SUMMARY
Background
Soft tissue sarcoma (STS) is a rare but serious side-effect of breast cancer radiotherapy, and rates are increasing in the US. We evaluated potential co-factors in two complimentary cohorts of US breast cancer survivors.
Methods
Eligibility criteria in both cohorts were one-year female breast cancer survivors (stage I-III) aged 20–84 at diagnosis. The Kaiser Permanente (KP) cohort included 16,004 women diagnosed 1990–2016 in Colorado, Northwest, or Washington, with detailed treatment data, and co-morbidities (including hypertension and diabetes at or before breast cancer diagnosis) from electronic medical records. The SEER 13 registries cohort included 457,300 women diagnosed 1992–2016 with initial treatment data (yes/no or unknown). The outcome of interest was any second thoracic STS (angiosarcomas and other subtypes) developed after breast cancer diagnosis. Risk factors for thoracic STS were assessed using multivariable Poisson regression models.
Findings
In the KP cohort, median follow-up was 6.2 years (IQR:3–10 years) and 19 women developed a thoracic STS (11 angiosarcomas, 8 other sub-types). Most (95%) thoracic STS occurred in women treated with radiotherapy (RR=8.1; 95%CI:1.1–60.4), but there was no relationship with prescribed dose, fractionation or boost. Anthracyclines were associated with an increased risk of angiosarcoma (RR=3.6, 95%CI:1.0–13.3) and alkylating agents with other sarcomas (RR=7.6, 95%CI:1.2–154.2). History of hypertension and diabetes were associated with approximately 5-times increased risk of angiosarcoma (RR=4.8, 95%CI:1.3–17.6 and 5.3, 95%CI:1.4–20.8, respectively). In the SEER cohort there were 430 subsequent thoracic STS (268 angiosarcomas) after a median follow-up of 8.3 years (IQR:4.3–13.9 years). Most (78%) cases occurred after radiotherapy (RR=3.0; 95%CI: 2.4–3.8) and; for angiosarcomas the RR for breast-conserving surgery+radiotherapy vs mastectomy+radiotherapy was 1.9 (95%CI:1.1–3.3). By 10 years after radiotherapy, cumulative incidence of thoracic STS was 0.20% in the KP cohort and 0.15% in SEER.
Interpretation
Radiotherapy was the strongest risk factor for thoracic STS in both cohorts. For angiosarcomas, there is growing evidence for a role of anthracyclines. This along with the novel findings for diabetes/hypertension warrant further investigation as potential targets for prevention strategies and increased surveillance.
Introduction
Soft tissue sarcoma (STS) is a rare but serious side-effect of breast cancer radiotherapy with poor prognosis and high mortality(1). There is some evidence that STS incidence rates are increasing in the US, possibly due to the increase in radiotherapy to treat breast cancer(2–4). Other treatments could also be involved; for example, a recent case series found that time to diagnosis of radiation-induced bone and STS was shorter in patients who also received chemotherapy(5). In childhood cancer survivors, anthracyclines have also been associated with an increased risk of STS and alkylating agents with bone sarcomas(6).
Despite these increasing rates, there are few recent studies of STS in breast cancer patients. Compared to other radiation-related subsequent malignancies these cancers occur at an older age and with a shorter latency period, which raises interesting questions about potential co-factors(2–4). Several case-series have described clinical characteristics and outcomes, but lack of an internal control population limits the ability to formally evaluate co-factors(5, 7, 8). Cancer registry studies enable large-scale population-level assessments; however, they lack treatment details such as specific chemotherapy agents, radiotherapy fields or information on other risk factors such as obesity(9,10).
The Kaiser Permanente (KP) Breast Cancer Survivors Cohort provides a distinctive opportunity to evaluate the risk of subsequent thoracic STS in relation to treatment and other medical conditions in a general community healthcare setting(11). This retrospective, electronic medical record linkage study of invasive, stage I-III breast cancer survivors diagnosed from 1990–2016 includes systematic follow-up for subsequent malignancies, rich treatment data including radiotherapy fields, prescribed dose and fractions, initial and subsequent chemotherapy including specific agents (anthracycline, alkylating agents and taxanes), and history of co-morbidities such as obesity and hypertension before or at breast cancer diagnosis. Due to the rarity of STS, we supplemented our study by evaluating the risk of subsequent thoracic STS in breast cancer survivors treated during the same time period in the US Surveillance, Epidemiology and End Results (SEER) 13 cancer registries. These two approaches provide both an in-depth evaluation of the role of treatment and co-morbidities and a large-scale population assessment of thoracic STS risk after breast cancer treatment.
Methods
Study Design and Participants
The KP Breast Cancer Survivors Cohort is a retrospective cohort of 16,004 women diagnosed with unilateral first primary invasive breast cancer (stages I-III) at age 20–84 years in three KP health systems: KP Colorado (01/01/1994–31/12/2014), KP Northwest (01/01/1990–31/12/2008) (which serves Oregon and Southwest Washington state) and KP Washington (01/01/1990–31/12/2016). Eligible subjects were women treated with surgery for their breast cancer, and alive and cancer free one year after diagnosis. For the current analysis, we excluded 64 patients with unknown radiation treatment status, leaving 15,940 eligible breast cancer survivors (Figure 1). Follow-up started one year after breast cancer diagnosis and ended at the date of the first of the following events: death, second cancer diagnosis (invasive or second in situ breast cancer), KP disenrollment, or end of study (31/12/2010 for KP Northwest; 31/12/2015 for KP Colorado; 31/12/2017 for KP Washington).
Figure 1 –
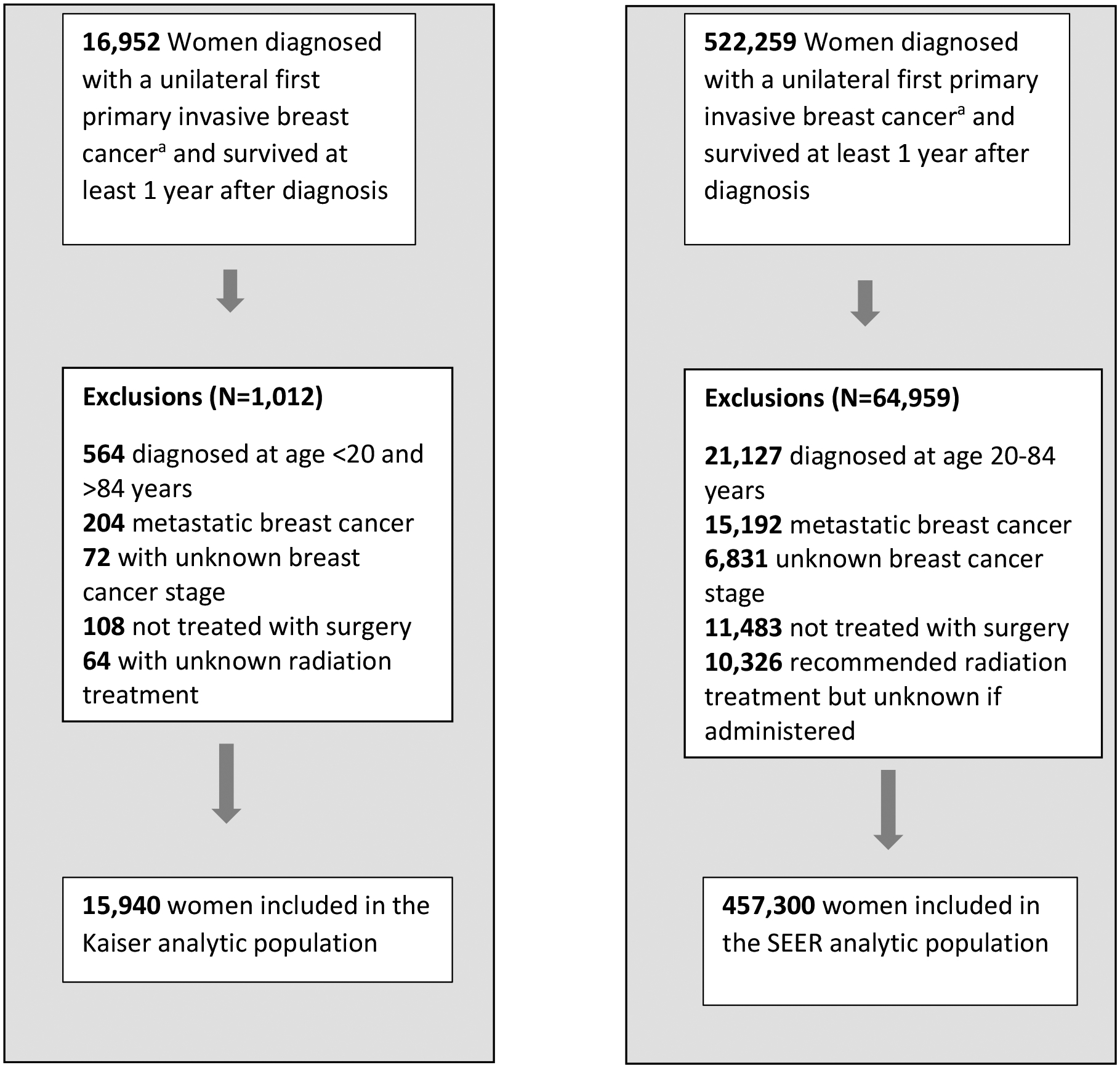
Diagram of selection of Kaiser and SEER analytic population of breast cancer survivors.
a Invasive Breast cancer includes ICD-O-3 site C500-C509 and ICD-O-3 histologies (8000–8001,8004–8005,8010–8013,8015,8020–8022,8030–8033,8035,8041, 8045–8046, 8050–8052, 8070–8072, 8074, 8082, 8123, 8130, 8140–8141, 8143, 8147, 8154, 8200–8201, 8211, 8230–8231, 8240, 8246, 8249, 8251, 8255, 8260, 8310, 8314–8315, 8320, 8323, 8343–8344, 8382, 8401, 8403, 8407, 8410, 8430, 8450, 8452–8453, 8470, 8480–8481, 8490, 8500–8501, 8502–8504, 8507, 8510, 8512–8514, 8520–8523, 8524–8525, 8530, 8540–8543, 8550, 8560, 8562, 8570–8575, 8743, 8800–8803, 8805, 8810–8811, 8830, 8832, 8836, 8840, 8850–8852, 8854, 8858, 8890–8891, 8895, 8900,8920, 8933, 8935, 8940, 8980, 8982, 8983, 8990, 9020, 9040–9041, 9044, 9120, 9180, 9183, 9220, 9231, 9540 and 9580).
