Abstract
Bacterial biofilms are a major healthcare concern resulting in refractory conditions such as chronic wounds, implant infections and failure, and multidrug-resistant infections. Aggressive and invasive strategies are employed to cure biofilm infections but are prone to long and expensive treatments, adverse side-effects, and low patient compliance. Recent strategies such as ultrasound-based therapies and antimicrobial nanomaterials have shown some promise in the effective eradication of biofilms. However, maximizing therapeutic effect while minimizing healthy tissue damage is a key challenge that needs to be addressed. We report here a combination treatment involving ultrasound and antimicrobial polymeric nanoparticles (PNPs) that synergistically eradicate bacterial biofilms. Ultrasound treatment rapidly disrupts biofilms and increases penetration of antimicrobial PNPs thereby enhancing their antimicrobial activity. This resulted in superior biofilm toxicity, while allowing for a 2- to 6- fold reduction in both the concentration of PNPs as well as the duration of ultrasound. Furthermore, we demonstrate that this reduction minimizes cytotoxicity towards fibroblast cells, while resulting in a 100- to 1000- fold reduction in bacterial concentration.
Keywords: Synergistic antimicrobial therapy, ultrasound treatment, polymeric nanoparticles, biofilm eradication, biofilm disruption, MRSA biofilm treatment
Graphical Abstract
Bacterial biofilm infections often result in refractory conditions such as chronic wounds, requiring aggressive and invasive treatment strategies with high costs and poor patient compliance. We report a combination treatment of ultrasound and antimicrobial polymeric nanoparticles which rapidly and effectively eradicates bacterial biofilms. Ultrasound treatment enhances the penetration of nanomaterials and the susceptibility of bacteria, resulting in synergistic antibacterial activity.
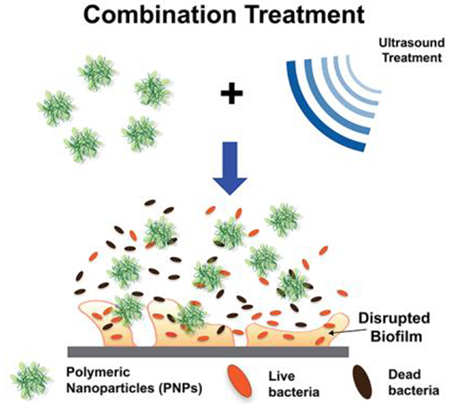
1. Introduction
Bacterial infections are a major healthcare concern, presenting several acute threats such as chronic wounds[1] and implant-associated infections and failure.[2, 3] Approximately 1.7 million hospital-acquired infections occur in the United States alone annually, resulting in a financial burden of 11 billion USD.[4] This challenge is further exacerbated by formation of bacterial biofilms, which are bacterial communities surrounded by a self-secreted extracellular polymeric substance (EPS).[5, 6] Biofilms promote the spread of infection and development of drug resistance by protecting bacteria from the host immune response and antimicrobial agents.[7, 8] Conventional therapies like antibiotics are unable to effectively penetrate through the EPS,[9, 10] are deactivated in the matrix,[11] and are rapidly removed from the microenvironment through efflux pumps.[12] Aggressive treatment strategies are often employed to treat biofilm infections involving surgical debridement of infected tissue, high doses of antibiotics or the use of last-resort antibiotics. [13, 14] These strategies result in low patient compliance due to long and expensive treatments with the possibility of adverse side-effects. Therefore, recent efforts have focused on developing minimally invasive strategies that can disrupt the biofilm to enhance the penetration of therapeutics, while minimizing side-effects.
External triggers including electric[15] or photomechanical fields,[16] have been utilized for treating bacterial biofilm infections through enhanced penetration of antibiotics or ablation. In this regard, ultrasound (US)-based treatments have gained interest for eradicating biofilms due to its enhanced tissue penetration and the ability to localize treatment to infected area.[17] Ultrasonic waves (frequency > 20 kHz) are pressure waves transmitted through the expansion and contraction of a medium.[18, 19] The ability of ultrasound to permeabilize tissue through thermal and cavitation effects is expected to result in enhanced penetration of antimicrobial agents into biofilms.[20] Heat generated from ultrasound has been utilized for ablation of infected tissue and for the localized release of drugs.[21, 22, 23, 24, 25] Cavitation, or the formation of microbubbles in the media during US treatment, results in shear forces inside bacterial cells causing pore formation, disruption of bacterial biofilms, and eventually to cell membrane disruption.[26] Several reports have also demonstrated that ultrasound enhanced the antimicrobial efficacy of commercial antibiotics, such as gentamicin, against E. coli biofilms by enhancing their penetration in vivo.[27, 28, 29] However, prolonged exposure to ultrasound leads to tissue damage, either due to the hyperthermic effect on surrounding tissue or due to the cavitation-associated damage to healthy cells, thereby contributing to delayed wound-healing. [30]
Nanomaterials have gained interest as antimicrobials owing to their tunable morphological and physicochemical properties such as size, shape, and surface chemistry.[31] Consequently, nanomaterials have been engineered to enhance biofilm penetration,[32] impart targeting or stimuli-responsive behavior,[33, 34] and facilitate localized delivery of antimicrobial agents.[35] For these reasons, nanotherapeutics are increasingly gaining interest as potential replacements for traditional antibiotics, particularly for the treatment of multi-drug resistant infections. For instance, cationic nanomaterials show enhanced penetration into biofilms and are also able to disrupt bacterial cell membranes due to the overall negative charge of the EPS. [36, 37] We have previously demonstrated the broad-spectrum antibacterial activity of polyoxanorborneneimide (PONI) – based polymers with a cationic trimethylammonium (TMA) headgroup. [38, 39] These antimicrobial polymeric nanoparticles (PNPs) are active against both Gram-positive and Gram-negative bacterial species, including dual-species biofilms. Furthermore, bacteria are unable to develop drug resistance against cationic PNPs due to their membrane disruption mechanism. However, prolonged exposure and higher doses of such cationic nanomaterials leads to disruption of healthy cell membrane leading to increased toxicity towards mammalian cells. Therefore, maximizing the therapeutic effect while minimizing host toxicity is a key challenge when designing antimicrobial nanomaterials.
Our strategy employs a combination of ultrasound and cationic antimicrobial polymeric nanoparticles (PNPs) for the rapid eradication of bacterial biofilms (as shown in Figure 1) with minimal toxicity to mammalian cells. We hypothesized that ultrasound treatment would enhance the antimicrobial activity of nanomaterials by rapidly disrupting the biofilm matrix and increasing the susceptibility of bacterial cells to the treatment. Furthermore, the disruption of the bacterial membrane due to the combined activity of acoustic cavitation and cationic PNPs allows us to reduce both the concentration of PNPs utilized and exposure to US treatment. For this study, we utilized the polymer PONI-C11-TMA (synthesis described in Figure S1). As shown in Figure 1(a), PONI-C11-TMA is based on a poly(oxanorborneneimide) (PONI) backbone, with a C11 alkyl sidechain and cationic trimethyl ammonium (TMA) headgroup and self-assembles into nanoparticles (size in Figure 1(b). As demonstrated in Figure S2, PNPs remained stable throughout the duration of ultrasound treatment, showing no significant change in size. Previous studies employ longer incubation times (~ 3hr) to enable penetration into and disruption of bacterial biofilms. This prolonged exposure as well as high concentrations, however, results in toxicity towards mammalian cells. In this study, we demonstrated that utilizing ultrasound in combination with PNPs results in rapid eradication of biofilms, with > 80% of the bacteria being eliminated within the first 30 min of combination treatment. This combination of ultrasound and PNPs was tested against biofilms of both Gram-positive and Gram-negative strains and was demonstrated to be effective. Furthermore, we observed synergistic or additive behavior in most combinations, resulting in a 2- to 6- fold reduction in both the concentration of PNPs and the duration of ultrasound treatment which minimizes the off-target effects of both treatments. We demonstrated through a co-culture model that this reduction in the concentration of PNPs and duration of ultrasound treatment resulted in minimized cytotoxicity to fibroblast cells, while resulting in a 100- to 1000- fold reduction in bacterial cell concentration.
