Abstract
Institutionalized and community-dwelling older adults have been greatly impacted by the coronavirus disease 2019 (COVID-19) pandemic with increased morbidity and mortality. The advent of vaccines and their widespread use in this population has brought about a dramatic turnaround in COVID-19 outcomes. The immunogenicity and effectiveness of the various vaccine options worldwide are discussed. Optimization of vaccine usage will still be important to maximize protection due to reduced initial immunity, development of variant strains, and fading of immunity over time. There are also lessons learned specific to older populations for future pandemics of novel pathogens.
Keywords: COVID-19 vaccine, Aging, Vaccine effectiveness, Immunogenicity, Geriatric, Older adults
Key points
-
•
Immunosenescence, inflammaging, and frailty are associated with the suboptimal immunogenicity of vaccines in older adults.
-
•
Immunogenicity is reduced in older individuals for both vaccine-specific antibody and T-cell levels.
-
•
Vaccine effectiveness to the current COVID-19 vaccines, however, is preserved in the older population despite reduced immunogenicity.
-
•
Being a vulnerable population, vaccination campaigns should continue to prioritize older adults for optimal protection against COVID-19.
Introduction
Institutionalized and community-dwelling older adults have been greatly impacted by the coronavirus disease 2019 (COVID-19) pandemic.1 Older adults have a higher incidence of hospitalizations and death compared with younger populations. Older adults, especially those with comorbidities, are specifically prone to having the severe disease when infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19.2 , 3 Thus, they are a population of utmost concern amid the pandemic, which has often led to their prioritization in vaccine recommendations.4 , 5 Despite the advent of vaccines, this group has experienced increased breakthrough infections.6 , 7 Whether this is due to inadequate vaccine coverage or vaccine ineffectiveness is unclear. Vaccination remains the most feasible and easily accessible preventive measure used for protection against COVID-19 among older adults. Thus, there is a significant need to optimize current vaccination measures to protect these populations against COVID-19.
Immune changes in older adults: clinical implications for vaccination
Older adults generate suboptimal immune responses to virtually all vaccines when compared with the younger adult population.8 Vaccines such as those for influenza, pneumococcus, and hepatitis B are less immunogenic and effective in adults older than 65 years.9 In addition, vaccine-induced antibodies tend to be more short-lived among this population.10
Immunosenescence and inflammaging are two established phenomena associated with the suboptimal immunogenicity of vaccines in older adults. In immunosenescence, the aging immune system is plagued with a progressive functional decline, which reduces its ability to mount an adequate response to new or previously encountered antigens in the form of vaccines or infections.11 This decline in cellular and humoral immunity is marked by reduced production of naive B and T cells, an increase in dysfunctional memory cells, and the involution of primary lymphoid organs such as the thymus.12 These physiologic changes make older adults more susceptible to infections as they continue to age.13 Furthermore, a characteristic reduction in the production of lymphocytes (lymphopoiesis) results in the impairment of the adaptive and innate immune response,14 which is also marked by the increased production of autoantibodies and low-affinity antibodies.10 Changes within the bone marrow, such as myelofibrosis, result in the production of short-lived and apoptosis-prone immune cells, with a shift toward myeloid precursor cells.15 These cumulative changes create “immune system fatigue” and dampen the ability of the immune system in older adults to be appropriately stimulated by neoantigens and previously encountered antigens alike.
Inflammaging is a progressive state of chronic, sterile low-grade inflammation that contributes to developing disease conditions associated with aging.16 Inflammaging is characterized by increased production of proinflammatory cytokines (such as interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF] α) with an increase in the ratio of Th17 cells, a proinflammatory subset of CD4+ T cells, to regulatory T cells. There is also the production of highly inflammatory late memory B cells with reduced telomerase activity, and reduced neutrophilic, monocytic, and dendritic cell functions such as chemotaxis, phagocytosis, signaling pathways, and intracellular killing via free radical production.11 Furthermore, elevated levels of TNF-α characteristic of inflammaging exist in the serum and resting B cells.17 As a result, inflammaging causes impairment in B cell function, and it has been reported that these systemically abundant proinflammatory cytokines tend to prevent optimal response to vaccines.18 This propensity toward a heightened anti-inflammatory response to low-grade chronic inflammation and suppression of acute inflammatory processes has clinical implications in the form of reduced reactions to vaccines observed in older adults.16
Frailty further contributes to the severity of complications suffered from disease and infections. This multifactorial syndrome is marked by a decline in physiologic function and increased susceptibility to environmental stressors,19 , 20 and it is a summation of an individual's functional status, mortality risk, and chronic medical conditions.21 The Frailty Index, an objective measure of frailty using key clinical and laboratory markers is often used to assess overall health status and risk stratification for serious disease complications among older adults.22 Generally, frailty is associated with aging and thus, predominantly occurs among older adults. Notably, a higher degree of frailty is associated with immune function decline, and consequently, the inability to mount an appropriate response to antigenic stimulation by either an infection or vaccine.
These resultant effects of aging on the immune system have diverse ramifications on response to vaccination among this age group. Consequences such as the ability of antigens from vaccines and infections to elicit an appropriate immune response (immunogenicity) and side effects of vaccines (reactogenicity) among these subjects differ from other age groups. These immunologic realities in this age group demonstrate the critical need for studies to best deploy available vaccines and/or engineer better ones.
Immune correlates of protection. Antibodies generally confer protection against infections, and specific antibody levels are often correlated with protection from such infections. Having an immune correlate of protection is very helpful for vaccine development and approval, allowing more rapid and less costly approvals for new vaccines or variations on current vaccines. Although specific correlates of protection from SARS-CoV-2 infection are yet to be established,23, 24, 25, 26 seroprotective levels of neutralizing and non-neutralizing antibodies are widely accepted as surrogates for protection in other viruses such as varicella zoster and hepatitis B virus.27 The discordance between immunogenicity and efficacy findings for older adults often observed in COVID-19 vaccine trials underscores the difficulty in defining specific immunologic correlates of clinical protection. Emerging variant strains pose an additional challenge because immune correlate may vary depending on the variant. All this taken together suggests that a well-defined immune correlate of protection across age and strain might not exist for COVID-19 vaccines.
