Abstract
Human use of marinescapes is rapidly increasing, especially in populated nearshore regions where recreational vessel traffic can be dense. Marine animals can have a physiological response to such elevated human activity that can impact individual health and population dynamics. To understand the physiological impacts of vessel traffic on baleen whales, we investigated the adrenal stress response of gray whales (Eschrichtius robustus) to variable vessel traffic levels through an assessment of fecal glucocorticoid metabolite (fGC) concentrations. This analysis was conducted at the individual level, at multiple temporal scales (1–7 days), and accounted for factors that may confound fGC: sex, age, nutritional status, and reproductive state. Data were collected in Oregon, USA, from June to October of 2016–2018. Results indicate significant correlations between fGC, month, and vessel counts from the day prior to fecal sample collection. Furthermore, we show a significant positive correlation between vessel traffic and underwater ambient noise levels, which indicates that noise produced by vessel traffic may be a causal factor for the increased fGC. This study increases knowledge of gray whale physiological response to vessel traffic and may inform management decisions regarding regulations of vessel traffic activities and thresholds near critical whale habitats.
Subject terms: Endocrinology, Animal physiology
Introduction
Marine ecosystems have experienced a dramatic rise in commercial and recreational shipping and vessel traffic1–3. These forms of transport can be associated with negative impacts on marine wildlife populations4,5, increased risk of mortality to cetaceans through ship strikes6,7, and increased noise levels in the marine environments8–10. Baleen whales are particularly vulnerable to acoustic disturbance caused by shipping and vessel traffic as it overlaps with the frequencies that are primarily used for communication, navigation and foraging11,12. Such frequency overlap can cause “acoustic masking” whereby transmission or reception of acoustic cues is degraded due to elevated ambient noise, which may potentially hinder a cetacean’s ability to perform important life history activities11. Therefore, elevated recurrence of both lethal and non-lethal interactions between vessel traffic and cetaceans may impact individual health and population viability.
Human-wildlife interactions can cause negative outcomes at short and long-term scales, yet longitudinal monitoring of impacts of human activities on long-lived wildlife populations can be difficult, often requiring considerable resources and contextual individual-level information (i.e., demographic data)13. Thus, studies have primarily focused on short-term behavioral responses to directly associate the cause (i.e., disturbance source) to the outcome (i.e., behavioral response)13,14. Multiple short-term impacts of vessel encounters on marine mammals have been documented, including altered behavioral states15–18, increased group cohesion13,19,20, and displacement15,21. Additionally, individuals may recognize vessels as a risk, and thus avoid them by employing other anti-predatory tactics17–19,21. However, many factors can influence the intensity of a response, including vessel movements19,21, distance to the vessel22, and number of vessels23, as well as the social context, quality of prey patches, health condition and history of previous vessel encounters by an individual24. Such factors may even encourage individuals to tolerate disturbances instead of escaping25. In this context, it is important to highlight that the absence of short-term behavioral shifts does not imply the absence of effects caused by vessel interactions or other stressors13,26, and highlights the need for studies on the physiological impacts of human disturbance to cetaceans, particularly across longer periods to help address potential consequences to population dynamics27,28.
Observations of individual physiological responses to disturbance can identify important potential shifts in homeostatic state. For example, vessel encounters have been correlated with the metabolic rates of bottlenose dolphins (Tursiops truncatus)21 and humpback whales (Megaptera novaeangliae)29 as determined through respiration rates and surfacing interval. Variations in stress-related hormone (e.g., glucocorticoids [GCs]: cortisol, corticosterone) concentrations relative to vessel abundance and related shifts in ambient noise have also been reported for killer whales (Orcinus orca)30 and North Atlantic right whales (NARWs; Eubalaena glacialis)31. Although these few studies on the physiological responses of marine mammals to vessel traffic have been conducted, assessments remain scarce. Baleen whale physiology, in particular, is one of the most poorly understood of all mammals given inherent difficulties in sample collection32,33. Nevertheless, new techniques enable noninvasive or minimally invasive methods to collect samples, including feces, respiratory vapor, and blubber31–35, which have proven to be reliable techniques to monitor baleen whale physiology.
In fact, fecal samples have been successfully collected from various baleen whale species, including NARWs33,36, humpback whales37,38, blue whales (Balaenoptera musculus)39,40, and gray whales (Eschrichtius robustus)41,42 to assess concentrations of steroid hormones (e.g., GCs, androgens, progestogens, mineralocorticoids)33,41 that are eventually cleared from blood circulation by the liver and excreted via the urine and feces43,44. The GC metabolites in feces tend to increase in concentration hours or days following a stress exposure, with the time lag determined by species-specific gut passage time43,44. Thus, fecal GCs (fGC) can be used to monitor physiological responses to acute disturbances provided the appropriate time lag to excretion can be determined (often 1 to 2 days in large mammals44).
Multiple factors can confound the assessment of hormone concentrations in feces42,45,46, such as sex, age, nutritional status, reproductive state, and environmental factors (e.g., temperature47), all of which should be accounted for at an individual level to reliably relate whale GCs to a specific anthropogenic disturbance. Hence, this study aimed to better understand how individual baleen whales physiologically respond to vessel traffic while controlling for multiple confounding factors. Furthermore, we investigate correlations between vessel traffic and ocean noise levels to add explanatory power to the root cause of elevated vessel traffic effects on fGC concentrations in gray whales. Our research focuses on a sub-group of the Eastern North Pacific (ENP) gray whale population called the Pacific Coast Feeding Group (PCFG), that forages along the Oregon coast, USA, between June and November48. In this study area, PCFG gray whales are exposed to varying degrees and sources of both vessel traffic15 and ocean noise49,50.
Our objectives are to (1) describe the relationship between vessel counts leaving the ports each day with Sound Pressure Levels (SPL) recorded by a hydrophone outside the port entrance, (2) determine the effects of vessel traffic and potential confounding variables (i.e., body condition, age, sex, time) on gray whale fGC concentrations, and (3) determine the time lag between vessel traffic and fGC concentration responses in gray whales, which likely represents the gut passage time. We hypothesize a correlation between vessel traffic and ocean noise levels and predict that both vessel traffic and ocean noise levels will be positively correlated with gray whale fGC concentrations. Given the data deficiency regarding physiological impacts of vessel traffic and resulting ocean noise on baleen whales, this study addresses a critical knowledge gap that may inform vessel traffic and acoustic disturbance policies (e.g., number of vessels, speed, proximity) to reduce impacts on individual physiology and population dynamics of baleen whales.
Results
Data were collected during three gray whale foraging seasons (May to October of 2016–2018) along the central coast of Oregon, USA. We analyzed 411 days of vessel count data during the 2016–2018 field seasons, and 1128 hours of acoustic recordings from a total of 235 days during the 2017–2018 seasons. Furthermore, a total of 67 gray whale fecal samples were collected from 15 different mature males (n = 27) and 14 mature females (n = 40) (Fig. 1; Appendix S1: Table S1; Fig. S1). Fourteen fecal sample concentrations were below the limit of detection (< LOD) for fGC, four for progestin metabolites (fP), and ten for androgen metabolites (fA), while 19 fecal samples had no corresponding Body Area Index (BAI; a metric of body condition derived from drone photogrammetry) information. Therefore, these samples had missing values (NA) in the linear mixed models (LMM) analyses for the respective variables. In total, we had variable sample sizes by year and sex (males: 4 in 2016, 7 in 2017, and 16 in 2018; females: 9 in 2016, 9 in 2017, and 22 in 2018). If we only consider the samples with detectable fGC concentrations, the sample size is reduced to 0 in 2016, 5 in 2017, and 13 in 2018 for males, and 6 in 2016, 9 in 2017, and 20 in 2018 for females (N = 53).
Figure 1.
