Abstract
Diffuse skeletal hyperostosis is a common spinal disorder, but its pathophysiology is mostly unclear. The disorder can lead to a variety of symptoms, but many patients remain relatively asymptomatic. We present a case demonstrating the development of bridging osteophytes on a series of magnetic resonance images. An elderly person's spine was scanned repeatedly due to non-specific back pain during the last 4 years and the consecutive images revealed the formation of a bony bridge in the lumbar spine. Extensive bone marrow edema was seen during the formation of the osteophyte, suggestive of an ongoing inflammatory process. This case underlines that the inflammatory reaction in diffuse skeletal hyperostosis can be intense and prolonged, and its role might be worth studying further.
Keywords: Diffuse skeletal hyperostosis, Forestier disease, Magnetic resonance imaging, Back pain, Bone marrow edema
Introduction
Diffuse skeletal hyperostosis (DISH) is a skeletal disorder characterized by calcification and ossification of ligaments and entheses. Usually, the affected region is the right-sided anterolateral thoracic spine, but the calcifications can occur in any joint [1]. Most patients with radiographical features of spinal DISH are asymptomatic, but they anyway suffer more from spinal pain, morning stiffness, and impaired mobility than individuals without DISH. Mechanical compression by large protruding osteophytes may cause symptoms (myelopathy, radiculopathy, dysphagia, etc.). The risk of complicated spinal fracture is also increased [2,3]. Traditionally, DISH is considered a non-inflammatory disease in contrast to axial spondyloarthropathy (AS), but the pathophysiological processes behind the ligament calcification and osteophyte formation are yet unknown [4,5]. DISH and AS seem to have certain similar features on magnetic resonance imaging (MRI), especially lesions of bone marrow edema (BME) or fatty deposition in vertebral corners suggesting, that inflammatory reaction has some role in the pathophysiology of DISH too [6]. The natural course of bridging osteophyte formation on computer tomography over time has been demonstrated by Yaniv et al. [7] and Kuperus et al. [8]. Here, we present a case demonstrating the natural course of bridging osteophyte formation and concomitant BME on MRI.
Case report
An elderly patient was admitted to the emergency department due to severe lower back pain after a fall. In addition to acute spinal fracture in the ankylosed spine, we noticed the patient's history of spinal imaging to be rather interesting. The patient had suffered from chronic spinal pain and was known to have DISH for more than 14 years. HLA-B27 was tested negative and the other laboratory tests were unremarkable. There were no signs of active or chronic inflammation in the sacroiliac joints. The symptoms were fluctuating; there had been phases of exacerbated back pain and better years between them. Besides axial back pain and stiffness, he suffered from radicular pains caused by osteophytes compressing nerve roots and atypical right-sided lower back and hip pain due to a large bone bridge in level L1-L2 protruding into the psoas muscle.
The first radiographical indications of DISH were seen 14 years ago. At this point, his thoracic spine was partly ankylosed and at the lumbar spine, there was a large bridging osteophyte at level L1-L2. Slowly the disease evolved to affect most of the thoracolumbar spine. The new bridging osteophytes were formed slowly, level by level, and not in straight ascending or descending order. The cervical spine has not been imaged.
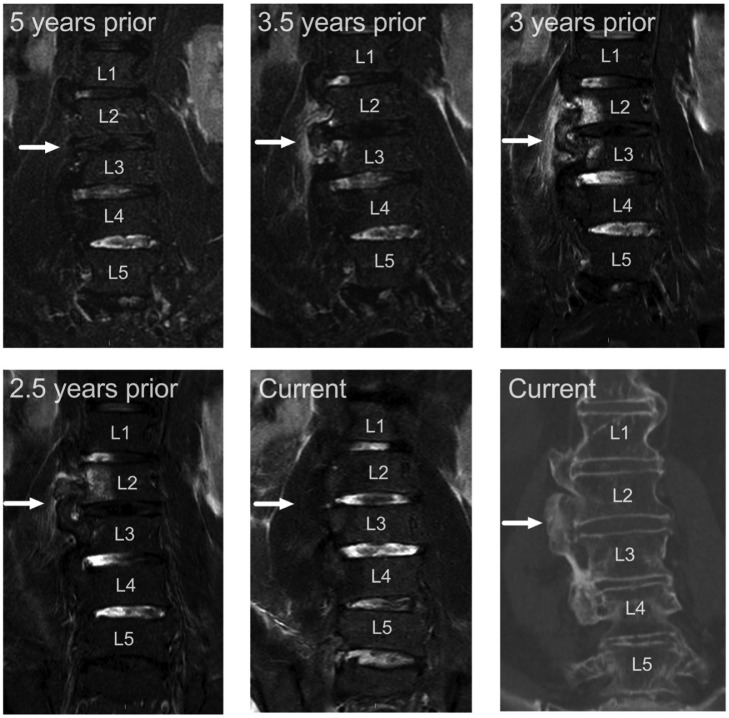
During the last 3 years, several lumbar spine MRIs were performed due to worsening lower back pain (Fig. 1). Those 4 consecutive MRI scans showed the formation of a new bridging osteophyte on a previously spared level. On levels L1-L2, L3-L4, and L4-L5, there has been typical bridging, right-sided anterolateral DISH osteophytes for 10 years, but within a year large new bridging osteophyte developed on levels L2-L3. Significant BME is seen in vertebrae L3 and L4 on the side of the growing osteophyte, in the newly formed bony bridge itself, and in the paravertebral soft tissue. In 16 months, the edema disappeared, and the osteophyte ceased to grow. The same kind of BME was seen also in older MRIs showing the evolution of osteophyte formation in levels L1-L2.
