Abstract
Background
Changes in tobacco products, use patterns, and assessment technology in the last 15 years led the Society for Research on Nicotine and Tobacco (SRNT) Treatment Research Network to call for an update to the 2003 SRNT recommendations for assessing abstinence in clinical trials of smoking cessation interventions.
Methods
The SRNT Treatment Research Network convened a group of investigators with decades of experience in conducting tobacco treatment clinical trials. To arrive at the updated recommendations, the authors reviewed the recommendations of the prior SRNT Workgroup as well as current literature. Ten additional experts in the field provided feedback on this paper and these recommendations.
Results
With respect to defining abstinence, the authors recommend: (1) continuing to use the definition of no use of combustible tobacco products (regardless of use of noncombustible tobacco products [e.g., snus] and alternative products [e.g., e-cigarettes]) and collecting additional data to permit alternate abstinence definitions; (2) no use of combustible or smokeless tobacco products; and (3) no use of combustible or smokeless tobacco products or alternative products, as appropriate for the research question being addressed. The authors also recommend reporting point prevalence and prolonged abstinence at multiple timepoints (end of treatment, ≥3 months after the end of treatment, and ≥6 months postquit or posttreatment initiation).
Conclusions
Defining abstinence requires specification of which products a user must abstain from using, the type of abstinence (i.e., point prevalence or continuous), and the duration of abstinence. These recommendations are intended to serve as guidelines for investigators as they collect the necessary data to accurately describe participants’ abstinence during smoking cessation clinical trials.
Implications
This paper provides updated recommendations for defining abstinence in the context of smoking cessation treatment clinical trials.
Introduction
In 2003, the Society for Research on Nicotine and Tobacco (SRNT) Treatment Research Network issued recommendations for assessing abstinence in clinical trials of smoking cessation interventions,1 updating 1986 recommendations.2 In 2017, SRNT Treatment Research Network members identified a need to update the recommendations in light of changes in smoking patterns (e.g., a higher prevalence of nondaily smoking3–7 and the high prevalence of polytobacco use8,9), the advent of new products (e.g., electronic cigarettes [e-cigarettes], heat-not-burn [HNB] products), and the development of new technologies to conduct tobacco use assessment (e.g., mobile applications, wearables). Similar to the 2003 recommendations,1 these recommendations are not intended to be either necessary or sufficient for all trials, nor are they the only valid measures for clinical trials. Further, these recommendations are not intended to take any position on the individual or public health effects of various products or abstinence outcomes. Rather, they are intended to serve as guidelines to enhance validity, consistency, and rigor across clinical trials and provide a common set of terms to be used in dissemination. A companion paper provides updated information and recommendations on biochemical verification of abstinence.10 Taken together, the two papers provide revised guidance to the research community on how to appropriately and rigorously assess smoking abstinence among combustible tobacco users.
The objective of this paper is to provide guidance for investigators regarding measurement of tobacco abstinence outcomes in smoking cessation trials. The emphasis of the paper is on abstinence from combustible cigarette smoking, including roll-your-own cigarettes. The measures might also apply to the use of other combustible products such as cigars, little cigars, or bidis, but more research on these products is needed. The focus on combustible cigarette smoking as an essential treatment outcome in tobacco treatment research is based on incontrovertible evidence of the harmful health effects of cigarette smoking.11 However, given important questions about the health effects of other tobacco products (e.g., HNB products) and alternative products (e.g., e-cigarettes),12–14 we acknowledge the need to consider their use in definitions of abstinence. The authors acknowledge and anticipate that these recommendations will need to be updated as the tobacco product landscape evolves and as more is understood about the risks and benefits of newer products.
To update the 2003 recommendations, in 2017 the SRNT Treatment Research Network convened a group of investigators experienced in conducting tobacco treatment clinical trials over the past several decades. To arrive at the updated recommendations, the authors reviewed the literature, although this was neither a systematic review nor a formal meta-analysis. In particular, three articles that recommended cessation treatment outcomes were examined: the recommendations of the prior SRNT Workgroup,1 the Russell Standard,15 and the revised Russell Standard.16
All three papers recommended the use of a prolonged abstinence outcome, allowing an initial 2-week grace period. The recommended abstinence duration was 6 or 12 months. The SRNT Workgroup and Revised Russell Standard also suggested using point prevalence as a secondary abstinence outcome, preferring 7-day point prevalence to 30-day point prevalence, although acknowledging that there may be special cases where 30-day point prevalence is preferred, such as in adolescents or other groups who may be using tobacco with lower frequency.17 The two Russell Standard guidelines recommended biochemically confirmed abstinence (expired carbon monoxide [CO] in West15 and cotinine in Cheung16; see Benowitz et al.’s companion paper10 for the current recommendations regarding biochemical verification). Finally, the authors considered the practical issues investigators face in the field. They held a series of conference calls and an in-person meeting at the 2018 SRNT Annual Meeting in Baltimore, MD. Additional experts in the field then reviewed the paper and its recommendations (see Acknowledgements) and the writing group addressed their comments prior to submission for peer review.
Defining Abstinence
Consistent definition of abstinence in smoking cessation clinical trials is critical to both comparing results across clinical trials and facilitating the merging of data for meta-analyses. Criteria for determining abstinence should include three dimensions: (1) specification of the products included and excluded in the investigators’ definition of abstinence; (2) measures of abstinence (including, potentially, a grace period and tolerance of any smoking after the quit day); and (3) duration of abstinence, which includes identifying the starting point of the timing of assessment (e.g., target quit day for aid-to-cessation trials, onset of the intervention for cessation-induction trials [see p. 10 for definitions]). Investigators should also decide whether the chosen definition of abstinence will include biochemical verification (see companion paper10 for further discussion). These updated guidelines address all three domains of the definition and measurement of abstinence, discuss old and new tools available to measure abstinence, and provide recommendations.