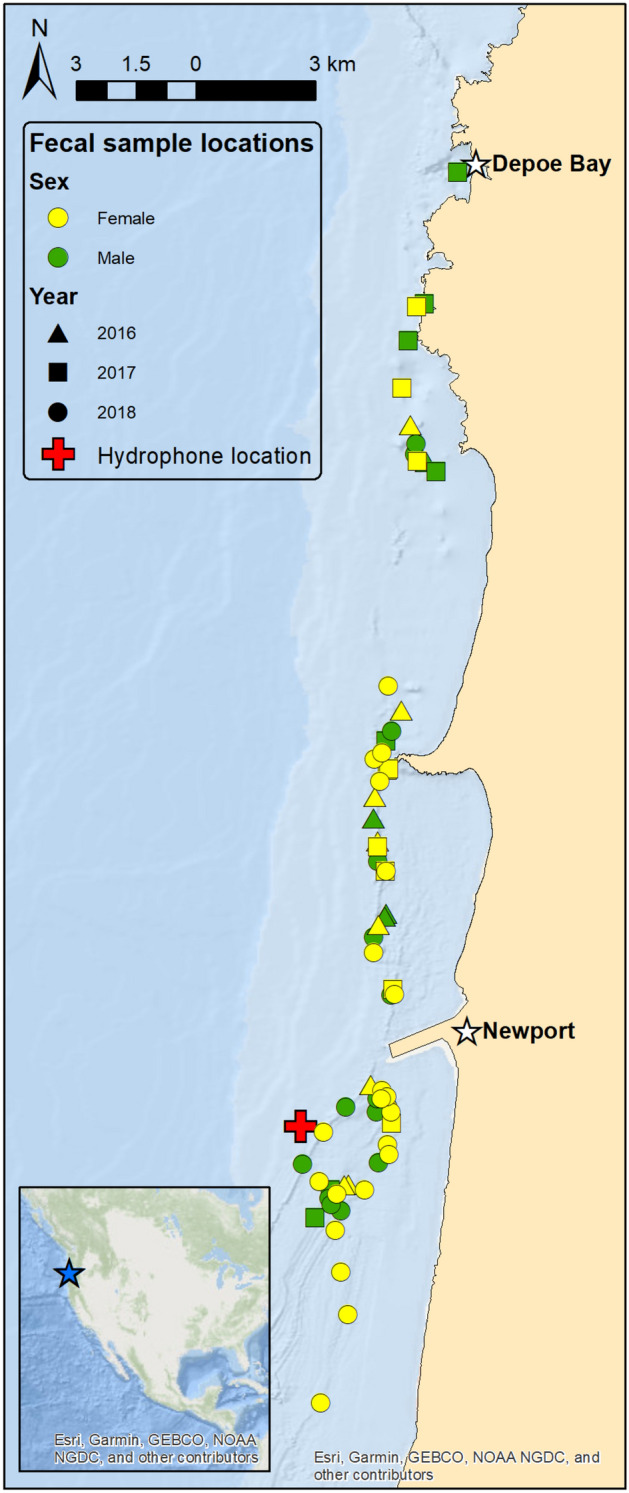
Location of field site off the central Oregon, USA coast (blue star on inset map), hydrophone deployment site (red cross), and gray whale fecal sample collections symbolized by color and shape to represent sex and year. The ports of Newport and Depoe Bay are represented by white stars. The Newport white star is also representing the anemometer station (NPOW3) from which the wind speed data were retrieved from. Figure created in ArcGIS software (version 10.8).
Photo-identification and sighting history analysis of the 29 gray whales that the 67 fecal samples came from and included in this study indicated that the mean number of days these whales were resident in the study area prior to sampling was 32.96 (SD = 39.57, min = 0, max = 120). Despite the large SD and that 20 of these individuals were not observed in our study system prior to the day of sample collection (min = 0 days), an assessment of the total number of days these sampled whales were observed in our study region across the entire sampling year also indicates high residency time (mean = 59.07, SD = 42.51, min = 1, max = 125). It is important to highlight that (1) a whale could have been in the study area before first recorded by our team, and (2) a whale may have left the study area after we first observed it in a year and then returned. Overall, these sighting results demonstrate high residency time of sampled whales within the study area and are therefore likely exposed to the noise, wind and vessel data assessed in this study.
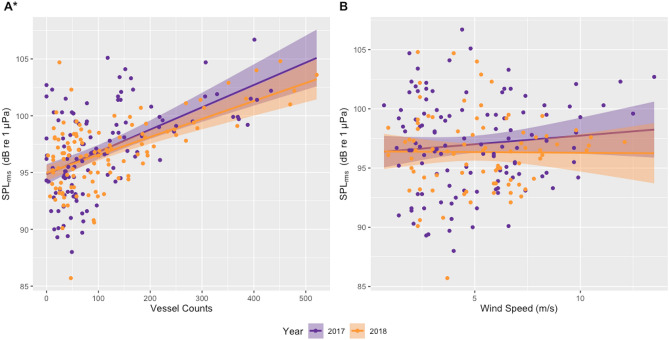
Daily counts of unique vessels from the two ports in the study area (Newport and Depoe Bay) were highly variable, ranging from 0 to 521 vessels counted per day, with higher mean numbers of vessels in July (mean = 135) and August (mean = 133). Underwater noise levels were positively correlated with vessel counts (2017: rate of change = 0.020, F1,114 = 43.72, R2 = 0.271, p < 0.001; 2018: rate of change = 0.016, F1,112 = 54.92, R2 = 0.323, p < 0.001; Fig. 2A). Most whales were sampled within 10 km of the hydrophone (44.62% < 5 km; 15.38% between 5 and 10 km; 40% > 10 km), indicating likely exposure to the vessel traffic noise recorded.
Figure 2.
Linear correlations between noise levels (daily median root mean square [rms] sound pressure level [SPL] in dB [re 1 μPa]; 50–1000 Hz) recorded on a hydrophone deployed outside the Newport harbor entrance during June to October of 2017 and 2018 and (A) vessel counts in Newport and Depoe Bay, Oregon, USA, and (B) daily median wind speed (m/s) from an anemometer station located on South Beach, Newport, Oregon, USA (station NWPO3). Asterisk indicates significant correlations between SPL and vessel counts in both years.
Daily median wind speed also fluctuated, ranging from 0.7 to 13.5 m/s, with higher median wind speeds occurring in July (5.95 m/s) and August (5.90 m/s). No correlation between wind speed and noise levels was observed in 2017 (rate of change = 0.146, F1,114 = 1.201, R2 = 0.002, p = 0.275) or 2018 (rate of change = − 0.012, F1,81 = 0.006, R2 = − 0.012, p = 0.937; Fig. 2B). Visual assessment of the temporal patterns in ambient sound level variation during the study periods (Fig. 3) indicates a strong, sharp onset maximum in underwater noise between 6 and 8 a.m. most days, which is coincident with peak recreational and sportfishing charter vessel traffic as boats transit out of the harbor to fishing grounds. A smaller peak in noise levels occurs at noon, which may represent a significant number of half-day fishing charters returning to the harbor around the same time. The absence of other similar peaks in noise levels during the day likely relates to the variable return times of vessels based on differences in fishing success (e.g., catch limits) or rising weather conditions and return distances and vessel speeds from the fishing grounds. Wind speeds in this study system typically begin to rise after 10 a.m. and peak between 3 and 4 p.m., which is an incongruous pattern with recorded ambient noise levels and further evidence that noise levels are dominated by vessel activity. Between midnight and 5 a.m., a steady decline of sound pressure levels to their lowest values is also noted as vessel traffic and wind speeds also reach their daily minimum.
Figure 3.
