Abstract
Objectives
To assess whether a single instance of low-level viraemia (LLV) is associated with the presence of drug resistance mutations (DRMs) and predicts subsequent virological failure (VF) in adults receiving ART in 30 communities participating in the Botswana Combination Prevention Project.
Methods
A total of 6078 HIV-1 C pol sequences were generated and analysed using the Stanford HIV drug resistance database. LLV was defined as plasma VL = 51–999 copies/mL and VF was defined as plasma VL ≥ 1000 copies/mL.
Results
Among 6078 people with HIV (PWH), 4443 (73%) were on ART for at least 6 months. Of the 332 persons on ART with VL > 50 copies/mL, 175 (4%) had VL ≥ 1000 copies/mL and 157 (4%) had LLV at baseline. The prevalence of any DRM was 57 (36%) and 78 (45%) in persons with LLV and VL ≥ 1000 copies/mL, respectively. Major DRMs were found in 31 (20%) with LLV and 53 (30%) with VL ≥ 1000 copies/mL (P = 0.04). Among the 135 PWH with at least one DRM, 17% had NRTI-, 35% NNRTI-, 6% PI- and 3% INSTI-associated mutations. Among the 3596 participants who were followed up, 1709 (48%) were on ART for ≥6 months at entry and had at least one subsequent VL measurement (median 29 months), 43 (3%) of whom had LLV. The OR of experiencing VF in persons with LLV at entry was 36-fold higher than in the virally suppressed group.
Conclusions
A single LLV measurement while on ART strongly predicted the risk of future VF, suggesting the use of VL > 50 copies/mL as an indication for more intensive adherence support with more frequent VL monitoring.
Introduction
The development of HIV-1 drug resistance mutations (DRMs) and inadequate adherence to ART remain ongoing barriers to sustained HIV viral load (VL) suppression in people with HIV (PWH) on ART.1 Resistance testing is important for preserving current regimens, choosing subsequent ART and predicting virological failure (VF). According to current WHO HIV treatment guidelines, VF is defined as two consecutive VLs of ≥1000 copies/mL after at least 6 months on ART.2 The U.S. Department of Health and Human Services 2019 guidelines define VF as VL > 200 copies/mL3 while the European AIDS Clinical Society (EACS) 2018 guidelines use a very strict threshold of VF as two consecutive VLs of >50 copies/mL after 6 months on treatment.4 In Botswana, the HIV treatment guidelines use a VF threshold of two consecutive HIV VLs of >400 copies/mL after at least 6 months on ART.5
The use of different VF thresholds has led to different definitions of LLV, resulting in contradictory data on the clinical significance of LLV.6 Several studies have found LLV to be associated with the emergence of MDR mutations7–10 and periods of LLV are linked with immune activation and stable CD4+ T cell counts.11–13 Furthermore, LLV strongly increases the risk of presenting with subsequent VF14 and impairing future ART options.15 Nevertheless, some studies have reported no association between very LLV (20–50 copies/mL) or LLV with negative immunological and virological consequences among individuals on ART.16,17 Recent WHO guidelines recommend maintaining ART with continued enhanced adherence counselling and repeat VL testing after 3 months among individuals with VL = 50–1000 copies/mL. Furthermore, ART switch is recommended for those receiving NNRTI-based regimens based on the clinical considerations and no adherence concerns.18 Currently, Botswana and WHO HIV treatment guidelines do not recommend drug resistance testing at LLV while the International Antiviral Society–USA panel recommend resistance testing at VL ≥ 200 copies/mL.19 Most commercial assays require HIV plasma VL > 1000 copies/mL for increased success rate and accurate results.20,21 However, there is increasing success of sequencing for lower plasma VL with in-house assays.22 In addition, proviral (integrated) DNA is also very useful in LLV,23 resulting in successful genotyping in >85% of persons with VL < 1000 copies/mL compared with 70% success with standard plasma HIV-1 RNA genotyping.24–29 Proviral DNA can also reveal previous archived DRMs.30
In Botswana, HIV drug resistance testing is only recommended for patients with two consecutive HIV VLs of ≥1000 copies/mL after at least 6 months of ART. Persons with VL = 400–999 copies/mL are not eligible for drug resistance testing. There are scanty data from sub-Saharan Africa on the prevalence of DRMs in individuals with LLV, or on whether LLV increases the risk of future VF. We thus evaluated the prevalence of LLV (using different VL thresholds for defining LLV) at a single timepoint in PWH on ART in Botswana, to determine the proportion of individuals with DRMs, stratified by HIV VL group, and to determine whether a single instance of LLV predicts subsequent VF.
Methods
Study population
Blood samples were collected from all consenting persons with HIV (regardless of ART status) aged 16–64 years who were enrolled in the Botswana Combination Prevention Project (BCPP) from 2013 to 2018 in 30 communities across central, northern and southern parts of Botswana.31 BCPP was a community-randomized trial evaluating whether a package of standard HIV prevention interventions could significantly reduce population-level HIV incidence over time, as previously described.32 The BCPP impact evaluation survey enrolled adults (16 years or older, regardless of HIV or ART status) residing in a random sample of approximately 20% of households from all 30 communities at study entry (baseline household survey, BHS) into a longitudinal cohort (annual household survey, AHS) that was followed for 30 months, as described elsewhere.33 In addition, in the 15 intervention communities, PWH who were receiving ART in local clinics were recruited to provide a one-time blood sample for HIV sequencing and metadata (but were not followed longitudinally). We included samples and data from all PWH in both the AHS cohort and from the clinics in this analysis.
Selection of study participants
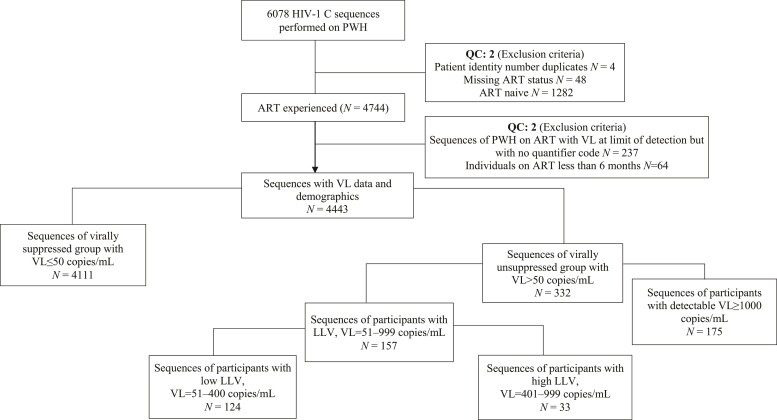
HIV-1 RNA VL was quantified using Abbott Realtime HIV-1 assay on the automated m2000rt/m2000sp system (Abbott Laboratories, Wiesbaden, Germany) with detection limits of 40 to 10 million copies/mL (we excluded from these analyses 228 participants whose entry VL assay had a lower limit of detection of 400 copies/mL). In this analysis, we include PWH who were on ART for at least 6 months, who had VL measurement at the first BCPP study visit and had available HIV sequence. These participants were classified as having VF (VL ≥ 1000 copies/mL, as per the current WHO definition)2 or LLV (VL = 51–999 copies/mL) or were virally suppressed (VL ≤ 50 copies/mL). LLV was further subcategorized into two groups: low LLV (51–400 copies/mL) and high LLV (401–999 copies/mL) (Figure 1).