Figure 1.
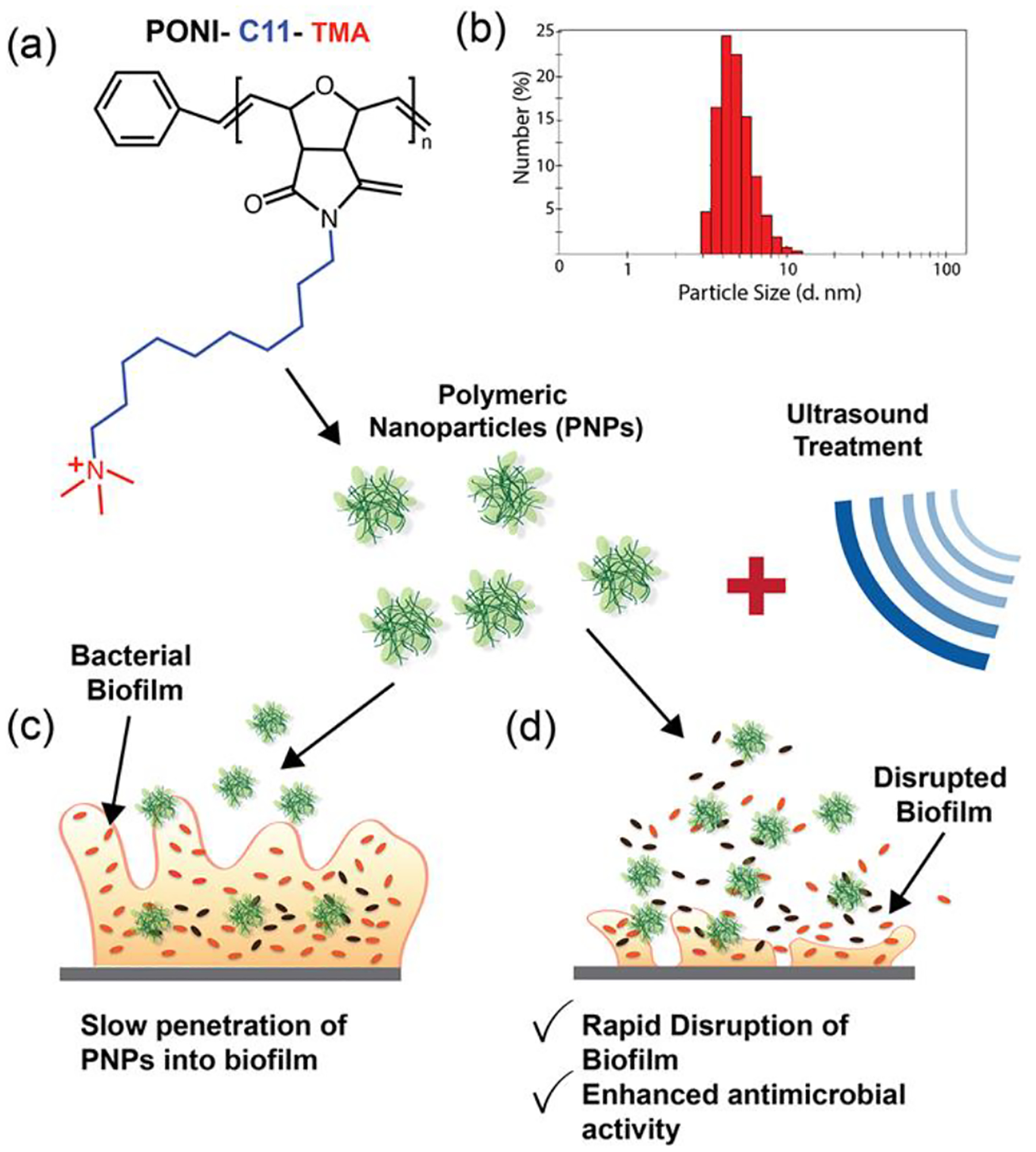
Schematic representation of effect of combination treatment on bacterial biofilms. (a) Chemical structure of cationic polymer PONI-C11-TMA with the poly(oxanorboroneneimide) backbone (in black), a C11 alkyl sidechain (in blue), and a cationic trimethyl ammonium headgroup (in red). (b) Hydrodynamic radius of PNPs, as measured by dynamic light scattering. (c) Scheme depicting slow penetration of PNPs into biofilms and (d) Scheme depicting rapid biofilm disruption and enhanced antibacterial activity due to ultrasound treatment.
2. Results and Discussion
2.1. Effect of Ultrasound Treatment on Biofilm Infections
Our initial studies focused on the effect of ultrasound treatment on bacterial biofilms by observing biofilms pre- and post-treatment using confocal microscopy. Protocols described by Müsken et al.[40] and Haney et al.[41] were modified and utilized for our experiments for growing and testing all bacterial biofilms. GFP-expressing MRSA were first grown to stationary phase in LB media. The culture tubes were then centrifuged to collect the bacteria and washed 3 times using a 0.85% NaCl solution followed by resuspension in PBS. Following this, the concentration of bacteria was determined by measuring the optical density of the suspension at 600 nm (OD600; 1 O.D600 = 109 cfu/mL). Seeding solutions were then prepared at 108 cfu/mL by diluting the bacteria in Tryptic Soy Broth (TSB) media. 2 mL of the seeding solution were then placed in a glass bottom confocal dish and incubated at 37 °C for 24 hr to generate MRSA biofilms. Mature biofilms were ~ 20 μm thick, as seen in Figure 2. Biofilms were then treated with ultrasound (conditions described in the Experimental Methods section) for varying durations. As shown in Figure 2, biofilm disruption begins ~40 s post exposure to ultrasound and is fully disrupted after about 300s of treatment. This demonstrated that short durations of exposure to ultrasound can cause significant disruption of the biofilm thereby enhancing penetration of antimicrobial agents.
Figure 2.