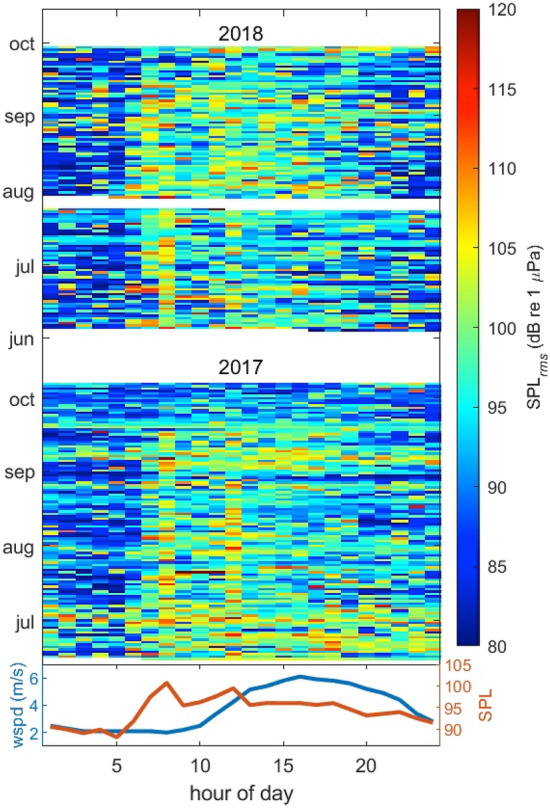
Median noise levels (root mean square sound pressure levels—SPLrms) for each hour of each day recorded on a hydrophone (50–10,000 Hz) deployed outside the Newport harbor entrance during June to October of 2017 (middle plot) and 2018 (upper plot), and hourly median noise level (SPL) against hourly median wind speed (lower plot) from an anemometer station located on South Beach, Newport, Oregon, USA (station NWPO3) over the same time period.
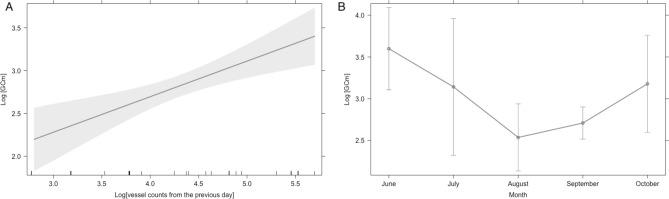
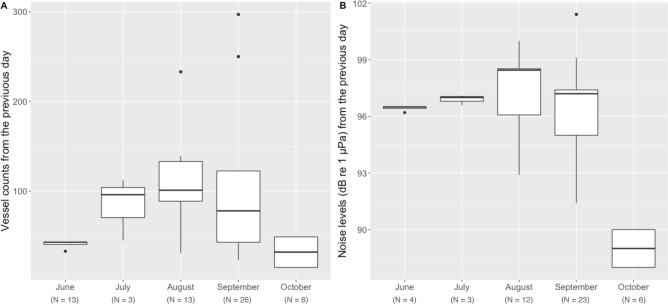
LMM analyses identified model 2 as the best model based on the lowest AIC. Model 2 included vessel counts from the previous day, sex, month, year, BAI, and fP and fA concentrations (Table 1, model 2; AIC = 58.55, df = 14, R2m = 0.64, R2c = 0.64), with vessel counts from previous day (F1,17 = 14.88, p = 0.001; Fig. 4A) and month (F1,17 = 4.12, p = 0.016) both significant (Table 2). Once the most influential temporal scale of vessel counts on fGC was determined (e.g., day before the sample collection), 17 additional LMMs were run with varied combinations of the fixed effects (Table 1, A–Q), but model 2 still displayed the lowest AIC. The months of August and September were significantly different from June in the model (Table 2). Although a linear regression between fGC concentrations and vessel counts of previous day, partitioned by month, found no significant correlation (p > 0.05), the plot (Fig. 4B) indicates that fGC was lower during the two months of August and September compared to June. Our posthoc analyses indicated no significant differences in vessel counts (ANOVA: F(4,56) = 1.701, p = 0.163; Fig. 5A) and significant differences in ambient noise levels (ANOVA: F(4,43) = 14.32, p < 0.001; Fig. 5B) on the previous day of fecal sample collection when partitioned by month. Yet, both vessel counts and ambient noise levels on the previous day of fecal sample collection display the same trend, with increased values in the months of August and September, and reduced values in the month of October.
Table 1.
Linear mixed model (LMM) selection parameters of gray whale fecal glucocorticoid metabolite (fGC) concentrations relative to vessel counts, sex, year, progestin metabolites (fP), androgen metabolites (fA) and body condition (Body Area Index: BAI).
| Models | DF | AIC | R2m | R2c |
|---|---|---|---|---|
| 0) fGC ~ sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 67.81511 | 0.45 | 0.49 |
| 1) fGC ~ vesselssameday + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 64.21275 | 0.54 | 0.54 |
| 2) fGC ~ vesselsprev1 + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 58.5528 | 0.64 | 0.64 |
| 3) fGC ~ vesselsprev2sum + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 59.86415 | 0.61 | 0.61 |
| 4) fGC ~ vesselsprev3sum + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 64.81003 | 0.5 | 0.5 |
| 5) fGC ~ vesselsprev4sum + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 68.19622 | 0.41 | 0.43 |
| 6) fGC ~ vesselsprev5sum + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 67.24901 | 0.45 | 0.56 |
| 7) fGC ~ vesselsprev6sum + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 68.38497 | 0.4 | 0.4 |
| 8) fGC ~ vesselsprev7sum + sex + month + year + fP + fA + BAI + (1|whale ID) | 14 | 68.10813 | 0.4 | 0.4 |
| A) fGC ~ vesselsprev1 + sex + month + year + fP + fA + (1|whale ID) | 13 | 91.82038 | 0.28 | 0.3 |
| B) fGC ~ vesselsprev1 + sex + month + year + fP + (1|whale ID) | 12 | 103.6393 | 0.2 | 0.2 |
| C) fGC ~ vesselsprev1 + sex + month + year + (1|whale ID) | 11 | 110.6568 | 0.1 | 0.1 |
| D) fGC ~ vesselsprev1 + sex + month + (1|whale ID) | 9 | 106.5958 | 0.08 | 0.08 |
| E) fGC ~ vesselsprev1 + sex + (1|whale ID) | 5 | 97.52082 | 0.05 | 0.05 |
| F) fGC ~ vesselsprev1 + month + (1|whale ID) | 8 | 104.8685 | 0.04 | 0.04 |
| G) fGC ~ vesselsprev1 + year + (1|whale ID) | 6 | 100.0222 | 0.05 | 0.05 |
| H) fGC ~ vesselsprev1 + fP + (1|whale ID) | 5 | 88.37007 | 0.13 | 0.13 |
| I) fGC ~ vesselsprev1 + fA + (1|whale ID) | 5 | 84.47714 | 0.09 | 0.09 |
| J) fGC ~ vesselsprev1 + (1|whale ID) | 4 | 95.57796 | 0.01 | 0.01 |
| K) fGC ~ vesselsprev1 + sex + month + year + fP + BAI + (1|whale ID) | 13 | 67.12452 | 0.54 | 0.68 |
| L) fGC ~ vesselsprev1 + sex + month + year + fA + BAI + (1|whale ID) | 13 | 60.16235 | 0.61 | 0.61 |
| M) fGC ~ vesselsprev1 + sex + month + year + BAI + (1|whale ID) | 12 | 71.2999 | 0.48 | 0.48 |
| N) fGC ~ vesselsprev1 + sex + month + BAI + (1|whale ID) | 10 | 67.72778 | 0.48 | 0.52 |
| O) fGC ~ vesselsprev1 + sex + BAI + (1|whale ID) | 6 | 65.35813 | 0.37 | 0.37 |
| P) fGC ~ vesselsprev1 + month + BAI + (1|whale ID) | 9 | 64.00439 | 0.49 | 0.51 |
| Q) fGC ~ vesselsprev1 + BAI + (1|whale ID) | 5 | 61.61131 | 0.37 | 0.37 |
All models used whale identification (whale ID) as a random factor to account for pseudoreplication. Models 1–8 included the same predictor variables except vessel counts, which was assessed at different temporal scales in each model and ranged from the count of vessels on the same day of the fecal sample collection (vesselssameday, model 1) to the count of vessel within the seven previous days (vesselsprev7sum, model 8). Model zero is the null model, which does not include vessels. Once the most influential temporal scale for vessel counts was determined (i.e., previous day; Model 2), 17 additional LMMs were run with varied combinations of the fixed effects (stepwise process, models A-Q; below the thin black line). Model in bold represents the selected model based on the lowest Akaike Information Criterion (AIC). The degrees of freedom for each model are indicated by DF. The fit of each model is represented by the marginal R2 (R2m; variance explained by fixed effects) and the conditional R2 (R2c; variance explained by both fixed and random effects).