Figure 1.
Flow chart showing how sequences were selected for drug resistance analysis. QC, quality control (not meeting any of inclusion criteria).
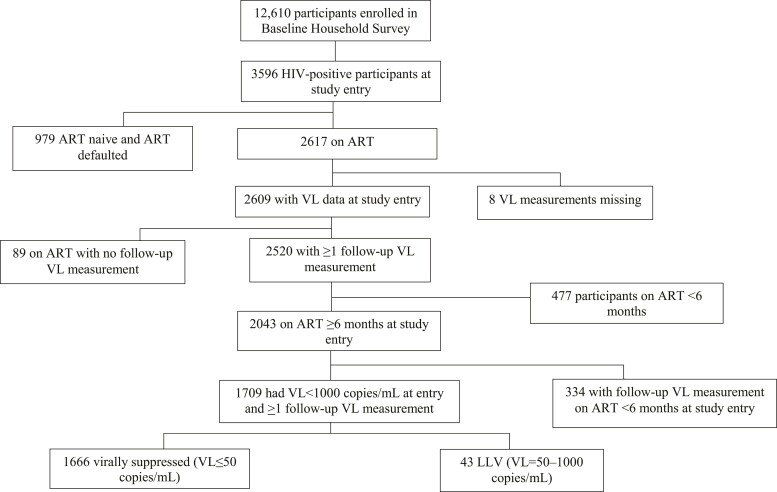
A subset of participants who either had viral suppression or LLV at enrolment and who had at least one subsequent VL measurement (through participation in the longitudinal AHS cohort) were included in the further analysis for predictive factors for VF (Figure 2).
Figure 2.
Flow chart showing how participants were selected for analysis of association of LLV at study entry with subsequent VF during the study follow-up.
Ethics
Ethics approval for the BCPP study was obtained from the Botswana Health Research and Development Committee and the CDC’s Institutional Review Boards (IRBs). BCPP study participants provided written informed consent and participants aged 16–17 years provided written informed assent with written permission from parents or guardians.
HIV viral sequences
In participants with VL > 50 copies/mL, HIV-1 C near-full length sequences were obtained by a long-range HIV genotyping technique from proviral DNA and viral plasma RNA, described elsewhere.24 More than 99% of our sequences were generated using proviral DNA from 2013–18. Failed first-round PCR was repeated at an annealing temp of 58°C instead of 62°C to increase the success. The first-round amplicon was used as a template for next-generation sequencing (NGS) and carried out using Illumina platforms MiSeq and HiSeq34 by the BioPolymers Facility at Harvard Medical School (Boston, USA; https:genome.med.harvard.edu/) in collaboration with the PANGEA HIV consortium at the Wellcome Trust Sanger Institute (Cambridge, UK; https://www.sanger.ac.uk).
HIV-1 C drug resistance analysis for participants with VL > 50 copies/mL
The HIV pol region was analysed for any DRMs associated with the highest levels of reduced susceptibility or those contributing to susceptibility to NRTIs, NNRTIs, PIs and integrase strand transfer inhibitors (INSTIs) according to the Stanford University HIV Drug Resistance Database.35 Using the Stanford HIV database, HIV DRMs with the highest level of reduced susceptibility to ARVs were classified as major DRMs. Hypermutations were screened and adjusted for at the nucleotide-position level. We excluded HIV DRMs that are associated with hypermutations in the prevalence of any DRM.
Statistical analysis
Descriptive statistics are reported as median (IQR) for continuous variables and compared among three groups using Kruskal–Wallis tests. Categorical variables are reported as percentages and compared using the chi-squared test. The prevalence of DRMs for different VL groups was estimated, with 95% CI, using the binomial exact method and compared using a comparison of proportion test.
We evaluated the association between LLV at entry and subsequent VF (and between the presence of at least one DRM at entry and subsequent VF). Only the subset of participants who were on ART for at least 6 months at enrolment and had VL measurements at two or more timepoints (at study enrolment and follow-up) were included in analyses of whether LLV predicts VF. Subsequent VF was defined as a single VL measurement of ≥1000 copies/mL at the end of the follow-up. We investigated the association between the following characteristics at entry and subsequent VF: VL group (suppressed VL versus LLV), LLV subgroups (low LLV and high LLV), the presence of major DRMs, ART regimen and duration on ART at the time of VF, sex and age. Both univariate and multivariate logistic regression models were performed. In the univariate analysis, all variables were adjusted for clustering by the community and all variables with P < 0.2 were included in the multivariate analysis. The LLV subgroup was excluded from multivariate analysis due to collinearity while those with major DRMs were excluded due to too few observations. Final multivariate models included VL group, age, sex and ART regimen and adjusted for clustering by the community. P values of <0.05 were considered statistically significant. All analyses were done using STATA version 15.
Results
The BCPP household survey enrolled 12 610 participants, 3596 of whom were HIV positive, 2901 (81%) of whom had HIV-1 sequence available. In addition, a total of 5022 PWH were enrolled from ART clinics to provide one-time blood draw, 3174 (63%) of whom had HIV-1 sequence available. Overall, 6078 HIV-1 sequences were available. In addition, 1709 PWH taking part in the AHS had a viral sequence result, at least one follow-up VL, and were on ART for at least 6 months at study entry and could hence be included in the analysis of subsequent VF.
Of the 6075 PWH who had HIV sequences available, 4443 (73%) were from persons on ART (for at least 6 months) at enrolment and were thus included in this analysis. The median age at enrolment was 41 years (IQR 35–49), 72% were female and the median duration of ART was 6.4 years (IQR 3.4–9.1). ART regimen was available for 4068 (92%), of whom 94% were on their initial regimen (60% on efavirenz-, 26.6% on nevirapine- and 7% on dolutegravir-based regimens, respectively) while 5% were on second-line therapy and 1% on salvage therapy. Among the 4443 participants on ART at enrolment who had VL data, 4111 (92%) were virally suppressed (VL ≤ 50 copies/mL) and 332 (8%) had detectable VLs of >50 copies/mL at entry. The study entry demographics of BCPP participants stratified by the VL group are shown in Table 1; female participants, older participants and those on ART for a longer period were more likely to have VLs of ≤50 copies/mL.
