Abstract
Purpose:
The study aims to (1) examine the spatiotemporal map of magnetoencephalography-evoked responses during an Auditory Memory Retrieval and Silent Repeating (AMRSR) task, and determine the hemispheric dominance for language, and (2) evaluate the accuracy of the AMRSR task in Wernicke and Broca area localization.
Methods:
In 30 patients with brain tumors and/or epilepsies, the AMRSR task was used to evoke magnetoencephalography responses. We applied Fast VEctor-based Spatial–Temporal Analyses with minimum L1-norm source imaging method to the magnetoencephalography responses for localizing the brain areas evoked by the AMRSR task.
Results:
The Fast-VEctor-based Spatial–Temporal Analysis found consistent activation in the posterior superior temporal gyrus around 300 to 500 ms, and another activation in the frontal cortex (pars opercularis and/or pars triangularis) around 600 to 900 ms, which were localized to the Wernicke area (BA 22) and Broca area (BA 44 and BA 45), respectively. The language-dominant hemispheric laterization elicited by the AMRSR task was comparable with the result from an Auditory Dichotic task result given to the same patient, with the exception that AMRSR is more sensitive on bilateral language laterization cases on finding the Wernicke and Broca areas.
Conclusions:
For all patients who successfully finished the AMRSR task, Fast-VEctor-based Spatial–Temporal Analysis could establish accurate and robust localizations of Broca and Wernicke area and determine hemispheric dominance. For subjects with normal auditory functionality, the AMRSR paradigm evaluation showed significant promise in providing reliable assessments of cerebral language dominance and language network localization.
Key Words: MEG, Auditory Memory Retrieval and Silent Repeating, Fast-VESTAL, Epilepsy, Language mapping
Functional brain mapping is critical in presurgical planning for epilepsy and brain tumor patients. The resected pathologic areas could be within, or close to the cortical areas that control sensorimotor or cognitive functions.1–3 The identification of language regions is a particular concern because of the risk of surgery-induced expressive or receptive aphasia.4–7 Expressive aphasia is caused by damage to anterolateral regions of the brain, including the inferior frontal region known as Broca area (BA 44 and BA 45). Receptive aphasia is often caused by neurologic damage to Wernicke area in the posterior part of the superior temporal gyrus (STG) of the dominant hemisphere (BA 22). Mapping the language areas in the brain is complicated because multiple brain regions are involved, and more importantly, cooperation and effort from the patients are needed.
Currently, the intraoperative electrical stimulation method is still the gold standard to localize language functions.8–10 The intracarotid amobarbital procedure (Wada) is the standard presurgical study to identify the language-dominant hemisphere, but is invasive, expensive, uncomfortable, and potentially inaccurate in cases where the sodium amytal injection cross-fills into the contralateral hemisphere.5,10,11 It is important to note that the Wada test routinely demonstrates language function in the right hemisphere.12 Therefore, there is a pressing need to supplement the Wada test and electrical stimulation method with other noninvasive modalities, such as magnetoencephalography (MEG) and functional MRI.
Various imaging tools have been applied in this field, including functional MRI,9,13,14 Positron emission tomography,15,16 transcranial magnetic stimulation,17 and MEG.6,17–24 Positron emission tomography is useful in the assessment of language networks in the brain, but the general signal-to-noise ratio is not high enough for a valid interpretation of individual data sets. Functional MRI offers considerable promise in the evaluation of cerebral dominance for language. However, the hemodynamic signals in functional MRI and positron emission tomography are indirect measures of the neuronal activity and may be contaminated by potential abnormal blood flow in the tissue, especially in blood-rich tumor tissue.
Magnetoencephalography is a noninvasive brain imaging technique that has been widely used in functional brain mapping. It provides millimeter spatial resolution with millisecond temporal resolution for characterizing focal brain activities.25 Magnetoencephalography has been successfully applied to language lateralization using various paradigms, and the Wada validation reported demonstrates high compatibility with MEG findings.5,6,26,27 Pirmoradi et al.28 reviewed 37 studies for epileptic/brain tumor patients under presurgical assessment and concluded that the best task to assess language comprehension in adults and children seems to be a word recognition task. Researchers and physicians are constantly testing the stimulus paradigm to reliably elicit Wernicke and Broca areas while keeping the study time reasonable.