Specification of Products
Specification of the products from which participants are required to abstain to meet the definition of abstinence is critical to rigorous assessment. Products should be decided a priori and reported in dissemination products (e.g., publications, presentations). The advent of new products such as e-cigarettes and HNB products, the lower cost of some products, and the popularity of other products, such as little cigars in the United States (that mimic combustible cigarettes) have accelerated the trend for tobacco users to use multiple products concurrently.9,18 Therefore, regardless of the products included in the definition of abstinence, it is important to document use of other tobacco products (e.g., HNB products, little cigars) or alternative products (e.g., e-cigarettes) in cigarette smoking cessation trials for a variety of reasons. First, use of other tobacco products may eliminate or moderate the health benefits of smoking cessation (e.g., switching from cigarettes to little cigars would still present the risks of combustible product use, and short- and long-term health effects of new products such as e-cigarettes and HNB products are not completely understood). Second, use of such products may influence smoking cessation outcomes; they may either facilitate abstinence (e.g., reduce craving) or undermine abstinence (e.g., by maintaining nicotine dependence or providing cues to smoke) from combustible cigarettes and thereby influence the estimation of treatment effects. Finally, use of noncigarette tobacco products or alternative products may affect the ability to validate abstinence biochemically. For instance, use of other combustible tobacco products (or other combusted substances) would alter levels of exhaled carbon monoxide (CO) and use of any product that delivers nicotine (including nicotine replacement therapy [NRT]) would alter cotinine results (see Benowitz et al.10 for further discussion).
Historically, the most common definition of abstinence in smoking cessation treatment studies has been no use of combustible cigarettes, without considering use of other tobacco or alternative products. However, with the rise in the use of other popular combustible products (e.g., cigars, little cigars, hookah) in countries such as the United States and England,19,20 an important definition of abstinence is abstinence from all combustible tobacco products. We note that this may create challenges for comparisons with previous studies where combustible cigarettes were the only product included in the abstinence definition. If this is a concern, investigators should assess abstinence from individual products. A second definition is abstinence from all combustible and smokeless tobacco products such as snus, chew (e.g., moist snuff, chewing tobacco), dissolvable tobacco, or HNB products. Finally, investigators could consider a third definition of abstinence that includes abstinence from all tobacco products and alternative products (e.g., e-cigarettes with or without nicotine). These additional outcomes could be explored in more thorough analyses to further understand the impact of various products on abstinence rates. It is important to note that our proposed definitions of abstinence for smoking cessation trials focus on tobacco and do not include abstinence from other combustible substances (e.g., marijuana), although use of other combustible substances may influence the choice of biochemical verification assay (see Benowitz et al., companion paper10). Further, we recommend that use of Food and Drug Administration (FDA) approved nicotine pharmacotherapies (e.g., nicotine patch, gum, lozenge) be documented but not be considered a violation of abstinence.
Including separate assessment of use of combustible, smokeless, and alternative products provides flexibility for data analysis so that a variety of outcomes can be considered. Assessment of other products typically occurs after assessment of cigarette use. For instance, “Have you smoked a cigarette, even a puff, in the last 7 days? Have you used any other combustible tobacco products, like cigars, little cigars, bidis, blunts, or hookah, in the last 7 days? Have you used any smokeless tobacco products, like smokeless tobacco, chew or snus, or HNB products in the last 7 days? Have you used any alternative products in the last 7 days, such as e-cigarettes? If yes, which products (provide list)?” Products continue to evolve, so investigators should use current, validated measures (e.g., PATH measures21 and recommended e-cigarette items22) whenever possible.
Below are brief descriptions and examples of other tobacco and alternative products. We include brief descriptions of how to quantify use. This can be challenging because some of these products are less likely than combusted cigarettes to be used daily (e.g., cigars), they might be shared (e.g., hookah), and specification of the amount may be less precise than counting cigarettes (e.g., smokeless tobacco). Therefore, quantifying both frequency and amount used may be particularly relevant for these tobacco and alternative products.
Noncigarette Combustible Products
Like cigarettes, these products involve combustion of tobacco leaves and inhalation of the smoke generated. This product group includes large cigars, little cigars, cigarillos, blunts, and bidis.20 However, quantifying use of these products can be difficult because some products are smoked intermittently (nondaily), the quantity of tobacco smoked varies widely from product to product and day to day, and users may limit the depth of inhalation, compared to cigarette smoking.21 Typical measurement of these products is similar to measurement of cigarette use—a count of products used and number of days in the past month.
Hookah (Waterpipe, Nargile, etc.)
A hookah is a waterpipe used to smoke combusted tobacco (or other substances). The pipe consists of one or more long flexible stems connected to a container of water or other liquid through which heated tobacco smoke is cooled and inhaled. Challenges to quantifying hookah exposure include that hookah may be smoked intermittently (e.g. nondaily), the amount of tobacco used at each session may vary as hookah tobacco is often shared with others, and users may limit the depth of inhalation.23 Therefore, there is no standard assessment strategy for quantifying hookah use. One approach for investigators to consider is documenting the number of days of hookah use in the last 30 days or in the last week.9
Smokeless Tobacco Products
A wide variety of smokeless products is available around the world, including single oral products (e.g., dipping tobacco, chewing tobacco, snus, dissolvable tobacco),24 oral tobacco mixed with other nontobacco products (e.g., gutka, naswar, toombak, tobacco paste, dic),25 and nasal products (e.g., snuff). These products vary widely in harm and dependence potential. Measurement of use may include counting the number of cans, pouches, or dips used daily/weekly and the nicotine content of cans or pouches, although this tobacco is sold loose in many lower- and middle-income countries.21
HNB Tobacco Products
HNB tobacco products are tobacco products that heat tobacco to a lower temperature than conventional cigarettes and produce an aerosol that contains nicotine and other chemicals. At this point, there is controversy over whether they are truly “smokeless.” 26–28 Investigators will need to determine whether they consider HNB combustible or smokeless products in their definition of abstinence until the data clarify this controversy. HNB devices like iQOS (Philip Morris International), Eclipse (Reynolds American), PLOOM (Japan Tobacco International), and Glo (British American Tobacco) are currently available in over 30 countries, including Japan, Switzerland, Russia, Canada, South Korea, Italy, New Zealand, and the UK.29–31 The companies that manufacture iQOS, Eclipse, and Glo have recently filed applications with the U.S. FDA to bring the products to the U.S. market.31 The first of the HNB products to receive FDA authorization for sale in the United States is the iQOS device and Marlboro brand tobacco sticks.32 These products can be quantified based on the number of tobacco sticks or pods used.
