Abstract
Studies focusing on children affected by HIV have shown that they have generally lower academic performance, however, few studies separate children who are HIV exposed and infected (CHEI) and those who are HIV exposed but uninfected (CHEU). Importantly, in rural sub-Saharan Africa, the majority of studies on CHEI and CHEU examine academic performance indirectly based on cognitive test scores. Therefore, studies assessing the effects of HIV on academic achievement directly for CHEI and CHEU are needed. This article evaluates the effects of HIV-infection on cognitive and academic performance by comparing CHEI (n=82) and CHEU (n=1045) aged 7-17 years old using cross-sectional data from an ongoing longitudinal study in a rural area of Zambia. Youth completed cognitive and academic assessments; their height and weight were assessed to generate Body Mass Index (BMI). Caregiver questionnaires provided information on youths’ years in school and household socio-economic status (SES). Results indicated that while HIV infection status did explain some of the variance in performance between CHEI and CHEU, age, BMI, years of schooling and SES accounted for additional variance. The effect of years of schooling on both cognitive and academic performance demonstrated that CHEI’s performance may be greatly improved by consistent school enrollment.
Keywords: HIV, CHEI, CHEU, youth, cognitive, academic, OVC
The HIV/AIDS epidemic has had devastating effects over much of sub-Saharan Africa (SSA) and the disease remains prevalent. Despite the effectiveness of mother-to-child transmission interventions, many children are living with HIV and, with improved medications, are reaching the life stage of working and contributing to the economy; however, their cognitive, behavioral, health and skill trajectories in various environments are not well understood. Children diagnosed with HIV may develop deficits as a direct result of HIV infection, as HIV may enter the central nervous system shortly after infection, affecting their neuropsychological health (Davis et al., 1992; Phillips et al., 2016; Wolters et al., 1995). Additionally, HIV exposed, e.g., through infected or deceased parent(s) or caregivers, but uninfected children (CHEU) have a higher risk of developmental challenges for reasons including being underweight, dropping out of school, lacking material needs and living in poor rural areas with reduced access to education (Nicholson et al., 2015). Both HIV exposed infected children (CHEI) and CHEU may therefore be vulnerable to developmental delays with long-term ramifications, yet few studies have compared their performance differences in terms of both cognitive and academic outcomes.
Previous research has examined cognitive and academic deficits of CHEI; however, most early studies (1988 to 2007) were carried out in Western nations, with only 13% conducted in Africa (Sherr et al., 2009). Studies indicated that HIV infection negatively effects children’s neurocognitive development, however, the heterogeneity of sample characteristics and assessments used render these findings tenuous (Sherr et al., 2009). Some studies in SSA have also revealed cognitive deficits in CHEI (Boivin et al., 1995). Many studies predict academic outcomes based on cognitive outcomes, but this has been shown to overpredict academic achievement scores (Garvie et al., 2014). Academic achievement has often been appraised from school enrolment and attendance (Guo et al., 2012; Parchure et al., 2016), being in the correct grade for age (Pufall et al., 2014; Henning et al., 2018; Shiau et al., 2020), school behavior (Guo et al., 2012), school connectedness (Sharp et al., 2018), and school completion (Guo et al., 2012; Pufall et al., 2014) rather than academic assessment scores. One recent study that did examine test scores for reading, mathematics and HIV knowledge only evaluated the difference between orphans and non-orphans (mostly due to HIV-related death of one or both parents) and did not take into account whether the child had HIV (Blevins & Kawata, 2019). Several studies demonstrate links between HIV and poorer academic performance; however, some studies have found none (Orkin et al., 2014; Pufall et al., 2014). This is likely due to the heterogeneity of variables used to predict academic performance as well as different confounding factors such as location of study, age, and HIV severity. Most studies show that children affected by HIV have lower academic performance than those unaffected, but do not separate CHEI and CHEU. Therefore, more SSA studies assessing the effects of HIV on academic achievement directly and separately in CHEI and CHEU are needed.