The Equivalent Current Dipole model (ECD) is the clinically approved MEG source localization method.6,7,25,29,30 It is satisfactory for modeling focal cortical activity such as epileptic spikes or evoked responses from primary sensory cortices, but may be limited for brain activity with a large number of neuronal sources. Furthermore, local MEG channel selections are required to localize Wernicke area and Broca area,8 which introduces subjective bias to the process. Distributed source modeling techniques19–21,31–33 are alternative approaches that can provide the spatial topography of task-related cortical activation. Hirata et al.27 reported a method using MEG and beamforming algorithm to lateralize the dominant language hemisphere and Broca area based on neuromagnetic oscillatory changes. Huang et al.20 reported using the L1-norm-based VEctor-based Spatial–TemporAL minimum L1-norm34,35 (Fast-VESTAL) algorithm to localize expressive language function using an object-naming task. In the present study, we applied the Fast-VESTAL on MEG evoked responses elicited by the Auditory Memory Retrieval and Silent Repeating (AMRSR) task and compared the result with the auditory dichotic task.36 We tried to study: (1) the spatiotemporal map of MEG-evoked responses during the AMRSR task and determine the hemispheric dominance for language using the Fast-VESTAL; and (2) the robustness of the AMRSR-VESTAL combination in localization for Wernicke and Broca area.
MATERIALS AND METHODS
Subjects
Thirty patients (6 epilepsy, 3 tumor/epilepsy patients, and 21 brain-tumor patients); 23 right-handed (15 male and 8 female), 6 left-handed (4 male and 2 female), and 1 ambidextrous right-handed predominant female, between 10 and 78 years old, participated in this study after providing informed consent. All had normal hearing function. The age distribution has a mean of 40, and SD of 17.
MEG Recording
Magnetoencephalography data were recorded using an Elekta Neuromag VectorView (Helsinki, Finland) with 102 magnetometer channels and 204 gradiometer channels, within a magnetically shielded room. Data were recorded from 0.1 to 330 Hz with a sampling rate of 1000 Hz. Two pairs of EOG (electrooculogram) electrodes were used to detect eye blinks and movements. An interval of 1200 ms poststimulus MEG-signal was selected when creating the averaged response from the raw data and a 300 ms prestimulus interval was used for noise estimation and baseline correction. Trials were rejected from analysis based on amplitude criteria to determine whether they were contaminated by artifacts. Our MEG machine has four bad sensors, and they were turned off during the recording. Magnetoencephalography raw data were first run through MaxFilter, also known as signal space separation,37,38 to remove external interference. The bad sensors were automatically detected and recovered virtually by Maxfilter.38,39 Next, residual artifacts because of eye movements and residual cardiac signals were removed via Independent Component Analysis using Fast-ICA (http://research.ics.aalto.fi/ica/fastica/).40
Stimulation Paradigm
Auditory stimuli were presented using STIM2 software (Compumedics) and delivered binaurally to the patient using Tubal Insert. Hearing thresholds were measured before the auditory study, and the auditory stimuli output level was set to 30 dB above the hearing threshold (average 80 dB).
Auditory function was assessed by presenting binaural 1 kHz tones to ensure patients had normal hearing ability, those with balanced auditory response would be further tested for the language studies.
The dichotic listening task36 was used to assess the language lateralization. In this task, the patient listens to pairs of different words, given simultaneously, one in each ear. The word list consisted of 60 pairs of abstract English nouns with scores of 3 or lower on the Concreteness Scale. The parameters were as follows: mean duration 500 ms, range 350 to 800 ms, with the interstimulus interval of 3 seconds. There were 2 types of word pairs: (1) 30 target pairs that were semantically related and (2) 30 nontarget pairs in which unrelated words were presented. To balance the auditory stimuli, each word pair was played at least 2 times, with left and right stimuli flipped. Therefore, the patient will hear a minimum of 120 word pairs in the dichotic listening task, so the total time for the dichotic task is around 6 minutes. If the words were semantically related, the patient was instructed to lift the left index finger.