Efficacy of current coronavirus disease 2019 vaccines among older adults
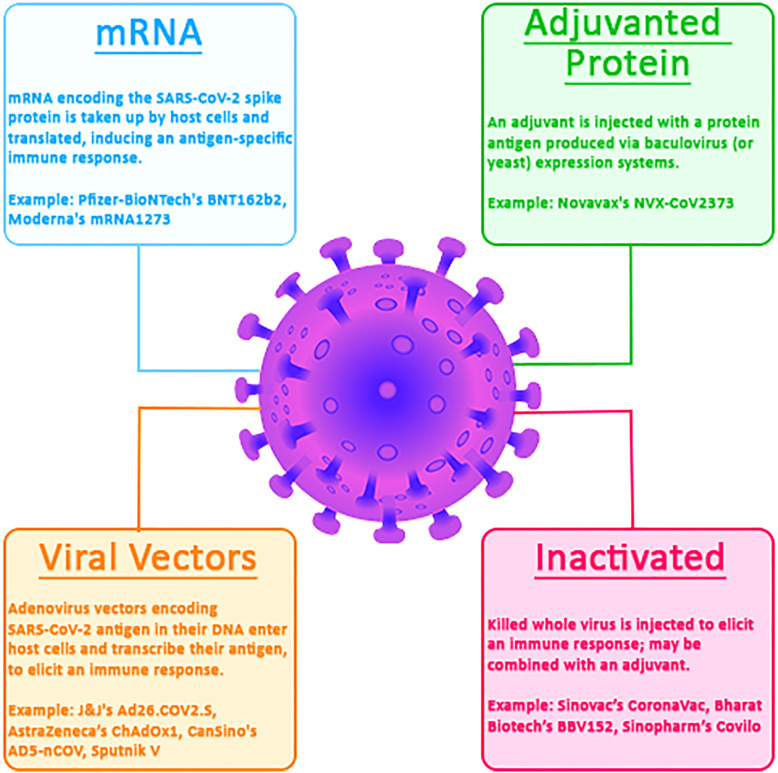
The rapid development of COVID-19 vaccines has helped mitigate the pandemic’s devastating effects across all eligible age groups, especially among vulnerable groups including older adults. The World Health Organization (WHO) registry contains more than 300 vaccines in development at different phases of clinical trials of which 11 have been approved for emergency use worldwide.28 These approved vaccines use different technologies (Fig. 1 ); they include the novel messenger RNA (mRNA) vaccines (Pfizer–BioNTech’s Comirnaty BNT162b2 and Moderna’s Spikevax mRNA-1273) and the non-mRNA vaccines such as adenovirus vector-based vaccines (Johnson & Johnson–Janssen’s Jcovden Ad26.COV2.S, CanSino’s Convidecia AD5-nCOV, and AstraZeneca’s Covishield and Vaxzevria AZD1222; ChAdOx1), adjuvanted protein vaccines (Novavax’s Covovax and Nuvaxovid NVX-CoV2373), and inactivated virus vaccines (Sinopharm’s Covilo, Sinovac’s CoronaVac, and Bharat Biotech’s Covaxin). These vaccines and the recently approved bivalent boosters have varying acceptance and usage in different parts of the world but have all remained effective in reducing morbidity and mortality among older adults, as well as other populations.29
Fig. 1.
COVID-19 vaccines according to technologies employed. All are WHO-approved except the viral vector sputnik V vaccine. mRNA, Messenger Ribonucleic Acid; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; J&J, Johnson and Johnson.
As is typical of vaccine development, initial safety and immunogenicity studies of many of these vaccines focused on middle-aged adults with much less older adult representation. The phase 3 approval trials, however, had better older adult representation with about 25% to 40% of subjects in an older age group category from greater than 55, 60, 65, or 70 years depending on the study (Table 1 ). This is a reasonable representation in the phase 3 trials. Results from these trials show that most COVID-19 vaccines did not have a significant initial difference in efficacy across the adult age spectrum (see Table 1). This observation suggests independence of vaccine efficacy (VE) from age as it applies to the COVID-19 vaccines in use. Rather than age, frailty may be a better predictor of the efficacy of certain COVID-19 vaccines.30 As explained earlier, immunosenescence has implications for the durability of vaccine-induced antibodies in older adults. Consequently, age and frailty are negatively associated with the durability of antibodies postvaccination, whereas a prior infection with SARS-CoV-2 increases durability regardless of age and frailty.31, 32, 33, 34
Table 1.
Summary of older age–specific vaccine efficacy data in phase 3 coronavirus disease 2019 vaccine trials
| Vaccine | Type | Number of Older Adult Participants (% of Total Participants) Age Cutoff | VE in Older Adults % (95% CI) | Overall Efficacy (95% CI) | Median Follow-Up (in days)a |
|---|---|---|---|---|---|
| BNT162b2 (Pfizer) | mRNA vaccine encoding spike glycoprotein | 15,921 (42.2) >55 y |
93.7 (80.6–98.8) | 95.0 (90.0–97.9) | 60 |
| mRNA-1273 (Moderna) | mRNA vaccine encoding spike glycoprotein | 7512 (25.8) ≥65 y |
86.4 (61.4–95.2) | 94.1 (89.3–96.8) | 63 |
| NVX-CoV2373 (NVX; Novavax) | Nanoparticle vaccine containing purified spike glycoprotein and adjuvant | 3910 (27.9) ≥65 y |
88.9 (20.2–99.7) | 89.7 (80.2–94.6) | 56 |
| ChAdOx-nCov19 (ChAd; AZD1222, AstraZeneca) | Replication-deficient chimpanzee adenovirus-vectored vaccine, expressing spike glycoprotein | 7238 (22.4) ≥65 y |
83.5 (54.2–94.1) | 74.0 (65.3–80.5) | 61 |
| Ad26.COV2.S (Ad26; Janssen) | Replication-deficient adenovirus vector vaccine constructed to encode a spike glycoprotein | 14,672 (33.5) ≥60 y |
66.2 (36.7–83.0) | 66.1 (55.0–74.8) | 58 |
| rAd26 and rAd5 vector-based (Sputnik V, Gam-COVID-Vac) | Heterologous rAd-based vaccine encoding spike glycoprotein | 2144 (10.8%) >60 y |
91.8 (67.1–98.3) | 91·6 (85.6–95.2) | 21 d after first dose |
| Inactivated whole virus vaccine (CoronaVac, Sinovac Biotech) | Inactivated whole SARS-CoV-2 | 43,774 (100%) ≥70 y |
55.4 (46.5–62.8) | Same | Not stated |
| BBV152 (Covaxin, Bharat Biotech Intl.) | Inactivated whole SARS-CoV-2 with Toll-like receptor 7/8 adjuvant adsorbed to alum | 1858 (10.9%) ≥60 y |
67.8 (8·0–90.0). | 77.8 (65·2–86.4) | 99 |
Nine of 11 WHO-approved COVID-19 vaccines shown as well as the Sputnik V vaccine.
Abbreviations: CI, confidence interval; rAd, recombinant adenovirus; VE, vaccine efficacy.
Median follow-up is counted after the second dose unless otherwise specified.
Like previous vaccines, the COVID-19 vaccines generated reduced cellular and humoral response when compared with the younger population.35 , 36 However, this initial reduction in immunogenicity does not necessarily translate into reduced protection for this population because exact immune correlates of protection are yet to be defined. More so, both real-world and trial-reported VE among these older adult populations compares impressively with those reported among other age groups further downplaying the effect of the disparity in observed immunogenicity. Although females have been reported to generate better immune responses to vaccines in general,37 , 38 such disparities have not been reported with the COVID vaccines currently in use.39 , 40 Of note, the authors caution that a head-to-head comparison of vaccine trials is not ideal due to variables, such as differences in trial settings and participants, symptomatic illness criteria, and predominant variants at the time trials were conducted.41
Effectiveness and Immunogenicity of the mRNA Vaccines in Older Adults
The mRNA vaccines, such as those manufactured by Pfizer (BNT162b2) and Moderna (mRNA-1273), were the earliest vaccines approved for emergency use in the United States and are the most widely used vaccines, especially in developed countries with cost, transport, and storage logistics being a major factor limiting their use in developing countries.