The Current Study
A longitudinal study (2015-2021) was conducted in Southern Province, Zambia, to follow growth trajectories of orphans and vulnerable children (OVCs) within 20 km of Macha Mission Hospital (MMH). Youths aged 7-17 were assessed on a set of cognitive skills and academic achievement. CHEI and CHEU OVCs were compared to determine whether these groups perform equally well. This study aims to fill a gap in the literature by comparing both cognitive and academic test scores of CHEI and CHEU in rural SSA, using direct assessments. It is expected that the cognitive and academic performance of CHEI’s will be poorer than CHEU’s, as most studies have found that increased HIV RNA and immune suppression are negatively associated with cognitive performance (Puthanakit et al., 2013; Rice et al., 2012; Ruel et al., 2012; Smith et al., 2006).
Methods
Sample
HIV OVCs were defined as youth (aged 7-17 years) reported as infected or affected by HIV (i.e., who either had one or both parents infected by HIV; who was either a single or double orphan due to HIV; or whose primary caregiver was diagnosed with HIV) at the time of screening. The screening tool used was a modified version of the Multiple Indicator Cluster Survey 3 (MICS3; UNICEF, 2009), which was applied to one household at a time to determine the presence of HIV OVCs. To ascertain the sample, 4,702 households in representative regions of the catchment area were screened. Among these households, 1,194 HIV OVCs were identified, recruited, consented, and assessed; of these youth, 82 were CHEI and 1112 were CHEU.
Regarding CHEI, information on when and how the child had become infected was not collected, but an ongoing cohort study of children with HIV infection being conducted at Macha has shown that 99% of pediatric HIV infections are due to mother-to-child transmission (Van Dijk et al, 2014). Although medical records were obtained for some of the CHEI (n = 71, average age at diagnosis = 5 years, all were on antiretroviral medication, ARVs), ARV usage and viral load for each diagnosed child were not included in this analysis due to missing data; not all children’s records were accessible and viral load was not ascertained routinely for all children. However, due to an active local HIV clinic, the majority of HIV-infected children in the area are on ARVs, have well-suppressed viral loads, and appear to be in good health. Regarding CHEU, the study did not ascertain when primary caregivers were diagnosed with HIV.
Materials/Instruments
All assessment instruments were previously used in a large-scale study on reading disability in this region and showed good psychometric properties (Hein et al., 2016; Mourgues et al., 2016; Reich et al., 2013). The standardized cognitive assessments had been adapted and piloted for appropriateness prior to use; the academic achievement tests and Home Environment Survey were developed specifically for use in rural Africa. Both types of assessments performed reliably in the study area (Cronbach’s α > 0.77).
Cognitive Assessments (3)
The materials used to measure cognitive ability were the Rapid Automatized Naming - Objects (RAN-O; Denckla & Rudel, 1976), Letter-Digit Span (LDSpan; WISC-IV, Wechsler, 2003), and Triangles (Tri; KABC-II, Kaufman & Kaufman, 2004) subtests. The RAN-O is a test of information processing speed; accuracy and time are recorded. LDSpan is a working memory task that requires participants to remember and repeat back (in forward or backward order) a list of 2-9 letters or numbers read to them. Triangles requires the participant to recreate a given abstract design using colored plastic shapes.
Academic Achievement Assessments (4)
The Zambian Achievement Test (ZAT) was used to measure the academic achievement of study participants (Stemler et al., 2009). The ZAT is comprised of four subtests: Reading Recognition (RR), which assesses letter matching, letter recognition, and components of phonological awareness; Reading Comprehension 1 (RC1), which assesses word level comprehension; Reading Comprehension 2 (RC2), which assesses sentence- and passage-level comprehension; and Mathematics, which assesses basic mathematics skills
Socio-economic Status (SES) and Years in School
Household SES and years in school were ascertained using data from the Home Environment Survey, which collected information from a parent/guardian regarding each child’s school history and home environment. The SES index was calculated as a fraction of twelve weighted indicators (vehicle, more than 50 cattle, a caregiver educated above secondary school level, a brick house, refrigerator, motorbike, stove, English spoken at home, television, meat eaten more than twice a month, a toilet, drinking clean water—i.e., tap or bottled). The indicators were weighted according to rankings collected from a sample of people living in the MMH catchment area. This index generates sample means consistent with the gross domestic product per capita estimates of the relative wealth of SSA countries, thus appears to provide an accurate representation of participant families’ SES (Fortson 2008).