The Auditory Memory Retrieval and Silent Repeating (AMRSR) task was used to assess language receptive and expressive functions, after the patient undergoes the dichotic task. Before the MEG scan, patient listens to and remembers 30 target words. Given the potential cognitive decline of epilepsy patients, the patients were asked to confirm that they did remember the target words. These 30 words were then mixed with 10 new distractor words in a random order, making a set of 40 total words. There was no significant difference between target and distractor word lists in word concreteness. During the MEG scan, the 40-words set was played 4 times continuously (in a different random order) and the patient was asked to silently repeat the target words, lifting the left index finger if a word a target word was recognized. The stimulus parameters were the same as the dichotic task, so for 160 trials, the total time of the AMRSR is about 8 minutes. The design of the Memory Retrieval portion of the AMRSR test was similar to the one proposed by Papanicolaou's group.8,18,23
Structural T1-MRI for MEG–MRI Registration and Boundary Element Method Forward Calculation
High-resolution 3D T1 MR images were collected for each subject, for cortical surface reconstruction, MRI-MEG registration, and inner skull extraction for boundary element method (BEM) calculation. Geometrical representation of each subject's cortical surface was reconstructed using Freesurfer (http://surfer.nmr.mgh.harvard.edu/).41,42 A realistic boundary element method head model was used for MEG forward calculation.43,44
Inverse MEG Source Imaging using Fast-VESTAL
Given the boundary element method forward model, one needs to solve the MEG inverse problems to localize neuronal sources. We applied the same method that was described by Huang et al.20,34,35 Here, the high-resolution Fast-VESTAL algorithm, with the second-order cone programming strategy was used to get single subject-based voxel-wise source magnitude images. The sensor–waveform covariance matrix was calculated from the 200 to 1000 ms poststimulus interval, and we used the −300 to 0 ms baseline interval for baseline corrections. A low-pass filter with a cutoff frequency of 50 Hz was applied on the sensor waveform for preprocessing, (same lowpass filter setting was used on ECD source modeling for Dichotic task). For each patient's Fast-VESTAL source magnitude image, voxel-wise F-tests were used to assess the variances between the poststimulus 200 to 1000 ms interval over the prestimulus interval of −300 to 0 ms for each grid node. The voxel-wise F-value maps were then constructed for each grid node. False discovery rate45 correction for multiple comparisons (corrected P = 0.01) was used. Only significant voxels that survived false discovery rate correction were displayed (i.e., in Figs. 1 and 2).
FIG. 1.
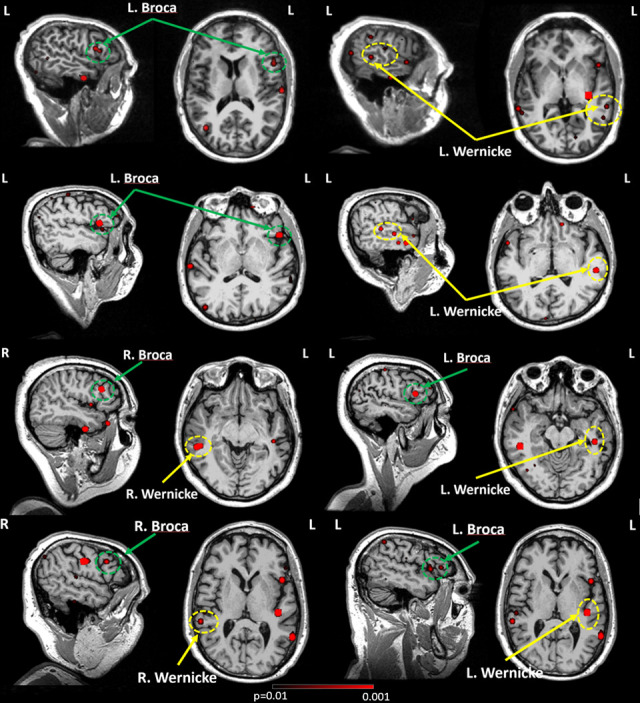
Source localization overlaid on individual MRIs. In each subject, an F-test was used to assess the statistical significance of the source magnitude (root mean square value) between a 200- and 1000-ms poststimulus interval and prestimulus baseline. The 1st and 2nd rows are for subjects (case #5 and case #20) with left hemisphere language dominance and the 3rd and 4th rows (case #10 and case #18) are for subjects with bilateral language dominance. Broca areas are in green dash circles, and Wernicke areas are in yellow dash circles.
FIG. 2.
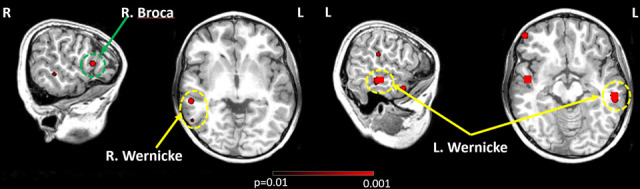
Case #24 source localization overlaid on MRIs, which is bilateral language dominant. Broca areas are in green dashed circles, and Wernicke areas are in yellow dashed circles.
Language Lateralization
Language lateralization tests conducted using the dichotic language task36 were analyzed using the methodology reported by Papanicalou,6 based on ECD model.25 Equivalent current dipole was fitted in the time window from 300 to 800 ms, via the manually selected MEG subarray of channels that cover the L and R temporal lobe. We keep the ECDs with goodness-of-fit > 85%. The laterality index was calculated using the formula (R − L)/(R + L), where R (or Rsum) represents the number of acceptable dipoles located in the right hemisphere and L (or Lsum) is the corresponding number on the left hemisphere. Index values between −0.1 and 0.1 were considered bilaterally symmetric activation, and if the index value is greater than 0.1 or less than −0.1 indicate right-hemisphere or left-hemisphere dominance,19,46 respectively.