This study was approved by the Institutional Review Boards of each participating institution. A waiver of written informed consent was granted based on the minimal risk of this electronic linkage-based research.
We identified a population-based cohort of women diagnosed with a first primary invasive breast cancer using the SEER-13 cancer registries from 01/01/1992 to 31/12/2016(12). To mirror the KP cohort, we restricted the SEER population-based cohort to women diagnosed at ages 20–84 years, breast cancer stage I-III, who had breast cancer surgery and had survived at least one year. We additionally excluded 10,326 patients with recommended radiotherapy but unknown if administered, leaving 457,300 eligible patients (Figure 1).
Follow-up started 1 year after breast cancer diagnosis until the earliest date of second primary cancer diagnosis, death, last follow-up, or end of study (31/12/2017).
Procedures
For the Kaiser cohort, electronic medical records (EMR) were used to ascertain patient characteristics, treatment for the breast cancer including type of surgery, radiotherapy, initial and subsequent chemotherapy and endocrine therapy. Detailed information on prescribed chemotherapy drugs was abstracted and grouped into three major classes: alkylating agents, anthracyclines and taxanes (details in footnotes of Table 1). Body mass index (BMI) was calculated using height and weight measurements between 2 years prior and 1 year after breast cancer diagnosis. Prevalence of selected comorbidities (hypertension, diabetes, and dyslipidemia) at breast cancer diagnosis were collected from the EMR based on ICD-9 and ICD-10 disease codes for KP Washington and Colorado as part of a sub-study on cardiovascular disease (Appendix p2).
Table 1 -.
Selected patient and clinical characteristics among women at the Kaiser Breast Cancer Survivors Cohort and among patients who developed subsequent thoracic soft tissue sarcomas
| Patient and Clinical characteristics | Overall cohort (N=15,940) | Thoracic soft tissue sarcomas | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N=19) | Angiosarcomas (N=11) | Other subtypes (N=8) | ||||||
| N | % | N | % | N | % | N | % | |
| Age at breast cancer diagnosis, years | ||||||||
| 20–54 | 5,354 | 33.5 | 8 | 42.1 | 2 | 18.2 | 6 | 75.0 |
| 55–64 | 4,474 | 28.0 | 5 | 26.3 | 4 | 36.4 | 1 | 12.5 |
| 65–84 | 6,112 | 38.2 | 6 | 31.6 | 5 | 45.5 | 1 | 12.5 |
| Median age at breast cancer diagnosis, years (IQR) | 61 (52–70) | 59 (44–74) | 62 (56–78) | 45 (40–55) | ||||
| Attained age, years | ||||||||
| 21–59 | 4,319 | 27.1 | 8 | 42.1 | 2 | 18.2 | 6 | 75.0 |
| 60–69 | 4,296 | 27.0 | 5 | 26.3 | 4 | 36.4 | 1 | 12.5 |
| 70–103 | 7,325 | 46.0 | 6 | 31.6 | 5 | 45.5 | 1 | 12.5 |
| Median attained age, years (IQR) | 68 (59–78) | 64 (54–83) | 69 (63–83) | 54 (46–61) | ||||
| Calendar year of breast cancer diagnosis | ||||||||
| 1990–1999 | 5,459 | 34.2 | 5 | 26.3 | 2 | 18.2 | 3 | 37.5 |
| 2000–2004 | 3,661 | 23.0 | 8 | 42.1 | 6 | 54.5 | 2 | 25.0 |
| 2005–2016 | 6,820 | 42.8 | 6 | 31.6 | 3 | 27.3 | 3 | 37.5 |
| Calendar year of follow-up | ||||||||
| 1991–2004 | 2,960 | 18.6 | 3 | 15.8 | 0 | 0.0 | 3 | 37.5 |
| 2005–2009 | 2,493 | 15.6 | 9 | 47.4 | 6 | 54.5 | 3 | 37.5 |
| 2010–2017 | 10,468 | 65.7 | 7 | 36.8 | 5 | 45.5 | 2 | 25.0 |
| Time since diagnosis to end of follow-up, years | ||||||||
| 1–4 | 6,480 | 40.7 | 8 | 42.1 | 4 | 36.4 | 4 | 50.0 |
| 5–9 | 4,908 | 30.8 | 8 | 42.1 | 6 | 54.5 | 2 | 25.0 |
| 10–27 | 4,552 | 28.6 | 3 | 15.8 | 1 | 9.1 | 2 | 25.0 |
| Median time since diagnosis, years (IQR) | 6.2 (3.3–10.8) | 5.5 (3.8–7.5) | 6.4 (4.8–7.5) | 5.0 (2.9–9.7) | ||||
| Breast cancer stage | ||||||||
| I | 9,317 | 58.5 | 7 | 36.8 | 6 | 54.5 | 1 | 12.5 |
| II | 5,326 | 33.4 | 10 | 52.6 | 4 | 36.4 | 6 | 75.0 |
| III | 1,297 | 8.1 | 2 | 10.5 | 1 | 9.1 | 1 | 12.5 |
| Race | 0.0 | 0.0 | ||||||
| White | 14,635 | 91 .4 | 17 | 89 .5 | 11 | 100.0 | 6 | 75.0 |
| Black | 453 | 2.8 | 1 | 5.3 | 0 | 0.0 | 1 | 12.5 |
| Othersa | 852 | 5.3 | 1 | 5.3 | 0 | 0.0 | 1 | 12.5 |
| Vital Status at the end of follow-up | ||||||||
| Alive | 11,627 | 72.9 | 9 | 47.4 | 4 | 36.4 | 5 | 62.5 |
| Deceased | 4,313 | 27.1 | 10 | 52.6 | 7 | 63.6 | 3 | 37.5 |
| Estrogen Receptor Status | ||||||||
| ER− | 2,659 | 16.7 | 5 | 26.3 | 3 | 27.3 | 2 | 25.0 |
| ER+ | 12,699 | 79.7 | 13 | 68.4 | 7 | 63.6 | 6 | 75.0 |
| Unknown | 582 | 3.7 | 1 | 5.3 | 1 | 9.1 | 0 | 0.0 |
| Initial radiotherapy | ||||||||
| No | 5,302 | 33.3 | 1 | 5.3 | 0 | 0.0 | 1 | 12.5 |
| Yes | 10,638 | 66.7 | 18 | 94.7 | 11 | 100.0 | 7 | 87.5 |
| Breast surgery type | ||||||||
| Breast conserving surgery | 9,744 | 61.1 | 15 | 79.0 | 10 | 90.9 | 5 | 62.5 |
| Mastectomy | 6,196 | 28.9 | 4 | 21.0 | 1 | 9.1 | 3 | 37.5 |
| Breast surgery type (Restricted to RT) | ||||||||
| RT, Breast conserving surgery | 8,959 | 84.2 | 15 | 83.3 | 10 | 90.9 | 5 | 71.4 |
| RT, Mastectomy | 1,679 | 15.8 | 3 | 16.7 | 1 | 9.1 | 2 | 28.6 |
| Any chemotherapy | ||||||||
| No | 9,171 | 57.5 | 7 | 36.8 | 6 | 54.5 | 1 | 12.5 |
| Yes | 6,769 | 42.5 | 12 | 63.2 | 5 | 45.5 | 7 | 87.5 |
| Any anthracyclines b | ||||||||
| No | 11,535 | 72.4 | 10 | 52.6 | 6 | 54.5 | 4 | 50.0 |
| Yes | 3,853 | 24.2 | 9 | 47.4 | 5 | 45.5 | 4 | 50.0 |
| Unknown | 552 | 3.5 | 0 | - | 0 | 0 | 0 | 0.0 |
| Any alkylating agents c | ||||||||
| No | 9,637 | 60.5 | 8 | 42.1 | 7 | 63.6 | 1 | 12.5 |
| Yes | 5,751 | 36.1 | 11 | 57.9 | 4 | 36.4 | 7 | 87.5 |
| Unknown | 552 | 3.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Any taxanes Regimen d | ||||||||
| No | 11,793 | 74.0 | 14 | 73.7 | 9 | 81.8 | 5 | 62.5 |
| Yes | 3,595 | 22.6 | 5 | 26.3 | 2 | 18.2 | 3 | 37.5 |
| Unknown | 552 | 3.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Endocrine therapy (ER+ only) e | ||||||||
| No | 2,206 | 17.4 | 3 | 23.1 | 1 | 14.3 | 2 | 33.3 |
| Yes | 10,493 | 82.6 | 10 | 76.9 | 6 | 85.7 | 4 | 66.7 |
| Hypertension history f | ||||||||
| No | 8,541 | 73.9 | 10 | 62.5 | 4 | 40.0 | 6 | 100.0 |
| Yes | 3,015 | 26.1 | 6 | 37.5 | 6 | 60.0 | 0 | 0.0 |
| Diabetes history f | ||||||||
| No | 10,535 | 91.2 | 13 | 81.3 | 7 | 70.0 | 6 | 100.0 |
| Yes | 1,021 | 8.8 | 3 | 18.8 | 3 | 30.0 | 0 | 0.0 |
| Dyslipidemia history f | ||||||||
| No | 9,652 | 83.5 | 14 | 87.5 | 8 | 80.0 | 6 | 100.0 |
| Yes | 1,904 | 16.5 | 2 | 12.5 | 2 | 20.0 | 0 | 0.0 |
| Body mass index (kg/m2) g | ||||||||
| ≤18 | 169 | 1.1 | 0 | - | 0 | - | 0 | - |
| 18–<25 | 4,060 | 25.5 | 4 | 21.1 | 1 | 9.1 | 3 | 37.5 |
| 25–<30 | 4,074 | 25.6 | 5 | 26.3 | 4 | 36.4 | 1 | 12.5 |
| 30–71 | 4,538 | 28.5 | 9 | 47.4 | 6 | 54.5 | 3 | 37.5 |
| Unknown | 3,099 | 19.4 | 1 | 5.3 | 0 | 0.0 | 1 | 12.5 |
Abbreviations: RT, Radiotherapy; BMI, body mass index; ER, estrogen receptor; IQR, interquartile range
Includes American Indian/Alaskan Native, Asian/Pacific Islanders and others
Anthracyclines includes doxorubicin and epirubicin.