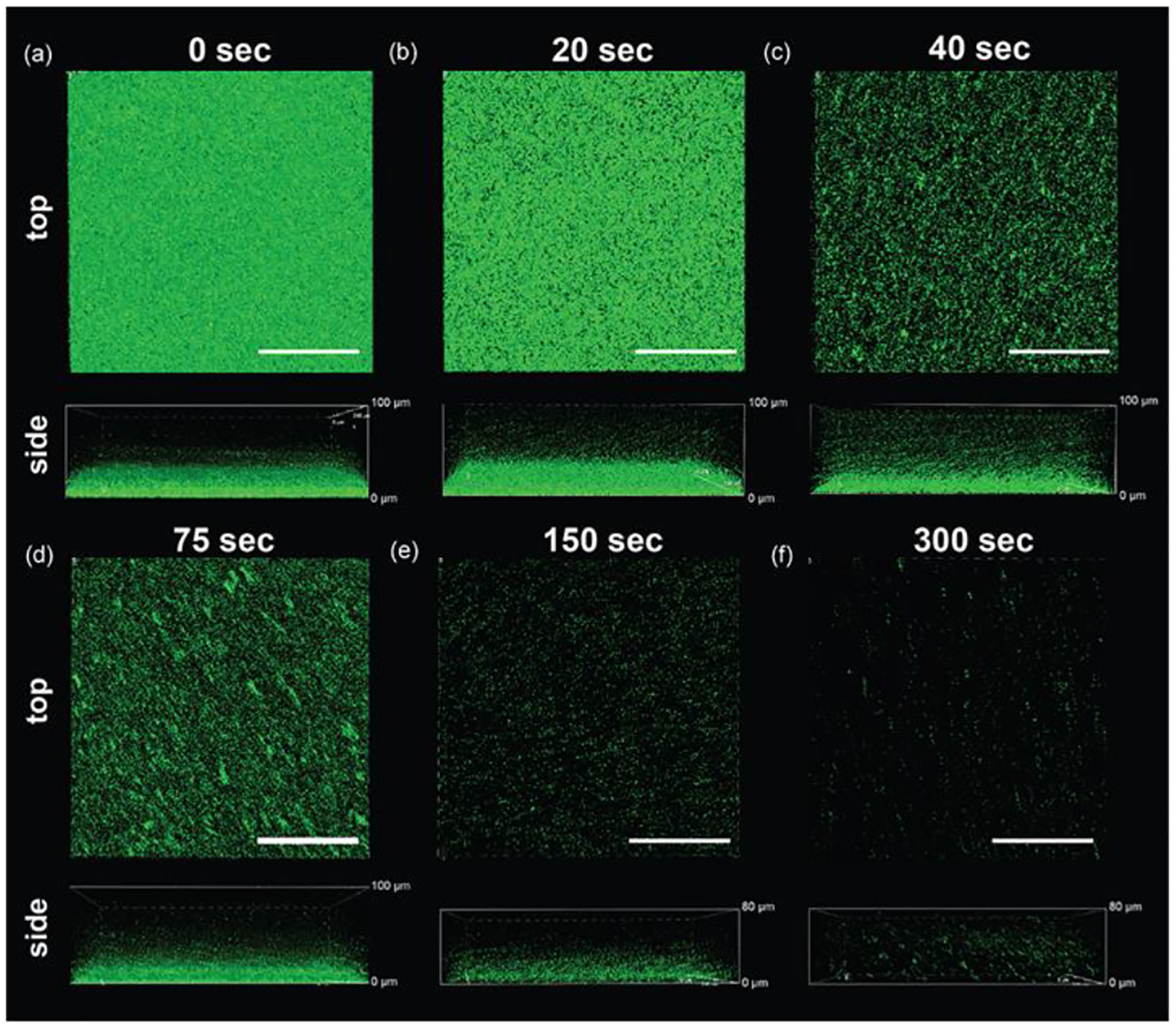
Top and side view confocal microscopy images demonstrating US-mediated biofilm disruption of MRSA-GFP biofilm. (a) Native untreated biofilm. Biofilms treated with ultrasound for (b) 20 s (c) 40 s (d) 75 s (e) 150 s and (f) 300 s. It can be seen that biofilm disruption begins at ~40s and is almost fully disrupted at ~300 s. Scale bar is 100 μm.
In addition to biofilm disruption, ultrasound treatment may also result in antimicrobial activity. We tested this on both Gram-positive and negative bacterial biofilms (results summarized in Figure 3). E. coli (CD2), P. aeruginosa (CD1006), MRSA (6169) and S. epidermidis (7073) biofilms were grown using the protocol described above. Mature biofilms were treated with ultrasound for varying durations. Following this, %bacterial viability was measured using alamarBlue assay and %toxicity was calculated and summarized in Figure 3. Ultrasound treatment resulted in ~80% toxicity across all strains, as seen in Figure 3 (a, b, c and d). This is primarily attributed to cavitation and mechanical disruption of bacterial cell membranes. This is further evidenced by the disrupted cell membranes of MRSA (Figure 3(e)), E. coli (Figure 3(f)) and P. aeruginosa (Figure 3(g)) observed through propidium iodide staining. Furthermore, as seen in Figure S3, the thermal effect from ultrasound treatment (increase in temperature from 25 °C to 40 °C) is not sufficient for antimicrobial activity. Additionally, ultrasound treatment over 600s did not result in sufficient changes Gram-positive strains were more resilient to the treatment. Both MRSA and S. epidermidis required ~600 s of ultrasound treatment to eliminate 60% of the bacteria, while E. coli and P. aeruginosa required ~200 s. This is explained by the presence of the peptidoglycan layer around Gram-positive bacteria which protects the cells from cavitation-associated membrane disruption.[42] Figure 3(e), (f) and (g) show fluorescence microscopy images of the dead bacteria stained by propidium iodide stain, which is further proof of the membrane disruption as a result of the ultrasound treatment.
Figure 3.