E-cigarettes
E-cigarettes, an alternative product, are battery-operated devices that aerosolize a solution for inhalation. The liquid may or may not contain nicotine (or other substances); if it does contain nicotine, the nicotine concentration may vary considerably. There are different generations of e-cigarettes and a multitude of device models and e-liquids that continue to evolve, including cigalikes, tank systems, modified devices (“mods”), and pod devices. Documenting e-cigarette use in the context of smoking cessation trials is important for consistency of interpretation of results across studies. This includes, but is not limited to, assessment of quantity (e.g., number of cartridges, milliliter of e-fluid), frequency, and nicotine concentration22.
It should be noted that the classification of these products may not be stable and could change based on new data. For example, HNB products are considered by many to be smokeless products. However, this categorization has been challenged (i.e., some believe that there is a small amount of combustion28) and could change in the future. There are also hybrid products that heat a liquid and the resulting aerosol is then drawn through tobacco (e.g., BAT’s Glo iFuse). Researchers should anticipate that new products will emerge on the market and plan to capture their use in an extant category or consider whether a new category or assessment item needs to be developed.
Finally, it is important to consider how users classify the product, rather than how researchers or regulators classify the product. For example, when asked about e-cigarettes, young people may not report JUUL use as they do not consider it to be an e-cigarette.33 Survey items querying e-cigarette use should include alternative names (such as e-cigs, vapes, vape pens, mods, and JUUL), use product images, or participants can be asked to bring in their products to improve the accuracy of product assessment22.
Measures of Abstinence
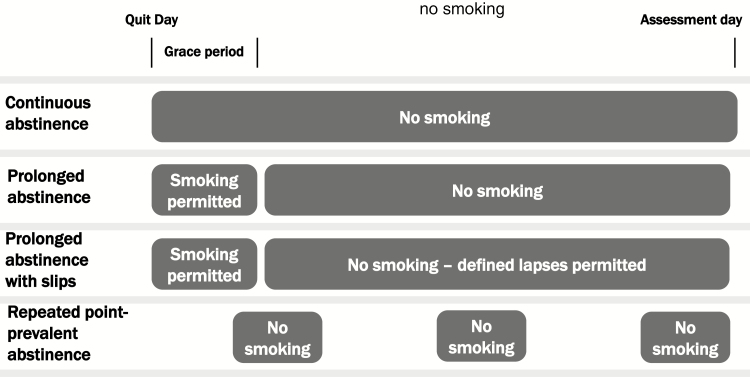
Following specification of products included in the abstinence definition, specific outcome measures should be selected a priori. There are a variety of outcomes to consider. Abstinence outcomes can be categorized as either point-prevalence abstinence (e.g., abstaining in the last 7 or 30 days), which is considered a “snapshot”, or extended abstinence (e.g., no smoking over a period of months). Options for defining extended abstinence include repeated point prevalence, prolonged, and continuous abstinence. Definitions vary in how they address the realities of the quitting process, including two principal features: (1) not all smokers are able to quit completely on their target quit day (TQD) and (2) some smokers who maintain long-term abstinence may have lapses (slips). Some definitions of extended abstinence outcomes allow participants an initial grace period (typically 1–2 weeks) to achieve abstinence if they are unable to quit on their TQD. Some definitions of extended abstinence also allow for a few lapses (but not relapse). Figure 1 compares the models of extended abstinence measures. Table 1 defines different types of abstinence with respect to duration, slips, and grace periods and Table 2 provides standardized outcome questions for cigarette abstinence based on the type of abstinence measure.
Figure 1.
Measures of extended abstinence. In this figure, “No smoking” may be defined by the product options outlined in Table 3.
Table 1.
Key Definitions
| Term | Definition |
|---|---|
| Outcomes related to abstinence | |
| Quit attempt/quit episode | A period of intentional abstinence (typically defined as >24 hours).2 Investigators should specify the duration of a quit attempt. |
| Abstinence | A period in which there is no use of combustible cigarettes. Investigators should clearly state whether this also means no use of any other combustible, smokeless, or alternative products. |
| Point-prevalence abstinence | Complete abstinence (“not even a puff”) during a designated time period (e.g., 7 or 30 d) prior to assessment |
| Repeated point-prevalence abstinence | Point-prevalence abstinence at each consecutive assessment point (e.g., 4, 8, and 12 wk). |
| Grace period | Initial period following the TQD during which smoking is permitted (e.g., 1–2 wk) |
| Continuous abstinence | Complete abstinence (“not even a puff”) beginning on the TQD (i.e., with no grace period) and lasting until the assessment |
| Prolonged abstinence | Complete abstinence (“not even a puff”) after an initial grace period; also known as sustained abstinence |
| Prolonged abstinence with lapses | Prolonged abstinence after a grace period, but some smoking is allowed (e.g., no more than 5 cigarettes15; fewer than seven consecutive days of smoking) |
| Outcomes related to resuming tobacco use after a period of abstinence | |
| Lapse (slip) | A smoking event following initial cessation that does not meet definition of relapse |
| Relapse | A return to regular smoking following a period of abstinence (i.e., seven consecutive days of smoking) |
| Types of smoking cessation trials | |
| Aid-to-cessation trials | A study design to test interventions for smokers ready to quit; TQD is set by study protocol, timing of abstinence assessments are based on the TQD, and end of treatment is specified |
| Cessation-induction trials | A study design to test treatments for all smokers, whether they are ready to quit or not (e.g., physician advice and smoking cessation consults for all inpatients who smoke), and variable quit day studies. These studies typically do not have a specified TQD and may not have a clear treatment end. |
TQD = target quit date.