Alkylating agents includes chlorambucil, cyclophosphamide, melphalan, mitomycin, and thiotepa.
Taxanes included doxetaxel and placlitaxel
Among 12,746 ER+ breast cancer patients
Restricted to 11,556 patients from Kaiser Colorado and Washington centers (16 thoracic sarcomas, 10 angiosarcomas and 6 other sarcomas). Comorbidities were ascertained using diagnosis codes before or at breast cancer diagnosis (See Supplementary Table 2 for details).
BMI calculated within 2 years prior through 1 year after the date of the initial breast cancer diagnosis
Patients were linked to tumor registries or SEER Puget-Sound (for KP Washington) to obtain tumor characteristics for the first primary breast cancer and all subsequent cancers. Vital status, date of death, and cause of death were determined from the registries and EMR with additional linkage to the National Death Index for patients with unknown cause of death for KP Colorado. We collected scanned copies of physical radiotherapy summaries for each patient treated with radiotherapy and developed a standardized protocol to manually abstract treatment details including the prescribed dose, fractionation, radiation type and energy. Radiotherapy fields included breast or chest wall (CW), supraclavicular lymph nodes (SCV), internal mammary lymph nodes (IMN), and posterior axillary boost (PAB). Treatments were categorized as: Breast/CW only, Breast/CW+SCV, Breast/CW+SCV/PAB, Breast/CW+SCV+IMN, Breast/CW+PAB+IMN, Breast/CW+IMN and other. We also reviewed available pathology reports to assess sarcoma location in relation to breast cancer (ipsilateral or contralateral).
Outcomes
For both cohorts, the outcome of interest was any second STS in the thoracic region (angiosarcoma and other STS subtypes) developed at least one year after breast cancer diagnosis (see Appendix p2 for subtype classification according to site/histology codes).
Statistical analysis
For the KP cohort, standardized incidence ratios (SIRs) and 95% Confidence Interval (CI) were calculated as the ratio between the observed and expected number of thoracic STS in the general population. The expected number of cases was calculated by multiplying the SEER-13 incidence rates of first thoracic STS in the female population by the corresponding age, race, and period-specific person-years. We reported results for all thoracic STS, angiosarcomas, and other STS sub-types (including unspecified). We also estimated survival after development of thoracic STS using the Kaplan-Meier method.
For both KP and SEER cohorts, we used multivariable Poisson regression analysis to evaluate the risk of subsequent thoracic STS in relation to patient characteristics and breast cancer treatment (surgery type, radiotherapy and chemotherapy). For the KP cohort, we also evaluated the risk in relation to chemotherapy classes of drugs and comorbidities (obesity, diabetes, hypertension and dyslipidemia). In the KP cohort, models were adjusted for radiotherapy (yes/no), attained age (<60, 60–69, 70+ years), study site, chemotherapy (yes/no) (all sarcomas), anthracyclines (yes/no) (angiosarcomas) and alkylating agents (yes/no) (other sarcomas).
For the SEER cohort, models were adjusted for attained age (<60, 60–69, 70+ years), radiotherapy (yes vs no/unknown) and chemotherapy (yes vs no/unknown). In both cohorts, adjustment factors were selected based on association with thoracic STS risk in the multivariable analysis (p<0.05).
For both cohorts, we estimated the cumulative incidence in the presence of competing risks of death and other second cancers(13). Additionally, we calculated age-adjusted incidence rates (2000 US Standard Population) for all angiosarcomas and thoracic angiosarcomas in the general US female population aged 20–84 years using SEER 13 and estimated the annual percent change (APC) from 1992 to 2017 and the trends in proportion that were subsequent malignancies.
All tests were 2-sided with statistical significance set at p<0.05. Data were analyzed using SEER*Stat 8.3.9 and Stata 17 (College Station, TX).
Role of the funding source
The funding organization had no role in the study design; the collection analysis or interpretation of the data; the writing of the report; or the decision to submit the manuscript for publication.
Results
In the KP cohort, there were 19 thoracic STS identified among the 15,940 breast cancer survivors, of which 11 were angiosarcomas and 8 had other subtypes. The median age at breast cancer diagnosis was 61 years (IQR:52–70 years) with 9,317 patients (58%) diagnosed at an early stage (stage 1) and 12,699 (80%) ER+ disease (Table 1). Initial treatment was most commonly breast conserving surgery (9,744 [61%] of 15,940), radiotherapy (10,638 [67%] of 15,940), chemotherapy (6,769 [42%] of 15,940) and 10,493 (80%) of 12,699 of ER+ cases received hormonal therapy. The median latent period for developing a second thoracic STS was 5.5 years (IQR: 3.8–7.5 years) and the median age at breast cancer diagnosis was 62 years (IQR: 56–78 years) for angiosarcomas compared to 45 years (IQR: 40–55 years) for the other STS subtypes (Table 1). Most (15 [79%] of 19) of the thoracic STS occurred in women treated with breast-conserving surgery and radiotherapy. Only 5% (1 of 19) of the thoracic STS cases had not received radiotherapy compared to 33% (5,302 of 15,940) of the cohort overall, and a higher proportion of STS cases had received chemotherapy compared to the overall cohort (12 [63%] of 19 vs 6,769 [42%] of 15,940). Angiosarcoma patients were more likely to have a history of hypertension (6 [60%] of 11 vs 3,015 [26%] of 11,556), diabetes (3 [30%] of 11 vs 1,021 [9%] of 11,556) and be obese (BMI:30-kg/m2) (6 [54%] of 11 vs 4,538 [28%] of 15,940) at breast cancer diagnosis than the cohort overall.
By the end of follow-up, 10 (53%) of 19 patients who developed a thoracic STS after breast cancer diagnosis had died and the 5-year overall survival for the angiosarcoma patients was only 42% (95%CI:14%−69%; 4 of 11 patients (Appendix p10). About half (6 of 11) of the patients who developed angiosarcoma had sarcoma-related deaths, whereas none of the patients who developed other thoracic STS subtypes had deaths attributed to the sarcoma diagnosis. Most of the STS were localized stage (12 [63%] of 19) and/or grade 2 or higher (11 [58%] of 19) (Appendix p3). After review of available pathology reports for 16 of the thoracic STS, we confirmed that 15 (94%) had the same laterality as the primary breast cancer.
Breast cancer patients in the KP cohort had a 12-times increased risk of developing a thoracic STS compared to the general female population (SIR=12.06, 95%CI:7.3–18.8), and for angiosarcomas the risk was increased 96-times (SIR=96.1, 95%CI:48.0–171.9). After radiotherapy, the cumulative incidence of developing a thoracic STS by 10 years was 0.21% (95%CI:0.12–0.34); 0.14% (95%CI:0.07–0.30) for angiosarcomas, and 0.06% (95%CI:0.02–0.14) for other sarcomas (Appendix p11).
Radiotherapy was associated with a significantly increased risk of developing a thoracic STS (RR=8.1, 95%CI:1.1–60.4). Anthracyclines were associated with a 3.6-times increased risk of angiosarcoma (RR=3.6, 95%CI:1.0–13.3) and alkylating agents with a 7.7-times increased risk of other STS subtypes (RR=7.7, 95%CI:1.2–150.8) (Table 2). Taxanes were not significantly associated with either sub-group of sarcomas. Patients who underwent breast-conserving surgery had a significantly higher risk of angiosarcoma (RR=7.1, 95%CI:1.3–131.6) than patients treated with mastectomy. Compared with standard fractionation, hypofractionated radiotherapy was not associated with the risk of angiosarcoma (RR=0.9, 95%CI:0.1–7.3). Due to small numbers the confidence intervals were very wide for several of these treatments.