Table 1.
Baseline characteristics of included participants
| Variable | Total | Suppressed | LLV | VL ≥ 1000 copies/mL | P valuea |
|---|---|---|---|---|---|
| N = 4443 | N = 4111 | N = 157 | N = 175 | ||
| District (N = 4443), n (%) | |||||
| Southern | 1230 (28) | 1121 (27) | 50 (32) | 59 (34) | 0.1 |
| Central | 1805 (41) | 1690 (41) | 59 (38) | 56 (32) | |
| Northern | 1408 (32) | 1300 (32) | 48 (31) | 60 (34) | |
| Sex (N = 4442), n (%) | N = 4110 | ||||
| Male | 1239 (28) | 1126 (27) | 53 (34) | 60 (34) | 0.03 |
| Female | 3203 (72) | 2984 (73) | 104 (66) | 115 (66) | |
| Age at enrolment (years), median (IQR) | 41 (35–49) | 42 (35–49) | 37 (31–45) | 34 (26–42) | <0.001b |
| ART regimen at enrolment (N = 4068), n (%) | N = 4068 | N = 3807 | N = 134 | N = 127 | <0.001 |
| First line | |||||
| Second line | 3807 (94) | 3589 (94) | 114 (85) | 104 (82) | |
| Deep salvage | 217 (5) | 183 (5) | 16 (12) | 18 (14) | |
| 44 (1) | 35 (1) | 4 (3) | 5 (4) | ||
| ARV regimen at enrolment (N = 3940), n (%) | N = 3940 | N = 3692 | N = 127 | N = 121 | |
| EFV-based | <0.001 | ||||
| NVP-based | 2364 (60) | 2226 (60) | 76 (60) | 62 (51) | |
| DTG-based | 1049 (27) | 1010 (27) | 18 (14) | 21 (17) | |
| LPV-based | 289 (7) | 257 (7) | 14 (11) | 18 (15) | |
| 238 (6) | 199 (5) | 19 (15) | 20 (17) | ||
| ART duration at enrolment (years), median (IQR) | 6.4 (3.4–9.1) | 6.5 (3.4–9.2) | 6.5 (4.0–9.9) | 5.0 (1.0–7.8) | <0.001b |
| VL (copies/mL), median (IQR) | 40 (40–40) | 40 (40–40) | 145 (74–346) | 19 574 (4219–65 319) | <0.001b |
EFV, efavirenz; NVP, nevirapine; DTG, dolutegravir; LVP, lopinavir.
P values were found using a chi-squared test for categorical variables and Kruskal–Wallis for continuous variables.
These P values were obtained from Kruskal–Wallis.
Prevalence of LLV and DRMs at baseline
Of the 332 participants with detectable VL (>50 copies/mL) on ART at entry, 157 (47%) had VL = 51–999 copies/mL and were categorized as having LLV. Within this LLV group, 124 (79%) had low LLV and 33 (21%) had high LLV.
One hundred and thirty-five (41%) of the 332 participants had at least one DRM. The prevalence and relative pattern of mutations were similar in the two VL groups (Table 2). Although a numerically higher proportion of individuals with VL ≥ 1000 copies/mL had at least one DRM (78; 45%) compared with the LLV group (57; 36%), we did not find statistically significant differences in the prevalence of DRMs between the two different VL groups overall, nor within drug class, with the exception of NRTI-associated DRMs.
Table 2.
Prevalence of HIV DRMs stratified by VL group at enrolment
| Resistance measure | Total | LLV (51–999 copies/mL) | VL ≥ 1000 copies/mL | P value |
|---|---|---|---|---|
| N = 332 | N = 157 | N = 175 | ||
| Any mutation, n (%) | 135 (41) | 57 (36) | 78 (45) | 0.09 |
| 95% CI: 35–46 | 95% CI: 29–44 | 95% CI: 37–52 | ||
| Resistance category, n (%) | ||||
| NRTI-associated | 55 (17) | 17 (11) | 38 (22) | 0.007 |
| NNRTI-associated | 116 (35) | 50 (32) | 66 (38) | 0.18 |
| PI-associated | 19 (6) | 7 (4) | 12 (7) | 0.23 |
| INSTI-associated | 10 (3) | 6 (4) | 4 (2) | 0.28 |
Numbers in the ‘Any mutation’ row are the number of participants with at least one mutation associated with any of the four drug classes (NNRTI, NRTI, PI and INSTI), Numbers in the ‘Resistance category’ rows are the number of participants with at least one mutation in any specific drug resistance class. Comparison of proportion test was used to test the difference in the prevalence of DRMs amongst the two VL groups. Proportions with 95% CI were estimated using the binomial exact method. Note that the sum of the number of persons with individual classes of mutations will exceed the number of persons with any mutation, as some people had more than one class of DRM.
Stratifying by LLV subgroup, the prevalence of any DRM was 43 (35%) and 14 (42%) in the low LLV and high LLV subgroups, respectively. In the low LLV subgroup, NNRTI-associated mutations (observed in 32% of persons with LLV) were most common, followed by NRTI-associated mutations (observed in 10%) while PI- and INSTI-associated mutations were found in 5% and 4%, respectively. In the high LLV subgroup, the prevalence of DRMs by class was 30% NNRTI, 12% NRTI, 3% PI and 6% INSTI (Table 3).
Table 3.
Prevalence of HIV DRMs stratified by LLV group at enrolment
| Resistance measure | Total | Low LLV | High LLV | P value |
|---|---|---|---|---|
| N = 157 | N = 124 | N = 33 | ||
| Any mutation, n (%) | 57 (36) | 43 (35) | 14 (42) | 0.46 |
| 95% CI: 28–43 | 95% CI: 26–44 | 95% CI: 25–61 | ||
| Resistance category, n (%) | ||||
| NRTI-associated | 17 (11) | 13 (10) | 4 (12) | 0.74 |
| NNRTI-associated | 50 (32) | 40 (32) | 10 (30) | 0.83 |
| PI-associated | 7 (4) | 6 (5) | 1 (3) | 0.63 |
| INSTI-associated | 6 (4) | 4 (3) | 2 (6) | 0.41 |
Numbers in the ‘Any mutation’ row are the number of participants with at least one mutation associated with any of the four drug classes (NNRTI, NRTI, PI and INSTI), Numbers in the ‘Resistance category’ rows are the number of participants with at least one mutation in any specific drug resistance class. Comparison of proportion test was used to test the difference in the prevalence of DRMs amongst the two VL groups. Proportions with 95% CI were estimated using the binomial exact method. Note that the sum of the number of persons with individual classes of mutations will exceed the number of persons with any mutation, as some people had more than one class of DRM.