Body Mass Index (BMI)
BMI was calculated from physical data. At assessment, children were weighed and their height measured. Their BMI was calculated using the standard formula of weight (in kg) divided by height (in meters) squared, BMI = w/h2.
Procedures
All study procedures were approved by the Yale University IRB prior to any engagement with participants, and subsequently by the University of Houston IRB after the move of the Primary Investigator of the study. IRB approval in Zambia was obtained from the Macha Research Trust IRB and permission given by the Zambia National Health Research Authority to conduct the study. Informed consent was obtained from parents/guardians when the family was invited to participate in the study after being screened for the presence of HIV OVC. Informed assents were obtained from children on the day of assessment, prior to their engagement. All children were administered the assessments one-on-one by a trained Zambian data collector fluent in both English and Chitonga. The Home Environment Survey was conducted one-on-one with the head of household or another senior household member. The language used was the participants’ native language, Chitonga.
Data Analysis
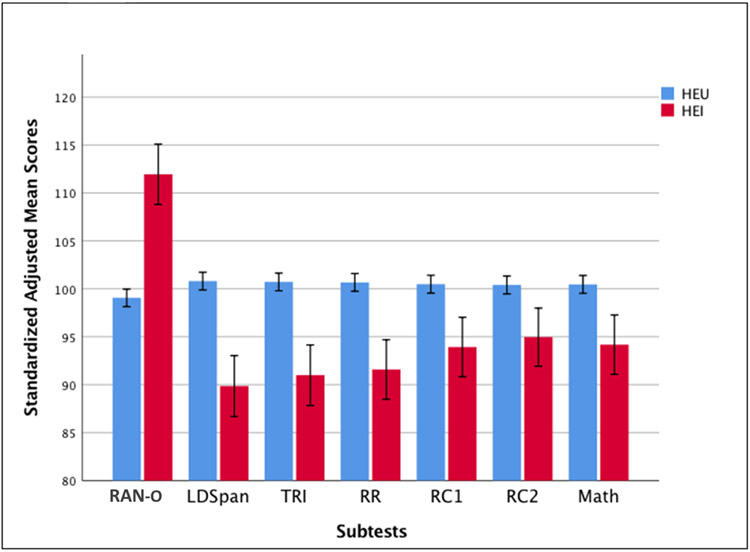
Raw summed scores of correct responses were generated for all assessments except RAN-O, for which the performance score is total time on task (lower scores indicate higher ability). The participants were grouped according to HIV status: CHEI and CHEU. Mean values for age, BMI, years in school and the mean weighted SES were calculated for each group, as well as mean performance values and standard deviations on all assessments. Pearson correlation coefficients were generated to evaluate relationships between test performance and covariates (age, sex, HIV status, BMI, years in school and SES). A multivariate analysis of covariance (MANCOVA) was carried out to examine the effect of HIV on children’s performance on both cognitive and academic assessments, controlling for age, BMI, years in school, and SES. This allowed us to test the statistical significance of the effect of HIV diagnosis on academic and cognitive performance after controlling for covariates. Due to missing data, the final sample size for the MANCOVA was CHEI = 79 and CHEU = 997. Sex was excluded due to its low correlation with student performance (Table 2). All analyses were carried out using raw performance scores; Figure 1, however, presents standardized scores adjusted for age and grade to allow uniformly scaled comparisons between subtests. The statistical package SPSS 25 (IBM, 2020) was used for all analyses.
Table 2.
