [PubMed] [Google Scholar]
- 36.Alikhan A, Sayed C, Alavi A et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. Part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol 2019; 81:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messenger AG, McKillop J, Farrant P et al. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol 2012; 166:916–26. [DOI] [PubMed] [Google Scholar]
- 38.Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol 2020; 183:990–8. [DOI] [PubMed] [Google Scholar]
- 39.Lee HH, Gwillim E, Patel KR et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol 2020; 82:675–82. [DOI] [PubMed] [Google Scholar]
- 40.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396:345–60. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020; 323:1945–60. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues M, Ezzedine K, Hamzavi I et al. Current and emerging treatments for vitiligo. J Am Acad Dermatol 2017; 77:17–29. [DOI] [PubMed] [Google Scholar]
- 43.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet 2015; 386:74–84. [DOI] [PubMed] [Google Scholar]
- 44.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost 2005; 94:362–5. [DOI] [PubMed] [Google Scholar]
- 45.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009; 361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duerr RH, Taylor KD, Brant SR et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006; 314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol 2011; 65:790–8. [DOI] [PubMed] [Google Scholar]
- 48.Meah N, Wall D, York K et al. The Alopecia Areata Consensus of Experts (ACE) study: results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol 2020; 83:123–30. [DOI] [PubMed] [Google Scholar]
- 49.Taieb A, Alomar A, Bohm M et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013; 168:5–19. [DOI] [PubMed] [Google Scholar]
- 50.Wollenberg A, Christen-Zach S, Taieb A et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereal 2020; 34:2717–44. [DOI] [PubMed] [Google Scholar]
- 51.Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr 2018; 6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aletaha D, Epstein AJ, Skup M et al. Risk of developing additional immune-mediated manifestations: a retrospective matched cohort study. Adv Ther 2019; 36:1672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneeweiss S, Glynn RJ, Tsai EH et al. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology 2005; 16:17–24. [DOI] [PubMed] [Google Scholar]
- 54.Desai RJ, Rothman KJ, Bateman BT et al. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 2017; 28:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 1994; 121:200–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cohort study design diagram.
Figure S2 The time to inflammatory bowel disease comparing patients with vs. without a chronic condition.
Table S1 International Classification of Diseases codes used to identify chronic inflammatory skin disease and inflammatory bowel disease.
Table S2 All baseline patient characteristics comparing patients with vs. without a chronic inflammatory skin disease, unadjusted.
Table S3 Exploratory analysis of the top 50 most common dermatological patient characteristics recorded in the group without chronic inflammatory skin disease, on the visit day that defined cohort entry.
Table S4 Follow-up time and reasons for censoring.
Table S5 All baseline characteristics and corresponding absolute standardized differences between patients with vs. without a chronic inflammatory skin disease, before and after propensity-score decile stratification.
Table S6 Incidence rate differences of inflammatory bowel disease, unadjusted.
Table S7 Hazard ratios (95% confidence intervals) of primary outcomes and the negative tracer outcome, with different confounder adjustment models.
Table S8 Hazard ratios for risk of irritable bowel disease in chronic inflammatory skin disease vs. non-chronic inflammatory skin disease, after additional adjustment for antibiotic use.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.