Eighty-four (25%) of 332 participants had at least one major DRM; 20% and 30% for LLV and VL ≥ 1000 groups, respectively (Table 4). Overall, the prevalence of at least one major DRM was significantly higher in the VL ≥ 1000 copies/mL group (30%) compared with the LLV group (30%); P = 0.04. The prevalence of at least one major DRM in each of the NNRTI and NRTI drug classes was significantly higher in the VL ≥ 1000 copies/mL group compared with the LLV group.
Table 4.
Prevalence of major HIV DRMs stratified by LLV group at enrolment
| Resistance measure | Total | LLV (51–999 copies/mL) | VL ≥ 1000 copies/mL | P value |
|---|---|---|---|---|
| (N = 332) | N = 157 | N = 175 | ||
| Any mutation, n (%) | 84 (25) | 31 (20) | 53 (30) | 0.04 |
| 95% CI: 21–30 | 95% CI: 14–27 | 95% CI: 24–38 | ||
| Resistance category, n (%) | ||||
| NRTI-associated | 54 (16) | 17 (11) | 37 (21) | 0.01 |
| NNRTI-associated | 47 (14) | 15 (10) | 32 (18) | 0.04 |
| PI-associated | 13 (4) | 7 (4) | 6 (3) | 0.6 |
| INSTI-associated | 7 (2) | 6 (4) | 1 (1) | 0.08 |
Numbers in the ‘Any mutation’ row are the number of participants with at least one mutation associated with any of the four drug classes (NNRTI, NRTI, PI and INSTI), Numbers in the ‘Resistance category’ rows are the number of participants with at least one mutation in any specific drug resistance class. Comparison of proportion test was used to test the difference in the prevalence of DRMs amongst the two VL groups. Proportions with 95% CI were estimated using the binomial exact method. Note that the sum of the number of persons with individual classes of mutations will exceed the number of persons with any mutation, as some people had more than one class of DRM.
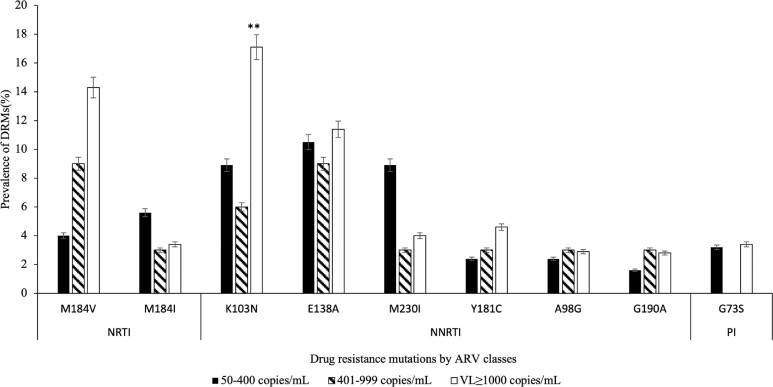
The predominant NRTI-associated mutations were M184I (4%) and M184 V (10%) (Figure 3). The NNRTI-associated mutations with prevalence of >2% were K103N (13%), E138A (11%), M230I (6%), Y181C (4%), A98G (3%) and G190A (2%). G173S (3%) was the most common PI-associated mutation. We didn’t record any INSTI-associated mutations with overall prevalence of >2% among 332 participants with VL > 50 copies/mL, although D232N was found in 2/33 (6%) of participants with high LLV, while E138K was detected in 3/124 (2%) of participants with low LLV.
Figure 3.
DRMs in adults on ART (N = 332) with detectable plasma HIV-1 RNA > 50 copies/mL, stratified by VL groups and drug classes (including only DRMs with an overall prevalence of >2%). ARV, antiretroviral. Note that prevalence of all INSTI-associated mutations was less than 2%. **Statistically higher compared with the whole LLV group (P = 0.01).
Of 135 participants with any DRM, 47% had one DRM, 19% had two DRMs, while 33% had at least three DRMs (Figure S1, available as Supplementary data at JAC Online). The proportions of PWH who had one DRM were 33 (58%) of 57 participants who had LLV and 31 (40%) of 78 individuals with VL ≥ 1000 copies/mL. The proportions of individuals with more than one DRM among LLV and VL ≥ 100 copies/mL groups was not statistically significant.
Predictive factors of VF
Among 3596 PWH in the BHS, 2617 were on ART and 2609 had baseline/enrolment VL measurement, of whom 97% (2520/2609) had at least one follow-up VL measurement and 81% (2043) were on ART for at least 6 months. A total of 84% (1709/2043) of those on ART for ≥6 months with VL < 1000 copies/mL at study entry had at least one follow-up VL measurement. Twelve (28%) of 43 persons with LLV at entry had subsequent VF compared with 12 (0.7%) of 1666 persons with VL ≤ 50 copies/mL at entry (unadjusted OR: 53; 95% CI: 19–150; P < 0.001; Table 5). In the adjusted analysis, being male, having LLV and being on second-line ART at enrolment were each associated with subsequent VF (Table 3). The adjusted OR of presenting with VF among individuals with LLV was 36 (95% CI: 10–137; P < 0.001). The OR of subsequent VF was 2.6 times higher among those with high LLV compared with those in the low LLV group (95% CI: 1.0–7.3; P = 0.07). The presence of major DRMs among participants with LLV at study enrolment did not increase the OR of VF.
Table 5.