Language lateralization tests conducted using the AMRSR were evaluated by the ROI-based small-scale index for Wernicke and Broca area.20 The voxel-wise Fast-VESTAL root mean square values for the 200 to 1000 ms poststimulus interval were first spatially coregistered to an MNI-152 brain-atlas template using a linear affine transformation via the FLIRT program in FSL (www.fmrib.ox.ac.uk/fsl/). Next, an ROI-based mask was constructed in this atlas and then transferred back to the subject's native coordinates, which contains STG (BA22), pars opercularis (BA 44), and pars triangularis (BA 45) in the left and right hemisphere. The ROI-based mask was applied to the voxel-wise Fast-VESTAL root mean square source images, and all activity within the mask was summed up for the left and right hemispheres, regardless of whether their F-value survived the false discovery rate correction. Using the same equation that was described by Papanicalou,6 the standard asymmetry index was calculated for Wernicke and Broca responses.
RESULTS
Patient information, and dichotic test and AMRSR task results are shown in Table 1. Of the 23 right-handed patients, 18 are left-hemisphere dominant, 5 have bilateral control. Among 6 left-handed patients, 3 have bilateral control, 3 are left-hemisphere dominant (columns 6 and 7 in Table 1). One patient is handedness ambiguous, with right hand preferred, shows bilateral language control. The MEG findings showed higher bilateral language representation comparing with the result reported by Knecht47 and Szaflarski48 with 21.7% of right-handed patients and 50% of left-handed patients considered bilateral dominance.
TABLE 1.
Summary of Characteristics in Patients Who Underwent Language Testing
| CaseNo. | Sex | Age | Diagnosis | Handedness | Dichotic ECD | AMRSR Fast-VESTAL | Wernicke Found? | Broca Found? | LI |
| 1 | F | 43 | Tumor | Rt | Lt | Lt | Y | Y | −0.31 |
| 2 | M | 59 | Tumor | Rt | Lt | Lt | Y | Y | −0.28 |
| 3 | M | 48 | Tumor | Rt | Lt | Lt | Y | Y | −0.36 |
| 4 | M | 61 | Tumor | Rt | Lt | Lt | Y | Y | −0.11 |
| 5 | M | 48 | Tumor | Rt | Lt | Lt | Y | Y | −0.19 |
| 6 | M | 48 | Tumor | Rt | Bilat | Bilat | Y | Y | −0.08 |
| 7 | M | 14 | Tumor | Rt | Lt | Lt | Y | Y | −0.49 |
| 8 | F | 45 | Tumor | Rt | Lt | Lt | Y | Y | −0.12 |
| 9 | M | 78 | Tumor | Rt | Lt | Lt | Y | Y | −0.23 |
| 10 | F | 28 | Tumor | Rt | Bilat | Bilat | Y | Y | −0.02 |
| 11 | M | 48 | Tumor | Rt | Lt | Lt | Y | Y | −0.17 |
| 12 | M | 33 | Tumor | Rt | Lt | Lt | Y | Y | −0.51 |
| 13 | F | 39 | Tumor | Rt | Lt | Lt | Y | Y | −0.39 |
| 14 | M | 50 | Tumor | Rt | Bilat | Bilat | Y | Y | 0.02 |
| 15 | M | 33 | Epilepsy | Rt | Lt | Lt | Y | Y | −0.22 |
| 16 | F | 10 | Tumor and epilepsy | Rt | Lt | Lt | Y | N | −0.37 |
| 17 | M | 36 | Tumor and epilepsy | Rt | Lt | Lt | Y | Y | −0.13 |
| 18 | M | 16 | Epilepsy | Rt | Bilal | Bilat | Y | Y | −0.01 |
| 19 | M | 58 | Tumor | Rt | Lt | Lt | Y | Y | −0.15 |
| 20 | F | 54 | Tumor | Rt | Lt | Lt | Y | Y | −0.16 |
| 21 | M | 56 | Epilepsy | Rt | Bilat | Bilat | Y | Y | −0.03 |
| 22 | F | 29 | Epilepsy | Rt | Lt | Lt | Y | Y | −0.38 |
| 23 | F | 38 | Epilepsy | Rt | Lt | Lt | Y | Y | −0.20 |
| 24 | F | 55 | Epilepsy | Lt | Lt | Bilat | Y | Y | 0.04 |
| 25 | M | 62 | Tumor | Lt | Bilat | Bilat | Y | N | 0.02 |
| 26 | M | 53 | Tumor | Lt | Lt | Lt | Y | Y | −0.10 |
| 27 | M | 19 | Tumor | Lt | Bilat | Bilat | Y | Y | −0.03 |
| 28 | M | 11 | Tumor | Lt | Bilat | Bilat | Y | Y | 0.07 |
| 29 | F | 30 | Tumor | Lt | Lt | Lt | Y | Y | −0.28 |
| 30 | F | 18 | Tumor and epilepsy | Ambi, Rt predominant | Bilat | Bilat | Y | Y | 0.01 |
Summary: Lateral Index for Right Handedness subject: Mean: −0.21261, SD: 0.151,364; Lateral Index for Left Handedness subject: Mean: −0.04667, SD: 0.128,944.