Table 2.
Assessment Items
| Abstinence Assessment Items | |
|---|---|
| Point-prevalence abstinence | In the last [7 or 30] days, have you smoked a cigarette, even a puff? |
| Repeated point-prevalence abstinence | In the last [7 or 30] days, have you smoked a cigarette, even a puff? This assessment is repeated over time (e.g., 4, 8, 12, and 26 wk). |
| Continuous abstinence | Have you smoked a cigarette, even a puff, since the target quit day? |
| Prolonged abstinence | Since [end of grace period], have you smoked a cigarette, even a puff? Grace periods typically range from 1 to 2 wk. |
| Prolonged abstinence with slips | After [the end of grace period] have you smoked more than 5 cigarettes? OR How many cigarettes have you smoked since [end of the grace period]? Grace periods typically range from 1 to 2 weeks. |
When considering which abstinence measures to select, it is important for investigators to consider what type of smoking cessation trial is being conducted. Proof of concept, efficacy, and effectiveness trials are typically considered aid-to-cessation trials and include a predetermined TQD. Pragmatic and population-level trials often include participants who are not yet ready to quit and can be categorized as cessation-induction trials because they are designed to increase quit attempts and/or abstinence (e.g., trials of provider advice or smoking cessation counseling or cessation pharmacotherapy for all inpatients or all clinic patients, typically including motivational protocols for those not ready to quit). In such studies, participants may be encouraged to set a TQD, but the timing may vary greatly across participants and, therefore, TQD is not a useful standardized milestone from which to measure abstinence. Thus, the type of trial will influence how and when abstinence is assessed (see below). It should also be noted that a serious quit attempt is defined as a period of intentional abstinence from smoking, typically lasting at least 24 hours34–36 (see Table 1). However, many reported quit attempts do not meet the 24-hour cutoff.37 Alternatively, some researchers define serious quit attempts as attempts where the smoker decided to try to make sure they never smoked again.36,38
Point-prevalence abstinence from cigarettes is defined as no smoking (“not even a puff”) during a specific prior interval (usually the last 7 or 30 days). Point-prevalence abstinence is sometimes considered to be a less rigorous measure than prolonged or continuous abstinence because it is less predictive of life-long abstinence.1,39 However, it has several advantages as an outcome measure. First, it is less subject to recall bias because it assesses a shorter timeframe. Second, it is less likely to be influenced by missing data such as when a participant is temporarily difficult to schedule for an assessment, but then returns to treatment or follow-up.40 Third, biochemical verification is more likely to be valid with 7-day point-prevalence abstinence than prolonged or continuous abstinence (see Benowitz et al.10). Finally, point-prevalence abstinence will more accurately reflect abstinence status for smokers who do not quit immediately, or have one or more early lapses, but then go on to refrain from smoking in the long term.40 This may be important in studies of treatments that help lapsing smokers become abstinent, even late in the treatment period (e.g., long-term NRT). One limitation of point-prevalence abstinence is that cessation starting later in the study period may not be associated with treatment, especially if abstinence is not established until treatment is over.
The U.S. Public Health Service Guidelines for Treating Tobacco Use and Dependence41 used 7-day point prevalence as the primary outcome variable for reporting evidence of efficacy of various interventions. The justification for this decision was that point-prevalence abstinence was the most commonly reported outcome measure in the studies analyzed. A PubMed search undertaken in September 2018 (limiting results to the past 5 years) resulted in 314 citations associated with the key words “point prevalence” and “abstinence” compared to 259 PubMed citations associated with “prolonged” or “continuous” and “abstinence”.
Multiple definitions have been used for extended abstinence (e.g., continuous abstinence, prolonged abstinence), which is anchored to the initiation of treatment/intervention and, thus, allows investigators to draw stronger causal inferences regarding treatment effects compared to point-prevalence abstinence, which is anchored to the endpoint. Continuous abstinence is defined as no smoking at all after the quit date and is conceptually the most rigorous definition of extended abstinence because it does not allow for any lapses at all, even early in the treatment process. It is also straightforward to assess with a single question with a yes/no response (i.e., “Have you smoked at all, even a puff, since your quit day?”). However, this measure may be unduly conservative (e.g., some smokers may take a few days to establish abstinence or may have a single slip) and, therefore, may underestimate treatment effects.
Prolonged abstinence (sometimes referred to as sustained abstinence) is defined as no smoking following an initial grace period (e.g., the first 7 days after the TQD or after treatment initiation). Many pharmaceutical industry trials allow a 4-week grace period42–44 and then examine continuous abstinence from week 9 to 12.45 This initial grace period gives smokers a few days to establish abstinence and allows treatment adjustments that reflect practical issues in clinical care (e.g., medication dose adjustment), in turn making prolonged abstinence a more realistic definition to use in clinical trials. The Cochrane Tobacco Addiction Group (TAG) prefers to use prolonged abstinence over point-prevalence abstinence for the primary outcome in their meta-analyses because they believe it to be a more rigorous measure.46–48 However, it is important to note that using prolonged abstinence as the outcome in meta-analyses can be challenging as both the duration of abstinence and the length of the grace period may vary widely across studies.
