Abstract
As an economical conjugated polymer, graphitic carbon nitride (g-C3N4) has recently attracted much attention due to its exciting chemical and thermal stability and easy availability. Herein, we constructed a metal-coordinated graphitic carbon nitride (M–g-C3N4) catalyst through simple impregnation and calcination methods and used it as a new heterogeneous catalyst for the efficient synthesis of bis (indolyl) methanes and trisindolines under mild conditions. This reaction is performed efficiently in water as an environmentally friendly solvent at ambient conditions. The ZnMo7O24/g-C3N4 nanocomposite was synthesized by a simple method by immobilizing Mo7O24(NH4)6·4H2O and ZnCl2 on the surface of g-C3N4 under hydrothermal conditions. It was characterized by FT-IR, EDS, and electronic scanning microscopy (SEM). The metal doping of Mo and Zn on the surface of graphitic carbon nitride leads to the formation of a green catalyst that gives good to excellent yields of products in short reaction times with an easy working procedure. In addition, the ZnMo7O24/g-C3N4 catalyst could be reused at least five runs without apparent loss of efficiency.
Subject terms: Environmental sciences, Solid Earth sciences, Chemistry
Introduction
The indole derivatives are important nitrogen-containing compounds due to their diverse pharmacological activities1,2. The indole alkaloids3,4, from lysergic5 acid to vincristine6 are one of the largest classes of alkaloids7, and they possess extended biological activity and drug discovery8. Among various reactions of indole9,10, the condensation reactions of indole with electron-deficient carbonyl compounds for the preparation of bis (indolyl) methanes and trisindolines has attracted and continues to attract interest in recent years11,12. In this context, various articles have focused on the preparation of target compounds employing homo and heterogeneous catalysts such as acidic ionic liquid immobilized on silica13, LiClO414, silica sulfuric acid15, magnetic metal–organic framework16, graphene17, Protic solvents18 and heteropoly acids19. Although these methods have some advantages, most have fundamental weaknesses, such as harsh reaction conditions, volatile organic solvents, toxic reagents and solvents, limited substrate scope, expensive reagents, and catalyst overload. In recent years, the literature has also documented various green protocols, such as organocatalyst20,21, ionic liquids22, deep eutectic solvents23, ultrasounds24, and Taurine25 for the efficient synthesis of indole derivatives.
Carbon nanomaterials have become a new research hotspot in sensors, drug delivery, photocatalysis, and energy-saving26,27. Graphite carbon nitrides (g-C3N4) as a fascinating conjugated polymer constructed from two-dimensional sheets with outstanding potential for catalytic and optoelectronic applications. Its physicochemical properties, such as resistance to acidic or basic media, extended chemical, and thermal stability, fascinating electronic properties, and unique structure, have elicited interdisciplinary research fascination28,29. g-C3N4 consists of earth-abundant carbon and nitrogen elements with a high degree of density and is the most stable allotrope of carbon nitrides in the ambient atmosphere30. It has rich surface properties due to its many nitrogen coordination sites suitable for catalytic applications31–34. In addition, many free amino groups on the C3N4 backbone made these compounds rich in electron lone pairs easily bound to metal ions25, doping g-C3N4 with metal and nonmetal ions showed significant improvement in their catalytic activity35–37. Furthermore, graphitic carbon nitride can easily be obtained under solid-state conditions without organic solvents38,39 from inexpensive materials such as melamine or urea derivatives40.
In continuation of our research by using green solvents and catalysts in organic transformations41,42 herein, we have reported a simple, mild, and general method for synthesizing indole derivatives in water in the presence of ZnMo7O24/g-C3N4 as a new separable and inexpensive heterogeneous composite.
Experimental
General
All chemicals, such as aldehydes, indole, ketones, isatin, Mo7O24(NH4)6·4H2O, and ZnCl2 were commercially available and used without further purifications. Solvents were purchased from commercial sources and distilled before use. The Buchi Melting point M-535 is used to determine melting temperatures.