The BNT162b2 mRNA vaccine is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a membrane-anchored SARS-CoV-2 full-length spike, stabilized in the prefusion conformation.42 This vaccine is administered in a 2-dose regimen 21 days apart. The early phase 2 trial that included 45 healthy adults aged 65 to 85 years reported a decline in immunogenicity with increasing age; however, this response exceeded that of convalescent subjects suggesting the superiority of vaccine-induced immunity over that of natural infection among this age group.42 Phase 3 trials reported an overall efficacy of 95.0% (90.0–97.9) after a median follow-up of 2 months with subgroup analysis revealing a consistent but slightly lower VE of 93.7% (80.6–98.8) among adults older than 55 years, 94.7% (66.7–99.9) among adults older than 65 years, and 100.0% (−13.1–100.0) in adults older than 75 years.43 The lower immunogenicity trend of the BNT1652b2 primary series observed among older adults seems to be reversed with the booster doses where they were reported to have mounted better responses.44 Phase 1 trial of the third dose of the Pfizer vaccine showed a better neutralization capacity in older adults compared with younger adults. Evaluating twelve 65- to 85-year-old participants over 1 month, the geometric mean ratio of neutralizing antibodies was consistently higher between the second and third dose for these older adults than their younger counterparts, underlining the efficacy of an additional dose in this population of older adults.44
The mRNA-1273 vaccine is a lipid nanoparticle-encapsulated mRNA-based vaccine that encodes the prefusion-stabilized full-length spike protein of the SARS-CoV-2 virus; it is administered in 2 doses, 28 days apart. A phase 2 open-label trial of the mRNA-1273 vaccine in adults aged 56 years and older showed a dose- and time-dependent robust immune response among these older adults.45 Measured binding and neutralizing antibodies were not dependent on age and were commensurate with levels earlier reported among adults aged 18 to 55 years.46 In phase 3 randomized, observer-blinded, placebo-controlled trial conducted at 99 centers across the United States, adults aged 65 years or older had a reported VE of 86.4% (61.4–95.2) against an overall VE of 94.1% (89.3–96.8).47
Although the Pfizer and Moderna trials were not designed to measure the impact on transmission explicitly, real-world studies have shown associated vaccine benefits in reducing incidences of SARS-CoV-2 infections. In a metadata study of 280 nursing homes (NH) across 21 states in the United States, Mor and colleagues48 used resident-level data to compare the rate of new resident infections as well as hospital transfers and/or deaths in facilities with early versus later vaccination clinics, adjusting for infection rates in each facility. Among early recipients of 2 doses of the mRNA vaccines, the investigators reported a magnitude of 5.2 fewer cases per 100 at-risk NH residents and an average cumulative reduction in hospitalization or death of 5 events per 100 infected residents per day up to 7 weeks.48 Similarly, in a large study involving residents of long-term care facilities in Israel, Muhsen and colleagues49 reported significantly lower rates of SARS-CoV-2 infection and hospitalization for severe COVID-19. Following up with residents up to 6 weeks after receiving a third dose of the BNT162b2 mRNA vaccine, the investigators observed an incidence ratio of 0.29 for overall infection and 0.20 for hospitalization, which corresponded to a relative rate reduction of 71% and 80%, respectively.50
Looking at real-world data, McConeghy and colleagues51 studied the additional role a fourth vaccine dose plays in reducing morbidity and mortality from COVID-19. Comparing a single mRNA COVID-19 vaccine booster dose, with a second booster dose, they found that a second booster dose provided additional protection against COVID-19-associated severe outcomes among NH residents during the Omicron period. Other smaller studies have reported similar protection among NH residents who have received the fourth vaccine dose to date.49 , 52
It is not clear what exact role and magnitude T cells play in protection from SARS-CoV-2, but they play a role in other viral infections in disease mitigation once infected. Owing to the prevailing realities of immunosenescence and inflammaging, older adults tend to have impaired vaccine-specific T-cell responses. Following 2 doses of the BNT162b2 mRNA vaccine, the frequencies of vaccine-specific IFNγ+ and IFNγ+IL-2+TNFα+ CD4+ and the frequency of specific CD8+ T cells were lower in COVID-19-naive older adults than in COVID-19-naive young adults.53 T-cell responses were the same in the older and younger populations that were vaccinated after prior COVID-19. Several studies specifically address issues related to immunosenescence and inflammaging. Palacios-Pedrero et al observed lower vaccine-specific responses in the older group, and interestingly found a correlation between naive CD4+ cells and reduced vaccine-induced CD4 cells. This finding supports a major component of immunosenescence: poorer response in the aged is due to loss of naive cells.54 Vitallé and colleagues55 demonstrated that older persons display less frequency and polyfunctionality of vaccine-induced T cells. Potentially demonstrating elements of both immunosenescence and inflammaging, they found that aging-related lower thymic function, altered T-cell homeostasis, proinflammatory monocyte profile, and altered dendritic cell features and function were associated with these reduced responses.
Effectiveness and Immunogenicity of the Non-mRNA Vaccines in Older Adults
The Novavax NVX-CoV2373 is a recombinant nanoparticle vaccine against SARS-CoV-2 that contains the full-length spike glycoprotein (S-protein) of the prototype strain plus Matrix-M adjuvant that is administered in 2-dose primary series 21 days apart. In a phase 3 randomized placebo-controlled trial conducted in the United Kingdom that included 1953 older adults aged between 65 and 84 years, the NVX-CoV2373 recorded a VE of 88.9% (20.2–99.7) followed up for a median of 56 days after the second dose. This VE compares impressively with the 89.8% VE reported for participants younger than 65 years.56 A similar phase 3 trial conducted in the United States and Mexico for a median follow-up of 64 days after the second dose reported a VE of 91% in participants at overall high risk for COVID-19, defined as subjects aged 65 years or older and those of any age with chronic health conditions or an increased risk for COVID-19 due to elevated exposure.57
Among older adults, in a phase 2 immunogenicity trial, a homologous booster dose of the NVX-CoV2373 vaccine administered approximately 6 months following the primary 2-dose series resulted in a significantly enhanced immunogenicity producing S-protein IgG and neutralization titers that were 4-fold higher than after the primary 2-dose series. Subgroup analysis for the ancestral Wuhan-Hu-1 strain showed slightly lower antibody responses in older adults (aged 60–84 years) than those in younger adults (aged 18–59 years).58
The Jansen’s Ad26.COV2.S vaccine is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length SARS-CoV-2 S-protein in a prefusion-stabilized conformation administered as a single-dose primary vaccination. In the initial phase 3 trial, participants aged 60 years or older had a VE of 76.3% (61.6–86.0) against moderate to severe-critical COVID-19 and a VE of 74.5% (57.9–84.3) against severity-adjusted symptomatic COVID-19, both higher than the overall VE reported in all age groups.59 Remarkably, although estimates of VE differed between older adults with or without coexisting conditions at short-term follow-up, they became similar with a longer follow-up time. The final analysis of the trial reported a VE of 55.0% (42.9–64.7) after 14 days of follow-up, which dropped to 46.6% (30.7–59.0) beyond 28 days among participants older than 60 years.60 This drop in efficacy between the primary and final analysis is believed to be due to more virulent circulating variants of SARS-CoV-2 that emerged after the primary analysis was carried out. In a double-blinded phase 3 randomized trial in which a homologous Ad26.COV2.S booster was administered 2 months after the primary series, efficacy against moderate to severe-critical COVID-19 among participants older than 60 years was 66.2% (−14.0–92.2) lower than the overall efficacy of 75.2% (54.6–87.3).61 However, the development of vaccine-induced thrombotic thrombocytopenia has limited the continued use of the Ad26.COV2.S vaccine either as a primary series or a booster62 and thus limited its further use among older adults; many of whom have comorbid conditions that predispose them to thrombotic events.