AMRSR, Auditory Memory Retrieval and Silent Repeating; ECD, Equivalent Current Dipole; Fast-VESTAL, Fast VEctor-based Spatial–Temporal Analyse.
However, inflection of the AMRSR task evoked a posterior to anterior sequence of cortical recruitment, consistent with classical models of language processing.32,33,49 Figure 3 is a typical AMRSR MEG recording sensor waveform plot. The sensors grouped in the yellow and green circles are close to the temporal and frontal lobes, respectively. A representative waveform was chosen and displayed. The earliest significant brain response was detected at about 100 ms poststimulus in the primary auditory cortex at the STG in the temporal lobe. Activation extended quickly to the posterior of the STG at around 240 ms, which is the classic Wernicke area, on Heschl gyrus and the planum temporale.14,15,17 That response peaks at about 340 ms. Broca area shows the greatest activity at about 690 ms. The MEG contour plots at 100, 340, and 690 ms are also shown. Three dipoles were fitted and projected onto the MEG sensor contour plots, and they are anatomically located from posterior to anterior. The 3 ECDs and the source waveform were superimposed on the 2D axial and sagittal MRI (Fig. 4). The yellow dipoles correspond to the early auditory response, elicited around 100 ms and located at the STG. The red dipoles located on the posterior STG correspond to the language reception with the source waveform peak at 340 ms. The green dipoles were fitted at 690 ms, and the locations are close to Broca area.
FIG. 3.
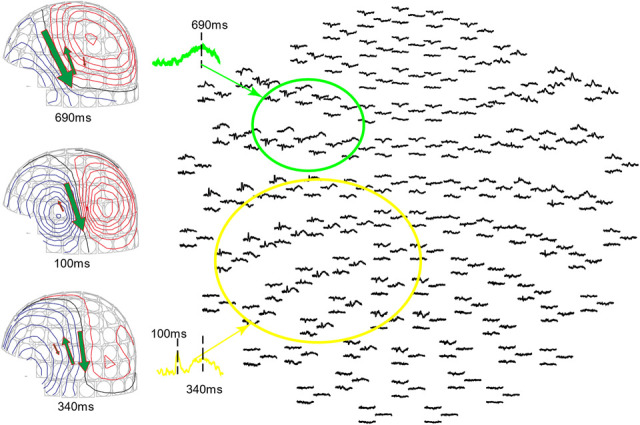
Magnetoencephalography recording waveform and contour plot of Auditory Memory Retrieval and Silent Reading Task. The sensor group in the green circle covers Broca area and the sensor group in the yellow circle covers the STG including Wernicke area. The contour plot at the middle-left shows the magnetic field and dipole location at 100 ms. The contour plot at the lower left shows the magnetic field and dipole location at 340 ms, where the posterior dipole has the largest current. The contour plot at the upper left shows the magnetic field and dipole location at 690 ms, where the anterior dipole has the largest current.
FIG. 4.
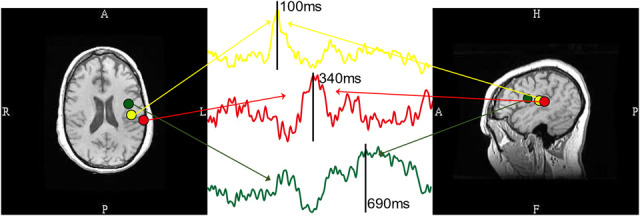
Magnetic source imaging (MSI) and dipole source waveform. Left and right images are fitting dipoles superimposed on axial and sagittal MRI images. Green, yellow and red dipoles correspond to the location of Broca area, primary auditory cortex, and Wernicke area, respectively. The dipole source waveforms are plotted in the middle image, with color-coded arrows pointing to the corresponding dipoles.