Preparation of ZnMo7O24/g-C3N4
The bulk g-C3N4 was prepared by thermal polymerization of melamine according to the reported procedure38. In detail, 20 g of melamine in 150 mL crucible is heated to 550 °C with a heating rate of 5 °C min−1 and kept at 550 °C for 3 h in an air atmosphere. The resultant light yellow agglomerates was ground by an agate mortar for the next steps. The ZnMo7O24/g-C3N4 composites were prepared by a facile chemical method. 0.5 g of g-C3N4 was dispersed in 50 mL deionized water using a stirrer for 10 min at room temperature. In the next step, 0.2 g of Mo7O24(NH4)6.4H2O was dispersed in 20 mL of deionized water under stirring. In another flask, 0.2 g of ZnCl2 was added to 20 mL of deionized water and dissolved by magnetic stirring. Then, Mo7O24(NH4)6.4H2O and ZnCl2 solutions were added to the g-C3N4 suspension, and a magnetic stirrer was used to stir the reaction mixture for 5 h at 90 °C. After completion of the reaction, the solvent was removed in a vacuum by rotary evaporator and was dried at room temperature for 12 h, and Blue-green powder ZnMo7O24/g-C3N4 catalysts were obtained (Fig. 1).
Figure 1.
Synthesis of ZnMo7O24/g-C3N4.
General procedure for the synthesis of bis‐indoles
Indole (1.0 mmol), aldehyde (0.5 mmol), and ZnMo7O24/g-C3N4 (15 mg) in deionized water (1.0 mL) were stirred well using a magnetic stirrer, and TLC assessed the progress of the reaction until the reaction completion. Then, ethyl acetate (10 mL) and water (10 mL) were added to the reaction mixture and centrifuged. The organic phase was removed under reduced pressure, and the crude product was purified by recrystallization in ethanol, ethyl acetate, or column chromatography to afford the corresponding products. All products were known and identified by melting point.
General procedure for the synthesis of trisindolines
A mixture of indole (1.0 mmol), isatin (0.5 mmol), and ZnMo7O24/g-C3N4 (30 mg) in deionized water (1.0 mL) conditions was stirred at room temperature, and TLC tracked the reaction progress. After completion, the reaction mixture was diluted with water and ethyl acetate and centrifuged to give the crude product after evaporation of ethyl acetate. The crude product was purified by silica gel column chromatography or recrystallized in ethanol or ethyl acetate to afford the corresponding pure trisindolines (Supplementary Information).
Results and discussion
The co-condensation procedure was used to synthesize pure g-C3N4. The ZnMo7O24/g-C3N4 nanocomposite was synthesized by immobilizing Mo7O24(NH4)6.4H2O and ZnCl2 on the surface of g-C3N4. The morphology and structure of nanocomposite were thoroughly characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FT-IR).
FTIR analysis was further carried out to identify the functional groups, and the results are shown in Fig. 2. The absorption peak at 3000–3500 cm−1 is related to the stretching vibration of NH and NH2 groups in the g-C3N4 or adsorb water from the environment. The prominent characteristic peaks in the area 1636, 1573, 1403, 1317, and 1235 cm−1 represent the stretching vibrations of s-triazine or tri-s-triazine of g-C3N4 in the sample. Besides, the strong absorption peak at 807 cm−1 is the bending vibration of the s-triazine rings system.
Figure 2.
FT-IR spectra of ZnMo7O24/g-C3N4.
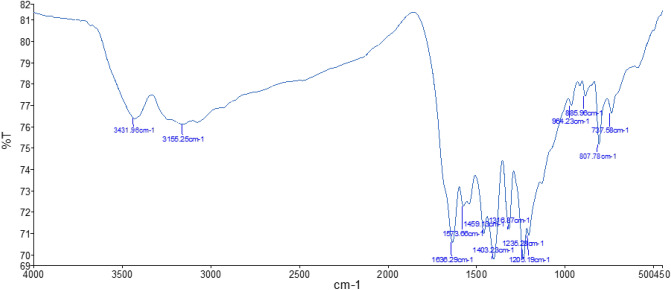
The typical SEM microscopy analysis is presented in Fig. 3 to investigate the new nanocomposite's morphology. The SEM spectrum of the ZnMo7O24/g-C3N4 catalyst indicates a series of thin sheets with wrinkles and irregular folding structures on the surface of g-C3N4.
Figure 3.
SEM images of ZnMo7O24/g-C3N4.