Correlations of child characteristics and assessment scores
| Age | Sex | HIV | BMI | Yrs in School |
SES | RON | LD Span |
Tri | RR | RC1 | RC2 | Math | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | ||||||||||||
| Sex | −0.01 | 1.00 | |||||||||||
| HIV | −0.05 | 0.01 | 1.00 | ||||||||||
| BMI | .62** | .08** | −.062* | 1.00 | |||||||||
| Yrs in School | .67** | 0.00 | −0.01 | .49** | 1.00 | ||||||||
| SES | .07* | 0.00 | 0.03 | .09** | .17** | 1.00 | |||||||
| RAN-O | −.53** | −0.03 | .12** | −0.36 | −.50** | −.89** | 1.00 | ||||||
| LDSpan | .56** | 0.04 | −.12** | .38** | .61** | .15** | −.58** | 1.00 | |||||
| Tri | .54** | −.12** | −.09** | .38** | .58** | .18** | −.53** | .69** | 1.00 | ||||
| RR | .58** | 0.03 | −.09** | .44** | .65** | .18** | −.59** | .78** | .70** | 1.00 | |||
| RC1 | .45** | 0.03 | −0.05 | .35** | .53** | .14** | −.46** | .64** | .55** | .74** | 1.00 | ||
| RC2 | .47** | 0.06 | −0.05 | .40** | .56** | .15** | −.44** | .59** | .55** | .70** | .75** | 1.00 | |
| Math | .63** | 0.02 | −0.06 | .49** | .70** | .22** | −.54** | .74** | .72** | .80** | .69** | .71** | 1.00 |
Notes.
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed); Sex = male (0) or female (1); HIV = HIV infected (1) or HIV uninfected (0); BMI = Body Mass Index; Yrs in school = number of years child attended school (not including extended periods of not attending school); SES = socio-economic status; RAN-O = Rapid Automatized Naming – Objects; LDSpan = Letter-Digit Span; Tri = Triangles subtest of the KABC; RR = Reading Recognition; RC1 = word-level reading comprehension; RC2 = sentence- and passage-level reading comprehension
Figure 1. Comparison of the standardized adjusted mean scores of CHEI and CHEU children’s performance on all assessments.
Notes. RAN-O, LDSpan, and Tri (cognitive) scores were adjusted for age; RR, RC1, RC2 and Math (academic) scores were adjusted for grade. CHEU = HIV exposed uninfected children; CHEI = HIV exposed infected children; RAN-O = Rapid Automatized Naming - Objects; LDSpan = Letter-digit span; Tri = Triangles subtest of the KABC; RR = Reading Recognition; RC1 = word-level reading comprehension; RC2 = sentence- and passage-level reading comprehension. The error bars indicate the 95% confidence intervals.
Results
The sample descriptive statistics for each group are presented in Table 1. These reflect the group mean differences between CHEI and CHEU in both covariates (age, BMI, years in school, and SES) and performance outcomes on the RAN-O, LDSpan and Triangles subtests (cognitive measures), and on the RR, RC1, RC2, and mathematics subtests (academic measures). CHEU consistently achieved higher mean scores as a group than CHEI on all performance measures. Although CHEI tend to be younger than CHEU and have relatively lower BMI, the two groups appear comparable with regard to years of school attendance and SES.
Table 1.
Descriptive statistics for CHEI and CHEU groups and assessment scores
| CHEI | CHEU | |
|---|---|---|
| N | 82 | 1045 |
| % Female | 47.56 | 45.74 |
| Covariates | mean (sd) | mean (sd) |
| Age | 11.70 (2.95) | 12.32 (3.16) |
| BMI | 16.44 (2.22) | 17.14 (2.96) |
| Years in school | 4.13 (2.98) | 4.27 (3.12) |
| Mean weighted SES index | 0.20 (0.12) | 0.19 (0.11) |
| Assessments | mean (sd) | mean (sd) |
| RAN-O | 102.41 (39.25) | 87.53 (32.94) |
| LDSpan | 15.02 (6.32) | 17.90 (6.48) |
| Tri | 11.85 (5.44) | 13.56 (5.02) |
| RR | 17.88 (7.49) | 20.67 (7.79) |
| RC1 | 5.98 (2.56) | 6.54 (2.79) |
| RC2 | 6.98 (3.10) | 7.62 (3.47) |
| Math | 18.06 (11.19) | 20.57 (11.16) |
Notes. CHEI = HIV exposed infected children; CHEU = HIV exposed uninfected children; BMI = Body Mass Index; SES = Socioeconomic Status; RAN-O = Rapid Automatized Naming - Objects; LDSpan = Letter-digit span; Tri = Triangles subtest of the KABC; RR = Reading Recognition; RC1 = word-level reading comprehension; RC2 = sentence- and passage-level reading comprehension
The correlation matrix (Table 2) reflects the relationships between the dependent performance variables, independent variable (HIV status/diagnosis) and covariates. Notably, all of the correlations are significant (at p < .01) except for some clear exceptions. Specifically, sex only showed a significant, but low and negative (R2 = −.12), correlation with the Triangles assessment. Due to this finding, sex was not included as a covariate in any further analyses. Additionally, HIV status did not correlate significantly with academic achievement, nor with years in school or SES. This is an important indication that HIV infection does not significantly affect school attendance, likely due to CHEI being on treatment, nor are CHEI segregated in poorer households. HIV status was, however, significantly negatively correlated with all cognitive subtests (for the RAN-O, higher scores reflect lower ability, hence the positive correlation) and with BMI, although these correlations were all relatively low (absolute R2 < .12). The adjusted mean scores for each subtest demonstrate that CHEU performed better than CHEI on all assessments; notably, the 95% confidence interval error bars do not overlap (Figure 1).
