Abstract
Brazil experienced one of the most prolonged periods of school closures, and reopening could have exposed students to high rates of SARS-CoV-2 infection. However, the infection status of students and school workers at the time of the reopening of schools located in Brazilian cities is unknown. Here we evaluated viral carriage by RT-PCR and seroprevalence of anti-SARS-CoV-2 antibodies (IgM and IgG) by immunochromatography in 2259 individuals (1139 students and 1120 school workers) from 28 schools in 28 Brazilian cities. We collected the samples within 30 days after public schools reopened and before the start of vaccination campaigns. Most students (n = 421) and school workers (n = 446) had active (qRT-PCR + IgM− IgG− or qRT-PCR + IgM + IgG−/+) SARS-CoV-2 infection. Regression analysis indicated a strong association between the infection status of students and school workers. Furthermore, while 45% (n = 515) of the students and 37% (n = 415) of the school workers were neither antigen nor antibody positive in laboratory tests, 16% of the participants (169 students and 193 school workers) were oligosymptomatic, including those reinfected. These individuals presented mild symptoms such as headache, sore throat, and cough. Notably, most of the individuals were asymptomatic (83.9%). These results indicate that many SARS-CoV-2 infections in Brazilian cities during school reopening were asymptomatic. Thus, our study highlights the need to promote a coordinated public health effort to guarantee a safe educational environment while avoiding exacerbating pre-existent social inequalities in Brazil, reducing social, mental, and economic losses for students, school workers, and their families.
Keywords: Asymptomatic SARS-CoV-2 infection, Anti-SARS-CoV-2 antibodies, Seroprevalence, School reopening
Asymptomatic SARS-CoV-2 infection; Anti-SARS-CoV-2 antibodies; Seroprevalence; School reopening.
1. Introduction
Many countries suspended in-person learning in schools as part of interventions to reduce SARS-CoV-2 transmission [1]. However, school closures are highly controversial in most countries [2, 3], as children and adolescents are less likely to experience life-threatening symptoms after severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) infection [4]. Moreover, school closure might adversely affect children's educational needs and mental health [5]. Although Brazil closed schools over prolonged periods of time [6], there is increasing evidence that this approach will result in many years of academic life lost [7]. In contrast, some countries, such as Sweden [8], have kept schools open throughout the pandemic [9], while others have closed only for short periods (e.g., two months in Germany [10]).
Brazil registered 29.8 million cases and 659,000 deaths due to COVID-19 by March 25th, 2022 [11,12] and is among the countries with the most extended periods of school closures [13, 14, 15]. Brazil, which has a population of 35.2 million children and adolescents of school age [16], reopened the schools for in-person classes on October 2020 [16]. Each state of the 5 Brazilian regions independently decided to reopen schools while there was still a high SARS-CoV-2 transmission across the country [17]. This period of school reopening provides a unique opportunity to document the SARS-CoV-2 infection status in students and school workers in Brazil since they may represent a group with a high prevalence of asymptomatic infections that could result in a high transmission rate [18].
Here, we investigated the occurrence of asymptomatic SARS-CoV-2 infections and seroprevalence in Brazil's first month of school reopening. The study was developed from November 17th, 2020, to January 21st, 2021, when Brazil was transitioning from the first to the second COVID-19 wave, which was more aggressive in this country compared to others around the globe [19, 20]. This period was marked by a phylogenetic diversity of SARS-CoV-2, with several virus variants emerging in South America, leading to the predominance of the Alpha (B.1.1.7) and Gamma (P.1) strains. At this time, the Omicron lineage (B.1.351, beta) had not been reported [19].
2. Methods
2.1. The observational cross-sectional approach
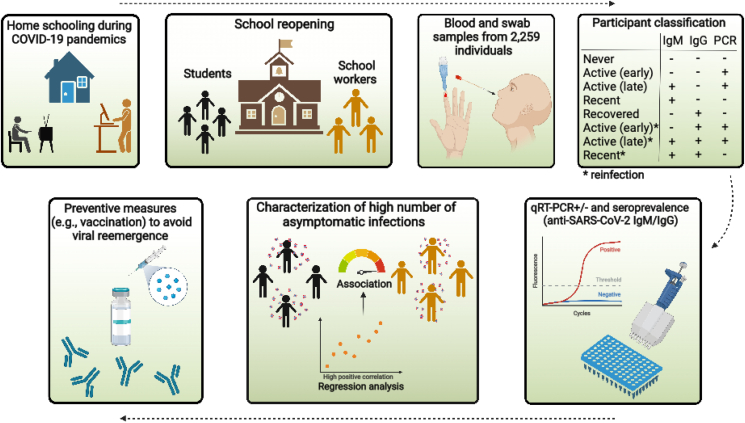
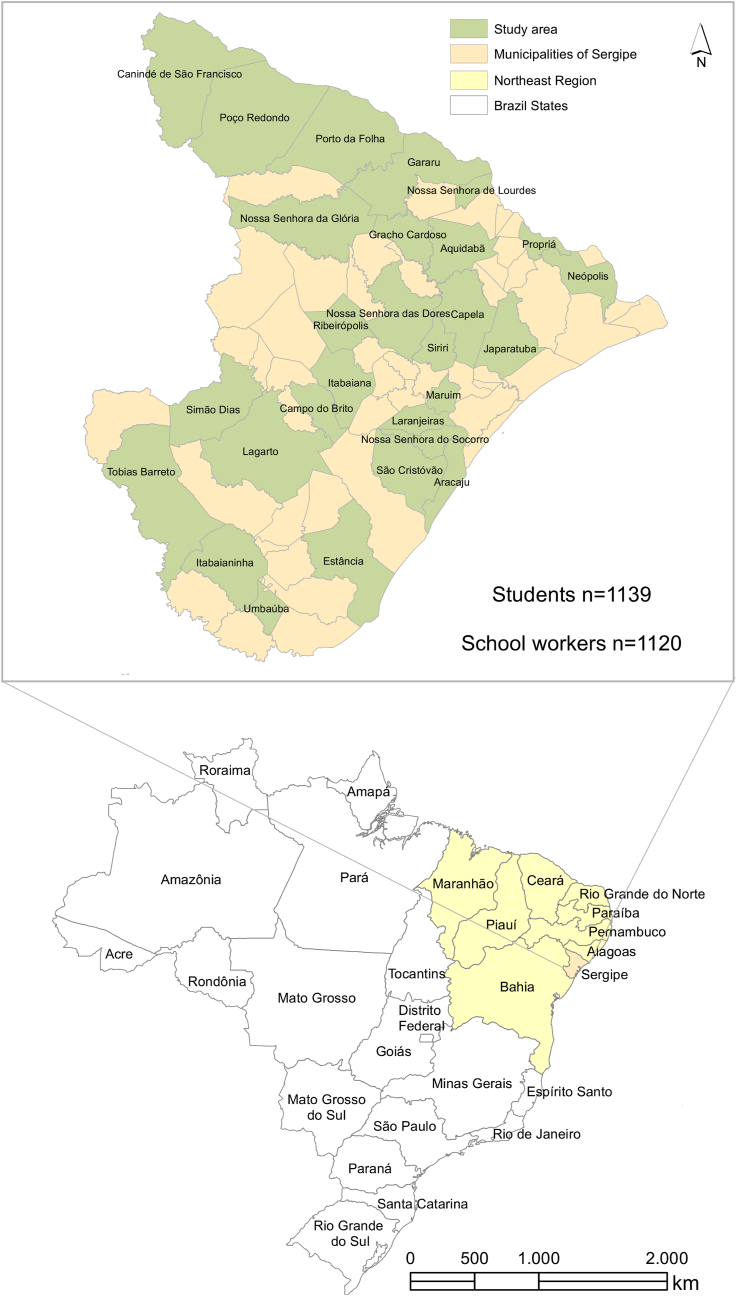