The ChAdOx1 nCoV-19 (AZD1222) is a replication-defective adenovirus-vectored vaccine expressing the full-length SARS-CoV-2 spike glycoprotein gene. The vaccine is administered in a prime-boost regimen 4 weeks apart and has been shown to have similar immunogenicity across age groups.63 After 15 days or more follow-up in a double-blind, randomized placebo-controlled phase 3 trial, older adults had an estimated VE of 83.5% (54.2–94.1) that was better than the overall 74.0% (65.3–80.5) observed for all age groups.64 A homologous booster dose of ChAdOx1 nCoV-19 given 6 to 8 months after completion of the primary series produced similar levels of boosting effect on anti-spike IgG and cellular responses after 28 days between older adults aged 70 years or older and adults aged between 18 and 69 years.65
The Gam-COVID-Vac (Sputnik V) is a recombinant adenovirus (rAd)-based vaccine that contains the full-length SARS-CoV-2 glycoprotein S gene. The vaccine is administered in a prime(rAd26)-boost(rAd5) regimen, 21 days apart. Despite the halt in its emergency use and approval by the WHO,66 the Sputnik V vaccine showed good efficacy among older adults. Although the initial safety and immunogenicity trial did not include adults older than 60 years,39 the phase 3 trials conducted in Russia had 2144 adults older than 60 years completing the study. At a median follow-up time of 48 days after the first dose, VE among this older age group was reported to be 91.8% (67.1–98.3), slightly higher than the overall efficacy of 91.6% (85.6–95.2).67
The CoronaVac (Sinovac Life Sciences) is an inactivated whole virus vaccine that has been shown to have immunogenicity and efficacy against COVID-19. CoronaVac, when administered in the standard 2-dose regimen 28 days apart, produces immunogenicity in adults aged 60 years and older, which is similar to adults aged 18 to 59 years, in a dose-dependent manner.68 , 69 A phase 3 trial of adults aged 70 years and older in Brazil found adjusted VE against hospital admissions was 55.5% (46.5% to 62.9%) and against deaths was 61.2% (48.9% to 70.5%) at greater than equal to 14 days after the second dose.70 A decline in effectiveness particularly in those older than 80 years was also reported. CoronaVac-induced antibodies tend to wane at a faster rate in older adults after the primary series but tend to be more durable after the third dose. For instance, older adults (aged 60 years or older) in a single-center phase 2 trial had an approximate decline of 10.7-fold in neutralizing antibodies 6 months after the second dose of the CoronaVac vaccine versus 6.8-fold observed for adults between 18 and 59 years. However, the rate of decline after a third dose given 8 months after the second dose was 2.5-fold among older adults versus 4.1-fold in the 18 to 59-year-old cohort over the same period.71
Although Covaxin (BBV152, Bharat Biotech International) does not seem to be currently in production or distribution, it was overwhelmingly the predominant vaccine initially used in India. The BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine formulated with a Toll-like receptor 7/8 agonist molecule adsorbed to alum (Algel-IMDG) administered in a 2-dose regimen 4 weeks apart.72 A total of 1858 older participants aged 60 years or older participated in the phase 3 trial and were found to have a modestly reduced VE to symptomatic disease of 67.8% (8.0–90.0) after a median follow-up of 99 days compared with 79.4% (66.0–88.2) VE in those younger than age 60 years.73
Hybrid Immunity from Vaccine and Infection
Hybrid immunity has been shown to confer some additional protection against SARS-CoV-2, even against the immune-escaping Omicron variants.74, 75, 76 Vaccination following a previous SARS-CoV-2 infection increases all SARS-CoV-2-specific immunologic parameters due to the activation of immunologic memory generated from prior exposure.77 This prior antigenic exposure has direct beneficial quantitative and qualitative effects on vaccine response in older adults. Vaccine-induced neutralizing and non-neutralizing antibodies are markedly increased among NH residents who had recovered from prior infection to the levels comparable to the younger comparator group36 and had more durable antibodies than their naive counterparts.78 , 79
This enhanced response seems to be even better with extended intervals between infection and vaccination. Fedele and colleagues80 found that a longer interval between previous SARS-CoV-2 infection and vaccination results in a higher antibody response 2 and 6 months postvaccination among NH residents. This finding is consistent with studies that observed an enhanced humoral response using a 2-dose SARS-CoV-2 regimen with extended intervals.81, 82, 83 Considering the initial reduced immune response documented among this aging population,36 it is thus interesting to note that an appropriate dosing interval, especially in convalescent elderly vaccinees, may overcome this initial diminished immune response to produce a robust response.81
Challenges of vaccination in older adults
Vaccine Uptake Among Older Adults
Despite the difficulties in achieving adequate vaccine compliance,84 older adults remain the target for vaccination policies.5 Early in the pandemic, age was associated with a lower willingness to receive a COVID-19 vaccine in the United States.85 However, there has been a dramatic turnaround in this trend with the older adult population accounting for the largest percentage of vaccinated individuals in the United States at the time of writing this review.86
Factors such as lower life expectancy, concerns about side effects, and efficacy historically influence vaccine uptake in this population. For COVID-19 vaccines, safety was the primary concern reported among unwilling older adults followed by doubts about their effectiveness and misinformation.87 Notable misinformation included the belief of long-lasting immunity once infected with SARS-CoV-2, protection by certain blood group types, and erroneous belief of magnets or chips implanted in vaccines among others.88 In a certain adult population aged 65 years and older in the United States, Nikolovski and colleagues89 reported a significant unwillingness to receive the COVID-19 vaccines among female subjects and African American subjects. These subjects were, however, willing to discuss this uncertainty/unwillingness with their healthcare providers, suggesting an essential rolehealthcare providers may play in the battle against vaccine hesitancy.90 In a community-based multidisciplinary study of more than 20,000 adults older than 60 years in Singapore, direct contact and clarity in communication using mutually understood languages as well as direct access and consultation with allergists were found to significantly increase the willingness of unvaccinated enrollees to receive the COVID-19 vaccine after a 3-month follow-up.88
Older adults who were willing to take the vaccine were likely to do so to protect themselves and others as well as contribute to ending the pandemic.90 Furthermore, influenza vaccination or a willingness to receive the vaccine was strongly associated with a willingness to receive COVID-19 vaccines among older adults.87 Thus, approaches that have been used in improving the uptake of influenza vaccines may be beneficial in increasing COVID-19 vaccine coverage among older adults.91
Reactogenicity in Older Adults