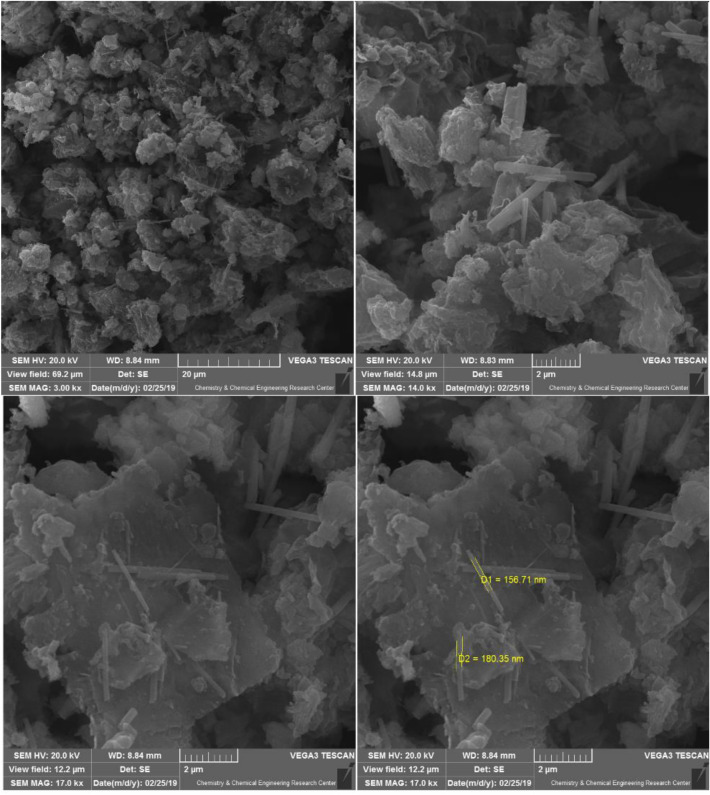
The energy dispersive spectroscopy (EDS) technique is used for the qualitative analysis of ZnMo7O24/g-C3N4. This pattern showed the Mo, Zn, and Cl elements are identified beside the C and N elements. As shown in Fig. 4, adopting Mo and Zn nanoparticles onto the g-C3N4 was efficacious. Also, the spectrum reveals that the scattering of these nanoparticles on the g-C3N4 substrates is uniform and acceptable.
Figure 4.
EDS spectrum of ZnMo7O24/g-C3N4.
After preparation and characterization of the ZnMo7O24/g-C3N4 composite, the catalytic activity of the composite was evaluated in the preparation of bis (indolyl) methanes via the reaction 2-methylindole (1.0 mmol) and aldehyde (0.5 mmol) in the presence of ZnMo7O24/g-C3N4 as a catalyst in deionized water (1.0 mL) to optimize reaction parameters (Table 1). The greener synthesis of bis (indolyl) methanes was carried out in a 5 mL three-necked round flask equipped with a magnetic stirrer, and the mixture was vigorously stirred at room temperature.
Table 1.
Optimization of the synthesis of bis (indolyl) methanes.
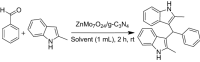
| |||
|---|---|---|---|
| Entry | ZnMo7O24/g-C3N4 (mg) | Solvents (1 mL) | Yields (%)a |
| 1 | 5 | Water | 67 |
| 2 | 10 | Water | 86 |
| 3 | 15 | Water | 95 |
| 4 | 20 | Water | 95 |
| 5 | 15 | Ethanol | 84 |
| 6 | 15 | DMF | 76 |
| 7 | 15 | THF | 72 |
| 8 | 15 | Toluene | 45 |
| 9 | 15 | Ethyl acetate | 44 |
| 10 | 15 | CH3CN | 56 |
| 11 b | 15 | Water | 32 |
| 12 c | 15 | Water | 75 |
| 13d | 15 | Water | 57 |
| 14e | 15 | Water | – |
| 15f | – | Water | – |
aIsolated yields.
bNa2Mo7O24.
cZnMo7O24 as a catalyst.
dZnCl2 catalyst.
eg-C3N4 as a catalyst.
fWithout catalyst.