At any given time point, prolonged abstinence yields equal or lower abstinence rates than point-prevalence abstinence. In practice, prolonged abstinence rates tend to be 50–60% of point-prevalence abstinence rates in the same trial.46 However, three reviews have found that prolonged abstinence is highly correlated with point-prevalence abstinence (r = 0.88)40 and the two measures provide very similar odds ratios for treatment efficacy,40,49 although absolute cessation rates differ. While the two types of abstinence outcomes are strongly related, there are other considerations when choosing an outcome. For instance, one of the disadvantages of prolonged abstinence is that it cannot be biochemically verified unless there are repeated biochemical assays, although strategies such as “bogus pipeline” studies have shown that telling participants that there will be biochemical verification (e.g., via exhaled CO) can increase the validity of self-report.50
Some definitions of prolonged abstinence tolerate lapses (but not relapses) to smoking (see Table 1). Lapses, also known as “slips,” can be defined by the pattern of smoking, the amount of smoking, or a combination of the two. For example, the “Russell Standard” abstinence definition, proposed by West and colleagues,15 is defined as self-report of smoking no more than five cigarettes following an initial grace period. This definition has been used in some trials but has not been widely adopted.16 Another common prolonged abstinence definition is abstinence that permits smoking on fewer than seven consecutive days and not in two consecutive weeks (i.e., not a relapse), although some investigators find it difficult to collect these data in practice.
Some definitions allow lapses after the grace period, but research shows that any lapse is highly predictive of a second lapse and a return to regular smoking,51–55 possibly due to reduced self-efficacy.56 Studies have shown that smoking on the quit day, even just a few cigarettes, and within the first 2 weeks of the quit attempt are highly predictive of smoking 6 months postquit.52,53,55,57 This suggests that the majority of smokers who smoke during the grace period will smoke after the grace period. Any abstinence definition that permits lapses should specify, a priori, the number and timing of the lapses that are permitted to enhance rigor.
Another less common definition is repeated point-prevalence abstinence, defined as point-prevalence abstinence at each consecutive assessment point (e.g., 4, 8, and 24 weeks). This definition has the advantage of avoiding recall bias and can be measured over an extended (or even indefinite) period of time.58,59 However, unlike prolonged abstinence, it does not assess daily smoking and some time periods are unlikely to be included in the assessments (e.g., examining 7-day point-prevalence abstinence at 3 months and 6 months will not account for 2 and half months of possible interval smoking).
Other abstinence outcomes that have been used to examine the smoking cessation process include latency to first lapse, latency to relapse or return to regular smoking, number of days of smoking, and longest duration of abstinence.56,60 Although not commonly used as primary outcome measures in smoking cessation trials, these outcomes are becoming more useful as technology enhances the capacity to collect daily use data and deliver just-in-time interventions that could be triggered by an initial lapse.61,62 Investigators may also want to consider reduction in cigarettes per day as an outcome as it may be a prelude to complete cessation.63 However, health benefits from smoking reduction may be limited by compensatory smoking64 and lack of durability, compromising the validity of smoking reduction as an outcome measure.
Duration of Abstinence
The scientific goals of any given study, as well as the study design, are key to determining the optimal duration of abstinence to measure, including when abstinence begins and how long it lasts. Study design typically dictates the abstinence start date (i.e., the anchor date). Smoking cessation study designs range from proof-of-concept studies designed to demonstrate whether a treatment is promising (i.e., produces early effects that might carry over to longer-term effects; e.g., 65), to highly controlled efficacy aid-to-cessation trials, to effectiveness trials in “real-world” settings,66 to very pragmatic aid-to-cessation or cessation-induction trials, in which there are few exclusion criteria and providers (rather than researchers) deliver interventions, with the focus on clinically meaningful primary outcomes. For aid-to-cessation trials, the timing of abstinence assessment is generally straightforward and the definition of abstinence is anchored to the TQD. For cessation-induction trials, abstinence may be tied to the TQD; however, it is more common practice to anchor on the date of intervention initiation (e.g., the date participants receive their first motivational intervention or receive their first medications in the mail). Investigators can also attempt to identify a participant’s identified quit day for variable quit date studies. This would allow investigators to measure floating prolonged abstinence, which defines abstinence starting from the date of the successful quit attempt rather than tying it to a fixed follow-up point or fixed target quit date.67 Such a definition is useful for cessation-induction trials (such as those that include all smokers, whether they are interested in quitting or not) or longitudinal care trials, in which smokers who relapse are encouraged to quit again. Use of treatment initiation date may also be relevant for effectiveness trials such as quitline or health care interventions in which treatment is delivered without setting a trial-determined quit date (e.g., the date they were referred to a quitline).
Abstinence assessments can occur during treatment, at the end of treatment, and after completion of treatment. Assessments during treatment and at the end of treatment have the advantage of testing short-term efficacy, which may be important for proof-of-concept studies. They are also practical because study duration will be shorter, requiring fewer resources. Research has also shown that the majority of relapses happen in the first weeks of a quit attempt and that this is when the strongest treatment effects are evident.68,69 It is ideal to include measurement at the end of treatment because there is potential to prolong some forms of treatment to maintain efficacy, consistent with other longitudinal care interventions common in medicine. Continued assessment following the end of treatment permits assessment of durability of the treatment effect or the health benefit of the intervention. Challenges to the feasibility of this approach include that some treatments have no set end of treatment (e.g., access to a smoking cessation web site), data collection takes longer to complete, and it may be harder to reach participants, especially among specific populations (e.g., smokers with substance use disorders, homeless smokers). Abstinence is generally measured at 6 or 12 months after a TQD (in an aid-to-cessation trial) or at a specified point after treatment initiation (in a cessation-induction trial). Six months post-TQD is the standard used by most meta-analyses (e.g., 46–48). Ultimately, the duration of abstinence is determined by the scientific objectives of the study (e.g., a shorter duration may be appropriate for pilot or feasibility studies) and needs to be specified a priori.