Generally, older adults tolerate vaccines better than younger adults.11 , 92 Considering that reactions result from immune system activation, it is not so surprising that older adults do not have as many side effects from vaccines because they generate less robust responses.12 In addition, reactions such as injection site pain, headache, and muscle pain may be more tolerable by older adults and as such, may be underreported among this age group.93 , 94 Accordingly, the COVID-19 vaccines currently in use have all demonstrated good safety profiles among older adults with reactogenicity mostly mild to moderate.63 , 95 For instance, there are fewer local and systemic reactions reported among older adults (aged ≥65 years) following the 2-dose mRNA-based COVID-19 vaccines compared with younger adults (aged <65 years).96
This reduced reactogenicity is consistent with findings among older adults who received the inactivated vaccines.97 A third dose of the BNT162b2 mRNA vaccine had mild to moderate side effects among participants aged 65 to 85 years in the Pfizer trials, like that of the second dose with no unsolicited adverse event.44 The Novavax trials reported a lower incidence of local and systemic reactions among participants older than 65 years compared with younger participants.56 Owing to the negative relationship between age, reactogenicity, and immunogenicity, it has often been hypothesized that reactogenicity could be related to immunogenicity. Certain groups found somewhat modest relationships between these 2 immunologic phenomena concerning the COVID-19 vaccines,93 , 98, 99, 100 whereas others did not observe any appreciable link between them.33 , 101 Differences in the study setting, analytical model, and manner of soliciting for vaccine reactions may have contributed to the differences in these findings. Nevertheless, older adults are known to report fewer postvaccination side effects and achieve lower antibody production compared with younger adults. This association needs further exploration through larger studies.
Proposed strategies to optimize vaccines for older adults
With the attendant reality of immunosenescence and inflammaging, a focused vaccine strategy that considers these immunologic effects could be helpful for older adults. A challenging feature of SARS-CoV-2 is its mutation ability leading to the production of more infectious and immune-escaping variants of concern. Although monovalent vaccines have some cross-variant activity,102 , 103 the bivalent vaccines recently approved for use have shown better neutralizing capacities and could provide better protection against the immune-evasive Omicron family.104 The initial bivalent vaccine approved by the European Union authorities adds the early Omicron BA1 strain to the Wuhan-containing current vaccine, and the version approved in the United States adds the more recent Omicron BA4/5. Chalkias and colleagues105 studied the immunogenicity of the bivalent BA1 vaccine in a population that had 40% of subjects aged 65 years or older. The investigators found superior anti-omicron neutralization compared with the monovalent vaccine.105 These data would suggest that the bivalent vaccine might be effective, but the clinical efficacy data of the bivalent vaccine are not yet available.
Overall, it is imperative that older adults are up to date with vaccinations because progressively significant benefits have been established with each extra dose administered in this age group.106 In a study of NH residents and healthcare workers, the authors observed that a booster dose is needed to achieve significant Omicron neutralization activity among this population even if they had an infection before the Omicron era.102 This observation is consistent with findings reported among younger adults.103 In the current Omicron era, this is essential to protect this age group from the possible devastating effects of infection with the immune-escaping Omicron variants. This additional dose was also shown to reduce transmission among NH residents compared with the primary vaccine series alone.107 Although the benefits of an extra dose on antibody levels abound among NH residents,79 cellular immunity seems to be impacted to a lesser degree among this population.108 , 109
Like the timing of vaccination after infection with SARS-CoV-2 as discussed earlier, modifications in the intervals between doses may help boost vaccine-induced immunity. Older adults 80 years or older receiving the 2-dose BNT162b2 mRNA vaccine at an extended interval of 11 to 12 weeks were found to produce a peak antibody response 3.5 times those that received the standard regimen 3 weeks apart. However, peak cellular responses were lower81; this is similar to findings in a younger healthcare worker cohort receiving the BNT162b2 vaccine.82 In like manner, an extended-interval protocol for the adenovirus-based ChAdOx1 vaccine has increased spike-specific antibody responses by 2.3-fold and improved VE across all age groups.110 Although this carries the risk of extending the period of partial vaccination, a single dose of the BNT162b2 vaccine produces favorable immunogenicity and clinical efficacy29 , 110, 111, 112 and is durable.113 Thus, the timing of subsequent doses could be targeted appropriately using the half-life of vaccine-induced antibodies.
An initial 3-dose series could be considered in this setting where the vaccine is a neoantigen vaccine because it is not boosting prior immunity but rather must generate a response from naive cells. A 3-dose series could allow several benefits as observed by our data and those of others. One benefit is the increased breadth of response that particularly occurs with the third dose providing better anti-Omicron immunity. The other benefit is that the third dose boosted antibody levels among the hyporesponders to the primary series; this is a subset of the older multimorbid NH resident population that produced negligible or very low vaccine-induced antibodies similar to the levels observed in immunocompromised individuals. Previously, immunocompromised adults received a Centers for Disease Control and Prevention recommendation for a 3-dose series.114 Thus, adopting a 3-dose vaccination schedule may be beneficial for this population of hyporesponders, and by extension, older adults; this would particularly apply to neoantigen vaccines. Alternatively, screening this population for possible hyporesponsiveness can help identify those who would need extra doses and thus, optimize protection for these adults.109 , 115 , 116
Moreover, heterologous boosting, which involves the administration of booster doses from a platform other than that of the primary series, has shown some effectiveness in enhancing protection against COVID-19. This strategy helps to maximize the quantity and breadth of vaccine-induced antibodies.117, 118, 119 This carries the potential for antibody diversity owing to the subtle differences that have been described between the 2 mRNA vaccines currently in use120 and even more, vaccines across different platforms.35 , 121 In a large cohort study among veterans, the incidence of infection and moderate-to-severe disease was significantly reduced among those who had been primed with an adenoviral vaccine and were boosted with an mRNA vaccine compared with those who had homologous vaccination with either the adenoviral or mRNA vaccines.122 More investigation is clearly needed in the older population to determine the optimal primary series, booster schedules, and booster vaccine products that could apply to both COVID-19 and new pathogen outbreaks.