The results of the MANCOVA revealed that, overall, significant differences between CHEI and CHEU were detected on all cognitive and academic dependent variables when controlling for age, BMI, years in school and SES. We report Pillai’s Trace (Garson, 2015; Olson, 1976) for the full model here: F(7, 1064) = 4.39, p = .000, partial η2 = .028. The main effect of diagnosis is reported in Table 3. Diagnosis appeared to play a pivotal role in both cognitive and academic performance, as its main effect is significant for all assessments apart from the reading comprehension assessments, RC1 and RC2. The contributing effects of the four covariates on each subtest are reported in Table 4. Significant contributing effects of years in school are shown for all subtests, of age for all except the RC2 subtest, of SES for all except the RAN-O subtests, and of BMI for only the RR, RC2 and Mathematics subtests.
Table 3.
The main effect of diagnosis on cognitive and academic performance
| F | p | partial η2 |
|
|---|---|---|---|
| RAN-O | 17.93 | .000 | .016 |
| LDSpan | 18.50 | .000 | .017 |
| Tri | 11.88 | .001 | .011 |
| RR | 14.93 | .000 | .014 |
| RC1 | 3.11 | .078 | .003 |
| RC2 | 2.00 | .156 | .002 |
| Math | 6.70 | .009 | .006 |
Notes. RAN-O = Rapid Automatized Naming - Objects; LDSpan = Letter-digit span; Tri = Triangles subtest of the KABC; RR = Reading Recognition; RC1 = word-level reading comprehension; RC2 = sentence- and passage-level reading comprehension
Table 4.
The partialed effects of the covariates on cognitive and academic performance
| Age | BMI | Years in School | SES | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p |
partial
η 2 |
F | p |
partial
η 2 |
F | p |
partial
η 2 |
F | p |
partial
η 2 |
|
| RAN-O | 61.35 | .000 | .054 | 0.31 | .557 | 0 | 74.32 | .000 | 0.065 | 0.79 | .373 | 0.001 |
| LD Span | 50.65 | .000 | .045 | 0.03 | .859 | 0 | 165.70 | .000 | 0.134 | 6.14 | .013 | 0.006 |
| Tri | 44.84 | .000 | .040 | 0.54 | .462 | 0 | 134.65 | .000 | 0.112 | 12.61 | .000 | 0.012 |
| RR | 36.28 | .000 | .033 | 5.64 | .018 | 0.005 | 238.51 | .000 | 0.182 | 13.72 | .000 | 0.013 |
| RC1 | 8.01 | .005 | .007 | 3.80 | .052 | 0 | 138.42 | .000 | 0.115 | 5.72 | .017 | 0.005 |
| RC2 | 4.22 | .040 | .004 | 13.43 | .000 | 0.012 | 163.31 | .000 | 0.132 | 6.13 | .013 | 0.006 |
| Math | 61.4 | .000 | .054 | 12.04 | .001 | 0.011 | 274.84 | .000 | 0.204 | 29.50 | .000 | 0.027 |
Notes. BMI = BMI = Body Mass Index; SES = Socioeconomic Status; RAN-O = Rapid Automatized Naming - Objects; LDSpan = Letter-digit span; Tri = Triangles subtest of the KABC; RR = Reading Recognition; RC1 = word-level reading comprehension; RC2 = sentence- and passage-level reading comprehension
Discussion
This evaluation of the effect of HIV infection on children’s development with regard to their cognitive capacities and academic achievement does have some limitations, particularly concerning facets of CHEI health and progress on treatment. Access to their complete medical records is challenging, and viral load is not routinely obtained. An additional weakness of this study is that the historical circumstances of CHEU are not known, such as when they may have become orphans or when their parents or caregivers acquired HIV. However, the analyses presented here provide a unique picture of how CHEI are faring cognitively and academically compared to CHEU peers.