Tools to Measure Abstinence
After choosing criteria of abstinence (the products from which participants must abstain, the abstinence outcome, and the duration of abstinence), it is critical to develop a rigorous protocol for assessing smoking behavior. Protocols should be publicly registered (e.g., registered at one of the sites listed at www.hhs.gov/ohrp/international/clinical-trial-registries/index.html) to provide access to details about abstinence measures. Fundamental issues related to the conduct of clinical trials to consider include: maintenance of masking of treatment condition among data collectors whenever possible and maintenance of separate tobacco treatment delivery and data collection study team personnel (see CONSORT70 for additional information). Separate treatment and assessment staff will help avoid participants’ potential misrepresentation of smoking status to coaches or counselors with whom they have developed a treatment relationship. Finally, investigators should prespecify methods for biochemically verifying self-reported abstinence and whether such assessment is warranted for the trial (see Benowitz et al.10) and management of missing abstinence outcome data.
Investigators traditionally use retrospective survey tools to assess participants’ reports of smoking behavior aggregated over a recent interval (e.g., “Have you smoked a cigarette, even a puff, in the last 30 days?”). Investigators may select among various data collection methods to assess self-reported smoking status including in-person visits, telephone surveys, online surveys, or smart phone technology.
Some methods can mitigate the limitations of recall bias, such as interactive voice response (IVR), ecological momentary assessment (EMA), and the Time-Line Follow Back (TLFB) method. Each tool has advantages and disadvantages, both with respect to response rates and accuracy.71,72 IVR, EMA, and TLFB permit collection of more granular smoking data. These data might include whether the participant smoked or not on each day during the interval being assessed, the number of cigarettes smoked per day, or other tobacco products used each day. Treatment use, such as the amount of NRT, can also be measured by these methods. IVR and EMA provide a clear time stamp for the data and are subject to the least amount of recall bias. The TLFB was originally developed to measure alcohol treatment outcomes73 but has been applied to tobacco use for interval durations up to 6 months.71,74 Recall of smoking over periods up to 30 days appear accurate but recall over longer periods is less reliable.72 For example, smokers typically fail to recall many quit attempts.75,76
Data collected via IVR, EMA, and TLFB provide investigators the ability to: identify initial cessation, lapses, and relapses; use different definitions of abstinence (e.g., real-time assessment might be more effective in tracking the use of all products included in the selected abstinence definition); measure the total number of days of abstinence; and assess smoking reduction. These data can also be helpful in understanding the mechanisms underlying treatment effects.
Recommendations
Based on the changes in the tobacco dependence treatment environment and current research, the authors have developed recommendations regarding the definition of abstinence, abstinence outcomes, and abstinence duration (see Table 3). They continue to include reporting abstinence from all combustible tobacco products for smoking cessation trials. However, in the current expanded tobacco product environment, a methodologically rigorous assessment includes measurement of abstinence from smokeless products and from alternative products (e.g., e-cigarettes with and without nicotine). The authors had strong consensus on the need to measure both abstinence from all combustible tobacco products and abstinence from other tobacco products and alternative tobacco products. Strengths of defining abstinence as abstinence from all combustible products include capacity for comparison to extant studies, relative ease of collecting information, use of other tobacco products and alternative tobacco products as cessation aids in some studies, and strong scientific evidence about the harmful health effects of combustible tobacco. The authors also agreed on the importance of collecting and reporting on data about other product use, which are needed to fill gaps in knowledge about potential risks and benefits.
Table 3.
Recommendations for Measures of Abstinence
| 1.Abstinence product definition.* We recommend using definition (a) for all smoking cessation trials and collecting data necessary to evaluate definitions (b) and (c): a.Abstinence from all combustible tobacco products. Abstinence from combustible tobacco products but “allowing” the use of noncombustible products and alternative products. b.Abstinence from combustible tobacco products and smokeless tobacco products. Abstinence from combustible and smokeless tobacco products but “allowing” use of alternative products. c.Abstinence from combustible tobacco products, smokeless tobacco products, and alternative products. |
| 2.Abstinence outcome measure. We recommend reporting (a) and (b): a.Point-prevalence abstinence (7 or 30 d). This is the preferred measure when using biochemical verification. b.Prolonged abstinence. This includes a grace period and may include some tolerance of lapses that reflect the realities of stopping smoking. |
| 3.Abstinence duration. We recommend reporting abstinence at multiple timepoints. Minimum data collection should include: a.End of treatment. This is appropriate if there is a clear end of treatment time point; however, some cessation-induction trials may manipulate treatment exposure or may not have a clear treatment termination date. b.A minimum of 3 mo after the end of treatment. This will provide an assessment of whether the treatment effect persists after treatment termination. c.A minimum of 6 mo post-TQD or posttreatment initiation. 6 mo post-TQD is the standard used by most meta-analyses. |
*All definitions permit the use of Food and Drug Administration approved pharmacotherapies that contain nicotine.
We recommend assessment of both point prevalence and prolonged abstinence and assessing abstinence at the end of treatment and a minimum of 3 months after the end of treatment. Whether point prevalence or prolonged abstinence is deemed the primary outcome should be based on study objectives and hypotheses.
Regardless of whether the definitions of prolonged abstinence allow or do not allow slips, we recommend investigators collect secondary outcome data on latency to first lapse and number of cigarettes smoked per day, especially on the lapse day, as this is predictive of relapse.52,60,77 If investigators are interested in the cessation process per se, rather than abstinence outcomes, we recommend using such data (i.e., initial lapse date) to conduct survival analyses to assess latency to lapse and relapse and the use of milestone analyses.60,78 Investigators may also want to consider other outcomes related to the cessation process, such as latency to set a TQD. The SRNT Biochemical Verification workgroup also recommends biochemical verification when feasible.10
In conclusion, the evolution of tobacco products and use patterns has prompted a reexamination of how investigators define, measure, and report abstinence, the key outcome of smoking cessation treatment trials. This paper provides investigators with guidance based on empirical and theoretical literature as well as pragmatic concerns. It is our hope that this guidance will increase the rigor and reproducibility of smoking cessation research findings and provide important insights into the cessation process. The recommendations will require reexamination periodically to ensure that they are consistent with the extant literature and evolution of tobacco and alternative products.