The traditional ECD model is not generally satisfactory for high cognitive tasks such as AMRSR. Fast-VESTAL provides accurate, reliable, and statistically significant results. Instead of finding sources at a single time point, Fast-VESTAL calculates the statistically significant variance of each voxel over the 200 to 1000 ms time window. The early auditory response window (50-200 ms) was not included in the fitting duration. This was omitted on purpose to not include sources close to the primary auditory cortex, which is very close to Wernicke area. In Fig. 1, four cases are presented, with 2 left-hemisphere dominant and 2 bilateral hemisphere control. The first row is a left-hemisphere dominant case, in which one can see that even though there is evidence of bilateral activation of Wernicke area, the expressive language location is only located at the left Broca area. The second row is another left hemisphere dominant case with clearly cortical activation in the left Wernicke and Broca areas. The third and fourth rows are bilateral control cases, in which one can find comparable location and strength sources on both hemispheres.
The AMRSR-VESTAL results are highly compatible with the dichotic language lateralization test, except for case #24. The subject is a left-handed female. Her dichotic result is left-hemisphere dominant, whereas the AMRSR-VESTAL result is bilateral language dominant. The source localization result is presented in Fig. 2. From the visual inspection, it is clear that the left Wernicke area is larger and more prominent than its right hemisphere counterpart, which is consistent with the asymmetry index: the Lsum of Wernicke area is 41% greater than the Rsum of Wernicke area. However, one could only find Broca area activation in the right hemisphere. Including the right Broca area in the Rsum balanced the LI result to bilateral language dominance.
Regarding the robustness of the AMRSR-VESTAL combination, the analysis of a sample of 30 patients shows promise. With the understanding that the dichotic task and ECD localization is the most common clinical approach to language lateralization with MEG, the AMRSR results matched 29/30 times. In addition, AMRSR has shown to be a powerful tool in locating Wernicke and Broca areas accurately. The task elicited Wernicke area 100% for the 30 patients and 93.3% for Broca area, and as such, both in 93.3% of cases.
DISCUSSION
In this paper, we introduced the AMRSR task for language area localization using MEG. By applying the Fast-VESTAL algorithm, we were able to determine the hemispheric dominance for language processing and consistently identify Wernicke area at the posterior STG and Broca area at pars opercularis and pars triangularis.
Overall, the spatiotemporal cortical response patterns supporting the AMRSR task involved distributed cortical networks. Cortical regions were recruited in a posterior to anterior sequence, and the pattern of response is consistent with previous MEG language studies.21,24,32,33,49 The data from the right-handed patients paint a laterality profile consistent with expectations based on published literature.5,7,28 We noticed 80% of the right-handed subjects showed left hemisphere language dominance, and other 5 right-handed patients are bilateral/symmetric language control. Among them, 4 have left side lesions/pathology, which could be the reason affecting language dominance. For the 6 left-handed patients, 50% are bilateral control and 50% are left-hemisphere dominant. Since the data sample are small, no statistically significant result could be addressed. Language dominance in left-handed or ambidextrous patients was validated in all 6 patients. This is clinically important because language dominance in left-handed or ambidextrous patients has considerable interindividual variation, and handedness does not serve as an indicator of language dominance.
We believe, comparing with the ECD method, the advantages of Fast-VESTAL are its ability to (1) localize multiple correlated sources without distorting activity, (2) avoid the subjective approach of selecting sub-array of MEG sensors and/or specifying the number of dipoles during an ECD approach in data with multiple sources such as the MEG language responses, (3) faithfully recover source time courses, and (4) generate accurate statistical maps of source images without signal leakage to other brain areas. It may lead to the result that the current study shows substantial improvement in spatial accuracy and resolution for localizing the responses of Broca area. Meanwhile, the AMRSR combines language interpretation, short-term memory retrieve and silent repeating. That is, one relatively simple task could reveal receptive and expressive language area. Our result shows robust Broca and Wernicke area localization, therefore making small-scale language lateralization more reliable. Further development of the noninvasive neuroimaging-based technique may render it a valuable adjunct for routine presurgical planning. It may have the possibility to serve as an alternative option to the Wada test. It can also act as a guide during neurosurgery to avoid important language areas or guide intracranial electrode placement. One limitation of our study is that we do not have complete records of surgical conformation from the patients who underwent surgeries. We are currently improving our clinical practice to collect more systematic information for the future. Meanwhile, we will integrate behavioral data collection in the future work.
In summary, using Fast-VESTAL analysis with the auditory AMRSR task allows MEG to serve as a powerful and effective presurgical tool for the localization of major language areas. The AMRSR task shows significant accuracy in locating the receptive and expressive language areas, and determining hemispheric dominance for language.
Footnotes
The authors have no conflicts of interest to disclose.