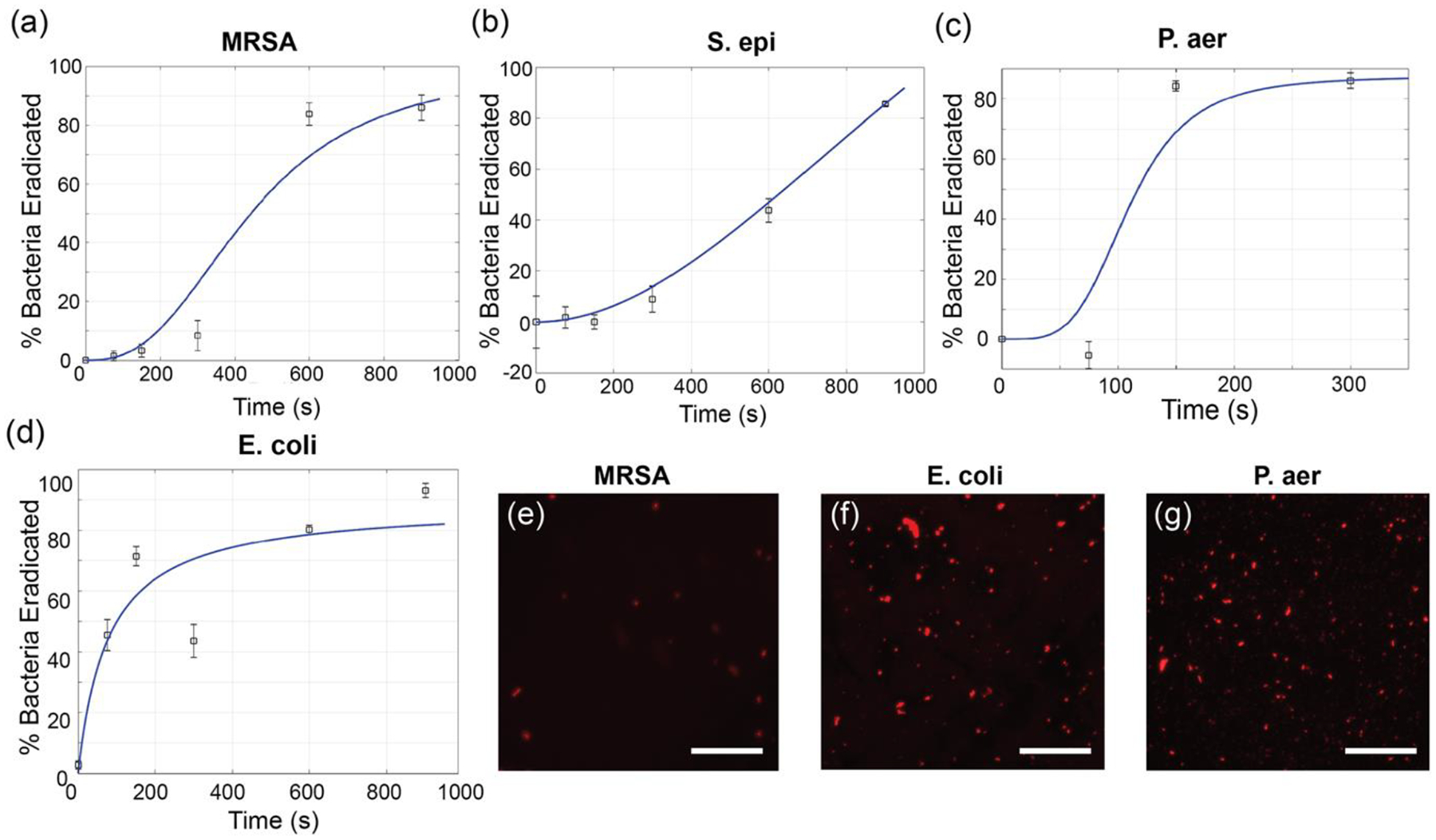
Broad-spectrum antibacterial activity of US treatment. Each graph represents %Bacteria Eradicated at different durations of ultrasound treatment for Gram-positive (a) MRSA and (b) S. epidermidis, and negative (c) P. aeruginosa and (d) E. coli biofilms. Error bars represent standard deviation (n=5). Solid blue line in (a), (b) and (c) show trends consistent with Hill Equation while (d) is consistent with Michelis-Menten Equation. Fluorescence images post pI staining for (e) MRSA (f) E. coli and (g) P. aeruginosa show dead bacteria. Scale bar is 50 μm.
2.2. Synergistic or Additive Combination therapy of Ultrasound and PNPs
We next studied the potential for ultrasound treatment to enhance the antibacterial activity of polymeric nanomaterials. We have previously demonstrated the broad-spectrum antibacterial activity of the cationic PNPs.[32, 33] Furthermore, bacteria were unable to develop resistance against PNPs owing to its membrane disruption mechanism. However, higher concentrations and longer treatment time have to be utilized for effectively eliminating biofilms using PNPs, which contributes to increased cytotoxicity. Ultrasound treatment is expected to rapidly disrupt the bacterial biofilm and also enhance the bacterial membrane permeability. For this reason, we hypothesized that a combination of ultrasound and PNPs will rapidly eliminate bacterial biofilms through a synergistic or additive behavior.
This combination therapy was tested on MRSA, E. coli and P. aeruginosa biofilms grown according to the protocol described previously. A modified protocol of checkerboard titrations was utilized to systematically study different combinations of PNPs and ultrasound treatment.[43, 44] The mature biofilms were then treated with a combination of PNP solution, concentration ranging from 0 to 0.25 mg/mL, and ultrasound ranging from 0 to 600 s. The biofilms were then incubated at 37 °C for 30 min and tested with alamarBlue to calculate bacterial cell viability. Figure 4 summarizes the results of %toxicity for each treatment condition in a heatmap where each cell represents a different treatment condition, and the color represents % toxicity. As seen in Figure 4(a), MRSA has limited susceptibility to ultrasound treatment or PNPs individually, exhibiting 80% toxicity at 600s of ultrasound or 0.5 mg/mL of PNP solution. However, a 2- to 6- fold reduction of ultrasound treatment time and 2- to 4- fold reduction of PNP concentration may be obtained through combination treatment for the same level of toxicity. Similarly, a 4- fold reduction in both ultrasound treatment time and PNP concentration was observed in the case of P. aeruginosa, as observed in Figure 4(b) while a 2- to 6- fold reduction in both ultrasound treatment time and PNP concentration was observed for E. coli. These results demonstrate that a combination treatment of ultrasound and PNPs has broad-spectrum activity and can be utilized against both Gram-positive and negative infections. Furthermore, we tested the prolonged effect of ultrasound treatment on the susceptibility of bacteria to PNPs by increasing the duration of administration of PNPs after ultrasound treatment. As seen in Figure S4, bacteria showed increased susceptibility towards PNPs up to 15 min post ultrasound treatment. Figure 4(d), (e) and (f) show fluorescence microscopy images of the dead bacteria stained by propidium iodide stain, which is further proof of the membrane disruption as a result of the combination treatment.
Figure 4.