Table 2 -.
Relative Risk (95%CI) for thoracic soft tissue sarcomas according to initial breast cancer treatment and selected patient and clinical characteristics among KP cohort of 15,904 women diagnosed with a first primary unilateral invasive breast cancer.
| Thoracic soft tissue sarcomas | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Overall (N=19) | Angiosarcomas (N=11) | Other sarcomas (N=8) | ||||
| N | PYR | RRa (95%CI) | N | RRb (95%CI) | N | RRc (95%CI) | |
| Age at breast cancer diagnosis, years | |||||||
| 20–54 | 8 | 35871.8 | 1.0 | 2 | 1.0 | 6 | 1.0 |
| 55–64 | 5 | 29799.5 | 1.4 (0.3–7.0) | 4 | 2.7 (0.2–29.2) | 2d | 0.7 (0.05–9.5) |
| 65–84 | 6 | 39632.1 | 2.3 (0.3–21.3) | 5 | 4.0 (0.2–74.4) | ||
| P | 0.74 | 0.63 | 0.78 | ||||
| Year of breast cancer diagnosis | |||||||
| 1990–1999 | 5 | 51806.9 | 1.0 | 2 | 1.0 | 3 | 1.0 |
| 2000–2004 | 8 | 27007.9 | 2.5 (0.8–7.6) | 6 | 4.0 (0.8–20.4) | 2 | 1.1 (0.2–6.9) |
| 2005–2016 | 6 | 26488.5 | 1.8 (0.5–6.0) | 3 | 1.9 (0.3–11.6) | 3 | 1.5 (0.3–7.9) |
| P | 0.27 | 0.18 | 0.91 | ||||
| Calendar year of follow-up | |||||||
| 1991–2004 | 3 | 37977.5 | 1.0 | ||||
| 2005–2009 | 9 | 30560.6 | 3.4 (0.9–12.7) | 6e | 1.0 | 6e | 1.0 |
| 2010–2017 | 7 | 36765.2 | 1.9 (0.5–7.8) | 5 | 1.0 (0.3–3.3) | 2 | 0.6 (0.1–3.1) |
| P | 0.14 | 0.96 | 0.52 | ||||
| Years since breast cancer diagnosis | |||||||
| 1–4 | 8 | 50062 | 1.0 | 4 | 1.0 | 4 | 1.0 |
| 5–27 | 11 | 55241.3 | 1.4 (0.5–3.5) | 7 | 1.5 (0.4–5.2) | 4 | 1.3 (0.3–5.3) |
| P | 0.51 | 0.55 | 0.74 | ||||
| Attained age, years | |||||||
| 21–59 | 8 | 31862.1 | 1.0 | 2 | 1.0 | 6 | 1.0 |
| 60–69 | 5 | 29213.5 | 0.8 (0.3–2.5) | 4 | 2.6 (0.5–14.2) | 2d | 0.3 (0.04–1.3) |
| 70–103 | 6 | 44227.7 | 0.9 (0.3–2.7) | 5 | 2.4 (0.4–13.4) | ||
| P | 0.93 | 0.47 | 0.11 | ||||
| Breast cancer stage | |||||||
| I | 7 | 65851.9 | 1.0 | 6 | 1.0 | 1 | 1.0 |
| II & III | 12 | 39451.4 | 2.3 (0.7–7.3) | 5 | 1.1 (0.3–4.2) | 7 | 5.6 (0.8–119.6) |
| P | 0.14 | 0.75 | 0.09 | ||||
| ER subtype | |||||||
| ER− | 5 | 16253.5 | 1.0 | 3 | 1.0 | 2 | 1.0 |
| ER+ | 13 | 83933.3 | 0.7 (0.2–2.0) | 7 | 0.5 (0.1–1.9) | 6 | 0.9 (0.2–4.7) |
| Unknown | 1 | 5116.5 | 1.1 (0.1–10.1) | 1 | 1.2 (0.1–11.8) | 0 | - |
| P | 0.71 | 0.5 | 0.11 | ||||
| Initial radiotherapy | |||||||
| No | 1 | 33596.5 | 1.0 | 0 | ne | 1 | 1.0 |
| Yes | 18 | 71706.9 | 8.1 (1.1–60.4) | 11 | 7 | 3.0 (0.4–24.3) | |
| P | 0.005 | 0.11 | |||||
| Any chemotherapy | |||||||
| No | 7 | 62046.7 | 1.0 | 6 | 1.0 | 1 | 1.0 |
| Yes | 12 | 43256.6 | 2.3 (0.8–6.3) | 5 | 1.5 (0.4–5.5) | 7 | 6.0 (0.7–53.0) |
| P | 0.1 | 0.52 | 0.06 | ||||
| Endocrine therapy (ER+ only) | |||||||
| No | 3 | 17230.8 | 1.0 | 1 | 1.0 | 2 | 1.0 |
| Yes | 10 | 66702.5 | 0.5 (0.1–1.8) | 6 | 1.0 (0.1–8.5) | 4 | 0.2 (0.03–1.0) |
| P | 0.31 | 0.97 | 0.09 | ||||
| Any Anthracyclines f | |||||||
| No | 10 | 78148.7 | 1.0 | 6 | 1.0 | 4 | 1.0 |
| Yes | 9 | 23170.9 | 2.5 (0.9–6.8) | 5 | 3.6 (1.0–13.3) | 4 | 1.7 (0.4–7.5)g |
| P | 0.07 | 0.06 | 0.46 | ||||
| Alkylating agents f | |||||||
| No | 8 | 65605.7 | 1.0 | 7 | 1.0 | 1 | 1.0 |
| Yes | 11 | 35714 | 2.2 (0.8–6.2) | 4 | 1.2 (0.3–4.7)h | 7 | 7.7 (1.2 – 150.8) |
| P | 0.11 | 0.74 | 0.03 | ||||
| Any taxanes f | |||||||
| No | 14 | 84236.7 | 1.0 | 9 | 1.0 | 5 | 1.0 |
| Yes | 5 | 17082.9 | 1.3 (0.4–3.8) | 2 | 1.1 (0.2–5.5) | 3 | 1.6 (0.4–7.4) |
| P | 0.64 | 0.91 | 0.52 | ||||
| Surgery type | |||||||
| Mastectomy | 4 | 38475.1 | 1.0 | 1 | 1.0 | 3 | 1.0 |
| Breast conserving surgery | 15 | 66828.2 | 2.6 (0.9–9.2) | 10 | 7.1 (1.3–131.6) | 5 | 1.3 (0.3–6.6) |
| P | 0.07 | 0.02 | 0.69 | ||||
| Surgery type (restricted to RT) | |||||||
| RT, Mastectomy | 3 | 8837 | 1.0 | 1 | 1.0 | 2 | 1 |
| RT, Breast conserving surgery | 15 | 62869.8 | 1.1 (0.3–5.1) | 10 | 2.7 (0.4–51.6) | 5 | 0.8 (0.2–5.7) |
| 0.86 | 0.32 | 0.79 | |||||
| Hypertension I,j | |||||||
| No | 10 | 60308.1 | 1.0 | 4 | 1.0 | 6 | ne |
| Yes | 6 | 16538.1 | 2.4 (0.8–6.8) | 6 | 4.8 (1.3–17.6) | 0 | |
| P | 0.12 | 0.02 | |||||
| Diabetes I,l | |||||||
| No | 13 | 71453.5 | 1.0 | 7 | 1.0 | 6 | ne |
| Yes | 3 | 5392.9 | 3.2 (0.9–11.5) | 3 | 5.3 (1.4–20.8) | 0 | |
| P | 0.11 | 0.04 | |||||
| Hypertension and Diabetesi | |||||||
| No Hypertension | 10 | 60308.3 | 1.0 | 4 | 1.0 | 6 | ne |
| Hypertension, No diabetes | 4 | 13370.1 | 2.0 (0.6–6.4) | 4 | 4.0 (1.0–16.3) | 0 | |
| Hypertension, diabetes | 2 | 3168 | 4.2 (0.9–19.8) | 2 | 8.4 (1.5–47.3) | 0 | |
| P | 0.21 | 0.04 | |||||
| Dyslipidemia i | |||||||
| No | 14 | 68073.7 | 1.0 | 8 | 1.0 | 6 | ne |
| Yes | 2 | 8772.7 | 1.1 (0.2–4.7) | 2 | 1.6 (0.3–7.6) | 0 | |
| P | 0.94 | 0.57 | |||||
| Body mass index (kg/m2) m | |||||||
| 18–<25 | 4 | 27227.3 | 1.0 | 1 | 1.0 | 3 | 1.0 |
| 25–<30 | 5 | 26318.2 | 1.3 (0.3–4.8) | 4 | 3.8 (0.4–33.8) | 1 | 0.4 (0.04–3.7) |
| 30–71 | 9 | 26905 | 2.2 (0.7–7.1) | 6 | 5.6 (0.7–46.8) | 3 | 1.1 (0.2–5.5) |
| P | 0.11 | 0.16 | 0.59 | ||||
| BMI continuous (per 5 kg/m 2 ) | 1.2 (0.9–1.7) | 1.3 (0.9–2.0) | 1.1 (0.6–1.8) | ||||
| P-trend | 0.22 | 0.16 | 0.79 | ||||
Abbreviations: PYR, Person-Year; RR; Relative Risk, CI; Confidence Interval, ER, Estrogen Receptor; BMI, body mass index; ne, Not estimated.