Figure 4.
(A) The effect of vessel counts in Newport and Depoe Bay (Oregon, USA) on the day previous to fecal sample collection on gray whale fecal glucocorticoid metabolite (fGC) concentrations, derived from linear mixed model results with whale identification as random effect (Table 2; Model 2). (B) The effect of month on the gray whale fecal glucocorticoid metabolite (fGC) concentrations, also derived from linear mixed model results with whale identification as random effect (Table 2; Model 2). Data collected between May and October of 2016, 2017 and 2018. Asterisk indicates significant correlations between the variables in both A and B. Note difference in axes scales between A and B.
Table 2.
Parameter estimates from the chosen generalized linear mixed model (model 2) for gray whale fecal glucocorticoid metabolite concentrations using vessel counts from the previous day, sex, month, year, progestin metabolites, androgen metabolites, and body condition (Body Area Index: BAI) as fixed effects and whale identification as random factor.
| Random effects | Variance | Standard deviation | Fixed effects | Estimate | Standard error | p value |
|---|---|---|---|---|---|---|
| Whale ID | 0.000 | 0.000 | Vessel counts from the previous day | 0.415 | 0.108 | 0.001* |
| Residual | 0.099 | 0.315 | Sex | − 0.270 | 0.179 | 0.150 |
| Year (2017) | 0.527 | 0.361 | 0.163 | |||
| Year (2018) | 0.577 | 0.372 | 0.140 | |||
| Progestin metabolites | 0.170 | 0.122 | 0.182 | |||
| Androgen metabolites | − 0.044 | 0.065 | 0.508 | |||
| Body Area Index | − 0.027 | 0.043 | 0.541 | |||
| Month (July) | − 0.457 | 0.482 | 0.356 | |||
| Month (August) | − 1.063 | 0.278 | 0.001* | |||
| Month (September) | − 0.890 | 0.272 | 0.004* | |||
| Month (October) | − 0.421 | 0.348 | 0.244 |
*Significance (p < 0.05).
Figure 5.
Boxplots displaying the variation in (A) vessel counts in Newport and Depoe Bay (Oregon, USA) on the day previous to fecal sample collection on gray whale fecal glucocorticoid metabolite (fGC), and (B) noise levels (daily median SPLrms, in dB [re 1 μPa]; 50 Hz - 1,000 Hz) recorded on a hydrophone deployed outside the Newport harbor entrance on the day previous to fecal sample collection summarized by month during 2017 and 2018. The boxplot upper and lower limits represent the 25% and 75% quantiles, the mid-lines indicate the medians, the whiskers represent minimum and maximum values (range), and the dots indicate outliers.
Discussion
This study demonstrates that increased vessel activity is a contributing factor in the stress physiology of gray whales. This finding is significant due to increasing human activities in oceans worldwide and should be incorporated into decision making by environmental managers when considering mitigation of impacts on baleen whale populations. Although the driver of this physiological response to vessel traffic remains undetermined, we hypothesize that elevated ocean noise from increased vessel traffic may be a causative factor of increased fGC concentrations. This hypothesis is based on a strong positive correlation between vessel counts from nearby ports and variable ambient noise levels (Fig. 2A), and between fGC concentrations and increased vessel counts (LMM; Table 2) from the previous day. Our assessment of the relationship between whale stress, vessel counts, and ambient noise occurred at varied temporal scales and accounted for confounding factors potentially influencing ambient sound levels (i.e., wind) and whale fGC concentrations, (i.e., body condition as an indicator of nutritional state, year, sex, age, and reproductive hormone concentrations). The amount of vessel traffic, and not wind speed, was correlated with underwater sound levels (50–1000 Hz) in our nearshore study area, which agrees with previous studies showing vessel traffic as the primary contributor to ocean noise in shallow coastal regions with significant fishing, recreation and tourism activities51–54. Our findings of a physiological response of a cetacean to elevated vessel traffic, with correlations to synoptic ocean noise levels, is important information for inclusion in the Population Consequences of Disturbance (PCoD)27 and Population Consequences of Multiple Stressors (PCoMS)28 frameworks to estimate impacts of disturbance events on population dynamics.
Our work highlights the utility of combining information on internal drivers (i.e., BAI, reproductive hormones) with external drivers (i.e., vessel counts, year) of mammalian “stress response”, since all these variables are known to affect physiological responses to acute stressors. Given the significant correlation between fGC and BAI in gray whales42, it was particularly important to include this nutritional index in our assessment of stress response. By incorporating multiple factors potentially impacting animal physiology into a single analysis, the unexplained variability of fecal hormone data can be better resolved to provide a clearer understanding of the major factors that affect stress responses in a field setting. While many questions remain regarding the drivers, timing, and repercussions of variable baleen whale hormone concentrations, we have demonstrated the feasibility of these methods and the correlation between vessel traffic and gray whale stress response.
Our analysis detected a statistically significant difference in fGC response by month (model 2; Table 2). The fGC response in gray whales was significantly lower in the months of August and September relative to June (Fig. 4B), which might be related to improved whale nutritive condition and/or prey availability during the later months of the foraging season. This hypothesis is supported by previous studies that determined increased body condition of gray whales in the later months of their foraging season55 and a correlation between body condition and fGC42. The increased fGC response observed in October (Fig. 4B) might be related to factors other than nutrition, such as reproductive cycles, but more data collection is needed to test these theories.
We assessed the influence of eight different temporal scales (between disturbance and fecal collection) of vessel counts on gray whale fGC concentrations and determined that vessel count on the day prior to sample collection best explained fGC data. Thus, we hypothesize that gut transit time of stress hormones in gray whales may occur within ~ 24 hours of the disturbance event. We recognize that whales likely moved location within the 24 hours prior to fecal sample collection, but these whales were not tagged, so we are unable to quantify the exact number of vessels encountered or the associated sound exposure level of individual whales in the days prior to sample collection. However, our non-invasive photo-identification analysis indicates that most whales have high residency time in the study region and therefore were likely exposed to the vessel traffic and measured noise or very similar levels encountered in the study area. Our approach to summarize and assess vessel traffic and possible associated noise levels across the entire study area on each day assumes all whales within the study area endure a common vessel and noise exposure level each day regardless of location. Yet, unaccounted for whale movement across the study area may obscure finer-scale relationships between variation in local vessel abundance and traffic and later whale excretion of GCs, potentially reducing correlations for longer time lags. However, our results align with previous studies indicating that large mammals generally have gut transit times between 12 hours and 4 days, with a ~ 24-hour lag common in large carnivores31,41,44.
A complete understanding of the impact of vessel traffic and its associated noise on whale physiology remains unclear, and other factors unaccounted for in this study may influence gray whale GC concentrations, such as contaminants56, predation events57, and infections58. Vessel traffic can affect whale physiology via multiple pathways, including behavioral disturbance and altered metabolic rates caused by increased risk of collision with vessels21,29, elevated energetic expenditure to call more loudly or missed acoustic signals from conspecifics and predators due to masking effects, or a combination of these and other possibilities. This population of gray whales does show signs of propeller strike41 and is also subject to whale watch activities with documented behavioral disturbance from vessel traffic within our study region15. Thus, disentangling the impacts of vessel traffic from other impacts will require further assessment and larger sample sizes, as well as analyzing other confounding variables in parallel.
In addition to a large and active sportfishing community, Newport and Depoe Bay harbors also support many large commercial fishing vessels and approximately five whale watch operations. The Newport channel is also dredged by a large U.S. Army Corps of Engineers vessel during summer months and the harbor is home to the NOAA Pacific Fleet of large research vessels. Unfortunately, these vessel activities were not available in the vessel count data from Oregon Department of Fish and Wildlife that focuses on the sportfishing effort from these ports. This unaccounted-for vessel traffic may partly explain the variation in data points around the trend lines between vessel counts and daily ambient sound levels (Fig. 2), and the unexplained variance in the linear model.