Both CHEI and CHEU are negatively affected by HIV but do show variable outcomes. In this study, the patterns of performance that emerged confirm previous findings (e.g., Shiau et al., 2020; Sherr et al., 2018), and suggest factors for further consideration, emphasizing the importance of context. As in other studies, the analyses showed that overall, the effect of HIV diagnosis on students’ cognitive and academic performance was statistically significant; yet its estimated effect, after controlling for the covariates of age, BMI, years of schooling and SES, appeared to be relatively small, indicating that HIV diagnosis explained ~3% of the variance in children’s performance. Also, in the analysis by subtest, HIV diagnosis explained more variance in children’s performance on cognitive tests (especially for RAN-O and LDspan) and RR (including phonological awareness) than in their performance on tests of higher-level academic skills of mathematics (small effect) and RC1/RC2 (no variance explained). This may reflect the more direct connections between HIV, neurological alterations, and cognitive development. While this study’s results agree with the majority of prior literature in that cognitive performance is significantly lower in CHEI than CHEU, it is one of only few studies that directly measured both academic and cognitive test performance. The results here illustrate that academic performance extrapolated or derived from cognitive performance may not be an accurate estimate of the true academic potential of the child, at least in some contexts.
Further explanatory information is provided by the analysis of the covariates. BMI, found in other studies to affect academic and cognitive performance (Stewart et al., 2013), appeared to have little bearing on the variance between CHEI and CHEU performance except in reading comprehension and mathematics. This may be related to healthier children’s ability to stay in school and develop the higher-level skills demanded by these domains. SES has often been associated with lower cognitive functioning (Christensen et al., 2014). In this study, SES explained some variance on all subtests except for RAN-O, but most prominently the variance in mathematics (~3%). Because mathematics involves skills that may be developed outside of school in activities of commerce (Ginsburg et al., 1981; Lave, 1988), this difference may reflect CHEU’s capability to engage more in such activities if their families do. The effect of the locally weighted SES is particularly notable since all study participants were from the same catchment area, sharing homogeneous characteristics.
The covariates of both age and years in school explained more variance than BMI or SES in both groups’ performance on most subtests. Age, like HIV diagnosis, was more closely related to cognitive and basic reading skills; this is not surprising as both sets of skills, which include phonological awareness, progress with child development. Years in school exhibited the most salient effect on the higher performance of CHEU across all subtests, primarily on reading-related and mathematics assessments, indicating the importance of school for the development of both cognitive and academic skills. Lowered school attendance of CHEI may be due to children with poorly treated or uncontrolled HIV being more likely to get sick and miss school more often. In addition, ARV treatment, while vital for children’s survival, may elicit a spectrum of adverse neurological effects, including nerve dysfunction outside of the central nervous system (CNS), and CNS side effects such as myopathy, insomnia, confusion, and nightmares (Thakur et al., 2019; Wilmshurst et al., 2018). These may lead to poor general functioning, low adherence to medication, or withdrawal from therapy, and missed school. In addition, stigma, which can lead to social exclusion and lower self-esteem, may link with lower class participation thereby interfering with learning (Anabwani et al., 2016). Stigma could also lead to diminished ambition and desire to perform well (Ntuli et al., 2020).