Figure 1 represents the overview of the cross-sectional approach described here in further detail. The cities where the study was conducted are located in the northeast (state of Sergipe) of Brazil, which showed the highest number of deaths in children and adolescents (Supplementary Figure 1). This study enrolled two thousand and fifty-nine individuals having in-person classes: 1139 students and 1120 school workers. All participants, demographic data, age, gender, risk factors, and symptoms are described in Supplementary Table 1. The median (interquartile range or IQR) age of students and school workers were 18.4 (17.8–19.6) and 41.4 (35.2–48.5) years, respectively (Supplementary Table 2). We only included in our study high school (teenagers) and adult high school students. Participants came from 28 cities in all seven administrative health regions of Sergipe (Figure 2). Municipality selection was based on including at least three cities for each administrative health region having the largest number of school communities. Demographic data were collected through a questionnaire, including age, gender, presence of risk factors, and symptoms compatible with COVID-19. A health care worker evaluated the participants at the testing time for the existence of COVID-19 symptoms. All enrolled individuals were tested for SARS-CoV-2 positivity by quantitative reverse transcription polymerase chain reaction (qRT-PCR – hereafter referred only as PCR) and anti-SARS-CoV-2 antibodies from November 17th, 2020, to January 21st, 2021. Since the different schools enrolled in our work reopened for in-person classes at different time points, we could synchronize the sample collection approximately 30 days after school reopening. Approximately 100 individuals from each municipality were tested for SARS-CoV-2 infection, and all individuals received their test results by e-mail. The health department of each municipality followed up on SARS-CoV-2-positive cases. No deaths or hospital admissions were reported during the time of this study. Fifty-three individuals were excluded because their PCR results were inconclusive or had missing additional information. Samples were collected under the Brazilian Health Regulatory Agency (ANVISA)/Oswaldo Cruz Foundation (FIOCRUZ)/Brazil biosafety protocol. All adult participants and parents or legal guardians of students provided signed consent to participate. The study was approved by the National Bioethics Committee of Brazil (CAAE 31018520.0.0000.5546).
Figure 1.
Graphic abstract and study approach. Study workflow and participant classification.
Figure 2.
Distribution of participants throughout the Brazilian municipalities. (a) The map of Sergipe indicates the municipalities of participating schools. (b) Map of Brazil highlighting the northeast region where Sergipe is located.
2.2. Calculating the mortality rate of children and adolescents across the Brazilian regions
The number of deaths due to COVID-19 in the Brazilian population under 18 years of age across all states was obtained from the national database of the Ministry of Health [21]. To calculate the COVID-19 national mortality rate by state, we obtained the number of inhabitants under 18 years of age from the Brazilian Institute of Geography and Statistics (IBGE) census (2019) [22]. ArcGis 10.4 software was utilized to represent the spatial distribution with cartographic bases of those Brazilian cities and states [23] and local mortality rates due to SARS-CoV-2. COVID-19 mortality rates were estimated using the 2019 IBGE census population under 18 years of age by State [22].
2.3. Assessment of anti-SARS-CoV-2 IgM and IgG antibodies
Anti-SARS-CoV-2 IgM and IgG antibodies were assessed in finger prick blood using lateral flow sandwich detection immunochromatography (Egens COVID-19 IgG/IgM Rapid Test Kit, Nantong Egens Biotechnology, China), according to the manufacturer's instructions, which was approved by ANVISA (Registration number: 814647500072). This test qualitatively detects IgM and IgG antibodies separately and has a sensitivity of 96.8% and specificity of 96.52% [24, 25].
2.4. Detection of SARS-CoV-2 positivity by real-time PCR and patient classification
Nasopharyngeal specimens were collected in Viral Transport Medium (VTM, Laborclin, Parana, Brazil) and transported to the Central Laboratory of Molecular Biology of Sergipe State (LACEN), Federal University of Sergipe, for PCR analysis. RNA extraction was performed using an automated magnetic bead-purification procedure (Quick-DNA/RNA™ Viral MagBead, Zymo Research, US). Testing for SARS-CoV-2 was conducted using a multiplex PCR assay (AllplexTM 2019-nCoV Assay, Seegene, South Korea) on the Applied Biosystems: QuantStudio 5 and 7500 Fast Food Safety Real-Time PCR Systems (Thermo Fisher Scientific, US).