Anthropogenic disturbance, including vessel traffic, can be stressful for marine mammals30,31, and often correlates with deterioration in overall health55,59,60, depressed immune systems, and increased mortality61. Despite our small sample size, we demonstrate correlations between vessel counts and whale stress-related hormone concentrations at an individual level and within short temporal periods (~ 24 hours). Furthermore, we provide initial evidence on a potential association between ocean noise originated from vessel traffic and fGC in gray whales. Our gray whale case study results can inform how other, less accessible baleen whale populations may physiologically respond to disturbance. However, it is important to note that our results are preliminary and not fully conclusive. A larger sample size is needed to confirm and further unravel patterns. Future work should build upon these documented associations between vessel traffic and stress responses in baleen whales to assess impacts on individual vital rates and subsequent population dynamics. Furthermore, future studies should directly explore potential correlations between stress responses in baleen whales and ocean noise. With continued assessment of physiological impacts of vessel disturbance and resulting ocean noise on baleen whales, environmental managers can develop effective regulations, such as determining thresholds for the number, speed and proximity of vessels to reduce sound levels near whale habitats and mitigate impacts on vulnerable and protected whale populations.
Methods
Gray whale data
All methods were carried out in accordance with relevant guidelines and regulations. This project was approved by the Oregon State University Institutional Animal Care and Use Committee (IACUC-2019-0008) and complies with the ARRIVE guidelines. All gray whale data collection was carried out under a research permit from NOAA/NMFS (#16011 and #21678, issued to John Calambokidis).
We used a small research vessel (5.4 m rigid-hulled inflatable boat) to collect gray whale data over the course of three foraging seasons (May to October from 2016 to 2018) along the central coast of Oregon, USA, including near the ports of Newport and Depoe Bay (Fig. 1). Gray whale visual surveys did not follow a standardized transect line, but rather prioritized maximizing whale encounters to collect individual-based data. Whale photographs were taken for individual photo-identification and drone-based videos were recorded for photogrammetry analysis when weather conditions and whale behavior were suitable (methods are fully described in62). We also opportunistically collected fecal samples at the whale sightings using two 300 µm nylon mesh dipnets (methods are fully described in41). Samples were transferred to sterile plastic jars and placed on ice until stored in a freezer (− 20 °C) for later analysis. Date, time, and location were documented for each fecal sample, as well as the matching photo for the specific individual.
We used Adobe Bridge software (version 8.0.1.282) for photo-identification analysis. Photographs were compared to long-term gray whale catalogs held by the Marine Mammal Institute at Oregon State University and Cascadia Research Collective (Olympia, WA, USA) to obtain individual sex and minimum age information based on date of first sighting. If sex was unknown, sex was determined through fecal genetic analyses (methods are fully described in55). Given the unknown movements of our study whales prior to fecal sample collection, we assessed the residency time (in days) of these whales within the study area to justify our assumption that the whales were exposed to the measured soundscape levels and vessel counts. Through photo-identification comparison, we summed the number of days a sampled whale was in the study area (1) prior to sample collection in that year, and (2) in total that year.
Images of whales flat and straight at the surface were extracted from drone video recordings using VLC software (version 2.2.8) and scored as good or poor quality based on pre-defined attributes55. Only images scored as good were measured in custom MATLAB (version 9.3.0.7, release 2017b) software, producing a series of ten morphometric attributes that describe the whale’s body condition. These metrics were assessed in R (version 3.5.063) to calculate a final metric called Body Area Index (BAI), which is a unitless and length-standardized metric of body condition that allows comparisons among individuals of different lengths and demographic units (e.g., calves and adults, or males and females55). We applied a coefficient of variance threshold of 5% for both whale length and BAI measurements to improve accuracy. Whales were assigned to a demographic unit based on sex and maturity status in each year55 based on fieldwork observations, photo-identification, and photogrammetry results. The BAI metric has been successfully implemented to document variation in body condition in this specific gray whale population across foraging seasons55.
Fecal samples were filtered, desalted, and freeze-dried41. Dried, processed samples were mixed and weighed to the nearest 0.2 g; samples below 0.02 g were excluded from the analysis to avoid inflated values ["small sample effect"; see 33,43]. Fecal hormone metabolites were extracted41 and quantified using commercial Enzyme-linked Immunosorbent Assay kits for cortisol (#ADI-900-071), progesterone (#ADI-900-011), and testosterone (#ADI-900-065) from Enzo Life Sciences, following the manufacturer's protocols (https://www.enzolifesciences.com). Since fecal samples reflect the metabolized products of the parent hormones, the cortisol kit quantifies fGC, the progesterone kit quantifies progestin metabolites (fP), and the testosterone kit quantifies androgen metabolites (fA).
Samples were run in duplicate in 2016–2017, and in triplicate in 2018. All samples were analyzed within 11 months of collection. Our quality assurance and quality control protocols include full standard curves in each assay, re-run of any sample with > 15% coefficient of variation (CV) between replicates, and re-run of any sample outside the percent-bound range of 15–85%. Samples not conforming to these standards were analyzed again until suitable values were obtained. Values < LOD were excluded from the analysis64. When multiple fecal samples were collected from the same individual in the same day, we applied the values from the sample with higher mass30. Gray whale fecal hormone assays have been validated for all hormones described in this study, and results exhibited excellent parallelism and accuracy as well as good match to known physiological state (age, sex, reproductive state)41.
Vessel traffic data
Daily counts of unique vessels from the ports of Newport and Depoe Bay during our three field seasons were obtained from the Oregon Department of Fish and Wildlife (ODFW) through video analysis at the ports. These daily vessel counts consist only of recreational craft, including commercial charters on fishing and crabbing trips, and private boats (e.g., private fishing trips, kayaks, row boats, and jet skis). Therefore, vessel activity for other purposes (e.g., whale watching, research, funerals, maintenance trips, commercial fishing, Coast Guard, and dredging) are not tracked by these counts and thus, are not accounted for in this analysis. However, during the time period of this study, no major seismic, sonar or marine construction occurred in our study area, and recreational vessel traffic in coastal areas has been found to correlate strongly with ambient noise levels9,54.
Acoustic data
Concurrent with gray whale data collection, acoustic data were recorded off the coast of Newport, Oregon, from 15 June to 8 October of 2017 and 5 June to 1 October of 2018 (no acoustic data is available from 2016). A passive acoustic monitoring (PAM) hydrophone system was deployed outside the Newport harbor entrance at 44.5932 N, −124.1029 W, in 20 m water depth, and 1.25 km from the coastline (Fig. 1). The custom PAM system consisted of an omni-directional hydrophone (International Transducer Corporation transducer model ITC1032) with sensitivity − 192 dB re μPa V−1 @ 1 m combined with a low-power 16-bit data acquisition system and preamplifier housed in a fiberglass composite pressure housing65. The PAM system was mounted on a weighted, semi-trawl protected aluminum frame 0.5 m above the seafloor with no sea surface expression. Data were recorded at 32 kHz sample rate on a 20% duty cycle (12 min of every hour). A low frequency cutoff was applied to avoid aliasing around the Nyquist frequency, resulting in acoustic measurements that included energy up to 13 kHz. Data analysis followed previously described methods65. Root mean square sound pressure levels (SPLrms) were calculated from 50 to 1000 Hz frequency band. This frequency range captures low frequency vessel-generated noise < 1000 Hz typical of commercial and recreational boats using the ports of Newport and Depoe Bay66, and the sound energy from wind-related processes down to 400 Hz67 while avoiding the increasing surface wind generated noise that scales with wind speed and frequency up to 10,000 Hz68. This range is also relevant for the acoustic sensitivity of gray whales and overlaps with two of the most frequent call types observed in the northeastern Pacific (known as “M1 and M3 calls”)69–71. Although audiograms for gray whales are not available, since they produce calls in this range it is likely that they are able to hear at, or are acoustically sensitive in the frequencies of the calls they produce.