Overall, the results of this study suggest that while HIV infection has a clear impact on cognitive and academic progress, important drivers of this difference may be years spent in school by CHEI and factors related to SES. The mechanisms underlying these covariates have not been examined here. Yet, this study has made in-roads into the practical ramifications of HIV infection on children in rural communities, addressing important contextual factors such as SES and BMI. It would be useful in future studies to compare CHEU and CHEI with a sample of HIV unexposed peers to determine whether academic performance is significantly weaker for all HIV affected children whether or not they are infected themselves. The progress of the children should also be studied longitudinally to determine whether long-term catch-up with uninfected peers in academics is possible, or whether CHEI continue to fall even further behind.
Acknowledgments
We would like to acknowledge the contributions of all of the children and families who participated in this research. We appreciate their time and support over the years. This research was supported by the National Institutes of Health, R01HD085836 (PI: Elena L. Grigorenko). Grantees undertaking such projects are encouraged to express freely their professional judgment. This article, therefore, does not necessarily reflect the position or policies of the funders, and no official endorsement should be inferred.
Footnotes
We have no known conflicts of interest to disclose.
References
- Anabwani G, Karugaba G, & Gabaitiri L (2016). Health, schooling, needs, perspectives and aspirations of HIV infected and affected children in Botswana: a cross-sectional survey. BMC pediatrics, 16(1), 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins BK, & Kawata K (2019). The orphan impact: HIV-AIDS and student test scores from sub-Saharan Africa. Educational Review, 1–24. [Google Scholar]
- Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK and Cutting WA, 1995. A preliminary evaluation of the cognitive and motor effects on pediatric HIV infection in Zairian children. Health Psychology, 14(1), p.13. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Schieve LA, Devine O, & Drews-Botsch C (2014). Socioeconomic status, child enrichment factors, and cognitive performance among preschool-age children: results from the Follow-Up of Growth and Development Experiences study. Research in developmental disabilities, 35(7), 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, … & Wiley CA (1992). Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology, 42(9), 1736–1736. [DOI] [PubMed] [Google Scholar]
- Denckla MB & Rudel RG (1976). Rapid automatized naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia, 14, 471–479. [DOI] [PubMed] [Google Scholar]
- Fortson JG (2008). The gradient in sub-Saharan Africa: socioeconomic status and HIV/AIDS. Demography, 45(2), 303–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson GD (2015). GLM multivariate, MANOVA & canonical correlation. Asheboro, NC: Statistical Associates Publishers. [Google Scholar]
- Garvie PA, Zeldow B, Malee K, Nichols SL, Smith RA, Wilkins ML, … & Pediatric HIV/AIDS Cohort Study (PHACS. (2014). Discordance of cognitive and academic achievement outcomes in youth with perinatal HIV exposure. The Pediatric infectious disease journal, 33(9), e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg HP, Posner JK, & Russell RL (1981). The Development of Mental Addition as a Function of Schooling and Culture. Journal of Cross-Cultural Psychology, 12(2), 163–178. [Google Scholar]
- Guo Y, Li X, Song Y, & Liu Y (2012). Bisexual behavior among Chinese young migrant men who have sex with men: implications for HIV prevention and intervention. AIDS care, 24(4), 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein S, Tan M, Reich J, Thuma PE, & Grigorenko EL (2016). School effects on non-verbal intelligence and nutritional status in rural Zambia. Learning and individual differences, 46, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning M, Kirk CM, Franchett E, Wilder R, Sezibera V, Ukundineza C, & Betancourt T (2018). Over-age and underserved: a case control study of HIV-affected children and education in Rwanda. Vulnerable Children and Youth Studies, 13(1), 81–93. [Google Scholar]
- IBM (2020). SPSS 25. Armonk, NY: IBM Corporation [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). K-ABC--Kaufman assessment battery for children, 2nd edition: Administration and scoring manual. San Antonio, TX: Pearson. [Google Scholar]
- Lave J (1988). Cognition in practice: Mind, mathematics and culture in everyday life. Cambridge University Press. [Google Scholar]