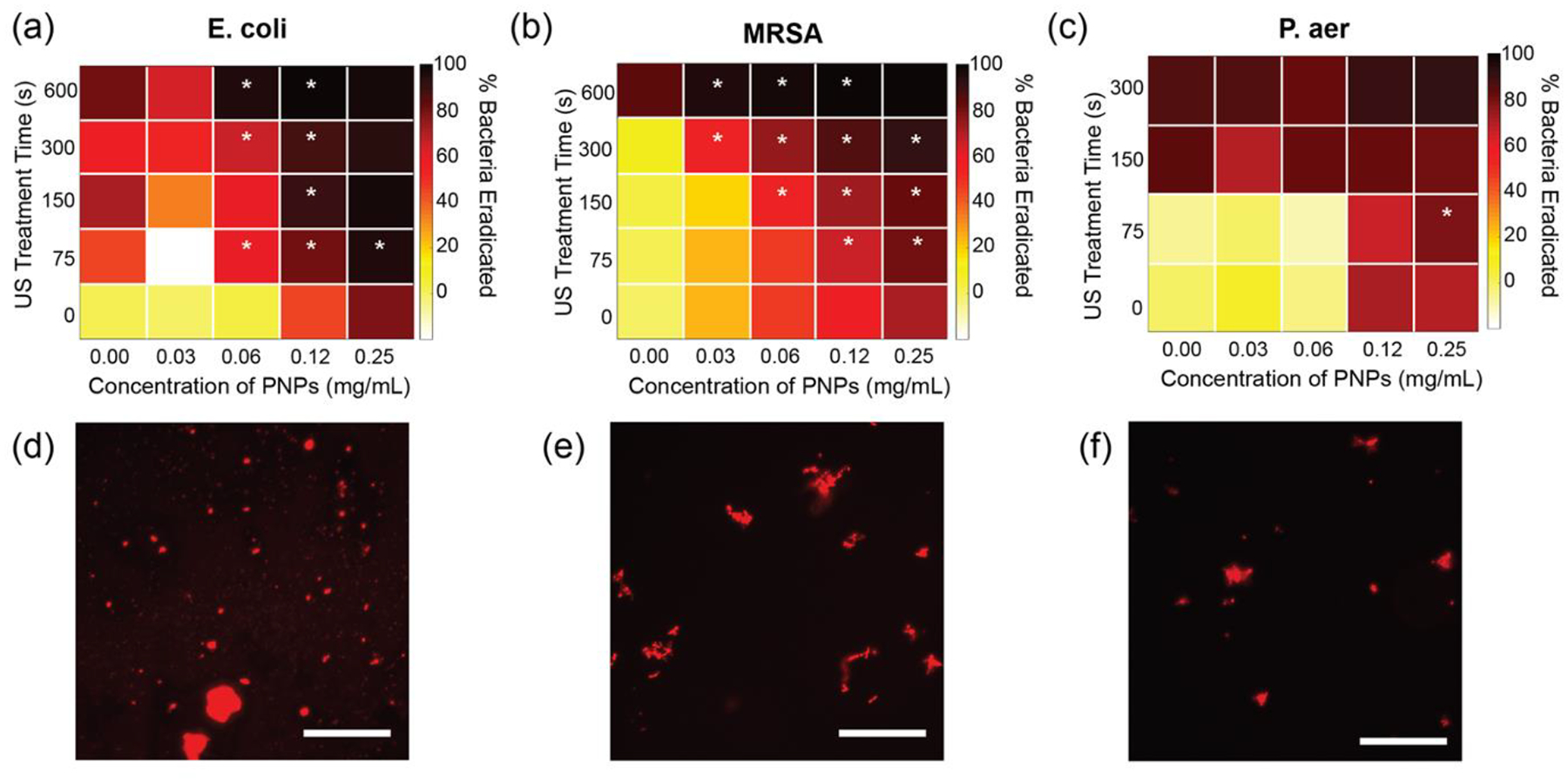
Antibacterial activity of combination therapy. % Bacteria Eradicated due to combination therapy, as measured by alamarBlue assay for (a) E. coli (b) MRSA and (c) P. aeruginosa biofilms. Conditions where synergy was observed are marked by *. E. coli and MRSA show synergy at several combinations and additivity for most others. P. aeruginosa shows mostly additive behaviour, presumably due to the strong antibiofilm effect of ultrasound treatment alone on P. aeruginosa biofilms. (d), (e) and (f) show fluorescence microscopy images of dead bacteria visualized by propidium iodide staining. Scale bar is 50 μm. 4–6 replicates were evaluated in each treatment group.
The additive, synergistic or antagonistic behavior of the combination treatments was evaluated using the Bliss independence model.[45] Scores for each condition are shown in Figure S5 and synergistic conditions are marked with an asterisks in Figure 4(a), (b) and (c). As expected, most combinations showed additive behavior. We observed that several synergistic conditions are observed for both MRSA and E. coli. In comparison, only one synergistic points are observed for P. aeruginosa. This is attributed to the significantly high susceptibility of P. aeruginosa to the ultrasound treatment alone (seen in Figure 3). It is also interesting to note that although MRSA biofilms are less susceptible to ultrasound alone, the combination treatment is especially effective against MRSA biofilms, with synergistic behavior observed for 12 treatment groups.
2.3. In vitro Biofilm and Fibroblast Co-culture Model to Study the Effect of Combination Treatment
We have demonstrated that the combination treatment is successful at reducing both the duration of ultrasound treatment as well as the concentration of the PNPs utilized, while preserving antimicrobial efficacy. This approach is not only beneficial for the rapid and efficient eradication of biofilms but can potentially limit side-target effects if it reduces the ‘dosing’ of each individual component. Ultrasound can be focused or directed to an area and therefore enables localized treatment of biofilm infections while minimizing significant damage to healthy tissue. However, prolonged exposure to ultrasound can result in cytotoxicity to healthy cells. On the other hand, PNPs also exhibit cytotoxicity at high concentrations that may be required to eradicate chronic biofilm infections. We therefore hypothesized that the combination treatment would allow us to preserve healthy cells, thereby promoting wound-healing by reducing both the intensity of ultrasound treatment and concentration of PNP utilized.
First, 3T3 fibroblast cells were cultured on collagen-coated wells of a 12 well plate. The collagen coating mimicked the extracellular matrix in native tissues and absorbed some energy from the ultrasound treatment, thereby protecting the cells.[46] As seen in Figure S6, significant differences in fibroblast cell viability was observed on uncoated vs. collagen-coated plates.
100k cells were seeded onto collagen-coated plates and allowed to grow overnight. Non-pathogenic E. coli DH5α bacteria were grown to log phase in LB media and then washed and harvested. Bacteria seeding solution of 108 cfu/mL was prepared in DMEM media and placed on top of the 3T3 cell culture. Plates were then incubated at 37 °C for 6 hr to allow biofilms formation. Following this, co-cultured were treated with a combination of ultrasound and PNPs as shown in Figure 5. 3T3 %viability was evaluated using the LDH cytotoxicity assay and colony-counting was performed to evaluate the bacterial viability. As seen in Figure 5(a), the majority of the cells survived the combination treatment at lower concentrations of PNPs. Although some cytotoxicity was observed at 0.06 mg/mL, ~60% of the cells still survive the combination treatment. 3T3 viability did not significantly change due to ultrasound treatment for any of the treatment groups. However, for the same treatment conditions, a 100- to 1000- fold reduction in the concentration of bacteria was observed, as shown in Figure 3(b). Ultrasound treatment results significant reduction as compared to PNP only for both lower concentrations of PNPs (0.007 mg/mL and 0.015 mg/mL). We therefore concluded that the combination treatment may be utilized to eradicate biofilm infections while minimizing significant tissue damage resulting from both ultrasound and PNP toxicity.
Figure 5.