Model adjusted for attained age (<60/60–69/70–103), study center, radiotherapy(y/n) and any chemotherapy (y/n) as appropriate.
Model adjusted for attained age (<60/60–69/70–103), study center, radiotherapy(y/n) and any anthracyclines (y/n) as appropriate.
Model adjusted for attained age (<60/60–69/70–103), study center, radiotherapy(y/n) and any alkylating agents (y/n) as appropriate.
Category merges all higher categories
Category merges all lower categories
Excluded patients with missing information on the respective class of drugs.
Risk associated with anthracyclines did not include adjustment for alkylating agents due to the high correlation between these drugs.
Risk associated with alkylating agents did not include adjustment for anthracyclines due to the high correlation between these drugs.
Before or at time of breast cancer diagnosis (restricted to 11,556 patients from Kaiser Colorado and Washington centers only)
Excluded patients with BMI <18 (N=168) and unknown BMI (N=3099).
There was no difference in prescribed doses or radiotherapy treatment fields between the patients who developed thoracic STS and those who did not (Table 3). Most patients were treated with 45–50Gy (15 [88%] of 17 and 7,002 [82%] of 8,632, respectively) in 1.8–2Gy fractions (15 [88%] of 17 and 6719 [86%] of 8,632, respectively), breast/CW RT only (14 [82.4%] of 17 and 6746 [78.2%] of 8,632, respectively) and most received a boost of electron fields (12 [70.6%] of 17 and 7261 [84.1%] of 8,632, respectively).
Table 3 -.
Details of radiotherapya received among breast cancer patients who developed thoracic sarcomas
| Thoracic sarcomas cases (N=17) | Angiosarcomas (N=10) | Non-cases (N=8632) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Number of Patients | 17 | 10 | 8632 | |||
| Radiotherapy fields | ||||||
| Breast/CW only | 14 | 82.4 | 8 | 80.0 | 6746 | 78.2 |
| Breast/CW + SCV | 2 | 11.8 | 1 | 10.0 | 1315 | 15.2 |
| Breast/CW + SCV/PAB | 1 | 5.9 | 1 | 10.0 | 426 | 4.9 |
| Breast/CW + SCV + IMN | 0 | - | 0 | - | 37 | 0.4 |
| Breast/CW + SCV/PAB + IMN | 0 | - | 0 | - | 19 | 0.2 |
| Breast/CW + region lymph nodes unknown | 0 | - | 0 | - | 46 | 0.5 |
| Breast/CW + IMN | 0 | - | 0 | - | 18 | 0.2 |
| Otherb | 0 | - | 0 | 25 | 0.3 | |
| Number of radiotherapy fields (any radiation type) | ||||||
| 1 | 3 | 17.6 | 1 | 10.0 | 901 | 10.4 |
| 2 | 12 | 70.6 | 8 | 80.0 | 5954 | 69.0 |
| 3+ | 2 | 11.8 | 1 | 10.0 | 1777 | 20.6 |
| Partial breast irradiation | ||||||
| No | 17 | 100.0 | 10 | 100.0 | 8602 | 99.7 |
| Yes | 0 | - | 0 | - | 30 | 0.3 |
| Received any boost to tumor bed | ||||||
| No | 5 | 29.4 | 2 | 20.0 | 1371 | 15.9 |
| Yes | 12 | 70.6 | 8 | 80.0 | 7261 | 84.1 |
| Hypofractionation c | ||||||
| No | 15 | 88.2 | 9 | 90.0 | 6787 | 86.4 |
| Yes | 2 | 11.8 | 1 | 10.0 | 1065 | 13.6 |
| Fractions | ||||||
| ≤16 | 2 | 11.8 | 1 | 10.0 | 1070 | 13.6 |
| 23 | 0 | - | 0 | - | 410 | 5.2 |
| 25 | 5 | 29.4 | 2 | 20.0 | 2035 | 25.9 |
| 28 | 10 | 58.8 | 7 | 70.0 | 4007 | 51.0 |
| Other (>=17 and <=33, excluding above) | 0 | - | 0 | - | 328 | 4.2 |
| Photon energy, MV | ||||||
| 4 | 0 | - | 0 | - | 983 | 13.4 |
| 6 | 8 | 53.3 | 4 | 44.4 | 4416 | 60.4 |
| 10 | 1 | 6.7 | 1 | 11.1 | 179 | 2.4 |
| 15 or 16 | 0 | - | 0 | - | 153 | 2.1 |
| 18 or 23 | 0 | - | 0 | - | 57 | 0.8 |
| 6 and 10 | 1 | 6.7 | 1 | 11.1 | 131 | 1.8 |
| 6 and 15 or 16 | 3 | 20.0 | 2 | 22.2 | 689 | 9.4 |
| 6 and 18 or 23 | 2 | 13.3 | 1 | 11.1 | 648 | 8.9 |
| Otherd | 0 | - | 0 | - | 55 | 0.8 |
| Dose, cGy | ||||||
| 4256 | 2 | 11.8 | 1 | 11.1 | 985 | 11.5 |
| 4500 | 0 | - | 0 | - | 487 | 5.7 |
| 4600 | 0 | - | 0 | - | 413 | 4.8 |
| 5000 or 5040 | 15 | 88.2 | 9 | 90.0 | 6102 | 71.5 |
| Other | 0 | - | 0 | - | 544 | 6.4 |
| Daily fractionated dose, cGy | ||||||
| 180 | 10 | 58.8 | 7 | 70.0 | 4591 | 58.5 |
| 200 | 5 | 29.4 | 2 | 20.0 | 2128 | 27.1 |
| ≥265 to ≤385 | 2 | 11.8 | 1 | 10.0 | 1065 | 13.6 |
| Other | 0 | - | 0 | - | 61 | 0.8 |
Abbreviations: CW, Chest wall; SCV, supraclavicular lymph nodes; IMN, internal mammary lymph nodes; PAB, posterior axillary boost; MV, megavoltage; cGy, centigray.
Includes only patients who received radiotherapy with known treatment details (8,632 out of 10,638 patients).
Includes brachytherapy to lumpectomy cavity and SCV + regional lymph nodes unknown but not chest wall.
≤16 fractions and daily fractionated dose ≥ 265 cGy
Includes electron only, brachytherapy (Ir-192 or Electronic) and other.
The relative risk of thoracic angiosarcomas for BMI >30 vs <25 was 5.6 (95%CI:0.7–46.8, p-trend=0.16). History of hypertension or diabetes were associated with a 5-times increased risk of angiosarcoma (RR=4.8, 95%CI:1.3–17.6 and 5.3, 95%CI:1.4–20.8, respectively). Patients with both a history of hypertension and diabetes had an 8-times increased risk of angiosarcoma (RR=8.4, 95%CI:1.5–47.3) compared to patients with neither condition.
There was an inverse relationship between anthracyclines use and history of hypertension and diabetes (Appendix p4). Patients without a history of hypertension or diabetes were less likely to receive anthracyclines than patients without a history of these comorbidities.
In the SEER cohort, there were 430 thoracic STS identified among the 457,300 breast cancer survivors, of which 268 were angiosarcomas and 162 had other subtypes. (Table 4). Characteristics were broadly similar to the Kaiser cohort with a median age at breast cancer diagnosis of 59 years (IQR:49–69 years) with most diagnosed at stage 1 (240,801 [53%] of 457,300 patients) and ER+ disease (334,691 [80%] of 417,313 patients with known ER status). Breast conserving surgery rates were also similar (262,568 [57%] of 457,300), but rates of other treatments were lower than in the KP cohort due to combination of no/unknown treatment; 55% (253,286 of 457,300) for radiotherapy, 39% (179,580 of 457,300) for chemotherapy and only 56% (147,526 of 334,691 ER+ patients) were known to have received hormonal therapy. Median age at breast cancer diagnosis was 65 years (IQR:54–71) in patients who developed a second angiosarcoma compared to 60 years (IQR:48–69 years) for the other STS subtypes. The angiosarcomas were diagnosed on average 7 years after the initial breast cancer (IQR: 5.3–9.4). They were more likely to occur among women with stage I breast cancer than stage II/III (p=0.02). Most of the thoracic STS occurred in women who had initial radiotherapy (231 [86%] of 268 angiosarcomas and 104 [64%] of 162 other thoracic STS) leaving 95 thoracic STS cases with no/unknown radiotherapy status. The majority of angiosarcomas (216 [80%] of 268) occurred in women who underwent breast-conserving surgery and radiotherapy. In multivariable models, radiotherapy was associated with a 5-times increased risk of developing angiosarcoma (RR=5.5, 95%CI:3.9–7.8). Women who had breast-conserving surgery were at significantly higher risk of developing an angiosarcoma than women treated with mastectomy (RR=3.7, 95%CI:2.3–6.0, Table 5). This risk was attenuated but remained significantly elevated when restricted to patients treated with radiotherapy (RR=1.9, 95%CI:1.1–3.3). There was no relationship with any initial chemotherapy or hormone therapy (yes vs no/unknown).
Table 4.