Acknowledgements
We would like to thank the six scientists who reviewed this paper prior to submission and offered their expert comments and suggestions: Tim Baker, Neal Benowitz, Dorothy Hatsukami, Jennifer McClure, Robin Mermelstein, and Robert West. We would also like to thank John R. Hughes for his consultation on this project and Leonie Brose and Andrea Weinberger for reviewing a draft of the paper on behalf of the SRNT Treatment Research Network Advisory Committee and Jasjit Ahluwalia and Suzanne Colby for reviewing a draft of the paper on behalf of the SRNT Board. This study is sponsored by the Society for Research on Nicotine and Tobacco (SRNT) Treatment Research Network
Contributor Information
Megan E Piper, Center for Tobacco Research and Intervention of Wisconsin, Department of Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, WI.
Christopher Bullen, National Institute for Health Innovation, School of Population Health, University of Auckland, Auckland, New Zealand.
Suchitra Krishnan-Sarin, Department of Psychiatry, Yale University School of Medicine, New Haven, CT.
Nancy A Rigotti, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Marc L Steinberg, Division of Addiction Psychiatry, Rutgers University, New Brunswick, NJ.
Joanna M Streck, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Anne M Joseph, Department of Medicine, University of Minnesota, Minneapolis, MN.
Funding
None noted.
Declaration of Interests
The authors have no conflicts of interest to declare.
References
- 1. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 2. Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Classification and assessment of smoking behavior. Health Psychol. 1986;5(suppl):3–11. [PubMed] [Google Scholar]
- 3. Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: an increasingly prevalent pattern. Arch Intern Med. 2009;169(19):1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotz D, Fidler J, West R. Very low rate and light smokers: smoking patterns and cessation-related behaviour in England, 2006-11. Addiction. 2012;107(5):995–1002. [DOI] [PubMed] [Google Scholar]
- 5. Lund M, Lund KE, Kvaavik E. Hardcore smokers in Norway 1996-2009. Nicotine Tob Res. 2011;13(11):1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Organizacion Panamericana de la Salud, Instituto Nacional de Salud Publica (MX). La encuesta global de tabaquismo en adultos. Mexico 2009. 2010. www.conadic.salud.gob.mx/pdfs/pie/GATS_2009.pdf. Accessed December 21, 2018.
- 7. Drope J, Schluger N, Cahn Z, et al. The Tobacco Atlas. 2018. https://files.tobaccoatlas.org/wp-content/uploads/2018/03/TobaccoAtlas_6thEdition_LoRes.pdf. Accessed June 19, 2019. [Google Scholar]
- 8. Sung HY, Wang Y, Yao T, Lightwood J, Max W. Polytobacco use and nicotine dependence symptoms among US adults, 2012-2014. Nicotine Tob Res. 2018;20(suppl_1):S88–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benowitz NL, Bernert JT, Jacob P, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 12. National Academy of Sciences Engineering and Medicine. Public health consequences of e-cigarettes. 2018. http://nationalacademies.org/hmd/Reports/2018/public-health-consequences-of-e-cigarettes.aspx. Accessed June, 19 2019.
- 13. World Health Organization. Tobacco: deadly in any form or disguise. 2006. www.who.int/tobacco/communications/events/wntd/2006/Tfi_Rapport.pdf. Accessed December 21, 2018.
- 14. Prignot JJ, Sasco AJ, Poulet E, Gupta PC, Aditama TY. Alternative forms of tobacco use. Int J Tuberc Lung Dis. 2008;12(7):718–727. [PubMed] [Google Scholar]
- 15. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303. [DOI] [PubMed] [Google Scholar]
- 16. Cheung KL, de Ruijter D, Hiligsmann M, et al. Exploring consensus on how to measure smoking cessation. A Delphi study. BMC Public Health. 2017;17(1):890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mermelstein R, Colby SM, Patten C, et al. Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine Tob Res. 2002;4(4):395–403. [DOI] [PubMed] [Google Scholar]
- 18. Richardson A, Xiao H, Vallone DM. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob Res. 2012;14(8):927–932. [DOI] [PubMed] [Google Scholar]
- 19. Maziak W, Ward KD, Afifi Soweid RA, Eissenberg T. Tobacco smoking using a waterpipe: a re-emerging strain in a global epidemic. Tob Control. 2004;13(4):327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Connor RJ. Non-cigarette tobacco products: what have we learnt and where are we headed? Tob Control. 2012;21(2):181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the population assessment of tobacco and health (PATH) study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearson JL, Hitchman SC, Brose LS, et al. Recommended core items to assess e-cigarette use in population-based surveys. Tob Control. 2018;27(3):341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blank MD, Brown KW, Goodman RJ, Eissenberg T. An observational study of group waterpipe use in a natural environment. Nicotine Tob Res. 2014;16(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chin N, Dozier A, Quinones Z, et al. A qualitative study of tobacco use in eight economically disadvantaged Dominican Republic communities. Glob Health Promot. 2017;24(4):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zervas E, Litsiou E, Konstantopoulos K, Poulopoulos S, Katsaounou P. Physical characterization of the aerosol of an electronic cigarette: impact of refill liquids. Inhal Toxicol. 2018; 30:1–6. [DOI] [PubMed] [Google Scholar]
- 27. Davis B, Williams M, Talbot P. iQOS: evidence of pyrolysis and release of a toxicant from plastic. Tob Control. 2019;28(1):34–41. [DOI] [PubMed] [Google Scholar]
- 28. Auer R, Concha-Lozano N, Jacot-Sadowski I, Cornuz J, Berthet A. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177(7):1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tabuchi T, Gallus S, Shinozaki T, Nakaya T, Kunugita N, Colwell B. Heat-not-burn tobacco product use in Japan: its prevalence, predictors and perceived symptoms from exposure to secondhand heat-not-burn tobacco aerosol. Tob Control. 2018;27(e1):e25–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simonavicius E, McNeill A, Shahab L, Brose LS. Heat-not-burn tobacco products: a systematic literature review. Tob Control. 2018;0:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization. Heated tobacco products (HTPs) information sheet. 2017. www.who.int/tobacco/publications/prod_regulation/heated-tobacco-products/en/. Accessed June 26, 2018.