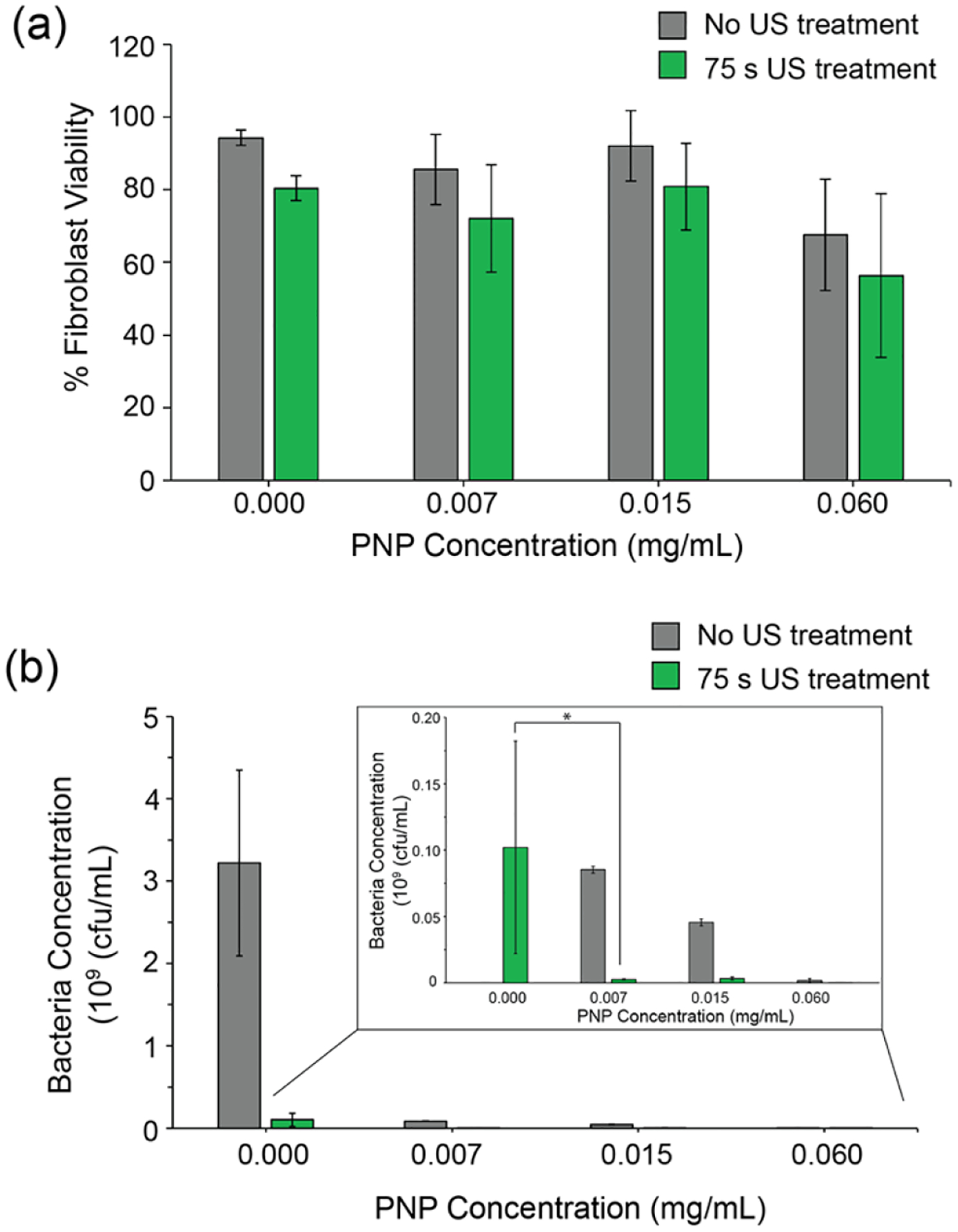
Effect of combination treatment on (a) fibroblast cells and (b) Non-pathogenic E. coli DH5α biofilms in an in vitro co-culture model. (a) % Fibroblast cell viability measured using LDH cytotoxicity assay shows that 60 to 80% of the cells survive the combination treatment. Error bars represent standard deviation (n=8). (b) 100- to 1000- fold reduction in bacteria concentration was observed during combination treatment. Bacteria concentration was measured by colony-counting. Error bars represent S.E.M. (n=4), (*) p < 0.05.
3. Conclusions
This study demonstrates the synergistic and additive therapeutic potential of combined ultrasound treatment and antimicrobial cationic PNPs. Ultrasound treatment rapidly disrupts bacterial biofilms and shows broad-spectrum antibacterial activity. Furthermore, when combined with our previously developed antimicrobial PNPs (PONI-C11-TMA) we observe enhanced antibacterial activity with a 2- to 6- fold reduction in the concentrations of PNPs and duration of ultrasound treatment. This combination treatment results in 100- to 1000- fold reduction in bacterial concentration with minimal toxicity to fibroblast cells. Taken together, this approach provides a rapid and effective method to eradicate biofilm infections, while minimizing tissue damage. Additionally, PNPs minimize the risk of developing drug resistant infections. Therefore, this combined approach provides a robust and effective strategy for treating refractory conditions like chronic wound infections, implant-associated infections, and multi-drug resistant infections. Polymer-based nanomaterials are widely utilized to encapsulate bioactive agents including antibiotics, phytochemicals, inorganic metals, proteins and nucleic acids that serve a wide variety of applications such as targeted therapy and rapid wound-healing. The activity of these agents may further be enhanced using ultrasound treatment through increased penetration of these agents into target site.
4. Experimental Methods
4.1. Materials
All solvents, reagents, and chemicals for synthesis of PNPs were obtained from Fisher Scientific and Sigma Aldrich and used without further purification unless otherwise specified. Bacteria isolates with the code CD were obtained from the Cooley Dickinson Hospital Microbiology Laboratory, Northampton, MA. MRSA (IDRL-6169) and S. epidermidis (IDRL-7073) was from the Infectious Diseases Research Laboratory at Mayo Clinic, Rochester MN. NIH-3T3 fibroblast cells were purchased from ATCC (ATCC CRL-1658). Luria broth, tryptic soy broth, DMEM (Dulbecco’s modified eagle medium) and agar were purchased from Fisher Scientific and used as received. AlamarBlue assay and CyQUANT LDH Cytotoxicity assay were purchased from Invitrogen on Fisher Scientific and used as suggested by the manufacturer. Rat-tail Collagen 1 solution (3–4 mg/mL) was purchased from ThermoFisher and used without further purification.
4.2. Bacterial and Biofilm Cultures
Growing bacteria for all experiments:
All bacteria strains utilized throughout this study (MRSA eGFP, CD2, IDRL-6169, CD1006, IDRL-7073 and E. coli DH5α) were first inoculated by transferring isolated colonies on agar plates into Luria broth (LB) media and grown to stationary phase overnight at 37 °C with aeration and agitation at 275 rpm. Bacteria were then collected and washed thrice through centrifugation at 3000 rpm for 5 min in 0.85% NaCl solution. Following this bacteria were resuspended in PBS media and their optical density (O.D600) was measured at 600 nm (1 O.D600 ~ 109 cfu/mL). Next, bacterial seeding solutions were prepared based on the experiment.
Confocal Imaging of Biofilm Disruption due to Ultrasound Treatment:
MRSA eGFP bacteria were grown and washed according to the protocol described above. A seeding solution of 108 cfu/mL of bacteria was prepared in Tryptic soy broth (TSB) containing 1mM of IPTG. Next, 2 mL of seeding solution each was placed in confocal dishes and allowed to incubate at 37 °C overnight to form biofilms. Mature biofilms were then washed once with PBS and then replaced with 2 mL of M9 media before ultrasound treatment. Treated biofilms were then washed with PBS to remove free-floating planktonic bacteria and imaged on a Zeiss LSM 510 Meta microscope, from the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, UMass Amherst, by using a 40× objective. The settings of the confocal microscope were as follows: green channel, λex = 488 nm and λem = LP 540 nm; red channel, λex = 560 nm and λem = LP 640 nm; blue channel, λex = 403 nm and λem = LP 495 nm. Emission filter: LP = high pass.
Propidium Iodide staining assay:
MRSA (6169), E. coli (CD2), and P. aeruginosa (CD1006) solutions were prepared in M9 media to a concentration of 108 cfu/mL. 2 mL of this solution was added to each well of a 12 well plate and treated with either ultrasound for 600 s(Figure 3) or a combination of 150 s ultrasound and 0.125 mg/mL of PNPs (Figure 4) and then then incubated for 30 min at 37 °C. Propidium iodide was then added to each treated bacteria solution such that the final PI concentration was 2 μM and then incubated in the dark at 37 °C for 30 min. 5 μL of each solution was then placed on a glass slide with a glass coverslip and observed with fluorescence microscopy, using an Olympus IX51 Inverted Phase Contrast Fluorescence Microscope with a 100W Mercury lamp and a QICAM fast 1394 digital camera from QICAM.