- Mourgues CV, Tan M, Hein S, Ojanen E, Reich J, Lyytinen H, & Grigorenko EL (2016). Paired associate learning tasks and their contribution to reading skills. Learning and Individual Differences, 46, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L, Chisenga M, Siame J, Kasonka L, & Filteau S (2015). Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC pediatrics, 15(1), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntuli B, Mokgatle M, & Madiba S (2020). The psychosocial wellbeing of orphans: The case of early school leavers in socially depressed environment in Mpumalanga Province, South Africa. Plos one, 15(2), e0229487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CL (1976). On choosing a test statistic in multivariate analysis of variance. Psychological bulletin, 83(4), 579–586. [Google Scholar]
- Orkin M, Boyes ME, Cluver LD, & Zhang Y (2014). Pathways to poor educational outcomes for HIV/AIDS-affected youth in South Africa. AIDS care, 26(3), 343–350. [DOI] [PubMed] [Google Scholar]
- Parchure R, Jori V, Kulkarni S, & Kulkarni V (2016). Educational outcomes of family-based HIV-infected and affected children from Maharashtra, India. Vulnerable Children and Youth Studies, 11(4), 332–338. [Google Scholar]
- Phillips N, Amos T, Kuo C, Hoare J, Ipser J, Thomas KG, & Stein DJ (2016). HIV-associated cognitive impairment in perinatally infected children: a meta-analysis. Pediatrics, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufall EL, Nyamukapa C, Eaton JW, Campbell C, Skovdal M, Munyati S, … & Gregson S. (2014). The impact of HIV on children's education in eastern Zimbabwe. Aids Care, 26(9), 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, … & PREDICT Study Group. (2013). Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. The Pediatric infectious disease journal, 32(5), 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Buchanan AL, Siberry GK, Malee KM, Zeldow B, Frederick T, … & Torre P III. (2012). Language impairment in children perinatally infected with HIV compared to children who were HIV-exposed and uninfected. Journal of developmental and behavioral pediatrics, 33(2), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, … & Kamya MR (2012). Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clinical Infectious Diseases, 54(7), 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Penner F, Marais L, & Skinner D (2018). School connectedness as psychological resilience factor in children affected by HIV/AIDS. AIDS care, 30(sup4), 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, Mueller J, & Varrall R (2009). A systematic review of cognitive development and child human immunodeficiency virus infection. Psychology, health & medicine, 14(4), 387–404. [DOI] [PubMed] [Google Scholar]
- Sherr L, Hensels IS, Tomlinson M, Skeen S, & Macedo A (2018). Cognitive and physical development in HIV-positive children in South Africa and Malawi: A community-based follow-up comparison study. Child: care, health and development, 44, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S, Arpadi SM, Burke M, Liberty A, Thurman C, Patel F, … & Kuhn L (2020). Educational delays among children living with perinatally-acquired HIV in Johannesburg, South Africa. AIDS care, 32(4), 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Malee K, Leighty R, Brouwers P, Mellins C, Hittelman J, … & Blasini I (2006). Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics, 117(3), 851–862. [DOI] [PubMed] [Google Scholar]
- Stemler SE, Chamvu F, Chart H, Jarvin L, Jere J, Hart L, & Grigorenko EL (2009). Assessing competencies in reading and mathematics in Zambian children. Multicultural psychoeducational assessment, 157–186. [Google Scholar]
- Stewart CP, Iannotti L, Dewey KG, Michaelsen KF, & Onyango AW (2013). Contextualising complementary feeding in a broader framework for stunting prevention. Maternal & child nutrition, 9, 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, & Birbeck GL (2019). Global HIV neurology: a comprehensive review. AIDS (London, England), 33(2), 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF, M. (2009). Multiple indicator cluster surveys. [Google Scholar]
- Van Dijk JH, Moss WJ, Hamangaba F, Munsanje B, & Sutcliffe CG (2014). Scaling-up access to antiretroviral therapy for children: a cohort study evaluating care and treatment at mobile and hospital-affiliated HIV clinics in rural Zambia. PloS one, 9(8), e104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children, 4th edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wilmshurst JM, Hammond CK, Donald K, Hoare J, Cohen K, & Eley B (2018). NeuroAIDS in children. Handbook of clinical neurology, 152, 99–116. [DOI] [PubMed] [Google Scholar]
- Wolters PL, Brouwers P, & Moss HA (1995). Pediatric HIV disease: effect on cognition, learning, and behavior. School Psychology Quarterly, 10(4), 305. [Google Scholar]