Selected patient and clinical characteristics of SEER cohort and among patients who developed subsequent thoracic soft tissue sarcomas a - SEER 13 registries, 1992–2017.
| Overall cohort (N=457,300) | Thoracic Angiosarcomas (N=268) | Other thoracic subtypes (N=162) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Age at breast cancer diagnosis, years | ||||||
| 20–54 | 178,204 | 39.0 | 68 | 25.4 | 61 | 37.7 |
| 55–64 | 115,815 | 25.3 | 65 | 24.3 | 46 | 28.4 |
| 65–84 | 163,281 | 35.7 | 135 | 50.4 | 55 | 34.0 |
| Median age at breast cancer diagnosis (IQR) | 59 (49–69) | 65 (54–71) | 60 (48–69) | |||
| Attained age, years | ||||||
| 21–59 | 124,949 | 27.3 | 51 | 19.0 | 51 | 31.5 |
| 60–69 | 117,667 | 25.7 | 65 | 24.3 | 42 | 25.9 |
| 70–108 | 214,684 | 46.9 | 152 | 56.7 | 69 | 42.6 |
| Median attained (IQR) | 69 (59–79) | 74 (62–79) | 60 (48–69) | |||
| Calendar year of breast cancer diagnosis | ||||||
| 1990–1999 | 135,163 | 29.6 | 123 | 45.9 | 68 | 42.0 |
| 2000–2004 | 91,848 | 20.1 | 65 | 24.3 | 43 | 26.5 |
| 2005–2016 | 230,289 | 50.4 | 80 | 29.9 | 51 | 31.5 |
| Year diagnosis to end of follow-up | ||||||
| 1–4 | 137,190 | 30.0 | 49 | 18.3 | 52 | 32.1 |
| 5–9 | 130,673 | 28.6 | 158 | 59.0 | 61 | 37.7 |
| 10–26 | 189,437 | 41.4 | 61 | 22.8 | 49 | 30.2 |
| Median time since breast cancer diagnosis (IQR) | 8.3 (4.3–13.9) | 7.1 (5.3–9.4) | 6.8 (4.2–11.0) | |||
| Breast cancer stage | ||||||
| I | 240,801 | 52.7 | 162 | 60.4 | 72 | 44.4 |
| II | 161,529 | 35.3 | 92 | 34.3 | 73 | 45.1 |
| III | 54,970 | 12.0 | 14 | 5.2 | 17 | 10.5 |
| Race | ||||||
| White | 363,908 | 79.6 | 233 | 86.9 | 118 | 72.8 |
| Black | 41,849 | 9.2 | 18 | 6.7 | 20 | 12.3 |
| Othersb | 49,921 | 10.9 | 17 | 6.3 | 24 | 14.8 |
| Unknown | 1,622 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| Vital status at the end of follow-up | ||||||
| Alive | 318,718 | 69.7 | 127 | 47.4 | 83 | 51.2 |
| Deceased | 138,582 | 30.3 | 141 | 52.6 | 79 | 48.8 |
| Estrogen Receptor Status | ||||||
| ER− | 82,622 | 18.1 | 38 | 14.2 | 37 | 22.8 |
| ER+ | 334,691 | 73.2 | 198 | 73.9 | 105 | 64.8 |
| Unknown | 39,987 | 8.7 | 32 | 11.9 | 20 | 12.3 |
| Initial Radiotherapy | ||||||
| No/unknown | 204,014 | 44.6 | 37 | 13.8 | 58 | 35.8 |
| Yes | 253,286 | 55.4 | 231 | 86.2 | 104 | 64.2 |
| Breast surgery type | ||||||
| Breast conserving surgery | 262,568 | 57.4 | 243 | 90.7 | 112 | 69.1 |
| Mastectomy | 194,292 | 42.5 | 25 | 9.3 | 50 | 30.9 |
| Surgery NOS | 440 | 0.1 | 0 | 0 | 0 | 0 |
| Breast surgery type (restricted to RT) | ||||||
| RT, Breast conserving surgery | 207,169 | 81.8 | 216 | 93.5 | 87 | 83.7 |
| RT, Mastectomy | 45,928 | 18.1 | 15 | 6.5 | 17 | 16.3 |
| RT, Surgery NOS | 189 | 0.1 | 0 | 0.0 | 0 | 0.0 |
| Initial chemotherapy | ||||||
| No/unknown | 277,720 | 60.7 | 192 | 71.6 | 97 | 59.9 |
| Yes | 179,580 | 39.3 | 76 | 28.4 | 65 | 40.1 |
| Endocrine therapy (ER+ only) c | ||||||
| No/unknown | 147,526 | 44.1 | 89 | 44.9 | 54 | 51.4 |
| Yes | 187,165 | 55.9 | 109 | 55.1 | 51 | 48.6 |
Abbreviations: RT, Radiotherapy; ER, estrogen receptor, IQR, interquartile range
Inclusion criteria: women diagnosed at ages 20–84 years with a first unilateral invasive breast cancer, stage I-III, had breast cancer surgery, and survived at least one year after diagnosis.
Includes American Indian/Alaskan Native, Asian/Pacific Islanders and others
Among 334,691 ER+ breast cancer patients
Table 5 -.
Relative Risk (95%CI)a for thoracic soft tissue sarcomas according to initial breast cancer treatment and selected patient and clinical characteristics among SEER cohort of 457,300 women diagnosed with a first primary unilateral invasive breast cancer - SEER 13 registries, 1992–2017.
| Characteristics | Thoracic sarcomas (N=430) | ||||||
|---|---|---|---|---|---|---|---|
| Overall (N=430) | Angiosarcomas (N=268) | Other sarcomas (N=162) | |||||
| N | PYR | RR (95%CI) | N | RR (95%CI) | N | RR (95%CI) | |
| Age at breast cancer diagnosis, years | |||||||
| 20–54 | 129 | 1706057.2 | 1.0 | 68 | 1.0 | 61 | 1.0 |
| 55–64 | 111 | 1008228.8 | 1.1 (0.8–1.6) | 65 | 1.0 (0.7–1.6) | 46 | 1.2 (0.7–2.0) |
| 65–74 | 130 | 792376.9 | 1.4 (0.9–2.1) | 93 | 1.5 (0.9–2.6) | 37 | 1.0 (0.5–2.1) |
| 75–84 | 60 | 402446.6 | 1.3 (0.8–2.1) | 42 | 1.5 (0.8–2.7) | 18 | 1.0 (0.5–2.2) |
| P | 0.46 | 268 | 0.20 | 0.88 | |||
| Year of breast cancer diagnosis | |||||||
| 1990–1999 | 191 | 1747144.4 | 1.0 | 123 | 1.0 | 68 | 1.0 |
| 2000–2004 | 108 | 993241.9 | 1.0 (0.8–1.2) | 65 | 0.9 (0.7–1.2) | 43 | 1.1 (0.7–1.6) |
| 2005–2016 | 131 | 1168723.2 | 1.0 (0.8–1.3) | 80 | 1.0 (0.7–1.3) | 51 | 1.1 (0.8–1.6) |
| P | 0.89 | 0.76 | 0.83 | ||||
| Time since breast cancer diagnosis, years | |||||||
| 1–4 | 113 | 1540701.8 | 1.0 | 58 | 1.0 | 55 | 1.0 |
| 5–9 | 208 | 1253377.7 | 2.1 (1.7–2.7) | 149 | 2.9 (2.1–3.9) | 59 | 1.2 (0.9–1.9) |
| 10–26 | 109 | 1115030.0 | 1.2 (0.9–1.5) | 61 | 1.2 (0.8–1.7) | 48 | 1.1 (0.8–1.7) |
| P | <0.001 | <0.001 | 0.41 | ||||
| Attained age, years | |||||||
| 21–59 | 103 | 1432255.0 | 1 | 51 | 1.0 | 52 | 1.0 |
| 60–69 | 107 | 1063026.1 | 1.3 (1.0–1.8) | 66 | 1.6 (1.1–2.3) | 41 | 1.1 (0.7–1.6) |
| 70–108 | 220 | 1413828.4 | 2.1 (1.7–2.7) | 151 | 2.8 (2.0–3.9) | 69 | 1.4 (1.0–2.1) |
| P | <0.001 | <0.001 | 0.14 | ||||
| Breast cancer stage | |||||||
| I | 234 | 2185881.0 | 1.0 | 162 | 1.0 | 72 | 1.0 |
| II | 165 | 1343478.3 | 1.5 (1.2–1.9) | 92 | 1.4 (1.0–1.8) | 73 | 1.9 (1.3–2.7) |
| III | 31 | 379750.1 | 1.0 (0.7–1.5) | 14 | 0.7 )0.4–1.3) | 17 | 1.5 (0.9–2.7) |
| P | <0.001 | 0.02 | 0.01 | ||||
| Race | |||||||
| White | 351 | 3174545.5 | 1.0 | 233 | 1.0 | 118 | 1.0 |
| Black | 38 | 315060.7 | 1.2 (0.9–1.7) | 18 | 0.9 (0.6–1.5) | 20 | 1.8 (1.1–2.9) |
| Othersb | 41 | 406319.6 | 1.0 (0.7–1.4) | 17 | 0.7 (0.4–1.1) | 24 | 1.7 (1.1–2.6) |
| P | 0.51 | 0.24 | 0.01 | ||||
| ER subtype | |||||||
| ER− | 75 | 698530.1 | 1.0 | 38 | 1.0 | 37 | 1.0 |
| ER+ | 303 | 2752519.1 | 0.9 (0.7–1.1) | 198 | 1.0 (0.7–1.4) | 105 | 0.7 (0.5–1.0) |
| Unknown | 51 | 458060.3 | 1.0 (0.7–1.5) | 32 | 1.2 (0.8–2.0) | 20 | 0.9 (0.5–1.5) |
| P | 0.25 | 0.56 | 0.16 | ||||
| Initial radiotherapy | |||||||
| No/unknown | 95 | 1774386.3 | 1.0 | 37 | 1.0 | 58 | 1.0 |
| Yes | 335 | 2134723.2 | 3.0 (2.4–3.8) | 231 | 5.5 (3.9–7.8) | 104 | 1.5 (1.1–2.0) |
| P | <0.001 | <0.001 | 0.01 | ||||
| Initial chemotherapy | |||||||
| No/unknown | 289 | 2408656.8 | 1.0 | 192 | 1.0 | 97 | 1.0 |
| Yes | 141 | 1500452.7 | 0.9 (0.7–1.1) | 76 | 0.8 (0.6–1.0) | 65 | 1.2 (0.8–1.6) |
| P | 0.29 | 0.05 | 0.40 | ||||
| Endocrine therapy (ER+ only) | |||||||
| No/unknown | 143 | 1355394.1 | 1.0 | 89 | 1.0 | 54 | 1.0 |
| Yes | 160 | 1397125.0 | 0.9 (0.7–1.1) | 109 | 0.9 (0.7–1.2) | 51 | 0.8 (0.6–1.2) |
| P | 0.23 | 0.40 | 0.37 | ||||
| Surgery type | |||||||
| Mastectomy | 75 | 1648429.8 | 1.0 | 25 | 1.0 | 50 | 1.0 |
| Breast-conserving surgery | 355 | 2256506.1 | 2.3 (1.7–3.1) | 243 | 3.7 (2.3–6.0) | 112 | 1.5 (1.0–2.3) |
| P | <0.001 | <0.001 | 0.05 | ||||
| Surgery type (restricted to RT) c | |||||||
| RT, Mastectomy | 32 | 320198.7 | 1.0 | 15 | 1.0 | 17 | 1.0 |
| RT, Breast-conserving surgery | 303 | 1812599.6 | 1.4 (0.9–2.0) | 216 | 1.9 (1.1–3.3) | 87 | 0.9 (0.5–1.6) |
| P | 0.08 | 0.01 | 0.79 | ||||
ER, Estrogen Receptor; BCS, Breast Conserving surgery; RT, radiotherapy.