Factors associated with VF among BCPP participants who had follow-up VL result
| Risk factor at study entry | VF at follow-up | Non-VF at follow-up (N = 1685) | Univariate OR | P value | Multivariate OR | P value |
|---|---|---|---|---|---|---|
| (N = 24) | (95% CI) | (95% CI) | ||||
| VL group, n (%) | ||||||
| Suppressed (N = 1666) | 12 (0.7) | 1654 (99.3) | 1 (ref) | <0.001 | 1 (ref) | <0.001 |
| LLV (N = 43) | 12 (27.9) | 31 (72.1) | 53.4 (19.0–150.0) | 36.1 (9.5–136.9) | ||
| LLV groups, n (%)a | ||||||
| Low LLV (N = 34) | 8 (24) | 26 (76) | 1 (ref) | b | ||
| High LLV (N = 9) | 4 (44) | 5 (56) | 2.6 (1.0–7.3) | 0.07 | ||
| Major DRM at LLV, n (%) | ||||||
| Absent (N = 27) | 5 (19) | 22 (81) | 1 (ref) | b | ||
| Present (N = 16) | 7 (44) | 9 (56) | 3.4 (1.0–17.1) | 0.13 | ||
| Sex n (%) | ||||||
| Female (N = 1296) | 14 (1) | 1282 (99) | 1 (ref) | 1 (ref) | 0.11 | |
| Male (N = 413) | 10 (2.4) | 403 (97.6) | 2.3 (0.89–5.8) | 0.09 | 2.4 (0.8–7.2) | |
| ART regimen | ||||||
| First line (N = 1507) | 15 (1) | 1492 (99) | 1 (ref) | 1 (ref) | ||
| Second line (N = 111) | 7 (6) | 104 (94) | 6.7 (2.6–17.3) | <0.001 | 2.6 (0.77–9.0) | 0.12 |
| Age at enrolment (years), median (IQR) | 35.6 (30.5–41.8) | 42.1 (36.5–50.2) | 0.94 (0.91–0.97) | <0.001 | 0.93 (0.89–0.98) | <0.01 |
| Duration on ART at VF, median (IQR) | 8.5 (7.6–12.8) | 8.2 (4.9–12.1) | 1.1 (0.91–1.3) | 0.36 | b |
All column variables represent those who experienced VF at follow-up while rows represent those who were virally suppressed or had LLV at study entry. REF, reference.
Low LLV was used as a reference.
Excluded from multivariate analysis because of collinearity or low numbers.
Discussion
We found that 4% of a representative population-based cross-sectional sample of adults on ART for at least 6 months in Botswana had LLV (50–999 copies/mL). Persons with LLV were less likely to have at least one major DRM (archived or in RNA) than persons with VL ≥ 1000 copies/mL, although the presence of any DRM (major or minor) did not differ between groups. Persons on ART who had LLV were much more likely to experience subsequent VF than persons with HIV-1 RNA ≤ 50 copies/mL.
The prevalence of LLV that we found is on the lower end of LLV (5.3%–27%) reported in several other countries in Africa, including South Africa,36 Kenya,37 a multi-country African cohort (Uganda, Tanzania, Nigeria and Kenya)38 and the PROMOTE longitudinal cohort (Uganda, Malawi, Zimbabwe and South Africa).39 There are several possible explanations for the lower prevalence of LLV in our study. First, the ART programme in Botswana is quite mature and monitors VL in all patients on ART. We used one VL of 51–999 copies/mL to define LLV, while other studies generally used more than one VL. Currently, Botswana HIV treatment guidelines have no clear guidelines on the management of LLV. In high-income settings, the interventions for LLV include intensified interventions such as adherence counselling, DRM testing and empirically changing ARV drugs on a case-by-case basis, upon detection of VL > 50 copies/mL, with emphasis on persistent LLV,40 suboptimal adherence to ART,41–43 detection of DRMs10 and subsequent VF.44,45
We did not find significant differences in the prevalence of at least one DRM (major or minor) by VL group. However, the prevalence of any major DRM was significantly higher in persons with VL ≥ 1000 copies/mL compared with those with LLV, as expected.46–48 One third (36%) of individuals with LLV had at least one DRM, which is at the lower end of what has been reported in the literature (19%–72%).37 The relative distribution of NNRTI-, NRTI-, PI- and INSTI-associated mutations stratified by VL was similar to that in persons with VL ≥ 1000 copies/mL, even though participants with DRM at LLV are not considered as failing ART in most resource-limited settings with annual VL monitoring.49 Our findings highlight that considerable levels of DRM are detectable at VLs of 51–999 copies/mL, with higher rates of NNRTI- and NRTI-associated mutations.9,10,50,51,52
Overall, our BCPP participants harboured relatively frequent resistance towards lamivudine, emtricitabine, abacavir, nevirapine and efavirenz in sequences that were derived primarily from proviral DNA. Most of these DRMs were likely selected by prior and ongoing ART, e.g. with efavirenz/emtricitabine/tenofovir disoproxil fumarate and cabotegravir/nevirapine. Some DRMs may also be from archived transmitted resistance mutations.53 Prior studies have reported accumulation of DRMs with persistent LLV1,37,42,50,54,55 and the potential for negative outcomes among persons remaining on non-suppressing therapy with low-level viral replication.43,45 These studies were in the era preceding dolutegravir-based ART when regimens with lower barriers to resistance were used. The impact of LLV in patients on dolutegravir-based ART remains unexplored, especially in resource-limited settings, but recent data indicate that patients on dolutegravir-based ART tend to have DRMs at low HIV VLs.56–58
We investigated the association between LLV and VF, age, gender, ART regimen and duration on ART among the subset of participants with follow-up VL data available. As in previous studies, being male and being on second-line ART were associated with VF.59–61 The risk of subsequent VF in participants with baseline LLV was 36-fold higher than in persons without baseline LLV in multivariate analysis, consistent with studies from South Africa36 and Canada.43 We used a single VL measurement to define LLV and a single VL result at the end of the study to define VF. We therefore could not differentiate LLV from viral blips nor identify participants who shifted from one VL group to another, although prior studies have also found viral blips to be associated with VF.62–64 The use of a single measurement of VL to define LLV was strongly associated with VF, starting with a lower range of LLV (51–200 copies/mL) in the HIV-1 C cohort in South Africa with 79% of LLV cases identified without confirmatory VL.36 Similarly, a study in China found that a single VL measurement above 400 copies/mL was associated with an increased risk of presenting with VF.65 Taken together, studies that reported the association of viral blip or unconfirmed LLV with VF support our findings. It is challenging to compare studies of LLV due to the use of different VF thresholds,2–4 LLV duration6,15,66–69 and number of VL measurements.6,36,38,40 However, our findings support the lowering of VF thresholds to VL > 50 copies/mL, as per the current EACS guidelines,4 and highlight the potential role of LLV as a warning of higher risk of VF. Study findings revealed that it is very important to closely monitor individuals with LLV by repeating VL testing upon detection of VL > 50 copies/mL with intensified adherence assessment and support.