Participants were classified according to the PCR and antibody tests results as having: (a) active (early) infection when PCR+, IgM−, and IgG−; (b) active late infection when PCR+, IgM+, and IgG−; (c) recent infection when PCR−, IgM+ and IgG−; (d) recovered infection when PCR−, IgM−, and IgG+; (e) early reinfections (PCR+, IgM−, and IgG+) and (f) late reinfection (PCR+, IgM+, and IgG+) and (g) never infected (PCR−, IgM−, and IgG−). None of the participants were vaccinated at the enrolment, as vaccinations started later (January 2021) [26].
2.5. Statistical analysis
The data obtained were analyzed using R [27] and RStudio [28]. Descriptive and inferential statistics were used to describe and analyze the data. Regression analysis was carried out using the R [27] packages glm.nb [29] and MASS [30] to evaluate the association between the infection status of students and school workers across the cities. We adopted a negative binomial distribution method that models counting variables for over-dispersed count outcomes. Residuals were estimated using the source envel_nbin.R [31]. The regression coefficient and p-value, corresponding to the relation between students and workers of each infection group, were plotted through a bubble-based heat map with Euclidian distance metric using the Morpheus web tool (https://software.broadinstitute.org/morpheus/) [32] (Supplementary Table 3). Likewise, we performed crude and adjusted Poisson regression analyses to calculate the Relative risk (RR) and respective 95% confidence intervals (CI) using stats packge [27].
2.6. Data visualization
The number of participants per infection group was visualized using the Circos plot (http://circos.ca/) [33]. We plotted the clinical data of each participant in a heatmap with k means clustering using the Morpheus webtool [32]. GraphPad Prisma (version 9.3.1; GraphPad Software, San Diego, Calif) [34] was used to generate the bar plots.
3. Results
3.1. Distribution of study participants according to infection status
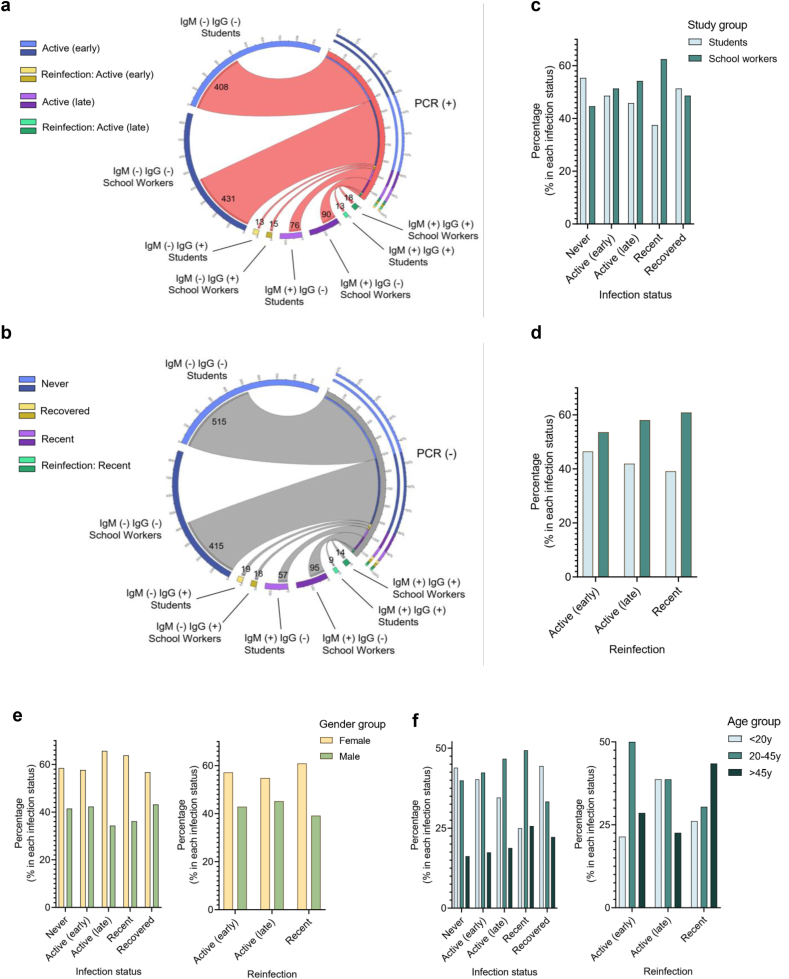
The detection of SARS-CoV-2 positivity and assessment of anti-SARS-CoV-2 IgM and IgG antibodies revealed that most of the PCR + individuals (students, n = 408 and school workers, n = 431) were IgM− and IgG−, thus, belonging to the active early infection status group (Figure 3a and Supplementary Table 2). They were followed by individuals belonging to the active late (PCR + IgM + IgG−) infection status group (students, n = 76; school workers, n = 90). A few individuals were reinfected with SARS-CoV-2 and presented either an early (PCR + IgM − IgG+) or late (PCR + IgM + IgG+) reinfection status. Furthermore, while most of the PCR- (non-active infection) individuals were never infected (PCR-IgM − IgG−: students, n = 515; school workers, n = 415), others were recently infected (PCR-IgM + IgG−: students, n = 57; school workers, n = 95), and a small number presented a recovered (PCR-IgM − IgG+) or recent reinfection status (PCR-IgM + IgG+) (Figure 3b). The percentage of students and school workers belonging to each infection status group was around 50% for all groups (Figures 3c and d).
Figure 3.
Distribution of study participants according to infection status. (a and b) Circos plots presenting the number of individuals in each study group, divided according to (a) positive PCR and (b) negative PCR results. (c–f) Bar graphs showing the percentage of individuals (students and school workers) in each infection and reinfection status group (c and d), according to (e) gender and (f) age.