A daily median SPLrms (50–1000 Hz) calculated from 6 a.m. to 7 p.m. Pacific Daylight Time provides a measure of the 50th percentile, or typical sound levels, associated with vessel activity at the harbor entrance during the busiest daytime hours of each day.
Wind speed data
Wind-generated surface noise also contributes to ocean soundscapes68. Therefore, we compiled local wind speed data during our study periods to assess and compare the contributions of wind and vessel traffic to recorded underwater ambient noise levels. Hourly wind speed data from an anemometer station located near the hydrophone on the South Beach jetty entrance to the port of Newport (station NWPO3, Newport, OR, −44.613 N, 124.067 W; Fig. 1) during our three field seasons were obtained from the NOAA National Data Buoy Center (NDBC). Times were converted to local Pacific Daylight Time. Hourly median wind speed (m/s) and a daily median wind speed value (m/s) from 6 a.m. to 7 p.m. were calculated to match noise level measurements from the deployed hydrophones.
Data analysis
Our goal was to assess if and how gray whale fGC concentrations vary relative to vessel counts, while simultaneously accounting for the effects of months, years, demographic units, other hormone metabolites, and body condition (BAI). Therefore, every fecal sample was matched with the BAI measurement of that individual from the same day or within ± 14 days of the fecal sample collection (no change in body condition within this window was detected; paired t-test using all BAI values of individuals assessed within 14 days in 2016, 2017 and 2018: n = 61, p = 0.86, df = 60, t = − 0.174).
Only mature males and non-pregnant, non-lactating mature females were included in this analysis to minimize the known impact of normal variation in fGC concentrations due to life history phases33,41,72. It was assumed that adult females swimming in close association with a calf were lactating females and that they were pregnant in the previous year, based on a gestation of 13 months73.
All statistical analyses were conducted in R software63 with a significance level of 0.05. Normality of all variables was tested using the Shapiro–Wilk normality test, with non-normal variables log-transformed (log-normal [value + 1]) before further analysis.
Linear regressions were performed using the lm function in R to test for correlations between (1) daily vessel count data from both ports and ocean noise (daily median SPLrms) in 2017 and 2018, and (2) ocean noise in 2017 and 2018 and daily median wind speed. To further explore the temporal patterns in underwater sound levels and any potential correlation with local wind patterns, the median noise levels for each hour of each day recorded by the Newport hydrophone (50–1000 Hz) in 2017 and 2018 were plotted in MATLAB (version 9.7.0.1190202–R2019b) alongside the hourly median wind speed over the same time period (Fig. 3).
Linear mixed models (LMM) were conducted using the lme4 package in R74, to assess the effects of vessel counts, month, year, sex, BAI, and other hormone metabolites on fGC concentrations. The ports of Newport and Depoe Bay are 22 km apart, which is within the daily travel range of a gray whale (L. Lemos, pers. obs.; based on field observations and photo-identification analysis). As daily vessel counts at Newport and Depoe Bay were positively correlated (rate of change = 3.176, F1,233 = 520.6, R2 = 0.689, p < 0.001), we assumed that vessel activity from both ports influences acoustic conditions within the study area. Therefore, we summed vessel counts from the two ports for analysis relative to fGC concentrations.
Due to uncertainty regarding gut transit time (which cannot be determined experimentally in mysticetes44), different time lags between vessel count and fecal collection were assessed in the LMMs, including the sum of vessel counts on the same day as fecal sample collection, and on the previous 1–7 days (based on known gut transit times in large mammals of ~ 12 hours to 4 days43,75,76). An additional null model (model 0; Table 1), which did not included vessel counts, was run to verify the effect of vessel traffic on gray whale fGC concentration. All models included whale identification as random effect to account for pseudoreplication. Model selection was based on the lowest Akaike’s information criterion (AIC77). It is important to highlight that most of the models (i.e., 1-4, 7-8, b-j, l-m, o, and q) displayed a singular fit message, which may indicate overfitting of the models. Despite limiting models to just one random effect (i.e., whale ID) and reducing the number of random effects included in the model, singular fits persisted. Since this warning message may also relate to the variance of one or more combinations of the effects being close to zero78, we believe that the singular fit is due to the low sample size while splitting the data between many relevant fixed and random effects. Therefore, exclusion of relevant variables may not necessarily resolve the issue; thus, we selected models based on AIC, parsimony, and inclusion of variables relevant for explaining fGC variation (Table 1). After the most influential temporal scale for vessel counts was determined, additional LMMs were run with varied combinations of the fixed effects. Model fit was evaluated by assessing the marginal R2 (R2m: variance explained by fixed effects) and the conditional R2 (R2c: variance explained by both fixed and random effects) using the MuMIn package in R79,80. F-statistics and p-values were obtained using the lmerTest package81. To assess the relationships between vessel counts and ambient noise levels from the previous day of fecal sample collection, we conducted additional ANOVAs between these variables and months. For these posthoc analyses, he had a smaller sample size based on only 2017 and 2018 data because no hydrophone data were available for 2016.
The code and associated data to run the LMMs, linear regressions and ANOVAs are deposited in the FigShare Digital Repository: 10.6084/m9.figshare.2117188682.
Supplementary Information
Acknowledgements
This work was supported by the NOAA National Marine Fisheries Service Office of Science and Technology Ocean Acoustics Program [2016 and 2017; 50-27], the Oregon Sea Grant Program Development funds [2018; RECO-40-PD], and the Office of Naval Research Marine Mammals and Biology program (Award N00014-20-1-2760). We are thankful for the support of Brazil’s Science Without Borders program, Brazil’s CNPq, and the Harvard Laspau Institute for financial aid and academic advising to LSL. We are also thankful for the support of the Mamie Markham Research Award (Hatfield Marine Science Center/OSU) and Cetacean Society International for awards given to LSL to support data and laboratory analyses. We thank the NOAA Pacific Marine Environmental Laboratory Acoustic Program for hydrophone and logistical support, Oregon Department of Fish and Wildlife for providing the vessel traffic data, John Calambokidis and Alie Perez (Cascadia Research Collective) for assistance with the photo-identification analysis, the OSU Marine Mammal Institute for logistical support, and Debbie Steel and Scott Baker (Cetacean Conservation and Genetic Laboratory/OSU) for assistance with the genetic analyses. We would also like to thank the Southwest Fisheries Science Center’s Marine Mammal Genetics Program for providing data on the genetic sex of some of the individuals included in this study. This research was conducted under the NOAA/NMFS permits #16011 and #21678 issued to John Calambokidis. Drone operations were conducted by a Federal Aviation Authority (FAA) certified private pilot with a Part107 license or under a Certificate of Authorization (2016-WSA-101-COA). We also thank two anonymous reviewers for their insightful feedback to improve this manuscript.
Author contributions
L.S.L. participated in fieldwork, conducted photo-identification and photogrammetry analyses, hormone assays, statistical analyses, and drafted the manuscript. J.H.H. led the acoustic components of the work, participated in fieldwork, contributed to interpretation of results, assisted with obtaining funding, and participated in study design. A.O. and A.S. conducted hormone validations and assisted with hormone assays. J.D.B. led whale photogrammetry method development. T.E.C. participated in fieldwork and contributed to field method developments. S.L.N. participated in fieldwork and participated in study design. S.E.L. oversaw hormone analyses and provided the assay kits. K.E.H. contributed to interpretation of results and participated in study design. J.H.H., A.O., A.S., J.D.B., T.E.C., S.L.N., S.E.L. and K.E.H. all critically revised the manuscript. L.G.T. envisioned the study, acquired funding for the research, designed and coordinated the project, lead the fieldwork, and drafted the manuscript. All authors give final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-14510-5.