Biofilm growth for all eradication studies:
MRSA (6169), E. coli (CD2), P. aeruginosa (CD1006) and S. epidermidis (7073) bacteria were grown and washed according to the protocol described above. A seeding solution of 108 cfu/mL of bacteria was prepared in Tryptic soy broth (TSB). Next, 1 mL of seeding solution was placed in each well of a 12-well plate and allowed to incubate at 37 °C overnight to form biofilms. Mature biofilms were washed once with PBS and then replaced with 2 mL of M9 media before ultrasound (Figure 3) or combination (Figure 4) treatment. Treated biofilms were resuspended through vigorous pipetting before transferring to multiple wells of a 96 well plate (90 μL per well, 3–4 replicates per treatment group).
%Toxicity Measurements:
%Biofilm Toxicity (100 - %viability) was measured through the alamarBlue assay using the protocol for suspension cells, provided by the supplier. Each well was then spiked with 10 μL of alamarBlue solution and incubated at 37 °C for 30 min. Fluorescence was measured at 560 nm Ex and 590 nm Em. %Bacterial cell viability was calculated with respect to untreated growth control and then subtracted from 100 to calculate %Toxicity.
4.3. Co-culture Model Studies
Fabrication of collagen-coated plates:
A modified version of the ThermoFisher protocol was utilized to prepare thick coatings. Rat-tail collagen 1 solution (3–4 mg/mL) was first diluted to 1 mg/mL in milliQ water. 1 mL of the collagen solution was placed into each well of a sterile 12 well plate. The plates were then incubated at 37 °C for 1 hr to allow for the formation of stable coatings on the plate. Excess collagen solution was removed after 1 hr and the plates were washed with PBS twice. Collagen-coated plates were utilized immediately for culturing cells.
Fibroblast cell culture:
Briefly, NIH-3T3 fibroblast cells (ATCC CRL-1658) were cultured in DMEM in the presence of 10% fetal bovine serum and 1% antibiotic solution. 100,000 cells/well were plated on uncoated and collagen-coated wells and allowed to grow overnight at 37 °C in a humidified atmosphere of 5% CO2.
Biofilm culture and treatment:
E. coli DH5α was to stationary phase overnight in LB media. Log-phase cultures were then prepared by spiking 160 μL of stationary phase in 3 mL of TSB media and allowed to grow for 3 hr in a shaker set at 37 °C, 300 rpm. Log-phase cultures were then washed and isolated per the protocol described above and diluted to 108 cfu/mL in antibiotic-free cell culture media (10% FBS + DMEM) to prepare seeding solutions. 1mL of the seeding solution was placed in each well of the fibroblast culture and allowed to incubate for 6 hr at 37 °C to prepare biofilms. Co-cultures were then treated with either a combination of ultrasound and PNPs, or individual treatments/ control groups and incubated at 37 °C for 30 min. Negative controls were co-culture groups that were left untreated. Positive controls (for LDH) were co-culture groups that were treated with Lysis buffer only.
Cell viability measurement:
3T3 viability was measured using the CyQUANT LDH cytotoxicity assay kit from Invitrogen. The protocol provided by the supplier was utilized for this assay. Briefly, the supernatant from treatment and control groups were harvested and placed in 96 well plates (50 μL × 4 per replicate (3) of each treatment/ control group). To these, 50 μL of the Reaction mixture (prepared by mixing the reactant and substrate mix) was added and mixed gently. Plates were then incubated for 30 min at 37 °C and finally 50 μL of the stop solution was added to each well. Absorbance of each well was measured at 490 and 680 nm. To determine LDH activity, first the absorbance at 680 nm was subtract from the absorbance at 490 nm for each well. %Cytotoxicity was calculated as 100 × ((Treatment group Activity – Negative Control Activity)/(Positive Control Activity – Negative Control Activity)). %Cell viability was calculated as 100 - %cytotoxicity.
Colony counting:
After collecting the supernatant for LDH assay, the remaining solution was homogenized through vigorous pipetting to resuspend the bacteria. Solutions were then collected and diluted up to 6 times (up to 10−6 of original solution). 10 μL from each dilution was then plated onto agar plates and allowed to grow overnight at 37 °C. Biofilm colonies in each plate were counted and multiplied with the appropriate dilution factor to calculate cfu/mL of bacteria that survived the treatment. Values were reported as Log10(cfu/mL).
4.4. Ultrasound Treatment
Ultrasound treatment was performed using a Vibracell 20 kHz 130-watt ultrasonic processor (VCX130) with a 6 mm probe at 35% amplitude for all studies. The duration for each treatment was varied from a range of 60 s to 900 s. It was ensured that at least 2 mL of media was present in the well prior to treatment.
4.5. Statistical Analysis
Data for Figures 3 and 4 were obtained by processing the fluorescence intensity data obtained through the Alamar Blue assay described above. Each treatment group involved at least 4 replicates. LDH analysis in Figure 5(a) was processed as described above in 4.3, and outliers were excluded on the basis of box plot analysis resulting in at least 8 remaining replicates per treatment group. Colony counting data (Figure 5(b)) was obtained on the basis of at least 4 replicates per treatment group. Bar graphs represent mean data with error bars representing ± S.D. except in the case of Figure 5(b) where error bars represent ± S.E.M. Bliss Synergy model was utilized in Figure 4 to evaluate synergistic or additive behavior. Significance (p-value) was calculated using two-sample, two-sided t-tests, wherever applicable. MATLAB (v, 2019a) was utilized for statistical analysis.
Supplementary Material
Acknowledgements
VR acknowledges support by the National Institutes of Health under R01 AI134770. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author. Supplementary data including synthesis protocol and characterization of PNPs, thermal effect of ultrasound treatment, prolonged bacterial susceptibility post US treatment, calculations for synergy and the effect of ultrasound on fibroblast cells cultured on collagen-coated plates.
Contributor Information
Sanjana Gopalakrishnan, Department of Chemistry, University of Massachusetts-Amherst, Amherst, Massachusetts, 01003, USA..
Aarohi Gupta, Department of Chemistry, University of Massachusetts-Amherst, Amherst, Massachusetts, 01003, USA..
Jessa M. V. Makabenta, Department of Chemistry, University of Massachusetts-Amherst, Amherst, Massachusetts, 01003, USA.
Jungmi Park, Department of Chemistry, University of Massachusetts-Amherst, Amherst, Massachusetts, 01003, USA..
John J. Amante, Department of Biomedical Engineering, University of Massachusetts, Amherst, Amherst, Massachusetts, 01003, USA.
Aritra Nath Chattopadhyay, Department of Chemistry, University of Massachusetts-Amherst, Amherst, Massachusetts, 01003, USA..
Dorcas Matuwana, Department of Biomedical Engineering, University of Massachusetts, Amherst, Amherst, Massachusetts, 01003, USA..
Cathal J. Kearney, Department of Biomedical Engineering, University of Massachusetts, Amherst, Amherst, Massachusetts, 01003, USA.
Vincent M. Rotello, Department of Chemistry, University of Massachusetts-Amherst, Amherst, Massachusetts, 01003, USA.