Poisson regression model adjusted for attained age (<60,60–69,70–108), radiotherapy (yes vs no/unknown) and chemotherapy (yes vs no/unknown) as appropriate
Includes American Indian/Alaskan Native, Asian/Pacific Islanders and others.
Restricted to 253,286 patients who received radiotherapy.
Minimally adjusted estimates (age/study center) of the Relative Risk of thoracic STS for both KP and SEER cohorts are presented in the Appendix p5–9 to illustrate the impact of additionally adjust for other treatment type.
At 10 years after breast cancer diagnosis, the cumulative incidence of developing a thoracic STS in the SEER cohort who received radiotherapy was 0.15% (95%CI:0.13–0.17%); 0.11% (95%CI:0.09–0.12%) for angiosarcomas and 0.04% (95%CI:0.03–0.05%) for other sarcomas (Appendix p11).
In the general US population, there was a significant increase in the rates of angiosarcoma from 1992–2017 (APC=2.47, 95%CI:1.47–3.51) and thoracic angiosarcoma (APC=3.11, 95%CI:1.63–4.60) (Appendix p12). This increase was mainly driven by the increasing proportion of subsequent thoracic angiosarcomas over time (44% [28 of 64 cases] in 1992–1996 to 71% [167 of 234 cases] in 2012–2017).
Discussion
In our KP cohort of 16,000 breast cancer survivors treated in a general community setting we found that 2 in 1,000 women developed a thoracic STS by 10 years after radiotherapy. About half of the angiosarcoma patients died from their cancer by the end of follow-up versus zero sarcoma-related deaths for the other STS sub-types. In this treatment era (1990–2016), most of the thoracic STS (70–80%) occurred in women treated with radiotherapy and breast-conserving surgery, but we did not find differences by radiotherapy fields, prescribed dose or fractions. Anthracyclines were related to a 3-times increased risk of thoracic angiosarcoma, and other thoracic STS were possibly related to alkylating agents. Also of clinical relevance, we observed a 5-times increased risk of angiosarcoma in survivors with a history of hypertension or diabetes.
In our parallel analysis of 460,000 breast cancer survivors in the general US population from the same treatment era, the patterns of thoracic STS were broadly similar in relation to breast conserving surgery and radiotherapy, lending external validity to our findings, with a cumulative incidence of 0.15% by 10 years after breast cancer diagnosis among patients who received radiotherapy. There was no association with initial chemotherapy but chemotherapy agents were not available so we could not evaluate the role of these specific agents.
In a Dutch cancer registry study of 180,000 breast cancer patients treated with radiotherapy in 1989–2015, 209 developed angiosarcoma(9). Overall, their angiosarcoma patterns were similar to our cohorts with 1 in 1,000 developing an angiosarcoma with a median age at breast cancer diagnosis of 65 years and median latency of 8 years. There was no relationship with chemotherapy (yes/no); however, they also did not examine specific chemotherapy agents. In a multi-center case series of radiation-induced sarcomas after first cancer treatment (51% after breast cancer), Zhang et al found that time to radiation-induced sarcoma diagnosis was significantly shorter in patients treated with anthracyclines or alkylating agents for their first cancer(median=10 vs 11 years)(5). This study included other first cancer types, but for breast cancer the median latency was 8 years. Unlike our study, they did not have a comparison group who did not develop angiosarcomas, so they were unable to formally assess the relationship. Anthracyclines and alkylating agents have previously been found to be associated with an increased risk of subsequent sarcoma in childhood cancer survivors(6).
Manual abstraction of radiotherapy summaries for the KP cohort enabled us to evaluate details of the radiotherapy including prescribed doses and treatment fields, but there was no clear relationship with thoracic STS risk. Breast cancer radiotherapy is evolving in the US with the American Society for Radiation Oncology recommendations for hypofractionation in 2011 and 2018(14, 15). A National Cancer Database study found that hypofractionation increased from 21% in 2012 to 59% in 2016 for patients who met the 2011 guidelines (age 50+, stage 1 and no chemotherapy)(16). Randomized trials established equivalent rates of recurrence after hypofractionation and lower rates of normal tissue effects including breast edema, but the impact on subsequent cancer risk is still uncertain(17, 18). In our KP cohort, only 14% of patients received hypofractionated radiotherapy with no evidence of a difference in sarcoma risk compared with standard fractionated radiotherapy, but longer follow-up of larger patient populations is needed to confirm this finding. In a UK audit of a case-series of 86 radiation-related angiosarcomas between 2000 and 2016, half of these patients received hypofractionated radiotherapy(7). Without a comparison population of women who did not develop angiosarcoma, this finding is difficult to interpret. Accelerated partial breast irradiation was uncommon in our patient population (0.3% by 2004–2007), but it has been hypothesized that it could decrease the risk of late effects(19).
In both our cohorts 70–80% of thoracic STS occurred in women who had breast-conserving surgery, rather than mastectomy, before their radiotherapy, and this proportion was even higher for angiosarcomas (80–90%). Similar findings were reported for angiosarcomas in the Dutch cohort8. Breast surgery causes damage to the lymphatic system, which could cause lymphedema in the breast, arms and/or chest(20). The greater area of skin exposed to radiation after breast-conserving surgery can result in some degree of edema in the conserved breast (transient and subclinical) that could predispose them to the development of angiosarcomas(21). The increasing rates of thoracic angiosarcomas in the general population in the US could be due to the increase in breast-conserving surgery and radiotherapy for breast cancer treatment.
To our knowledge, this is the first study to assess history of cardiovascular risk factors in subsequent STS risk. We found a striking effect for hypertension and diabetes before breast cancer diagnosis as possible novel risk factors for angiosarcoma. These are also likely risk factors for lymphedema after breast cancer treatment(22–24). There is some evidence that antihypertensive medications such as calcium blockers increase the risk of lymphedema(25–27). A pathway from diabetes and hypertension (or antihypertensive medications) to lymphedema and to thoracic angiosarcoma is a possible biologic mechanism for our findings. Cardiovascular treatment data were collected in KP Washington as part of the Commonly Used Medications and Breast Cancer Outcomes (COMBO) study(28). In the year prior to breast cancer diagnosis 10% had used calcium blockers and during follow-up after diagnosis this increased to 21% (29% during an average of 6 years of follow-up).