References
- 1.McDonald MA, Hildebrand JA, Wiggins SM. Increases in deep ocean ambient noise in the Northeast Pacific west of San Nicolas Island, California. J. Acoust. Soc. Am. 2006;120:711–718. doi: 10.1121/1.2216565. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MB, Solomon S. A coming boom in commercial shipping? The potential for rapid growth of noise from commercial ships by 2030. Mar. Policy. 2016;73:119–121. [Google Scholar]
- 3.McCarthy E. International regulation of underwater sound: establishing rules and standards to address ocean noise pollution. Kluwer Academic Publishers; 2004. [Google Scholar]
- 4.Weilgart LS. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007;85:1091–1116. [Google Scholar]
- 5.Bas AA, et al. Marine vessels alter the behaviour of bottlenose dolphins Tursiops truncatus in the Istanbul Strait, Turkey. Endanger. Species Res. 2017;34:1–14. [Google Scholar]
- 6.Laist DW, Knowlton AR, Mead JG, Collet AS, Podesta M. Collisions between ships and whales. Mar. Mammal Sci. 2001;17:35–75. [Google Scholar]
- 7.Douglas AB, et al. Incidence of ship strikes of large whales in Washington State. J. Mar. Biol. Assoc. U. K. 2008;88:1121–1132. [Google Scholar]
- 8.Hildebrand JA. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 2009;395:5–20. [Google Scholar]
- 9.Hermannsen L, et al. Recreational vessels without Automatic Identification System (AIS) dominate anthropogenic noise contributions to a shallow water soundscape. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-51222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman NR, Price A. Low frequency deep ocean ambient noise trend in the Northeast Pacific Ocean. J. Acoust. Soc. Am. 2011;129:EL161–EL165. doi: 10.1121/1.3567084. [DOI] [PubMed] [Google Scholar]
- 11.Erbe C, Reichmuth C, Cunningham K, Lucke K, Dooling R. Communication masking in marine mammals: a review and research strategy. Mar. Pollut. Bull. 2016;103:15–38. doi: 10.1016/j.marpolbul.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Erbe, C. et al. The effects of ship noise on marine mammals: a review. Front. Mar. Sci.6 (2019).
- 13.Bejder L, Samuels A, Whitehead H, Gales N. Interpreting short-term behavioural responses to disturbance within a longitudinal perspective. Anim. Behav. 2006;72:1149–1158. [Google Scholar]
- 14.Sutherland WJ. The importance of behavioural studies in conservation biology. Anim. Behav. 1998;56:801–809. doi: 10.1006/anbe.1998.0896. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan FA, Torres LG. Assessment of vessel disturbance to gray whales to inform sustainable ecotourism. J. Wildl. Manag. 2018;82:896–905. [Google Scholar]
- 16.Pirotta E, Merchant ND, Thompson PM, Barton TR, Lusseau D. Quantifying the effect of boat disturbance on bottlenose dolphin foraging activity. Biol. Conserv. 2015;181:82–89. [Google Scholar]
- 17.Dans SL, Degrati M, Pedraza SN, Crespo EA. Effects of tour boats on dolphin activity examined with sensitivity analysis of Markov chains. Conserv. Biol. 2012;26:708–716. doi: 10.1111/j.1523-1739.2012.01844.x. [DOI] [PubMed] [Google Scholar]
- 18.Christiansen F, Rasmussen M, Lusseau D. Whale watching disrupts feeding activities of minke whales on a feeding ground. Mar. Ecol. Prog. Ser. 2013;478:239–251. [Google Scholar]
- 19.Nowacek SM, Wells RS, Solow AR. Short-term effects of boat traffic on Bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar. Mammal. Sci. 2001;17:673–688. [Google Scholar]
- 20.Bejder L, Dawson SM, Harraway JA. Responses by Hector’s dolphins to boats and swimmers in Porpoise Bay, New Zealand. Mar. Mammal Sci. 1999;15:738–750. [Google Scholar]
- 21.Lusseau D. Male and female bottlenose dolphins Tursiops spp. have different strategies to avoid interactions with tour boats in Doubtful Sound. New Zealand. Mar. Ecol. Prog. Ser. 2003;257:267–274. [Google Scholar]
- 22.Williams R, Trites AW, Bain DE. Behavioural responses of killer whales (Orcinus orca) to whale-watching boats: opportunistic observations and experimental approaches. J. Zool. 2002;256:255–270. [Google Scholar]
- 23.Barr K. Impacts of tourist vessels on the behaviour of dusky dolphins (Lagenorhynchus obscurus) at Kaikoura. University of Otago; 1997. [Google Scholar]
- 24.Blumstein DT, Fernández-Juricic E, Zollner PA, Garity SC. Inter-specific variation in avian responses to human disturbance. J. Appl. Ecol. 2005;42:943–953. [Google Scholar]
- 25.Bejder L, Samuels A, Whitehead H, Finn H, Allen S. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 2009;395:177–185. [Google Scholar]
- 26.Gill JA, Norris K, Sutherland WJ. Why behavioural responses may not reflect the population consequences of human disturbance. Biol. Conserv. 2001;97:265–268. [Google Scholar]
- 27.National Research Council . Marine Mammal Populations and Ocean Noise: Determining When Noise Causes Biologically Significant Effects. National Academies Press; 2005. [Google Scholar]
- 28.National Academies of Sciences Engineering and Medicine . Approaches to Understanding the Cumulative Effects of Stressors on Marine Mammals. London: The National Academies Press; 2017. [Google Scholar]
- 29.Sprogis KR, Videsen S, Madsen PT. Vessel noise levels drive behavioural responses of humpback whales with implications for whale-watching. Elife. 2020;9:e56760. doi: 10.7554/eLife.56760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayres KL, et al. Distinguishing the impacts of inadequate prey and vessel traffic on an endangered killer whale (Orcinus orca) population. PLoS ONE. 2012;7:e36842. doi: 10.1371/journal.pone.0036842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolland RM, et al. Evidence that ship noise increases stress in right whales. Proc. R. Soc. B Biol. Sci. 2012;279:2363–2368. doi: 10.1098/rspb.2011.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt KE, et al. Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv. Physiol. 2013;1:cot006–cot006. doi: 10.1093/conphys/cot006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt KE, Rolland RM, Kraus SD, Wasser SK. Analysis of fecal glucocorticoids in the North Atlantic right whale (Eubalaena glacialis) Gen. Comp. Endocrinol. 2006;148:260–272. doi: 10.1016/j.ygcen.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Dunstan, J., Gledhill, A., Hall, A., Miller, P. & Ramp, C. Quantification of the hormones progesterone and cortisol in whale breath samples using novel, non-invasive sampling and analysis with highly-sensitive ACQUITY UPLC and Xevo TQ-S. Waters Application Note: Waters Corporation 1–8 (2012).
- 35.Champagne CD, et al. Blubber cortisol qualitatively reflects circulating cortisol concentrations in bottlenose dolphins. Mar. Mammal Sci. 2017;33:134–153. [Google Scholar]
- 36.Rolland RM, Hunt KE, Kraus SD, Wasser SK. Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen. Comp. Endocrinol. 2005;142:308–317. doi: 10.1016/j.ygcen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Roman J, McCarthy JJ. The whale pump: marine mammals enhance primary productivity in a coastal basin. PLoS ONE. 2010;5:e13255. doi: 10.1371/journal.pone.0013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt KE, Robbins J, Buck CL, Bérubé M, Rolland RM. Evaluation of fecal hormones for noninvasive research on reproduction and stress in humpback whales (Megaptera novaeangliae) Gen. Comp. Endocrinol. 2019;280:24–34. doi: 10.1016/j.ygcen.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Lefebvre KA, Bargu S, Kieckhefer T, Silver MW. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon. 2002;40:971–977. doi: 10.1016/s0041-0101(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 40.Valenzuela-Molina M, Atkinson S, Mashburn K, Gendron D, Brownell RL. Fecal steroid hormones reveal reproductive state in female blue whales sampled in the Gulf of California, Mexico. Gen. Comp. Endocrinol. 2018;261:127–135. doi: 10.1016/j.ygcen.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Lemos LS, et al. Assessment of fecal steroid and thyroid hormone metabolites in eastern north Pacific gray whales. Conserv. Physiol. 2020;8:coaa110. doi: 10.1093/conphys/coaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemos LS, et al. Stressed and slim or relaxed and chubby? A simultaneous assessment of gray whale body condition and hormone variability. Mar. Mammal Sci. 2021 doi: 10.1111/mms.12877. [DOI] [Google Scholar]
- 43.Palme R, Fischer P, Schildorfer H, Ismail MN. Excretion of infused 14C-steroid hormones via faeces and urine in domestic livestock. Anim. Reprod. Sci. 1996;43:43–63. [Google Scholar]
- 44.Wasser SK, et al. Non-invasive measurement of thyroid hormone in feces of a diverse array of avian and mammalian species. Gen. Comp. Endocrinol. 2010;168:1–7. doi: 10.1016/j.ygcen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Palme R. Non-invasive measurement of glucocorticoids: advances and problems. Physiol. Behav. 2019;199:229–243. doi: 10.1016/j.physbeh.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Goymann W. On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 2012;3:757–765. [Google Scholar]
- 47.Romero LM, Wingfield JC. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford Series in Behavioral Neuroendocrinology. Oxford University Press; 2016. p. 614. [Google Scholar]
- 48.Calambokidis, J., Laake, J. L. & Klimek, A. Updated Analysis of Abundance and Population Structure of Seasonal Gray Whales in the Pacific, Vol. 2010 (2012).