Our study had several limitations. We could not establish whether detected DRMs were present at prior LLV events or were newly accumulated DRMs during LLV periods, as we assessed LLV and viral sequence at only one timepoint. In addition, the vast majority of our sequences were from proviral DNA, and the mutations identified in the proviral DNA might not be identical to those in the plasma HIV-1 RNA. However, studies have shown relative concordance in DRMs between the two34,70–72 and US clinical guidelines support the use of proviral DNA genotyping for VL = 200–1000 copies/mL but interpretation with caution.3 We adjusted for hypermutations to avoid the overestimation of mutations. Due to the lack of more frequent VL measurements during the follow-up, we could not identify individuals who may have shifted from one VL group to another, and we only had follow-up VL for a subset of participants on ART who had viral sequences at baseline (as only some PWH with sequences in BCPP were enrolled in the longitudinal survey cohort). At the time of enrolment there were few participants (n = 64) who were on ART for <6 months (1.4%). However, the majority of these had suppressed VL (66%; 42/64) and 34% had LLV (27%; 17/64 and 8%; 5/64 had low LLV and high LLV, respectively). We excluded all these participants from the analysis. Our study was performed before the widespread rollout of dolutegravir-based ART in Botswana; only 7.0% of our participants were taking dolutegravir and studies in populations taking dolutegravir-based regimens are warranted. However, our study had the advantage of the large sample size and the sampling design of the BCPP cohort providing good representativeness of the Botswana population. The other strength of our study is that we were able to detect DRMs from both plasma and proviral DNA in settings of LLV, where diagnosis of DRMs using plasma is limited by VL thresholds of >1000 copies/mL with most commercially available genotyping kits.20
In conclusion, in a well-characterized large population-based group of adults with HIV on ART in Botswana, persons with VF had higher prevalence of major DRMs than persons with LLV (although similar prevalence of any DRM). A single episode of LLV was strongly associated with subsequent VF among these individuals who were predominantly taking NNRTI-based ART. Our findings show that any detectable VL between 50 and 1000 copies/mL leads to poorer treatment outcomes, therefore we highlight that it is very important that persons with LLV are provided with enhanced adherence support and more frequent VL monitoring.
Supplementary Material
Acknowledgements
We thank all the study participants. We thank the BCPP study team for their contribution to this study. We thank the Ministry of Health and Wellness, Botswana Harvard AIDS Institute Partnership, CDC Botswana and US CDC for their excellent support and contributions to the study. We acknowledge the contributions of the PANGEA-HIV Consortium Steering Committee (Helen Ayles, Lucie Abeler-Dörner, David Bonsall, Rory Bowden, Max Essex, Sarah Fidler, Christophe Fraser, Kate Grabowski, Tanya Golubchik, Ravindra Gupta, Richard Hayes, Joshua Herbeck, Joseph Kagaayi, Pontiano Kaleebu, Jairam Lingappa, Vladimir Novitsky, Sikhulile Moyo, Deenan Pillay, Thomas Quinn, Andrew Rambaut, Oliver Ratmann, Janet Seeley, Deogratius Ssemwanga, Frank Tanser and Maria Wawer) for generation of HIV sequences used for this paper.
Contributor Information
Ontlametse T Bareng, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Sikhulile Moyo, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Melissa Zahralban-Steele, Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Dorcas Maruapula, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Biological Sciences, Faculty of Science, University of Botswana, Gaborone, Botswana.
Tsotlhe Ditlhako, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Baitshepi Mokaleng, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Patrick Mokgethi, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Wonderful T Choga, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Division of Human Genetics, Department of Pathology, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Natasha O Moraka, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Division of Medical Virology, Stellenbosch University, Cape Town, South Africa.
Molly Pretorius-Holme, Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Madisa O Mine, Botswana Ministry of Health and Wellness, Gaborone, Botswana.
Elliot Raizes, U.S. Centers for Disease Control and Prevention, Atlanta, USA.
Kesaobaka Molebatsi, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Statistics, University of Botswana, Gaborone, Botswana.
Modisa S Motswaledi, School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Irene Gobe, School of Allied Health Professions, Faculty of Health Sciences, University of Botswana, Gaborone, Botswana.
Terence Mohammed, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Tendani Gaolathe, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Roger Shapiro, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Mompati Mmalane, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Joseph M Makhema, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Shahin Lockman, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Brigham and Women’s Hospital, Boston, MA, USA.
Max Essex, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Vlad Novitsky, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Simani Gaseitsiwe, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
the PANGEA consortium:
Helen Ayles, Lucie Abeler-Dörner, David Bonsall, Rory Bowden, Max Essex, Sarah Fidler, Christophe Fraser, Kate Grabowski, Tanya Golubchik, Ravindra Gupta, Richard Hayes, Joshua Herbeck, Joseph Kagaayi, Pontiano Kaleebu, Jairam Lingappa, Vladimir Novitsky, Sikhulile Moyo, Deenan Pillay, Thomas Quinn, Andrew Rambaut, Oliver Ratmann, Janet Seeley, Deogratius Ssemwanga, Frank Tanser, and Maria Wawer
Funding
The BCPP Impact Evaluation was funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC, cooperative agreements U01 GH000447 and U2G GH001911). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies. O.T.B. was supported by the Fogarty International Center (Grant # 5D43TW009610). S.G. and W.T.C. were partially supported by H3ABioNet. H3ABioNet is supported by the National Institutes of Health Common Fund (U41HG006941). H3ABioNet is an initiative of the Human Health and Heredity in Africa Consortium (H3Africa) programme of the African Academy of Science (AAS). S.M. and S.G. were partly supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (grant # DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (grant #107752/Z/15/Z) and the UK government. PANGEA-HIV is funded primarily by the Bill & Melinda Gates Foundation (BMGF). The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. The funders had no role in the study design, data collection and decision to publish, or in the preparation of the manuscript.
Transparency declarations
None to declare.
Author contributions
S.G., O.T.B. and S.M. conceived the study and prepared the first draft. R.S. and E.R. reviewed the manuscript and provided comments. O.T.B., S.G., S.M. and M.S.M. finalized the manuscript based on feedback from other authors. S.G., S.M., O.T.B., V.N., M.E., T.G., J.M.M. and S.L. collected or prepared the data. S.M., S.G., V.N., M.Z.S., D.M., B.M., T.D., O.T.B. and P.M. conducted and supervised the laboratory experiments. O.T.B., S.M., S.G. and V.N. analysed the sequences. O.T.B., N.O.M., I.G., W.T.C., K.M. and S.M. did the statistical analysis and data presentation. S.M., S.G., T.G., M.P.H., J.M.M., M.O.M., T.M., M.E. and S.L. helped provide overall guidance to the conduct of the study. M.P.H., J.M.M., M.M., V.N., S.M., S.G., M.E. and S.L. were involved in the origination and development of the concept of the study.
Data availability
All relevant data are presented within the paper and the Supplementary data, figures and tables. HIV-1 sequences and associated clinical data are available on reasonable request through the PANGEA consortium (www.pangea-hiv.org). BCPP data are available at https://data.cdc.gov/Global-Health/Botswana-Combination-Prevention-Project-BCPP-Publi/qcw5-4m9q.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online.