3.2. SARS-CoV-2 positivity and seroprevalence by age and sex
We next evaluated the proportion of participants with SARS-CoV-2 and positive seroprevalence by sex and age. There was a higher percentage of females than males across all infection status groups (never, active, recent, recovered, and reinfection) (Figure 3e). Likewise, except for a higher percentage of older individuals with recent reinfection, younger individuals (<20 and 20–45 years of age) predominated across all groups (Figure 3f and Supplementary Table 4).
3.3. The association between different infection status groups in the schools
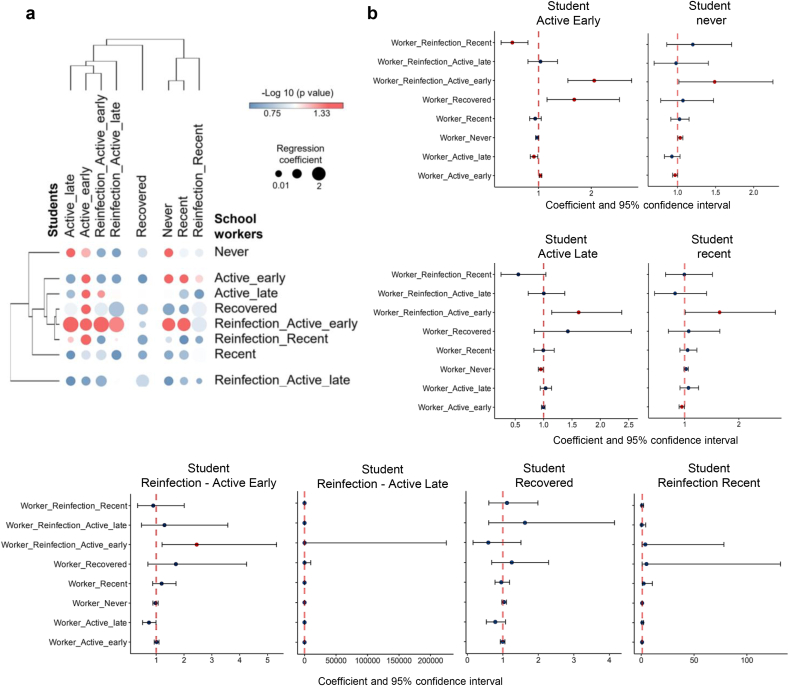
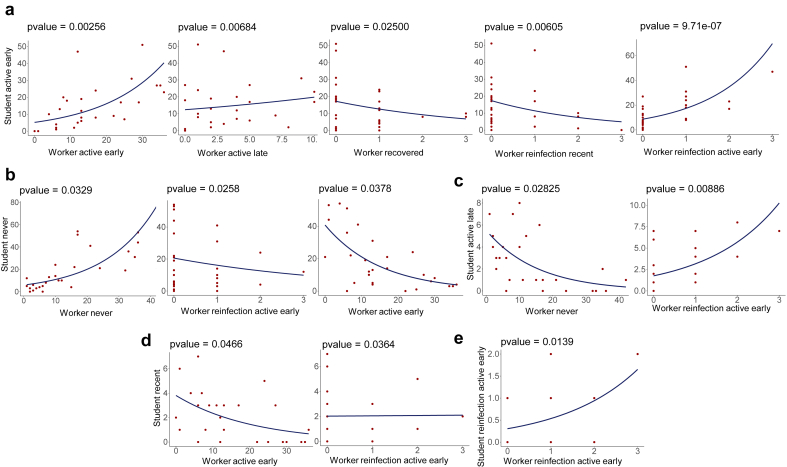
To evaluate the association between the number of students and school workers according to the SARS-Cov-2 infection status, we performed a negative binomial regression based on the number of students and school workers from each city. This approach revealed a statistically significant association between several groups of students and school workers according to their infection status. Figures 4a and b provide a hierarchical cluster and graphic overviews, respectively, of all associations (statistically significant [both negative and positive] and non-significant associations) between students and school workers. At the same time, Figure 5 details the association distribution of those statistically significant. In general, the regression analysis revealed strong positive or negative associations between those individuals belonging to the same or related infection status category. For instance, the group of students designated as active early infected presents a positive statistical association with workers active early and workers reinfection active early (Figure 5a).
Figure 4.
Regression analysis of different infection status groups according to the distribution of participants in the municipalities. (a) Bubble heatmap showing the overall relationship between students and workers infection status groups. The size of the circles indicates the value of the regression coefficient, and the color represents the –log 10 of p-values. (b) Forest plots showing confidence interval and negative binomial regression coefficients across infections status groups of workers compared to students. Dots represent the individual result of the regression coefficient with the 95% confidence interval indicated by horizontal lines, and both results are expressed as exponential values. Red dots correspond to significant associations with a p-value < 0.05. Except for the association between Student Reinfection - Active Late versus the other groups (second bottom graphic from left to right), all other graphics are shown in exponential values.
Figure 5.
Distribution of students and workers of different infection status groups per municipality through negative binomial regression. (a–e) The negative binomial distribution was used to model the count of the participants in each infection status group for each municipality in the state of Sergipe. The y-axis corresponds to the count of students and the x-axis to the number of workers per municipality.
Furthermore, there is a strong association between the group of students and workers never infected (Figure 5b) and between student active late versus worker reinfection active early (Figure 5c). The same strong relationship was detected between student reinfection active early with workers reinfection active early (Figure 5e). Likewise, there is a significant negative relationship, for example, among students never infected with workers active early (Figure 5b) or between the groups student active late with worker never (Figure 5c) as well as student recent with worker active early (Figure 5d). Comparable results were obtained by relative risk analysis, which showed significant associations between students and school workers who were IgM+, PCR+, never and active early recent infection, and reinfection active early (Table 1).