- 49.Dahlheim ME. Bio-acoustics of the Gray Whale (Eschrichtius robustus) University of British Columbia; 1987. [Google Scholar]
- 50.López-Urbán A, Thode A, Durán CB, UrbáN-R J, Swartz S. Two new grey whale call types detected on bioacoustic tags. J. Mar. Biol. Assoc. U. K. 2018;98:1169–1175. [Google Scholar]
- 51.Kaplan MB, Mooney TA. Ambient noise and temporal patterns of boat activity in the US Virgin Islands National Park. Mar. Pollut. Bull. 2015;98:221–228. doi: 10.1016/j.marpolbul.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 52.Erbe C. Underwater noise of whale-watching boats and potentital effects on killer whales (Orcinus orca), based on an acoustic impact model. Mar. Mammal Sci. 2002;18:394–418. [Google Scholar]
- 53.Pine MK, Jeffs AG, Wang D, Radford CA. The potential for vessel noise to mask biologically important sounds within ecologically significant embayments. Ocean Coast. Manag. 2016;127:63–73. [Google Scholar]
- 54.Pine MK, et al. A Gulf in lockdown: How an enforced ban on recreational vessels increased dolphin and fish communication ranges. Glob. Chang. Biol. 2021;00:1–10. doi: 10.1111/gcb.15798. [DOI] [PubMed] [Google Scholar]
- 55.Soledade Lemos L, Burnett JD, Chandler TE, Sumich JL, Torres LG. Intra- and inter-annual variation in gray whale body condition on a foraging ground. Ecosphere. 2020;11:e03094. [Google Scholar]
- 56.Trumble SJ, Robinson EM, Berman-Kowalewski M, Potter CW, Usenko S. Blue whale earplug reveals lifetime contaminant exposure and hormone profiles. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16922–16926. doi: 10.1073/pnas.1311418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barrett-Lennard LG, Matkin CO, Durban JW, Saulitis EL, Ellifrit D. Predation on gray whales and prolonged feeding on submerged carcasses by transient killer whales at Unimak Island, Alaska. Mar. Ecol. Prog. Ser. 2011;421:229–241. [Google Scholar]
- 58.Dailey MD, Gulland FMD, Lowenstine LJ, Silvagni P, Howard D. Prey, parasites and pathology associated with the mortality of a juvenile gray whale (Eschrichtius robustus) stranded along the northern California coast. Dis. Aquat. Organ. 2000;42:111–117. doi: 10.3354/dao042111. [DOI] [PubMed] [Google Scholar]
- 59.Le Boeuf BJ, Perez-Cortes MH, Urbán RJ, Mate BR, Ollervides UF. High gray whale mortality and low recruitment in 1999: Potential causes and implications (Eschrichtius robustus) J. Cetacean Res. Manag. 2000;2:85–99. [Google Scholar]
- 60.Wikelski M, Cooke SJ. Conservation physiology. Trends Ecol. Evol. 2006;21:38–46. doi: 10.1016/j.tree.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Burek KA, Gulland FMD, O’Hara TM. Effects of climate change on Arctic marine mammal health source. Ecol. Appl. 2018;18:S126–S134. doi: 10.1890/06-0553.1. [DOI] [PubMed] [Google Scholar]
- 62.Burnett JD, et al. Estimating morphometric attributes of baleen whales with photogrammetry from small UASs: a case study with blue and gray whales. Mar. Mammal Sci. 2019;35:108–139. [Google Scholar]
- 63.R Core Team. R: a language and environment for statistical computing. (2019).
- 64.Wood MD, Beresford NA, Copplestone D. Limit of detection values in data analysis: Do they matter? Radioprotection. 2011;46:85–90. [Google Scholar]
- 65.Haxel JH, Dziak RP, Matsumoto H. Observations of shallow water marine ambient sound: the low frequency underwater soundscape of the central Oregon coast. J. Acoust. Soc. Am. 2013;133:2586–2596. doi: 10.1121/1.4796132. [DOI] [PubMed] [Google Scholar]
- 66.Parsons MJG, Erbe C, Meekan MG, Parsons SK. A review and meta-analysis of underwater noise radiated by small (<25 m length) vessels. J. Mar. Sci. Eng. 2021;9:827. [Google Scholar]
- 67.Hildebrand JA, Frasier KE, Baumann-Pickering S, Wiggins SM. An empirical model for wind-generated ocean noise. J. Acoust. Soc. Am. 2021;149:4516–4533. doi: 10.1121/10.0005430. [DOI] [PubMed] [Google Scholar]
- 68.Knudsen VO, Alford RS, Emling JW. Underwater ambient noise. J. Mar. Res. 1948;7:410–429. [Google Scholar]
- 69.Dahlheim M, Castellote M. Changes in the acoustic behavior of gray whales Eschrichtius robustus in response to noise. Endanger. Species Res. 2016;31:227–242. [Google Scholar]
- 70.Guazzo RA, et al. Migratory behavior of eastern North Pacific gray whales tracked using a hydrophone array. PLoS ONE. 2017;12:e0185585. doi: 10.1371/journal.pone.0185585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guazzo RA, et al. Migrating eastern North Pacific gray whale call and blow rates estimated from acoustic recordings, infrared camera video, and visual sightings. Sci. Rep. 2019;9:12617. doi: 10.1038/s41598-019-49115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rolland RM, Hunt KE, Kraus SD, Wasser SK. Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen. Comp. Endocrinol. 2005;142:308–317. doi: 10.1016/j.ygcen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Perryman WL, Lynn MS. Evaluation of nutritive condition and reproductive status of migrating gray whales (Eschrichtius robustus) based on analysis of photogrammetric data. J. Cetacean Res. Manag. 2002;4:155–164. [Google Scholar]
- 74.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- 75.Wasser SK, et al. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- 76.Hunt KE, Trites AW, Wasser SK. Validation of a fecal glucocorticoid assay for Steller sea lions (Eumetopias jubatus) Physiol. Behav. 2004;80:595–601. doi: 10.1016/j.physbeh.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 77.Burnham KP, Anderson DR, Huyvaert KP. Aic model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011;65:23–35. [Google Scholar]
- 78.Gomes DGE. Should I use fixed effects or random effects when I have fewer than five levels of a grouping factor in a mixed-effects model? PeerJ. 2022;10:e12794. doi: 10.7717/peerj.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013;4:133–142. [Google Scholar]
- 80.Barton, K. MuMIn: Multi-Model Inference. R Package. version 1.43.17 (2020).
- 81.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: tests in linear mixed effects models. J. Stat. Softw. 2017;82:1–26. [Google Scholar]
- 82.Lemos, L. S. et al. Effects of vessel traffic and ocean noise on gray whale stress hormones, FigShare, Dataset. 10.6084/m9.figshare.21171886 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.