References
- 1. Tobin NH, Learn GH, Holte SE et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 2005; 79: 9625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017. https://www.who.int/publications/i/item/9789241550062. [PubMed]
- 3. Department of Human Health Services . Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2019. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
- 4. European AIDS Clinical Society . EACS Guidelines. 2018. https://www.eacsociety.org/media/2018_guidelines-9.1-english.pdf.
- 5. Botswana Ministry of Health . Botswana Integrated HIV Clinical Care Guidelines. 2016. https://www.childrenandaids.org/sites/default/files/2017-04/Botswana_Integrated-HIV-Clinical-Care-Guidelines_2016.pdf.
- 6. Joya C, Won SH, Schofield C et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 2019; 69: 2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleman S, Söderbärg K, Visco-Comandini U et al. Drug resistance at low viraemia in HIV-1-infected patients with antiretroviral combination therapy. AIDS 2002; 16: 1039–44. [DOI] [PubMed] [Google Scholar]
- 8. Cozzi-Lepri A, Phillips AN, Ruiz L et al. Evolution of drug resistance in HIV-infected patients remaining on a virologically failing combination antiretroviral therapy regimen. AIDS 2007; 21: 721–32. [DOI] [PubMed] [Google Scholar]
- 9. Jordan MR, Winsett J, Tiro A et al. HIV drug resistance profiles and clinical outcomes in patients with viremia maintained at very low levels. World J AIDS 2013; 3: 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackie NE, Phillips AN, Kaye S et al. Antiretroviral drug resistance in HIV-1-infected patients with low-level viremia. J Infect Dis 2010; 201: 1303–7. [DOI] [PubMed] [Google Scholar]
- 11. Karlsson AC, Younger SR, Martin JN et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS 2004; 18: 981–9. [DOI] [PubMed] [Google Scholar]
- 12. Nettles RE, Kieffer TL, Kwon P et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA 2005; 293: 817–29. [DOI] [PubMed] [Google Scholar]
- 13. Sklar PA, Ward DJ, Baker RK et al. Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS 2002; 16: 2035–41. [DOI] [PubMed] [Google Scholar]
- 14. Maggiolo F, Callegaro A, Cologni G et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr 2012; 60: 473–82. [DOI] [PubMed] [Google Scholar]
- 15. Cohen C. Low-level viremia in HIV-1 infection: consequences and implications for switching to a new regimen. HIV Clin Trials 2009; 10: 116–24. [DOI] [PubMed] [Google Scholar]
- 16. Helou E, Shenoi S, Kyriakides T et al. Characterizing patients with very-low-level HIV viremia: a community-based study. J Int Assoc Provid AIDS Care 2017; 16: 261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen G-J, Sun H-Y, Chang S-Y et al. Incidence and impact of low-level viremia among people living with HIV who received protease inhibitor- or dolutegravir-based antiretroviral therapy. Int J Infect Dis 2021; 105: 147–51. [DOI] [PubMed] [Google Scholar]
- 18. WHO . Guidelines: updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. 2021. https://apps.who.int/iris/handle/10665/340190. [PubMed]
- 19. Günthard HF, Calvez V, Paredes R et al. Human Immunodeficiency Virus Drug Resistance: 2018 Recommendations of the International Antiviral Society-USA Panel. Clin Infect Dis 2019; 68: 177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosemary A, Chika O, Jonathan O et al. Genotyping performance evaluation of commercially available HIV-1 drug resistance test. PLoS One 2018; 13: e0198246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santoro MM, Fabeni L, Armenia D et al. Reliability and clinical relevance of the HIV-1 drug resistance test in patients with low viremia levels. Clin Infect Dis 2014; 58: 1156–64. [DOI] [PubMed] [Google Scholar]
- 22. Steegen K, Demecheleer E, De Cabooter N et al. A sensitive in-house RT-PCR genotyping system for combined detection of plasma HIV-1 and assessment of drug resistance. J Virol Methods 2006; 133: 137–45. [DOI] [PubMed] [Google Scholar]
- 23. Villalobos C, Ceballos ME, Ferrés M et al. Drug resistance mutations in proviral DNA of HIV-infected patients with low level of viremia. J Clin Virol 2020; 132: 104657. [DOI] [PubMed] [Google Scholar]
- 24. Novitsky V, Zahralban-Steele M, McLane MF et al. Correction for Novitsky et al., Long-range HIV genotyping using viral RNA and proviral DNA for analysis of HIV drug resistance and HIV clustering. J Clin Microbiol 2016; 54: 1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chew CB, Potter SJ, Wang B et al. Assessment of drug resistance mutations in plasma and peripheral blood mononuclear cells at different plasma viral loads in patients receiving HAART. J Clin Virol 2005; 33: 206–16. [DOI] [PubMed] [Google Scholar]
- 26. Coovadia A, Hunt G, Abrams EJ et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis 2009; 48: 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacLeod IJ, Rowley CF, Thior I et al. Minor resistant variants in nevirapine-exposed infants may predict virologic failure on nevirapine-containing ART. J Clin Virol 2010; 48: 162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neogi U, Shet A, Sahoo PN et al. Human APOBEC3G-mediated hypermutation is associated with antiretroviral therapy failure in HIV-1 subtype C-infected individuals. J Int AIDS Soc 2013; 16: 18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y, Etemad B, Dele-Oni R et al. Drug resistance mutations in HIV provirus are associated with defective proviral genomes with hypermutation. AIDS 2021; 35: 1015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noë A, Plum J, Verhofstede C. The latent HIV-1 reservoir in patients undergoing HAART: an archive of pre-HAART drug resistance. J Antimicrob Chemother 2005; 55: 410–2. [DOI] [PubMed] [Google Scholar]
- 31. Gaolathe T, Wirth KE, Holme MP et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3: e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wirth KE, Gaolathe T, Pretorius Holme M et al. Population uptake of HIV testing, treatment, viral suppression, and male circumcision following a community-based intervention in Botswana (Ya Tsie/BCPP): a cluster-randomised trial. Lancet HIV 2020; 7: e422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makhema J, Wirth KE, Pretorius Holme M et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019; 381: 230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moyo S, Gaseitsiwe S, Zahralban-Steele M et al. Low rates of nucleoside reverse transcriptase inhibitor and nonnucleoside reverse transcriptase inhibitor drug resistance in Botswana. AIDS 2019; 33: 1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rhee S-Y, Gonzales MJ, Kantor R et al. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res 2003; 31: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hermans LE, Moorhouse M, Carmona S et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18: 188–97. [DOI] [PubMed] [Google Scholar]
- 37. Kantor R, DeLong A, Schreier L et al. HIV-1 second-line failure and drug resistance at high-level and low-level viremia in Western Kenya. AIDS 2018; 32: 2485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esber A, Polyak C, Kiweewa F et al. Persistent low-level viremia predicts subsequent virologic failure: is it time to change the third 90? Clin Infect Dis 2019; 69: 805–12. [DOI] [PubMed] [Google Scholar]