Z. Ji and R. R. Song first authorship, authors engaged in equal contribution.
Supported in part by Merit Review Grants from the U.S. Department of Veterans Affairs Dr. Mingxiong Huang and Dr. Roland Lee (P.I.: M.X.H., I01-CX002035-01, NURC-007-19S, I01-CX000499, MHBA-010-14F, I01-RX001988, B1988-I, NURC-022-10F, NEUC-044-06S).
Contributor Information
Zhengwei Ji, Email: z2ji@health.ucsd.edu.
Ryan R. Song, Email: ryanrsong@gmail.com.
Ashley Robb Swan, Email: arobb@health.ucsd.edu.
Annemarie Angeles Quinto, Email: adangeles@health.ucsd.edu.
Roland R. Lee, Email: rrlee@health.ucsd.edu.
REFERENCES
- 1.Mäkelä JP, Kirveskari E, Seppä M, et al. Three-dimensional integration of brain anatomy and function to facilitate intraoperative navigation around the sensorimotor strip. Hum Brain Mapp 2001;12:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grummich P, Nimsky C, Pauli E, Buchfelder M, Ganslandt O. Combining fMRI and MEG increases the reliability of presurgical language localization: a clinical study on the difference between and congruence of both modalities. Neuroimage 2006;32:1793–1803. [DOI] [PubMed] [Google Scholar]
- 3.Mäkelä JP, Forss N, Jääskeläinen J, Kirveskari E, Korvenoja A, Paetau R. Magnetoencephalography in neurosurgery. Neurosurgery 2006;59:493–510. [DOI] [PubMed] [Google Scholar]
- 4.Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 2003;61:699–701. [DOI] [PubMed] [Google Scholar]
- 5.Breier JI, Simos PG, Zouridakis G, et al. Language dominance determined by magnetic source imaging: a comparison with the Wada procedure. Neurology 1999;53:938–945. [DOI] [PubMed] [Google Scholar]
- 6.Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg 1999;90:85–93. [DOI] [PubMed] [Google Scholar]
- 7.Salmelin R. Clinical neurophysiology of language: the MEG approach. Clin Neurophysiol 2007;118:237–254. [DOI] [PubMed] [Google Scholar]
- 8.Papanicolaou AC, Simos PG, Castillo EM, et al. Magnetoencephalography: a noninvasive alternative to the Wada procedure. J Neurosurg 2004;100:867–876. [DOI] [PubMed] [Google Scholar]
- 9.Fernández G, Specht K, Weis S, et al. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology 2003;60:969–975. [DOI] [PubMed] [Google Scholar]
- 10.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. Experimental and clinical observations. J Neurosurg 1960;17:266–282. [DOI] [PubMed] [Google Scholar]
- 11.Mikuni N, Takayama M, Satow T, et al. Evaluation of adverse effects in intracarotid propofol injection for Wada test. Neurology 2005;65:1813–1816. [DOI] [PubMed] [Google Scholar]
- 12.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 1989;71:316–326. [DOI] [PubMed] [Google Scholar]
- 13.Jäncke L, Specht K, Shah JN, Hugdahl K. Focused attention in a simple dichotic listening task: an fMRI experiment. Brain Res Cogn Brain Res 2003;16:257–266. [DOI] [PubMed] [Google Scholar]
- 14.Szaflarski JP, Gloss D, Binder JR, et al. Practice guideline summary: use of fMRI in the presurgical evaluation of patients with epilepsy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2017;88:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price CJ, Wise RJ, Warburton EA, et al. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain 1996;119:919–931. [DOI] [PubMed] [Google Scholar]
- 16.Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within “Wernicke's area”. Brain 2001;124:83–95. [DOI] [PubMed] [Google Scholar]
- 17.Rezaie R, Schiller KK, Embury L, Boop FA, Wheless JW, Narayana S. The clinical utility of transcranial magnetic stimulation in determining hemispheric dominance for language: a Magnetoencephalography comparison study. J Clin Neurophysiol 2020;37:90–103. [DOI] [PubMed] [Google Scholar]
- 18.Billingsley-Marshall RL, Simos PG, Papanicolaou AC. Reliability and validity of functional neuroimaging techniques for identifying language-critical areas in children and adults. Dev Neuropsychol 2004;26:541–563. [DOI] [PubMed] [Google Scholar]
- 19.Hirata M, Kato A, Taniguchi M, et al. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. Neuroimage 2004;23:46–53. [DOI] [PubMed] [Google Scholar]
- 20.Huang CW, Huang MX, Ji Z, et al. High-resolution MEG source imaging approach to accurately localize Broca's Area in patients with brain tumor or epilepsy. Clin Neurophysiol 2016;127:2308–2316. [DOI] [PubMed] [Google Scholar]
- 21.Raghavan M, Li Z, Carlson C, et al. MEG language lateralization in partial epilepsy using dSPM of auditory event-related fields. Epilepsy Behav 2017;73:247–255. [DOI] [PubMed] [Google Scholar]