Table 1.
| Comparisons | RRc (CI95%) | p-value | RRa (CI 95%) | p-value |
|---|---|---|---|---|
| School Workers vs Students | ||||
| IgM (+) | 1.42 (1.17–1.71) | <0.001 | 1.00 (0.73–1.36) | 0.999 |
| IgG (+) | 1.22 (0.86–1.73) | 0.269 | 0.76 (0.41–1.41) | 0.387 |
| PCR (+) | 1.10 (1.01–1.20) | 0.031 | 1.22 (1.04–1.44) | 0.016 |
| Never | 0.81 (0.74–0.90) | <0.001 | 0.82 (0.69–0.96) | 0.015 |
| Active early | 1.07 (0.96–1.19) | 0.216 | 1.32 (1.08–1.63) | 0.008 |
| Active late | 1.21 (0.90–1.63) | 0.201 | 1.06 (0.65–1.72) | 0.823 |
| Recent | 1.68 (1.22–2.31) | 0.001 | 0.94 (0.58–1.53) | 0.806 |
| Recovered | 1.01 (0.53–1.93) | 0.976 | 1.58 (0.41–6.02) | 0.502 |
| Reinfection active early | 1.16 (0.56–2.44) | 0.684 | 0.35 (0.13–0.92) | 0.034 |
| Reinfection active late | 1.40 (0.69–2.84) | 0.353 | 2.73 (0.52–14.40) | 0.237 |
| Reinfection recent | 1.57 (0.68–3.61) | 0.288 | 0.44 (0.10–1.93) | 0.279 |
RRc – Crude Relative Risk. RRa – Adjusted Relative Risk. CI95% – 95% Confidence Interval. Relative risks were estimated by Poisson regression with robust standard errors with adjustment for age, sex, comorbidities, symptoms, and contact with SARS-CoV-2 infected individuals in the past month.
3.4. Distribution of study participants according to the presence of symptoms and contact with SARS-CoV-2 infected individuals
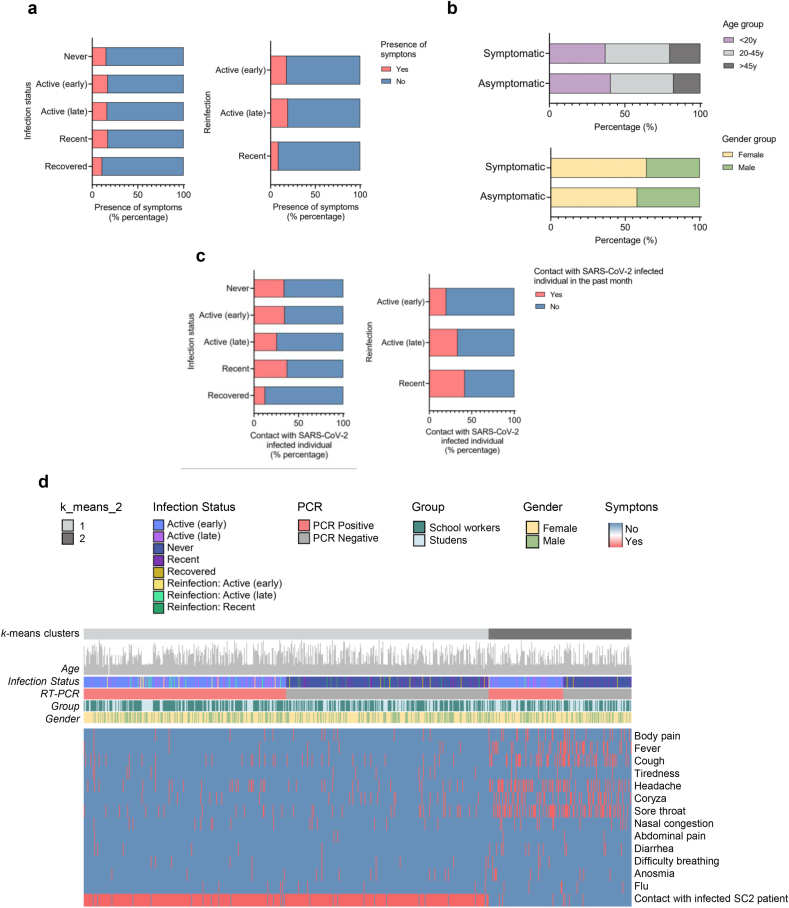
Next, we performed a descriptive analysis regarding the presence or absence of clinical manifestation related to COVID-19 in students or school workers and if these individuals had contact with known SARS-CoV-2 infected individuals in the past month. While most of the individuals belonging to all infection status groups were asymptomatic, only a few (16.1%: 362 individuals of the 2206 tested) participants were oligosymptomatic (Figure 6a and Supplementary Table 5). They showed mild respiratory symptoms such as body pain, coryza, cough, headache, nasal congestion, sore throat, and/or low fever. In addition, most asymptomatic and symptomatic individuals were between 20 and 45 years of age and females (Figure 6b). Furthermore, a small number of individuals in each infection status group reported having had contact with known SARS-CoV-2 infected individuals in the past month (Figure 6c and Supplementary Table 5).
Figure 6.
Distribution of study participants according to the presence of symptoms and contact with SARS-CoV-2 infected individuals. (a) Percentage of study participants according to infection status and presence of mild respiratory symptoms. (b) Percentage distribution of symptomatic and asymptomatic individuals by age and gender. (c) Percentage of study participants according to infection status with and without contact with known SARS-CoV-2-infected individuals in the month previous to the study. (d) Heatmap showing the presence of each symptom according to age, infection status, PCR result, study group, and gender, considering only participants who reported symptoms or contact with SARS-CoV-2 positive individual (regardless of the presence of symptoms) as total number (n = 728; students = 287; school workers = 441).