- 39. Atuhaire P, Hanley S, Yende-Zuma N et al. Factors associated with unsuppressed viremia in women living with HIV on lifelong ART in the multi-country US-PEPFAR PROMOTE study: a cross-sectional analysis. PLoS One 2019; 14: e0219415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leierer G, Grabmeier-Pfistershammer K, Steuer A et al. Factors associated with low-level viraemia and virological failure: results from the Austrian HIV Cohort Study. PLoS One 2015; 10: e0142923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Podsadecki TJ, Vrijens BC, Tousset EP et al. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis 2007; 196: 1773–8. [DOI] [PubMed] [Google Scholar]
- 42. Li JZ, Gallien S, Do TD et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother 2012; 56: 5998–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swenson LC, Min JE, Woods CK et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014; 28: 1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laprise C, de Pokomandy A, Baril J-G et al. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57: 1489–96. [DOI] [PubMed] [Google Scholar]
- 45. Gonzalez-Serna A, Min JE, Woods C et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis 2014; 58: 1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grant-McAuley W, Fogel JM, Galai N et al. Antiretroviral drug use and HIV drug resistance in female sex workers in Tanzania and the Dominican Republic. PLoS One 2020; 15: e0240890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Etta EM, Mavhandu L, Manhaeve C et al. High level of HIV-1 drug resistance mutations in patients with unsuppressed viral loads in rural northern South Africa. AIDS Res Ther 2017; 14: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dagnra AY, Vidal N, Mensah A et al. High prevalence of HIV-1 drug resistance among patients on first-line antiretroviral treatment in Lomé, Togo. J Int AIDS Soc 2011; 14: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. National AIDS & STI Control Program, Ministry of Health Kenya . Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infections in Kenya. 2016. http://www.prepwatch.org/wp-content/uploads/2016/08/Guidelines-on-ARV-for-Treating-Preventing-HIV-Infections-in-Kenya.pdf.
- 50. Delaugerre C, Gallien S, Flandre P et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One 2012; 7: e36673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Assoumou L, Charpentier C, Recordon-Pinson P et al. Prevalence of HIV-1 drug resistance in treated patients with viral load >50 copies/mL: a 2014 French nationwide study. J Antimicrob Chemother 2017; 72: 1769–73. [DOI] [PubMed] [Google Scholar]
- 52. Doyle T, Smith C, Vitiello P et al. Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis 2012; 54: 724–32. [DOI] [PubMed] [Google Scholar]
- 53. Zaccarelli M, Santoro MM, Armenia D et al. Genotypic resistance test in proviral DNA can identify resistance mutations never detected in historical genotypic test in patients with low level or undetectable HIV-RNA. J Clin Virol 2016; 82: 94–100. [DOI] [PubMed] [Google Scholar]
- 54. Taiwo B, Gallien S, Aga E et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis 2011; 204: 515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gallien S, Delaugerre C, Charreau I et al. Emerging integrase inhibitor resistance mutations in raltegravir-treated HIV-1-infected patients with low-level viremia. AIDS 2011; 25: 665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seatla KK, Avalos A, Moyo S et al. Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana. AIDS 2018; 32: 1899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seatla KK, Maruapula D, Choga WT et al. HIV-1 subtype C drug resistance mutations in heavily treated patients failing integrase strand transfer inhibitor-based regimens in Botswana. Viruses 2021; 13: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. George JM, Kuriakose SS, Dee N et al. Rapid development of high-level resistance to dolutegravir with emergence of T97A mutation in 2 treatment-experienced individuals with baseline partial sensitivity to dolutegravir. Open Forum Infect Dis 2018; 5: ofy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Penot P, Héma A, Bado G et al. The vulnerability of men to virologic failure during antiretroviral therapy in a public routine clinic in Burkina Faso. J Int AIDS Soc 2014; 17: 18646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hailu GG, Hagos DG, Hagos AK et al. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS One 2018; 13: e0196259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Samizi FG, Panga OD, Mulugu SS et al. Rate and predictors of HIV virological failure among adults on first-line antiretroviral treatment in Dar Es Salaam, Tanzania. J Infect Dev Ctries 2021; 15: 853–60. [DOI] [PubMed] [Google Scholar]
- 62. Sörstedt E, Nilsson S, Blaxhult A et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis 2016; 16: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Farmer A, Wang X, Ganesan A et al. Factors associated with HIV viral load “blips” and the relationship between self-reported adherence and efavirenz blood levels on blip occurrence: a case–control study. AIDS Res Ther 2016; 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grennan JT, Loutfy MR, Su D et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012; 205: 1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang T, Ding H, An M et al. Factors associated with high-risk low-level viremia leading to virologic failure: 16-year retrospective study of a Chinese antiretroviral therapy cohort. BMC Infect Dis 2020; 20: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fleming J, Mathews WC, Rutstein RM et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS 2019; 33: 2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taramasso L, Magnasco L, Bruzzone B et al. How relevant is the HIV low level viremia and how is its management changing in the era of modern ART? A large cohort analysis. J Clin Virol 2020; 123: 104255. [DOI] [PubMed] [Google Scholar]
- 68. Wirden M, Todesco E, Valantin M-A et al. Low-level HIV-1 viraemia in patients on HAART: risk factors and management in clinical practice. J Antimicrob Chemother 2015; 70: 2347–53. [DOI] [PubMed] [Google Scholar]
- 69. Gaifer Z, Boulassel M-R. Low-level viremia predicts virological failure in HIV-infected Omani patients receiving antiretroviral therapy. J Int Assoc Provid AIDS Care 2020; 19: 2325958220979817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Derache A, Shin H-S, Balamane M et al. HIV drug resistance mutations in proviral DNA from a community treatment program. PLoS One 2015; 10: e0117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bon I, Alessandrini F, Borderi M et al. Analysis of HIV-1 drug-resistant variants in plasma and peripheral blood mononuclear cells from untreated individuals: implications for clinical management. New Microbiol 2007; 30: 313–7. [PubMed] [Google Scholar]
- 72. Novitsky V, Wester CW, DeGruttola V et al. The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in southern Africa. AIDS Res Hum Retroviruses 2007; 23: 868–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are presented within the paper and the Supplementary data, figures and tables. HIV-1 sequences and associated clinical data are available on reasonable request through the PANGEA consortium (www.pangea-hiv.org). BCPP data are available at https://data.cdc.gov/Global-Health/Botswana-Combination-Prevention-Project-BCPP-Publi/qcw5-4m9q.