Summary
Vaccination remains a key tool in protecting older adults against severe outcomes of COVID-19. Thus, this population should remain of high priority for vaccination campaigns and must be kept up to date on additional doses for optimal protection. Vaccine protocols and formulations could be adapted to cater to the attendant immune changes in this population. Considering the high likelihood of hyporesponders to vaccination in this group, appropriate screening measures should be put in place. Finally, vaccination protocols should be tailored to maximize the benefits in this population and, with the robust benefits of a third dose among older adults, a 3-dose regimen with modified intervals should be considered, as well as heterologous boosting.
Clinics care points
-
•
Due to the high risk of SARS-CoV-2 infection in the older population, they should remain a high priority for vaccination campaigns and must be kept up to date on additional doses for optimal protection.
-
•
A 3-dose primary series enhances antibody production and breadth significantly providing better cross-variant protection and could be considered.
-
•
Older adults who report no vaccine side effects and/or have never been infected with SARS-CoV-2 should be prioritized when screening for vaccine responsiveness.
Acknowledgement
This work was supported by NIH AI129709-03S1, U01 CA260539-01, and CDC 200-2016-91773.
References
- 1.COVID-19 nursing home data - centers for Medicare & Medicaid Services data. 2022. https://data.cms.gov/covid-19/covid-19-nursing-home-data Accessed October 25, 2022. [PubMed]
- 2.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 3.Remelli F., Volpato S., Trevisan C. Clinical Features of SARS-CoV-2 Infection in Older Adults. Clin Geriatr Med. 2022;38(3):483–500. doi: 10.1016/j.cger.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 vaccination Program Operational Guidance | CDC. 2022. https://www.cdc.gov/vaccines/covid-19/covid19-vaccination-guidance.html Accessed September 15, 2022.
- 5.Dooling K., McClung N., Chamberland M., et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams C., Al-Bargash D., MacAlintal C., et al. Coronavirus Disease 2019 (COVID-19) Outbreak Associated With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) P.1 Lineage in a Long-Term Care Home After Implementation of a Vaccination Program—Ontario, Canada, April–May 2021. Clin Infect Dis. 2022;74(6):1085–1088. doi: 10.1093/cid/ciab617. [DOI] [PubMed] [Google Scholar]
- 7.Lafuente-Lafuente C., Rainone A., Guérin O., et al. COVID-19 Outbreaks in Nursing Homes Despite Full Vaccination with BNT162b2 of a Majority of Residents. Gerontology. 2022:1–9. doi: 10.1159/000523701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger B. Vaccines for the elderly: Current use and future challenges. Immun Ageing. 2018;15(1):1–8. doi: 10.1186/s12979-017-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osterholm M.T., Kelley N.S., Sommer A., et al. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 10.Siegrist C.A., Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 11.Ciabattini A., Nardini C., Santoro F., et al. Vaccination in the elderly: The challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Crooke S.N., Ovsyannikova I.G., Poland G.A., et al. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16(1):1–16. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawelec G. Age and immunity: What is “immunosenescence”. Exp Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Linton P.J., Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 15.Kirkland J.L., Tchkonia T., Pirtskhalava T., et al. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37(6):757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi C., Garagnani P., Parini P., et al. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 17.Frasca D., Diaz A., Romero M., et al. High TNF-α levels in resting B cells negatively correlate with their response. Exp Gerontol. 2014;54:116–122. doi: 10.1016/j.exger.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElhaney J.E., Kuchel G.A., Zhou X., et al. T-cell immunity to influenza in older adults: A pathophysiological framework for development of more effective vaccines. Front Immunol. 2016;7(FEB):41. doi: 10.3389/fimmu.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergman H., Ferrucci L., Guralnik J., et al. Frailty: An Emerging Research and Clinical Paradigm—Issues and Controversies. Journals Gerontol Ser A. 2007;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strandberg T.E., Pitkälä K.H. Frailty in elderly people. Lancet. 2007;369(9570):1328–1329. doi: 10.1016/S0140-6736(07)60613-8. [DOI] [PubMed] [Google Scholar]
- 21.Fried L.P., Ferrucci L., Darer J., et al. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. Journals Gerontol Ser A. 2004;59(3):M255–M263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earle K.A., Ambrosino D.M., Fiore-Gartland A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 25.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. 2021;397(10283):1421–1423. doi: 10.1016/S0140-6736(21)00782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asamoah-Boaheng M., Goldfarb D.M., Karim M.E., et al. The Relationship Between Anti-Spike SARS-CoV-2 Antibody Levels and Risk of Breakthrough COVID-19 Among Fully Vaccinated Adults. J Infect Dis. 2022 doi: 10.1093/INFDIS/JIAC403. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plotkin S.A. Correlates of Protection Induced by Vaccination. Clin Vaccin Immunol. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO – COVID19 Vaccine Tracker. https://covid19.trackvaccines.org/agency/who/ Accessed October 20, 2022.
- 29.Bernal J.L., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/BMJ.N1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semelka C.T., Partnership C-19 C.R., DeWitt M.E., et al. Frailty and COVID-19 mRNA Vaccine Antibody Response in the COVID-19 Community Research Partnership. Journals Gerontol Ser A. 2022;77(7):1366–1370. doi: 10.1093/gerona/glac095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyer A.H., Noonan C., McElheron M., et al. Previous SARS-CoV-2 Infection, Age, and Frailty Are Associated With 6-Month Vaccine-Induced Anti-Spike Antibody Titer in Nursing Home Residents. J Am Med Dir Assoc. 2022;23(3):434–439. doi: 10.1016/j.jamda.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Søgaard O.S., Reekie J., Johansen I.S., et al. Characteristics associated with serological COVID-19 vaccine response and durability in an older population with significant comorbidity: the Danish Nationwide ENFORCE Study. Clin Microbiol Infect. 2022;28(8):1126–1133. doi: 10.1016/j.cmi.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller L., Andrée M., Moskorz W., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73(11):2065–2072. doi: 10.1101/2021.03.03.21251066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canaday D.H., Oyebanji O.A., Keresztesy D., et al. Significant Reduction in Vaccine-Induced Antibody Levels and Neutralization Activity Among Healthcare Workers and Nursing Home Residents 6 Months Following Coronavirus Disease 2019 BNT162b2 mRNA Vaccination. Clin Infect Dis. 2022;75(1):e884–e887. doi: 10.1093/cid/ciab963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros G.X., Sasahara G.L., Magawa J.Y., et al. Reduced T cell and antibody responses to inactivated coronavirus vaccine among individuals above 55 years old. Front Immunol. 2022;13:666. doi: 10.3389/fimmu.2022.812126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canaday D.H., Carias L., Oyebanji O.A., et al. Reduced BNT162b2 Messenger RNA Vaccine Response in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Naive Nursing Home Residents. Clin Infect Dis. 2021;73(11):2112–2115. doi: 10.1093/cid/ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleina S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voigt E.A., Ovsyannikova I.G., Kennedy R.B., et al. Sex differences in older adults’ immune responses to seasonal influenza vaccination. Front Immunol. 