Finally, we carried out a hierarchical clustering analysis of oligosymptomatic individuals plus the participants who had contact with SARS-CoV-2 infected individuals (both with and without symptoms). Approximately half of the participants who reported having contact with known SARS-CoV-2 infected individuals were PCR+ or PCR−. These individuals formed the group exhibiting fewer symptoms at the time of our study (Figure 6d). A possible explanation for this apparent discrepancy is that this group of individuals might have presented the symptoms of SARS-CoV-2 before our research. Meanwhile, the individuals who reported no contact with known SARS-CoV-2 infected individuals clustered in the heatmap due to a higher presence of symptoms. Since around half of them showed a PCR positive test, it indicates that these participants were infected by the SARS-CoV-2 from other asymptomatic individuals later than those reporting to have contact with known SARS-CoV-2 infected individuals.
4. Discussion
School closure is a preventive measure adopted as a critical non-pharmaceutical intervention during epidemics of respiratory infections [35, 36] such as influenza and other coronavirus outbreaks (e.g., due to severe acute respiratory syndrome or the Middle East respiratory syndrome) [5]. Considering the need to assess SARS-CoV-2 infection status and seroprevalence during school reopening, we conducted PCR and serological tests with students and school workers during the first month of school reopening in 28 Brazilian cities. We found a high number of asymptomatic SARS-CoV-2 infected individuals among students and school workers in our study cohort. This finding suggests that, despite the long period of school closure in Brazil, this preventive measure was insufficient in avoiding the SARS-CoV-2 presence among Brazilian students and school workers, which was detected in loco during the first month of return to classroom lessons. However, the impact of the extended school closure on students’ education and mental health [5] remains to be investigated in Brazil. In addition, since all Brazilian states closed their schools for classroom lessons, we cannot comparatively determine whether this measure reduced the velocity of SARS-CoV-2 spread among students and school workers. Whether school closure significantly impacted transmission in the general population was beyond the scope of this study, thus, requiring future investigation.
The effectiveness of school closures is being debated worldwide [5, 37, 38]. For instance, a review conducted by Viner and co-workers [39] suggests that school closures would prevent only 2–4% of deaths, much less than other social distancing interventions [43]. Another study has shown that school reopening did not increase COVID-19 incidence or mortality on average, up to 12 weeks after school reopening [40]. For instance, school closures in Brazil decreased social contact between students and school staff. Still, it may have shifted social contacts to other non-school sites, increasing contact between students and adults as well as between students from different schools. This observation might partially explain the high number of asymptomatic active or recently infected students and school workers at the school reopening and their high positive associations across the cities. For example, since schools reopened in Brazil when the community transmission was still at high levels, our data suggest that students and school workers may have been exposed before they returned to school [41]. For instance, despite public campaigns promoting mitigation measures for COVID-19 prevention, such as rigorous social distancing, some participants reported that they had contact with known infected persons in the past month, thus, reinforcing the need to promote safer strategies to reopen schools in future pandemic contexts.
Of note, a small number of individuals in each infection group reported having had contact with known SARS-CoV-2 infected individuals in the past month. Hence, it indicates that symptomless transmission [42] silently drove viral spread among our study population. The high number of asymptomatic SARS-CoV-2 infections observed in the tested individuals from Brazilian cities is in agreement with the fact that the SARS-CoV-2 infections increased rapidly in the school-age population in England following the emergence of a novel variant of concern presenting elevated transmission rates [43]. However, these results differ from the low general prevalence of SARS-CoV-2 positivity reported in students during the reopening of schools in other high-income countries with elevated incidence of SARS-CoV-2 infections in the general population, such as Germany [44, 45], Switzerland [46], and Italy [47]. The reasons for this disparity are unclear and remain to be investigated. Indeed, the incidence of SARS-CoV-2 infection varied widely over time and between countries due to reasons that need to be clarified [48].
Our study, however, has limitations. For instance, we performed data mining, extracting and discovering the patterns of the infection status of students and school workers during school reopening in Brazilian cities without an epidemiological investigation and confirmation of the genomic sequencing of SARS-CoV-2 to confirm transmission. Another limitation involves the diagnostic test (Nantong Egens Biotechnology CO, Ltd., China) used to detect anti-SARS-CoV-2 IgM and IgG, which has 96.9% sensitivity and 100% specificity [49]. Thus, the individuals classified as never positive might have had the infection. Still, they were classified as false-negative for various reasons, such as the inability of the assay to detect the antibodies or testing too soon after infection (during the immunological window). In addition, while most SARS-CoV-2-infected individuals seroconvert, others do not. We hypothesize that their T cells killed the virus without developing IgG seropositivity and neutralizing antibodies following primary infection, as previously reported [50, 51, 52]. Moreover, the reopening of the schools in Brazil was not accompanied by a systematic screening and surveillance of SARS-CoV-2 infections in schools as in European countries [46]. Thus, future studies are required to determine whether the high incidence of asymptomatic infections that we found in the cities of the state of Sergipe reflects the situation in other Brazilian states, such as those from the southern region. To reach this goal would allow locally assessing the frequency and transmission of asymptomatic or oligosymptomatic infections at schools [25, 53]. Another limitation is that most of the students tested were teenagers. Hence, since a variation in seroprevalence by age has been reported by multiple countries and settings [3], the inclusion of individuals with a broader age range could have provided a distribution by age and a possible age-dependent interpretation of the infection status across the population of students in the Brazilian schools. We also do not have longitudinal data to determine the course of the infection status at schools during the COVID-19 pandemic. In this context, the effect of comorbidities and the appearance of novel SARS-CoV-2 variants could also have brought important insights to understanding the population studied and the dynamics of the viral spread, respectively.
In conclusion, although Brazil had one of the most extended school closures in the world [6], the school reopening was marked by a high number of asymptomatic SARS-CoV-2 infections among students and school workers. This fact has a significant public health implication, and future studies are essential to determine whether the school closure effectively reduced SARS-CoV-2 transmission [5] and the incidence of severe infections. For instance, it is particularly crucial to address this issue in Brazil since there is no data on the contribution of school closures to the transmission of SARS-CoV-2 infection. In addition, many students at public schools live in social vulnerability, depending on school meals to fulfill their nutritional needs. The educational loss with this extended closure is possibly, the most significant loss for them in a situation where school quality and performance are often not considered satisfactory. Thus, our study highlights the need to promote a coordinated public health effort to guarantee education [16] while avoiding exacerbating pre-existent social inequalities in Brazil, reducing social, mental, and economic losses for students, school workers, and their families.