Also, the inverse relationship between anthracyclines use and history of hypertension and diabetes would negatively confound the relationship between diabetes/hypertension and angiosarcoma risk. We have adjusted for anthracyclines (yes/no) in the multivariate model, however, if the chemotherapy doses were lower in patients with these pre-existing conditions there could be residual confounding towards the null. Further evaluation of treatment for and history of hypertension, diabetes, lymphedema, cholesterol and angiosarcoma risk is warranted. The strengths of our KP cohort include detailed treatment data including chemotherapy agents, prescribed radiation dose, fractionation and history of comorbidities. Limitations include lack of information on intensity and duration of chemotherapy and other potential risk factors including genetic susceptibility, axillary lymph-node dissection, and lymphedema diagnosis. The main limitation, however, is the population size for studying these rare late-effects which resulted in wide confidence intervals, especially for STS sub-types, and the risks from multiple testing. We confirmed broadly similar patterns of thoracic STS in our parallel analysis in SEER-13, however, which had much larger patient numbers. This comparison also highlighted the limitations of SEER including the evidence of greater misclassification of radiotherapy(29), with no ability to differentiate between no receipt and unknown radiotherapy and no specific chemotherapy agents. Our study has potential implications for clinical practice. The growing evidence that anthracyclines can cause solid tumors, including STS suggests that these risks need to be factored into the risk-benefit calculation. Also, if the pathway to thoracic angiosarcoma development is through breast lymphedema, which is influenced by common co-morbidities like hypertension, obesity and diabetes, then monitoring of patients who develop this acute side-effect is possibly warranted. A number of strategies have been recommended to decrease the risk of lymphedema after breast cancer treatment(30). Our cumulative absolute risk estimates, which were broadly consistent in both cohorts, though also show that these are still very rare subsequent neoplasms.
In conclusion, we found that breast cancer survivors who received radiotherapy, anthracyclines, had breast-conserving surgery, and had a history of hypertension and/or diabetes before breast cancer diagnosis were at greatest risk of developing a thoracic angiosarcoma. The potential role of these co-morbidities in the etiology and prevention of this rare, but life-threatening disease warrants further investigation regarding their role in causing these sarcomas and as potential targets for future prevention strategies and increased surveillance.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Survivors of breast cancer have increased risks of thoracic STS due to radiotherapy with poor prognosis and high mortality. Incidence rates of these radiation-induced sarcomas are increasing in the U.S., possibly due to the increased use of radiotherapy to treat breast cancer. We searched the scientific literature in PubMed for peer-reviewed studies until September 30, 2021, for studies of subsequent STS after breast cancer treatment. Search terms included “soft tissue sarcoma”, “angiosarcoma”, “radiation-induced sarcomas”, and “breast cancer” with no language or date restriction. Factors associated with increased risk of STS include genetic susceptibility, older age, chronic lymphedema, and possibly chemotherapy, but it is unclear what other factors contribute to this increased risk. There were few recent studies of STS in breast cancer patients. Most studies were case series describing clinical characteristics and outcomes, but they lacked an internal control population which limited the ability to formally evaluate risk factors. Cancer registry studies enable large-scale population-level assessments; however, they lack treatment details such as specific chemotherapy agents, radiotherapy fields or information on other risk factors, such as comorbidities.
Added value of this study
To our knowledge, this is the first study to assess comorbidities in subsequent STS risk. We found that breast cancer survivors who received radiotherapy, anthracyclines, had breast-conserving surgery, and had a history of hypertension and/or diabetes at the time of their breast cancer diagnosis were at greatest risk of developing a thoracic angiosarcoma.
Implications of all the available evidence
Our study has potential implications for clinical practice. The striking effect for hypertension and diabetes at the time of breast cancer diagnosis is a novel finding, and the potential role of these comorbidities in the etiology and prevention of this life-threatening disease warrants further investigation. These factors might be potential targets for future prevention strategies and identification of patients who should receive increased surveillance.
Acknowledgements:
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the US National Cancer Institute (NCI). Authors employed by NIH include L HS Veiga PhD, J B Vo PhD, R E Curtis MA, M M Mille PhD, C Lee PhD, C Ramin PhD, C Bodelon PhD, G L Gierach PhD, and A Berrington de Gonzalez DPhil. Data collected at Kaiser Permanente Colorado was supported by contracts from NCI (HHSN 261201800469P, HHSN 261201700708P, HHSN 261201600711P) and a subcontract with RTI International (HHSN 26120090017C). Data collected at Kaiser Permanente Washington was supported by NCI grants (1R01CA1205621 and P01CA154292) and NCI contracts (HHSN 261201700564P, HHSN75N91019P00076, HHSN 5N91020P00327). Data collected at Kaiser Permanente Northwest was supported by several NCI subcontracts with RTI International (Nos. 20-312-0212208, 17-312-0212208). EJ Aiello Bowles was also supported by a NCI grant (R50CA211115). Cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by Contract No. N01-CN-67009 and N01-PC-35142 from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington.
Fundings
US National Cancer Institute and National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: All authors declare no competing interests
Data sharing statement:
Data from this study, including individual participant data, are not available for sharing. Summary statistical data will be available from the corresponding author upon reasonable request with the permission of the contributing Kaiser centers.
References
- 1.Buehler D, Rice SR, Moody JS, Rush P, Hafez GR, Attia S, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37(5):473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922–30. [DOI] [PubMed] [Google Scholar]
- 3.Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the U.S. : an analysis of 12,114 cases. Cancer. 2008;113(3):616–27. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich AU, Reisenbichler ES, Heller DR, LeBlanc JM, Park TS, Killelea BK, et al. Characteristics and Long-Term Risk of Breast Angiosarcoma. Ann Surg Oncol. 2021;28(9):5112–8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang AY, Judson I, Benson C, Wunder JS, Ray-Coquard I, Grimer RJ, et al. Chemotherapy with radiotherapy influences time-to-development of radiation-induced sarcomas: a multicenter study. Br J Cancer. 2017;117(3):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson TO, Whitton J, Stovall M, Mertens AC, Mitby P, Friedman D, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99(4):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks J, George J, Potter S, Gardiner MD, Ives C, Shaaban AM, et al. Breast Angiosarcoma Surveillance Study: UK national audit of management and outcomes of angiosarcoma of the breast and chest wall. Br J Surg. 2021;108(4):388–94. [DOI] [PubMed] [Google Scholar]
- 8.Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28(12):2064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rombouts AJM, Huising J, Hugen N, Siesling S, Poortmans PM, Nagtegaal ID, et al. Assessment of Radiotherapy-Associated Angiosarcoma After Breast Cancer Treatment in a Dutch Population-Based Study. JAMA Oncol. 2019;5(2):267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrington de Gonzalez A, Kutsenko A, Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res. 2012;2(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramin C. Risk of second primary cancers among breast cancer patients treated in the modern era: The Kaiser Breast Cancer Survivors Cohort. 2021.
- 12.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Plus Data with Additional Fields and Case Listing, 13 Registries (excl AK), Nov 2019 Sub (1992–2017) - Linked To County Attributes - Total U.S., 1969–2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.
- 13.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. The Stata Journal. 2004;4:103–12. [Google Scholar]
- 14.Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8(3):145–52. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Bentzen SM, Correa CR, Hahn CA, Hardenbergh PH, Ibbott GS, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81(1):59–68. [DOI] [PubMed] [Google Scholar]
- 16.Woodward SG, Varshney K, Anne PR, George BJ, Willis AI. Trends in Use of Hypofractionated Whole Breast Radiation in Breast Cancer: An Analysis of the National Cancer Database. Int J Radiat Oncol Biol Phys. 2021;109(2):449–57. [DOI] [PubMed] [Google Scholar]
- 17.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–20. [DOI] [PubMed] [Google Scholar]
- 18.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–94. [DOI] [PubMed] [Google Scholar]
- 19.Mukesh M, Harris E, Jena R, Evans P, Coles C. Relationship between irradiated breast volume and late normal tissue complications: a systematic review. Radiother Oncol. 2012;104(1):1–10. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg. 2018;7(4):379–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monroe AT, Feigenberg SJ, Mendenhall NP. Angiosarcoma after breast-conserving therapy. Cancer. 2003;97(8):1832–40. [DOI] [PubMed] [Google Scholar]
- 22.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15. [DOI] [PubMed] [Google Scholar]
- 23.Jammallo LS, Miller CL, Singer M, Horick NK, Skolny MN, Specht MC, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat. 2013;142(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetzig N, Gill PG, Espinoza D, Mister R, Stockler MR, Gebski VJ, et al. Sentinel-Lymph-Node-Based Management or Routine Axillary Clearance? Five-Year Outcomes of the RACS Sentinel Node Biopsy Versus Axillary Clearance (SNAC) 1 Trial: Assessment and Incidence of True Lymphedema. Ann Surg Oncol. 2017;24(4):1064–70. [DOI] [PubMed] [Google Scholar]
- 25.Stolarz AJ, Lakkad M, Klimberg VS, Painter JT. Calcium Channel Blockers and Risk of Lymphedema among Breast Cancer Patients: Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2019;28(11):1809–15. [DOI] [PubMed] [Google Scholar]
- 26.Ridner SH, Dietrich MS. Self-reported comorbid conditions and medication usage in breast cancer survivors with and without lymphedema. Oncol Nurs Forum. 2008;35(1):57–63. [DOI] [PubMed] [Google Scholar]
- 27.Togawa K, Ma H, Sullivan-Halley J, Neuhouser ML, Imayama I, Baumgartner KB, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16(4):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau DM, Yu O, Chubak J, Wirtz HS, Bowles EJ, Fujii M, et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat. 2014;144(2):405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118(2):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockson SG. Lymphedema after Breast Cancer Treatment. N Engl J Med. 2018;379(20):1937–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study, including individual participant data, are not available for sharing. Summary statistical data will be available from the corresponding author upon reasonable request with the permission of the contributing Kaiser centers.