- 32. U.S. Food & Drug Administration. FDA permits sale of IQOS Tobacco Heating System through premarket tobacco product application pathway. 2019. www.fda.gov/news-events/press-announcements/fda-permits-sale-iqos-tobacco-heating-system-through-premarket-tobacco-product-application-pathway. Accessed June 19, 2019.
- 33. Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control. 2019;28(1):115–116. [DOI] [PubMed] [Google Scholar]
- 34. PhenX. Smoking quit attempts protocol. 2018. https://s.details.loinc.org/LOINC/62608-5.html?sections=Web. Accessed December 21, 2018.
- 35. Stoddard J, Delucchi K, Muñoz R, et al. Smoking cessation research via the internet: a feasibility study. J Health Commun. 2005;10(1):27–41. [DOI] [PubMed] [Google Scholar]
- 36.IARC. IARC Handbooks of Cancer Prevention, Tobacco Control, Vol. 12. Methods for Evaluating Tobacco Control Policies; 2008. [Google Scholar]
- 37. Hughes JR, Callas PW. Definition of a quit attempt: a replication test. Nicotine Tob Res. 2010;12(11):1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. West R. Feasibility of a national longitudinal study (‘The Smoking Toolkit Study’) to monitor smoking cessation and attempts at harm reduction in the UK. 2006. https://file:///C:/Users/wt2/Desktop/West%202006.pdf. Accessed December 21, 2018.
- 39. Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23–41. [DOI] [PubMed] [Google Scholar]
- 40. Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob Res. 2010;12(7):756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 42. Tonstad S, Farsang C, Klaene G, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24(10):946–955. [DOI] [PubMed] [Google Scholar]
- 43. Tønnesen P, Tonstad S, Hjalmarson A, et al. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med. 2003;254(2):184–192. [DOI] [PubMed] [Google Scholar]
- 44. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568. [DOI] [PubMed] [Google Scholar]
- 45. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
- 46. Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2015;10:CD009670. [DOI] [PubMed] [Google Scholar]
- 47. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor GMJ, Dalili MN, Semwal M, Civljak M, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2017;9:CD007078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Velicer WF, Prochaska JO. A comparison of four self-report smoking cessation outcome measures. Addict Behav. 2004;29(1):51–60. [DOI] [PubMed] [Google Scholar]
- 50. Roese NJ, Jamieson DW. Twenty years of bogus pipeline research: a critical review and meta-analysis. Psychol Bull. 1993;114:363–375. [Google Scholar]
- 51. Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–379. [DOI] [PubMed] [Google Scholar]
- 52. Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. [DOI] [PubMed] [Google Scholar]
- 53. Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch Intern Med. 1997;157(3):335–340. [PubMed] [Google Scholar]
- 54. Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addict Behav. 1990;15(2):105–114. [DOI] [PubMed] [Google Scholar]
- 55. Nides MA, Rakos RF, Gonzales D, et al. Predictors of initial smoking cessation and relapse through the first 2 years of the lung health study. J Consult Clin Psychol. 1995;63(1):60–69. [DOI] [PubMed] [Google Scholar]
- 56. Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J Abnorm Psychol. 2012;121(1):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yeh VM, McCarthy DE, Baker TB. An ecological momentary assessment analysis of prequit markers for smoking-cessation failure. Exp Clin Psychopharmacol. 2012;20(6):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cropsey KL, Jackson DO, Hale GJ, Carpenter MJ, Stitzer ML. Impact of self-initiated pre-quit smoking reduction on cessation rates: results of a clinical trial of smoking cessation among female prisoners. Addict Behav. 2011;36(1-2):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shiffman S, Scharf DM, Shadel WG, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74(2):276–285. [DOI] [PubMed] [Google Scholar]
- 61. Nahum-Shani I, Hekler EB, Spruijt-Metz D. Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychol. 2015;34S:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-Time Adaptive Interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med. 2018;52(6):446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med. 2015;13:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hatsukami DK, Joseph AM, Lesage M, et al. Developing the science base for reducing tobacco harm. Nicotine Tob Res. 2007;9(suppl 4):S537–S553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl). 2006;184(3-4):628–636. [DOI] [PubMed] [Google Scholar]
- 66. Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454–463. [DOI] [PubMed] [Google Scholar]
- 67. Aveyard P, Wang D, Connock M, Fry-Smith A, Barton P, Moore D. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res. 2009;11(5):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. [DOI] [PubMed] [Google Scholar]
- 69. Rosen LJ, Galili T, Kott J, Goodman M, Freedman LS. Diminishing benefit of smoking cessation medications during the first year: a meta-analysis of randomized controlled trials. Addiction. 2018;113(5):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Griffith SD, Shiffman S, Heitjan DF. A method comparison study of timeline followback and ecological momentary assessment of daily cigarette consumption. Nicotine Tob Res. 2009;11(11):1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychol. 2009;28(5):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. [DOI] [PubMed] [Google Scholar]
- 74. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. [DOI] [PubMed] [Google Scholar]
- 75. Berg CJ, An LC, Kirch M, et al. Failure to report attempts to quit smoking. Addict Behav. 2010;35(10):900–904. [DOI] [PubMed] [Google Scholar]
- 76. Gilpin E, Pierce JP. Measuring smoking cessation: problems with recall in the 1990 California tobacco survey. Cancer Epidemiol Biomarkers Prev. 1994;3(7):613–617. [PubMed] [Google Scholar]
- 77. Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmacology (Berl). 1992;108(4):495–500. [DOI] [PubMed] [Google Scholar]
- 78. Japuntich SJ, Leventhal AM, Piper ME, et al. Smoker characteristics and smoking-cessation milestones. Am J Prev Med. 2011;40(3):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]