Declarations
Author contribution statement
Lysandro P. Borges, Adriana G. Guimarães, Ricardo Q. Gurgel, Paula P. Freire, Niels Olsen Saraiva Camara, Vera Lúcia Garcia Calich, Lasse M. Giil, Hans D Ochs, Lena F. Schimke, Aline F. Martins, Otavio Cabral-Marques, Íkaro D. C. Barreto: Conceived and designed the experiments; analyzed and interpreted the data; wrote the paper.
Aline S. A. Lopes, Dennyson Leandro M. Fonseca, Daniela R. V. Souza, José Melquiades de Rezende Neto, Kezia A. dos Santos, Igor L. S. Matos, Grazielly B. da Invenção, Brenda M. Oliveira, Aryanne A. Santos, Daniele Almeida Soares, Marco A. O. Goes, Pamela C. de Jesus, Mércia S. F. de Souza: Performed the experiments; contributed reagents, materials, analysis tools or data.
Cliomar A. dos Santos, Desirée Rodrigues Plaça, Igor Salerno Filgueiras, Alexandre H. C. Marques, Gabriela Crispim Baiocchi, William Cabral- Miranda, Gustavo Cabral de Miranda, Rodrigo Nalio Ramos, Helder I Nakaya, Vanderson Rocha, Luis E. Cuevas: Analyzed and interpreted the data.
Funding statement
This work was supported by Public Ministry of Labor, Federal Public Ministry, and State Public Ministry (grant 06.20.01.0078).
Otavio Cabral-Marques was supported by São Paulo Research Foundation (FAPESP grants 2018/18886-9, 2020/01688-0 and 2020/07069-0).
Paula Paccielli Freire was supported by São Paulo Research Foundation (FAPESP grant 2020/09146-1).
Igor Salerno Filgueiras was supported by the coordination for the improvement of higher education personnel–Brazil (CAPES) (finance code 001).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Lysandro P. Borges, Email: lysandro.borges@gmail.com.
Otavio Cabral-Marques, Email: otavio.cmarques@usp.br.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Wu J.T., Mei S., Luo S., et al. A global assessment of the impact of school closure in reducing COVID-19 spread. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2022:380. doi: 10.1098/rsta.2021.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson I. Schools do not need to close to reduce COVID-19 but other measures are advisable. Acta Paediatr. Int. J. Paediatr. 2021;110:2296–2297. doi: 10.1111/apa.15951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaythorpe K.A.M., Bhatia S., Mangal T., et al. Children’s role in the COVID-19 pandemic: a systematic review of early surveillance data on susceptibility, severity, and transmissibility. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-92500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control . Ecdc; Stock: 2020. COVID-19 in Children and the Role of School Settings in COVID-19 Transmission; p. 31. [Google Scholar]
- 5.Viner R.M., Russell S.J., Croker H., et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc. Heal. 2020;4:397–404. doi: 10.1016/S2352-4642(20)30095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Bank Education COVID-19 School Closures Map. https://www.worldbank.org/en/data/interactive/2020/03/24/world-bank-education-and-covid-19 (accessed March 5, 2022)
- 7.Christakis D.A., Van Cleve W., Zimmerman F.J. Estimation of US children’s educational attainment and years of life lost associated with primary school closures during the coronavirus disease 2019 pandemic. JAMA Netw. Open. 2020;3:1–12. doi: 10.1001/jamanetworkopen.2020.28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweden Keeps Schools Open during the Covid-19 Pandemic: Results of the Situation | Eurydice. https://eacea.ec.europa.eu/national-policies/eurydice/content/sweden-keeps-schools-open-during-covid-19-pandemic-results-situation_en (accessed March 5, 2022)
- 9.Lopes-Junior L.C., Siqueira P.C., Maciel E.L.N. School reopening and risks accelerating the COVID-19 pandemic: a systematic review and meta-analysis protocol. PLoS One. 2021;16:1–10. doi: 10.1371/journal.pone.0260189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freundl V., Lergetporer P., Zierow L. Germany’s education policy during the COVID-19 crisis. Z. für Polit. (Neue Folge) 2021;31:109–116. [Google Scholar]
- 11.COVID-19 Data Explorer – Our World in Data. https://ourworldindata.org/explorers/coronavirus-data-explorer (accessed March 26, 2022)
- 12.COVID-19/csse_covid_19_data at master CSSEGISandData/COVID-19. https://github.com/CSSEGISandData/COVID-19/tree/master/csse_covid_19_data (accessed March 26, 2022)
- 13.Campos de Lima E.E., Gayawan E., Baptista E.A., Queiroz B.L. Spatial pattern of COVID-19 deaths and infections in small areas of Brazil. PLoS One. 2021;16:1–12. doi: 10.1371/journal.pone.0246808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candido D.S., Claro I.M., de Jesus J.G., et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369:1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decreto No 40539 DE 19/03/2020 – Estadual – Distrito Federal – LegisWeb. https://www.legisweb.com.br/legislacao/?id=391027 (accessed March 5, 2022)
- 16.Barberia L.G., Bastos L.S., de Sousa T.C.M. School reopening and COVID-19 in Brazil. Lancet Reg. Heal. – Am. 2022;5 doi: 10.1016/j.lana.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barberia L.G., Bastos L.S., Moraes T.C., Sousa D. The COVID-19 Resource centre Is Hosted on Elsevier Connect, the company’s public news and information. 2020. Since January 2020 Elsevier Has Created a COVID-19 Resource centre with Free Information in English and Mandarin on the Novel Coronavirus COVID-19; pp. 2020–2022. [Google Scholar]
- 18.Ma Q., Liu J., Liu Q., et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4:1–18. doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeiser F.A., Donida B., da Costa C.A., et al. First and second COVID-19 waves in Brazil: a cross-sectional study of patients’ characteristics related to hospitalization and in-hospital mortality. Lancet Reg. Heal. Am. 2022;6 doi: 10.1016/j.lana.2021.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallapaty S. Kids and COVID: why young immune systems are still on top. Nature. 2021;597:166–168. doi: 10.1038/d41586-021-02423-8. [DOI] [PubMed] [Google Scholar]