2019;10:180. doi: 10.3389/fimmu.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabal K.A., Ben-Amram H., Beiruti K., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 MRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance. 2021;26(6):2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapaka R.R., Hammershaimb E.A., Neuzil K.M. Are Some COVID-19 Vaccines Better Than Others? Interpreting and Comparing Estimates of Efficacy in Vaccine Trials. Clin Infect Dis. 2022;74(2):352–358. doi: 10.1093/cid/ciab213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh E.E., Frenck R.W., Falsey A.R., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falsey A.R., Frenck R.W., Walsh E.E., et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N Engl J Med. 2021;385(17):1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson E.J., Rouphael N.G., Widge A.T., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baden L.R., Sahly HM El, Essink B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mor V., Gutman R., Yang X., et al. Short-term impact of nursing home SARS-CoV-2 vaccinations on new infections, hospitalizations, and deaths. J Am Geriatr Soc. 2021;69(8):2063–2069. doi: 10.1111/jgs.17176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muhsen K., Maimon N., Mizrahi A.Y., et al. Association of Receipt of the Fourth BNT162b2 Dose With Omicron Infection and COVID-19 Hospitalizations Among Residents of Long-term Care Facilities. JAMA Intern Med. 2022;182(8):859–867. doi: 10.1001/jamainternmed.2022.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhsen K., Maimon N., Mizrahi A., et al. Effects of BNT162b2 Covid-19 Vaccine Booster in Long-Term Care Facilities in Israel. N Engl J Med. 2022;386(4):399–401. doi: 10.1056/NEJMc2117385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McConeghy K.W., White E.M., Blackman C., et al. Effectiveness of a Second COVID-19 Vaccine Booster Dose Against Infection, Hospitalization, or Death Among Nursing Home Residents — 19 States, March 29–July 25, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(39):1235–1238. doi: 10.15585/mmwr.mm7139a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grewal R., Kitchen S.A., Nguyen L., et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378 doi: 10.1136/BMJ-2022-071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demaret J., Corroyer-Simovic B., Alidjinou E.K., et al. Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 mRNA Vaccine in Older People. Front Immunol. 2021;12:4639. doi: 10.3389/fimmu.2021.778679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palacios-Pedrero M.Á., Jansen J.M., Blume C., et al. Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Nat Aging. 2022;2(10):896–905. doi: 10.1038/s43587-022-00292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vitallé J., Pérez-Gómez A., Ostos F.J., et al. Immune defects associated with lower SARS-CoV-2 BNT162b2 mRNA vaccine response in aged people. JCI Insight. 2022;7(17) doi: 10.1172/JCI.INSIGHT.161045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heath P.T., Galiza E.P., Baxter D.N., et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunkle L.M., Kotloff K.L., Gay C.L., et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N Engl J Med. 2022;386(6):531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallory R.M., Formica N., Pfeiffer S., et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2022;22(11):1565–1576. doi: 10.1016/S1473-3099(22)00420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadoff J., Gray G., Vandebosch A., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadoff J., Gray G., Vandebosch A., et al. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N Engl J Med. 2022;386(9):847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardt K., Vandebosch A., Sadoff J., et al. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022;0(0) doi: 10.1016/s1473-3099(22)00506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coronavirus (COVID-19) Update: FDA Limits Use of Janssen COVID-19 vaccine to certain individuals | FDA. 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals Accessed October 20, 2022.
- 63.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falsey A.R., Sobieszczyk M.E., Hirsch I., et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munro A.P.S., Janani L., Cornelius V., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Webster P. Russian COVID-19 vaccine in jeopardy after Ukraine invasion. Nat Med. 2022 doi: 10.1038/d41591-022-00042-y. Accessed September 20, 2022. [DOI] [PubMed] [Google Scholar]
- 67.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Z., Hu Y., Xu M., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y., Zeng G., Pan H., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranzani O.T., Hitchings M.D.T., Dorion M., et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:2015. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xin Q., Wu Q., Chen X., et al. Six-month follow-up of a booster dose of CoronaVac in two single-centre phase 2 clinical trials. Nat Commun. 2022;13(1):1–7. doi: 10.1038/s41467-022-30864-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ella R., Vadrevu K.M., Jogdand H., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21(5):637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ella R., Reddy S., Blackwelder W., et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui D.S. Hybrid immunity and strategies for COVID-19 vaccination. Lancet Infect Dis. 2022;0(0) doi: 10.1016/s1473-3099(22)00640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carazo S., Skowronski D.M., Brisson M., et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Infect Dis. 2022;0(0) doi: 10.1016/s1473-3099(22)00578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malato J., Ribeiro R.M., Leite P.P., et al. Risk of BA.5 Infection among Persons Exposed to Previous SARS-CoV-2 Variants. N Engl J Med. 2022;387(10):953–954. doi: 10.1056/NEJMc2209479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crotty S. Hybrid immunity. Science (80- ) 2021;372(6549):1392–1393. [Google Scholar]
- 78.Katz M.J., Heaney C.D., Pisanic N., et al. Evaluating immunity to SARS-CoV-2 in nursing home residents using saliva IgG. J Am Geriatr Soc. 2022;70(3):659–668. doi: 10.1111/jgs.17660. [DOI] [PubMed] [Google Scholar]
- 79.Vanshylla K., Tober-Lau P., Gruell H., et al. Durability of omicron-neutralising serum activity after mRNA booster immunisation in older adults. Lancet Infect Dis. 2022;22(4):445–446. doi: 10.1016/S1473-3099(22)00135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fedele G., Palmieri A., Damiano C., et al. Humoral immunity induced by mRNA COVID-19 vaccines in Nursing Home Residents previously infected with SARS-CoV-2. Aging Clin Exp Res. 2022:1–8. doi: 10.1007/s40520-022-02239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parry H., Bruton R., Stephens C., et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. npj Vaccin. 2022;7(1):1–5. doi: 10.1038/s41541-022-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Payne R.P., Longet S., Austin J.A., et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184(23):5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tauzin A., Gong S.Y., Beaudoin-Bussières G., et al. Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022;30(1):97–109.e5. doi: 10.1016/j.chom.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bridges C.B., Hurley L.P., Williams W.W., et al. Meeting the Challenges of Immunizing Adults. Vaccine. 2015;33:D114–D120. doi: 10.1016/j.vaccine.2015.09.054. [DOI] [PubMed] [Google Scholar]
- 85.Kreps S., Prasad S., Brownstein J.S., et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw Open. 2020;3(10):e2025594. doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.COVID-19 vaccination coverage and vaccine Confidence among adults | CDC. 2021. https://www.cdc.gov/vaccines/imz-managers/coverage/covidvaxview/interactive/adults.html Accessed September 15, 2022.