- 22.Bowyer SM, Zillgitt A, Greenwald M, Lajiness-O’Neill R. Language mapping with Magnetoencephalography: an update on the current state of clinical research and practice with considerations for clinical practice guidelines. J Clin Neurophysiol 2020;37:554–563. [DOI] [PubMed] [Google Scholar]
- 23.Papanicolaou AC, Rezaie R, Simos PG. The auditory and association cortex and language evaluation methods. Handb Clin Neurol 2019;160:465–479. [DOI] [PubMed] [Google Scholar]
- 24.Youssofzadeh V, Babajani-Feremi A. Mapping critical hubs of receptive and expressive language using MEG: a comparison against fMRI. Neuroimage 2019;201:116029. [DOI] [PubMed] [Google Scholar]
- 25.Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa Ov. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 1993;65:413–497. [Google Scholar]
- 26.Bowyer SM, Moran JE, Mason KM, et al. MEG localization of language-specific cortex utilizing MR-FOCUSS. Neurology 2004;62:2247–2255. [DOI] [PubMed] [Google Scholar]
- 27.Hirata M, Goto T, Barnes G, et al. Language dominance and mapping based on neuromagnetic oscillatory changes: comparison with invasive procedures. J Neurosurg 2010;112:528–538. [DOI] [PubMed] [Google Scholar]
- 28.Pirmoradi M, Béland R, Nguyen DK, Bacon BA, Lassonde M. Language tasks used for the presurgical assessment of epileptic patients with MEG. Epileptic Disord 2010;12:97–108. [DOI] [PubMed] [Google Scholar]
- 29.Szymanski MD, Perry DW, Gage NM, et al. Magnetic source imaging of late evoked field responses to vowels: toward an assessment of hemispheric dominance for language. J Neurosurg 2001;94:445–453. [DOI] [PubMed] [Google Scholar]
- 30.Van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang 2006;97:279–293. [DOI] [PubMed] [Google Scholar]
- 31.Dale AM, Liu AK, Fischl BR, et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 2000;26:55–67. [DOI] [PubMed] [Google Scholar]
- 32.Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E. Spatiotemporal maps of past-tense verb inflection. Neuroimage 2003;19:91–100. [DOI] [PubMed] [Google Scholar]
- 33.Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron 2003;38:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang MX, Dale AM, Song T, et al. Vector-based spatial-temporal minimum L1-norm solution for MEG. Neuroimage 2006;31:1025–1037. [DOI] [PubMed] [Google Scholar]
- 35.Huang MX, Huang CW, Robb A, et al. MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. Neuroimage 2014;84:585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald JD, Chong BW, Lewine JD, et al. Integration of preoperative and intraoperative functional brain mapping in a frameless stereotactic environment for lesions near eloquent cortex. J Neurosurg 1999;90:591–598. [DOI] [PubMed] [Google Scholar]
- 37.Taulu S, Kajola M, Simola J. Suppression of interference and artifacts by the signal space separation method. Brain Topogr 2004;16:269–275. [DOI] [PubMed] [Google Scholar]
- 38.Taulu S, Simola J, Kajola M. MEG recordings of DC fields using the signal space separation method (SSS). Neurol Clin Neurophysiol 2004;2004:35. [PubMed] [Google Scholar]
- 39.Song T, Gaa K, Cui L, Feffer L, Lee RR, Huang M. Evaluation of signal space separation via simulation. Med Biol Eng Comput 2008;46:9239–32. [DOI] [PubMed] [Google Scholar]
- 40.Hyvärinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw 2000;13:411–430. [DOI] [PubMed] [Google Scholar]
- 41.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 42.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 43.Huang MX, Song T, Hagler DJ, Jr, et al. A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage 2007;37:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosher JC, Leahy RM, Lewis PS. EEG and MEG: forward solutions for inverse methods. IEEE Trans Biomed Eng 1999;46:245–259. [DOI] [PubMed] [Google Scholar]
- 45.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 46.Hinkley LBN, Witte ED, et al. Optimizing magnetoencephalographic imaging estimation of language lateralization for simpler language tasks. Front Hum Neurosciences 2020;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knecht S, Dräger B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain 2000;123:2512–2518. [DOI] [PubMed] [Google Scholar]
- 48.Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology 2002;59:238–244. [DOI] [PubMed] [Google Scholar]
- 49.Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol 2001;11:202–208. [DOI] [PubMed] [Google Scholar]