- 21.Ministério da Saúde – Organizações – Opendatasus. https://opendatasus.saude.gov.br/organization/ministerio-da-saude (accessed March 5, 2022)
- 22.IBGE . 2019. Municípios E Para as Unidades da Federação Brasileiros Com Data de Referência em 1o de Julho de 2019 Agosto de 2019; pp. 1–16. [Google Scholar]
- 23.IBGE | Censo 2010. https://censo2010.ibge.gov.br/ (accessed March 5, 2022)
- 24.Santos de Melo M., Pinto Borges L., Raguer Valadão de Souza D., et al. Anti-SARS-CoV-2 IgM and IgG antibodies in health workers in Sergipe, Brazil. medRxiv. 2020 [Google Scholar]
- 25.Crowe J., Schnaubelt A.T., Schmidtbonne S., et al. Assessment of a program for SARS-CoV-2 screening and environmental monitoring in an urban public school district. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vacinação Contra a Covid-19 Começa Em Todo O País. https://agenciabrasil.ebc.com.br/saude/noticia/2021-01/vacinacao-contra-covid-19-comea-em-todo-o-pais (accessed March 5, 2022)
- 27.Wickham H. second ed. 2019. Advanced R. [Google Scholar]
- 28.RStudio - RStudio. https://rstudio.com/products/rstudio/ (accessed May 2, 2020)
- 29.glm.nb Function – RDocumentation. https://www.rdocumentation.org/packages/MASS/versions/7.3-55/topics/glm.nb (accessed March 18, 2022)
- 30.CRAN – Package MASS. https://cran.r-project.org/web/packages/MASS/index.html (accessed March 18, 2022)
- 31.Paula GA. MODELOS DE REGRESS˜AOREGRESS˜ REGRESS˜AO Com Apoio Computacional. .
- 32.Morpheus. https://software.broadinstitute.org/morpheus/ (accessed March 26, 2022)
- 33.Krzywinski M., Schein J., Birol I., et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prism - GraphPad. https://www.graphpad.com/scientific-software/prism/ (accessed March 5, 2022)
- 35.Cauchemez S., Valleron A.J., Boëlle P.Y., Flahault A., Ferguson N.M. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452:750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 36.Mossong J., Hens N., Jit M., et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas J., Kneale D., O’Mara-Eves A., Rees R. School closure in response to epidemic outbreaks: systems-based logic model of downstream impacts. F1000Research. 2020;9:352. doi: 10.12688/f1000research.23631.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies N.G., Klepac P., Liu Y., et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 39.Viner R.M., Mytton O.T., Bonell C., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichand G., Alberto Doria C., Cossi Fernandes J.P., Leal Neto O. Reopening schools in the pandemic did not increase COVID-19 incidence and mortality in Brazil. SSRN Electron. J. 2021 doi: 10.1001/jamahealthforum.2021.5032. Published online April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buss L.F., Prete C.A., Abrahim C.M.M., et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371:288–292. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen A.L., Popescu S.V. SARS-CoV-2 transmission without symptoms. Science. 2021;371:1206–1207. doi: 10.1126/science.abf9569. [DOI] [PubMed] [Google Scholar]
- 43.Mensah A.A., Sinnathamby M., Zaidi A., et al. SARS-CoV-2 infections in children following the full re-opening of schools and the impact of national lockdown: prospective, national observational cohort surveillance, July-December 2020, England. J. Infect. 2021;82:67–74. doi: 10.1016/j.jinf.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Körner R.W., Weber L.T. Prevalence of COVID-19 among children and adolescents while easing lockdown restrictions in cologne, North rhine-Westphalia, Germany. Klin. Pädiatr. 2021;233:135–140. doi: 10.1055/a-1341-9530. [DOI] [PubMed] [Google Scholar]
- 45.Hoehl S., Kreutzer E., Schenk B., et al. Longitudinal testing for respiratory and gastrointestinal shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in day care centers in Hesse, Germany. Clin. Infect. Dis. 2021;73:e3036–e3041. doi: 10.1093/cid/ciaa1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kriemler S., Ulyte A., Ammann P., et al. Surveillance of acute SARS-CoV-2 infections in school children and point-prevalence during a time of high community transmission in Switzerland. Front. Pediatr. 2021;9:159. doi: 10.3389/fped.2021.645577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandini S., Rainisio M., Iannuzzo M.L., Bellerba F., Cecconi F., Scorrano L. A cross-sectional and prospective cohort study of the role of schools in the SARS-CoV-2 second wave in Italy. Lancet Reg. Heal. - Eur. 2021;5 doi: 10.1016/j.lanepe.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louca S. SARS-CoV-2 infections in 165 countries over time. Int. J. Infect. Dis. 2021;111:336–346. doi: 10.1016/j.ijid.2021.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurgel R.Q., de Sá L.C., Souza D.R.V., et al. SARS-CoV-2 has been circulating in northeastern Brazil since February 2020: evidence for antibody detection in asymptomatic patients. J. Infect. 2021;82:186–230. doi: 10.1016/j.jinf.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Immune responses and immunity to SARS-CoV-2. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses (accessed Aug 6, 2022)
- 51.Post N., Eddy D., Huntley C., et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021:371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panovska-Griffiths J., Kerr C.C., Stuart R.M., et al. Determining the optimal strategy for reopening schools, the impact of test and trace interventions, and the risk of occurrence of a second COVID-19 epidemic wave in the UK: a modelling study. Lancet Child Adolesc. Heal. 2020;4:817–827. doi: 10.1016/S2352-4642(20)30250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.