- 87.Basta N.E., Sohel N., Sulis G., et al. Factors Associated With Willingness to Receive a COVID-19 Vaccine Among 23,819 Adults Aged 50 Years or Older: An Analysis of the Canadian Longitudinal Study on Aging. Am J Epidemiol. 2022;191(6):987–998. doi: 10.1093/aje/kwac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moosa A.S., Wee Y.M.S., Jaw M.H., et al. A multidisciplinary effort to increase COVID-19 vaccination among the older adults. Front Public Heal. 2022;10:2526. doi: 10.3389/fpubh.2022.904161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikolovski J., Koldijk M., Weverling G.J., et al. Factors indicating intention to vaccinate with a COVID-19 vaccine among older U.S. adults. PLoS One. 2021;16(5):e0251963. doi: 10.1371/journal.pone.0251963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nichol K.L., Zimmerman R. Generalist and Subspecialist Physicians’ Knowledge, Attitudes, and Practices Regarding Influenza and Pneumococcal Vaccinations for Elderly and Other High-Risk Patients: A Nationwide Survey. Arch Intern Med. 2001;161(22):2702–2708. doi: 10.1001/archinte.161.22.2702. [DOI] [PubMed] [Google Scholar]
- 91.Malosh R., Ohmit S.E., Petrie J.G., et al. Factors associated with influenza vaccine receipt in community dwelling adults and their children. Vaccine. 2014;32(16):1841–1847. doi: 10.1016/j.vaccine.2014.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hervé C., Laupèze B., Giudice G Del, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccin. 2019;4(1):1–11. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oyebanji O.A., Wilson B., Keresztesy D., et al. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin Exp Res. 2021;33(11):3151–3160. doi: 10.1007/s40520-021-01987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.King A., Daniels J., Lim J., et al. Time to listen: a review of methods to solicit patient reports of adverse events. BMJ Qual Saf. 2010;19(2):148–157. doi: 10.1136/qshc.2008.030114. [DOI] [PubMed] [Google Scholar]
- 95.Mathioudakis A.G., Ghrew M., Ustianowski A., et al. Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey. Life. 2021;11(3):249. doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chapin-Bardales J., Gee J., Myers T. Reactogenicity following Receipt of mRNA-Based COVID-19 Vaccines. JAMA - J Am Med Assoc. 2021;325(21):2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 97.Wan E.Y.F., Wang Y., Chui C.S.L., et al. Safety of an inactivated, whole-virion COVID-19 vaccine (CoronaVac) in people aged 60 years or older in Hong Kong: a modified self-controlled case series. Lancet Heal Longev. 2022;3(7):e491–e500. doi: 10.1016/S2666-7568(22)00125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braun E., Horowitz N.A., Leiba R., et al. Association between IgG antibody levels and adverse events after first and second Bnt162b2 mRNA vaccine doses. Clin Microbiol Infect. 2022;0(0) doi: 10.1016/j.cmi.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hermann E.A., Lee B., Balte P.P., et al. Association of Symptoms After COVID-19 Vaccination With Anti–SARS-CoV-2 Antibody Response in the Framingham Heart Study. JAMA Netw Open. 2022;5(10):e2237908. doi: 10.1001/jamanetworkopen.2022.37908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Held J., Esse J., Tascilar K., et al. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®) Vaccines. 2021;9(10):1063. doi: 10.3390/vaccines9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hwang Y.H., Song K.H., Choi Y., et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med. 2021;36(6):1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Canaday D.H., Oyebanji O.A., White E., et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. eBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Beltran W.F., Denis KJ. St., Hoelzemer A., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chalkias S., Harper C., Vrbicky K., et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N Engl J Med. 2022;387(14):1279–1291. doi: 10.1056/NEJMOA2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chalkias S., Eder F., Essink B., et al. Safety, immunogenicity and antibody Persistence of a bivalent Beta-containing booster vaccine. Nat Med. 2022 doi: 10.21203/RS.3.RS-1555201/V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blain H., Tuaillon E., Gamon L., et al. Strong Decay of SARS-CoV-2 Spike Antibodies after 2 BNT162b2 Vaccine Doses and High Antibody Response to a Third Dose in Nursing Home Residents. J Am Med Dir Assoc. 2022;23(5):750–753. doi: 10.1016/j.jamda.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prasad N., Derado G., Nanduri S.A., et al. Effectiveness of a COVID-19 Additional Primary or Booster Vaccine Dose in Preventing SARS-CoV-2 Infection Among Nursing Home Residents During Widespread Circulation of the Omicron Variant — United States, February 14–March 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(18):633–637. doi: 10.15585/mmwr.mm7118a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giménez E., Albert E., Zulaica J., et al. Severe Acute Respiratory Syndrome Coronavirus 2 Adaptive Immunity in Nursing Home Residents Following a Third Dose of the Comirnaty Coronavirus Disease 2019 Vaccine. Clin Infect Dis. 2022;75(1):e865–e868. doi: 10.1093/cid/ciac223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Praet JT Van, Vandecasteele S., Roo A De, et al. Dynamics of the Cellular and Humoral Immune Response After BNT162b2 Messenger Ribonucleic Acid Coronavirus Disease 2019 (COVID-19) Vaccination in COVID-19-Naive Nursing Home Residents. J Infect Dis. 2021;224(10):1690–1693. doi: 10.1093/infdis/jiab458. [DOI] [PubMed] [Google Scholar]
- 110.Voysey M., Clemens S.A.C., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skowronski D.M., De Serres G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2021;384(16):1576–1578. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 113.Doria-Rose N., Suthar M.S., Makowski M., et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clinical Guidance for COVID-19 vaccination | CDC. 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html Accessed October 25, 2022.
- 115.Witkowski W., Gerlo S., Smet E De, et al. Humoral and Cellular Responses to COVID-19 Vaccination Indicate the Need for Post-Vaccination Testing in Frail Population. Vaccines. 2022;10(2):260. doi: 10.3390/vaccines10020260. Page 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Castro M.C., Singer B. Prioritizing COVID-19 vaccination by age. Proc Natl Acad Sci U S A. 2021;118(15) doi: 10.1073/pnas.2103700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mok C.K.P., Chen C., Yiu K., et al. A Randomized Clinical Trial Using CoronaVac or BNT162b2 Vaccine as a Third Dose in Adults Vaccinated with Two Doses of CoronaVac. Am J Respir Crit Care Med. 2022;205(7):844–847. doi: 10.1164/rccm.202111-2655LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28(3):486–489. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takano T., Sato T., Kotaki R., et al. Heterologous booster immunization with SARS-CoV-2 spike protein after mRNA vaccine elicits durable and broad antibody responses. Research Square. 2022 doi: 10.21203/RS.3.RS-2014078/V1. [DOI] [Google Scholar]
- 120.Kaplonek P., Cizmeci D., Fischinger S., et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci Transl Med. 2022;14(645):2311. doi: 10.1126/scitranslmed.abm2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clemens S.A.C., Weckx L., Clemens R., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mayr F.B., Talisa V.B., Shaikh O., et al. Effectiveness of Homologous or Heterologous Covid-19 Boosters in Veterans. N Engl J Med. 2022;386(14):1375–1377. doi: 10.1056/NEJMc2200415. [DOI] [PMC free article] [PubMed] [Google Scholar]