References
- [1].Wolcott RD; Ehrlich GD JAMA 2008, 299 (22), 2682–2684. [DOI] [PubMed] [Google Scholar]
- [2].Van Epps JS; Younger JG Shock 2016, 46 (6), 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Donlan RM Emerg. Infect. Dis 2001, 7 (2), 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Römling U; Kjelleberg S; Normark S; Nyman L; Uhlin BE; Åkerlund BJ Intern. Med 2014, 276 (2), 98–110. [DOI] [PubMed] [Google Scholar]
- [5].Flemming HC; Wingender J Nat. Rev. Microbiol 2010, 8 (9), 623–633. [DOI] [PubMed] [Google Scholar]
- [6].Arciola CR; Campoccia D; Montanaro L Nat. Rev. Microbiol 2018, 16 (7), 397–409. [DOI] [PubMed] [Google Scholar]
- [7].Sharma D; Misba L; Khan AU Antimicrob. Resist. Infect. Control 2019, 8 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ciofu O; Moser C; amp P; Jensen strup; Hamp N. Nat. Rev. Microbiol 2022, 1–15. [DOI] [PubMed] [Google Scholar]
- [9].Cao B; Christophersen L; Thomsen K; Sønderholm M; Bjarnsholt T; Jensen PØ; Høiby N; Moser CJ Antimicrob. Chemother 2015, 70 (7), 2057–2063. [DOI] [PubMed] [Google Scholar]
- [10].Cao B; Christophersen L; Kolpen M; Jensen PØ; Sneppen K; Høiby N; Moser C; Sams T PLoS One 2016, 11 (4), e0153616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kranjec C; Angeles DM; Mårli MT; Fernández L; García P; Kjos M; Diep DB Antibiot. 2021, 10 (2), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Soto SM Virulence. 2013, 4 (3), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Namgoong S; Jung SY; Han SK; Kim AR; Dhong ES J. Plast. Surg. Hand Surg 2020, 54 (1), 47–54. [DOI] [PubMed] [Google Scholar]
- [14].Wu H; Moser C; Wang HZ; Høiby N; Song ZJ Int. J. Oral Sci 2015, 7 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Soukos SN, Socransky SS, Mulholland SE, Lee S, Doukas AG Pharm. Res 2000, 17, 405–409. [DOI] [PubMed] [Google Scholar]
- [16].Del Pozo JL, Rouse MS, Patel R The International Journal of Artificial Organs. 2008, 31(9), 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hayes BT; Merrick MA; Sandrey MA; Cordova ML J. Athl. Train 2004, 39 (3), 230. [PMC free article] [PubMed] [Google Scholar]
- [18].LuTheryn G; Glynne-Jones P; Webb JS; Carugo D Microb. Biotechnol 2020, 13 (3), 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nakonechny F; Nisnevitch M; Nakonechny F; Nisnevitch M Adv. Funct. Mater 2021, 31 (44), 2011042. [Google Scholar]
- [20].Oberli MA, Schoellhammer CM, Langer R, Blankschtein D Ther Deliv. 2014, 5(7), 843–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O’Brien WD Prog. Biophys. Mol. Biol 2007, 93 (1–3), 212–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ouyang J; Tang Z; Farokhzad N; Kong N; Kim NY; Feng C; Blake S; Xiao Y; Liu C; Xie T; et al. Nano Today 2020, 35, 100949. [Google Scholar]
- [23].Huebsch N, Kearney CJ, Zhao X, Kim J, Cezar CA, Suo Z and Mooney DJ, Proc. Natl. Acad. Sci. U. S. A, 2014, 111, 9762–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ahmad T, McGrath S, Sirafim C, Do Amaral RJFC, Soong SL, Sitram R, Turkistani S, Santarella F and Kearney CJ, Biomater. Sci, 2021, 9, 4278–4288. [DOI] [PubMed] [Google Scholar]
- [25].Kearney CJ, Skaat H, Kennedy SM, Hu J, Darnell M, Raimondo TM, Mooney DJ, Adv. Healthc. Mater, 2015, 4, 1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kimmel E Crit. Rev. Biomed. Eng 2006, 34 (2), 105–161. [DOI] [PubMed] [Google Scholar]
- [27].Rediske AM; Roeder BL; Brown MK; Nelson JL; Robison RL; Draper DO; Schaalje GB; Robison RA; Pitt WG Antimicrob. Agents Chemother 1999, 43 (5), 1211–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peterson RV; Pitt WG Colloids Surfaces B Biointerfaces 2000, 17 (4), 219–227. [Google Scholar]
- [29].Rediske AM; Roeder BL; Nelson JL; Robison RL; Schaalje GB; Robison RA; Pitt WG Antimicrob. Agents Chemother 2000, 44 (3), 771–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ebbini ES; Ter Haar G 2015, 31 (2), 77–89. [DOI] [PubMed] [Google Scholar]
- [31].Makabenta JMV; Nabawy A; Li CH; Schmidt-Malan S; Patel R; Rotello VM 2020. 19 (1), 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mahmoudi M; Serpooshan V ACS Nano 2012, 6 (3), 2656–2664. [DOI] [PubMed] [Google Scholar]
- [33].Wu J; Li F; Hu X; Lu J; Sun X; Gao J; Ling D ACS Cent. Sci 2019, 5 (8), 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koo H; Allan RN; Howlin RP; Stoodley P; Hall-Stoodley L Nat. Rev. Microbiol 2017, 15 (12), 740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li CH; Landis RF; Makabenta JM; Nabawy A; Tronchet T; Archambault D; Liu Y; Huang R; Golan M; Cui W; et al. Mater. Horizons 2021, 8 (6), 1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carmona AM; Id R Int. J. Environ. Res. Public Heal 2018, 15 (7), 1408. [Google Scholar]
- [37].Gupta A; Mumtaz S; Li CH; Hussain I; Rotello VM Chem. Soc. Rev 2019, 48 (2), 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gupta A; Landis RF; Li CH; Schnurr M; Das R; Lee YW; Yazdani M; Liu Y; Kozlova A; Rotello VM J. Am. Chem. Soc 2018, 140 (38), 12137–12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Makabenta JMV; Park J; Li CH; Chattopadhyay AN; Nabawy A; Landis RF; Gupta A; Schmidt-Malan S; Patel R; Rotello VM Molecules 2021, 26 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Müsken M, Fiore SD, Römling U, Häussler S Nat. Protoc 2010, 5, 1460–1469. [DOI] [PubMed] [Google Scholar]
- [41].Haney EF, Trimble MJ, Hancock RE W. Nat. Protoc 2021, 16, 2615–2632. [DOI] [PubMed] [Google Scholar]
- [42].Monsen T; Lövgren E; Widerström M; Wallinder L J. Clin. Microbiol 2009, 47 (8), 2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Odds FC, Journal of Antimicrobial Chemotherapy, 2003, 52(1), 1. [DOI] [PubMed] [Google Scholar]
- [44].Gupta A, Saleh NM, Das R, Landis RF, Bigdeli A, Motamedchaboki K, Campos AR, Pomeroy K, Mahmoudi M, Rotello VM Nano Futures, 2017, 1, 015004. [Google Scholar]
- [45].Tang J, Wennerberg K and Aittokallio T, Front. Pharmacol, 2015, 6, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kanta J, Cell Adh. Migr, 2015, 9, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