Fig. 1.
Evolution of a bridging osteophyte on the right side of the L2-L3 levels, from a 5-year follow-up period. Images are coronal fat-suppressed T2-weighted MR images, except the bottom right corner represents non-contrast CT.
Discussion
Although the exact etiology of DISH is unclear, it is associated with higher age, diabetes, obesity, hypertension, and hyperuricemia amongst some other metabolic derangements [2]. Genetic, vascular, and mechanical factors influencing new bone formation have also been proposed [2,9]. Although DISH is thought to be a non-inflammatory disease by nature, it seems that osteophyte formation can often be companied by prolonged BME, suggesting an ongoing inflammatory reaction [6]. Besides the similarities in BME patterns of DISH and AS, the rate of new bone formation is quite uniform in those diseases [10]. Mader et al. recently discussed the potential inflammatory processes in DISH suggesting that inflammation might have a more fundamental role in DISH pathogenesis than previously thought [11].
Our case demonstrates the ongoing inflammation during the new bone formation in DISH. We also noted the cessation of active inflammation after completion of the bridging osteophytes. Still, it is not known if the local inflammation is inducing the osteophyte formation or is it the sequelae. Analogous to AS, there may be a sustained predisposition to inflammatory exacerbations in DISH leading to the formation of new bone and bridging osteophytes. Factors activating the active inflammatory phases in DISH are not yet understood, but in the future, they might be of help in slowing down the new bone formation and relieving the symptoms. Sometimes the amount of inflammation is so remarkable, that it raises a question about spondylitis or even neoplasm. In these cases, computed tomography better showing the bony structure may have an eye-opening role.
The case also shows the periodical progression in new bone formation level by level with active and inactive phases. The slow, but inevitable progression is consistent with the previous findings about the evolution of DISH [7,8]. The most widely used diagnostic criteria for spinal DISH by Resnick and Niwayama [1] includes bridging of at least 4 adjacent vertebral bodies, but with normal or only mildly decreased intervertebral disc height and without severe sclerosis or erosion in facet joints or sacroiliac joints. However, these criteria have been criticized to underestimate changes in the early stage of the disease, and newer criteria for early or developing DISH have been proposed [12,13]. The new criteria by Kuperus et al. [13] include 3 stages: no DISH, early DISH, and definite DISH. Three adjacent segments with almost complete bone bridges or one complete bone bridge with incomplete, developing bone bridges in the adjacent segments are considered early DISH, while 3 adjacent levels with complete bone bridges are considered definite DISH. These criteria do not exclude the possibility of the co-existence of DISH and AS in the clinical setting. The need for different diagnostic criteria for the research and clinical settings has also been discussed [14]. This case underlines the relevance of these criteria for early-phase disease due to the progressive nature of DISH. We also know that the symptoms may emerge at just one level with extensive osteophyte formation.
Although anecdotal, this case presents longitudinal MRI features highlighting the role of inflammation in the forming of bridging osteophytes in DISH. The pathophysiology of the disease is not understood and needs to be studied further, but it seems that the inflammation must not be overlooked.
Patient consent
Informed consent was obtained from the subject described in this report.
Acknowledgments
We obtained permission from the hospital district board for this study and informed consent was obtained from the subject described in this report.
Footnotes
Competing Interests: The authors declare that they have no conflict of interest.
All authors have contributed to the study design and drafting or revising the article. The authors have accepted the final version to be published and agree to be accountable for the work.
References
- 1.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH) Radiology. 1976;119(3):559–568. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 2.Mader R, Verlaan J-J, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol. 2013;9(12):741–750. doi: 10.1038/nrrheum.2013.165. [DOI] [PubMed] [Google Scholar]
- 3.Taljanovic MS, Hunter TB, Wisneski RJ, Seeger JF, Friend CJ, Schwartz SA, et al. Imaging characteristics of diffuse idiopathic skeletal hyperostosis with an emphasis on acute spinal fractures: review. AJR Am J Roentgenol. 2009;193(3 Suppl) doi: 10.2214/AJR.07.7102. S10-0, Quiz S20-4. [DOI] [PubMed] [Google Scholar]
- 4.Forestier J, Rotes-Querol J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9(4):321–330. doi: 10.1136/ard.9.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le HV, Wick JB, Van BW, Klineberg EO. Diffuse idiopathic skeletal hyperostosis of the spine: pathophysiology, diagnosis, and management. JAAOS J Am Acad Orthop Surg. 2021;29(24):1044–1051. doi: 10.5435/JAAOS-D-20-01344. [DOI] [PubMed] [Google Scholar]
- 6.Latourte A, Charlon S, Etcheto A, Feydy A, Allanore Y, Dougados M, et al. Imaging findings suggestive of axial spondyloarthritis in diffuse idiopathic skeletal hyperostosis. Arthritis Care Res. 2018;70(1):145–152. doi: 10.1002/acr.23244. [DOI] [PubMed] [Google Scholar]
- 7.Kuperus JS, Buckens CF, Šprem J, Oner FC, de Jong PA, Verlaan J-J. The natural course of diffuse idiopathic skeletal hyperostosis in the thoracic spine of adult males. J Rheumatol. 2018;45(8):1116–1123. doi: 10.3899/jrheum.171091. [DOI] [PubMed] [Google Scholar]
- 8.Yaniv G, Bader S, Lidar M, Herman A, Shazar N, Aharoni D, et al. The natural course of bridging osteophyte formation in diffuse idiopathic skeletal hyperostosis: retrospective analysis of consecutive CT examinations over 10 years. Rheumatology. 2014;53(11):1951–1957. doi: 10.1093/rheumatology/ket335. [DOI] [PubMed] [Google Scholar]
- 9.Mori K, Yayama T, Nishizawa K, Nakamura A, Mimura T, Imai S. Aortic pulsation prevents the development of ossification of anterior longitudinal ligament toward the aorta in patients with diffuse idiopathic skeletal hyperostosis (DISH) in Japanese: results of chest CT-based cross-sectional study. J Orthop Sci. 2019;24(1):30–34. doi: 10.1016/j.jos.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Baraliakos X, Listing J, Buschmann J, von der Recke A, Braun J. A comparison of new bone formation in patients with ankylosing spondylitis and patients with diffuse idiopathic skeletal hyperostosis: a retrospective cohort study over six years. Arthritis Rheum. 2012;64(4):1127–1133. doi: 10.1002/art.33447. [DOI] [PubMed] [Google Scholar]
- 11.Mader R, Pappone N, Baraliakos X, Eshed I, Sarzi-Puttini P, Atzeni F, et al. Diffuse idiopathic skeletal hyperostosis (DISH) and a possible inflammatory component. Curr Rheumatol Rep. 2021;23(1):6. doi: 10.1007/s11926-020-00972-x. [DOI] [PubMed] [Google Scholar]
- 12.Kuperus JS, de Gendt EEA, Oner FC, de Jong PA, Buckens SCFM, van der Merwe AE, et al. Classification criteria for diffuse idiopathic skeletal hyperostosis: a lack of consensus. Rheumatology. 2017;56(7):1123–1134. doi: 10.1093/rheumatology/kex056. [DOI] [PubMed] [Google Scholar]
- 13.Kuperus JS, Oudkerk SF, Foppen W, Hoesein FAM, Gielis WP, Waalwijk J, et al. Criteria for early-phase diffuse idiopathic skeletal hyperostosis: development and validation. Radiology. 2019;291(2):420–426. doi: 10.1148/radiol.2019181695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuperus JS, Mohamed Hoesein FAA, de Jong PA, Verlaan JJ. Diffuse idiopathic skeletal hyperostosis: etiology and clinical relevance. Best Pract Res Clin Rheumatol. 2020;34(3) doi: 10.1016/j.berh.2020.101527. [DOI] [PubMed] [Google Scholar]