The first finding indicated that the synthesis of bis(indolyl)methanes in the presence of the ZnMo7O24/g-C3N4 (15 mg) in deionized water (1.0 mL) was accomplished within 120 min with quantitative yields. (Table 1, entry 3). First, the amount of ZnMo7O24/g-C3N4 on the model reaction was optimized, and the results are shown in Table 1. The maximum yields of 95% were obtained when the loaded amount of composite was 15 mg (Table 1, entry 3). As the loaded amounts of composite increased to 20 mg, the reaction yields did not increase (Table 1, entry 4). While the amount of composite is reduced to 5 and 10 mg, increased reaction time was needed to achieve the optimal results (Table 1, entries 1–2). Furthermore, composite elements such as g-C3N4 (Table 1, entry 14) and ZnMo7O24 (Table 1, entry 12) Na2Mo7O24 (Table 1, entry 11) ZnCl2 (Table 1, entry 13) gave reduced yields. The model reaction was performed in different polar and nonpolar solvents (Table 1, entries 5–10) to optimize reaction conditions. The model reaction in organic solvents such as ethanol, dimethylformamide, and tetrahydrofuran in the presence of ZnMo7O24/g-C3N4 (15 mg) formed the expected product in lower yield.
The general nature of the procedure was confirmed by using structurally various aromatic and aliphatic aldehydes bearing electron-withdrawing and electron-donating substituents in the reaction with indole derivatives under the optimized conditions (Table 2). As seen in Table 2, different electron-donating or electron-withdrawing groups in the benzaldehyde ring proceeded well with 2-methylindole or indol, which gives good to excellent yields under short reaction times. It is necessary to mention that no remarkable reactivity differences were observed. In other words, the aromatic aldehydes with the electron-donating groups increased the yield slightly. They gave well to excellent results, while electron-withdrawing benzaldehyde derivatives did not reduce the reactivity. As an exception, the interaction of 2-hydroxy benzaldehyde and 2-methylindole yielded a lower yield (70%) than the other aldehydes. In addition, cyclohexanone generated the corresponding product in only a moderate yield under identical reaction conditions.
Table 2.
The synthesis of bis (indolyl) methanes using ZnMo7O24/g-C3N4 as the catalyst.
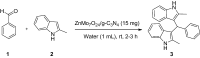
| |||||
|---|---|---|---|---|---|
| Entry | Aldehydes (R-CHO) | Indole | Products | M.P. (°C) | Yields (%)a |
| 1 | C6H5- | 2-Methylindole | 3a | 120–121 118–12013 | 95 |
| 2 | C6H5- | 1-Methylindole | 3b | 182–184 181–18313 | 96 |
| 3 | 3-OMe-C6H4 | 2-Methylindole | 3c | 237–239 236–23815 | 93 |
| 4 | 4-Cl-C6H4 | 2-Methylindole | 3d | 229–230 228–23014 | 90 |
| 5 | 4-Me-C6H4 | 2-Methylindole | 3e | 217–218 217–21914 | 95 |
| 6 | 4-OMe-C6H4 | 2-Methylindole | 3f. | 194–195 194–19614 | 79 |
| 7 | 2,4-Cl-C6H3 | 1-Methylindole | 3 g | 136–138 210–21214 | 94 |
| 8 | Thiophene-2- | 1-Methylindole | 3 h | 147–149 148–15019 | 89 |
| 9 | 4-NO2-C6H4 | 2-Methylindole | 3i | 238–241 240–24219 | 91 |
| 10 | C6H5- | Indole | 3j | 52–53 52–5317 | 95 |
| 11 | 4-Me-C6H4 | Indole | 3 k | 219–221 220–22117 | 95 |
| 12 | 3-NO2-C6H4 | Indole | 3 l | 214–216 216–21819 | 85 |
| 13 | 2,4-Cl-C6H3 | Indole | 3 m | 103–105 103–10618 | 94 |
| 14 | 4-CO2Me-C6H4 | Indole | 3n | 219–221 219–22119 | 91 |
| 15 | Cyclohexanone | Indole | 3o | 184–186 184–18714 | 73 |
aIsolated yields.
The heterocyclic spirooxindole skeleton, such as isatin containing core structure, has different biological activities and can function as synthons for naturally occurring alkaloids and pharmaceutically important drug molecules15,16. Encouraged by this success, we extended this reaction of substituted isatin with indole derivatives to obtain trisindoline compounds with ZnMo7O24/g-C3N4 as the catalyst (Fig. 5). Initially, indole (1.0 mmol) and isatin (0.5 mmol) reacted in the presence of ZnMo7O24/g-C3N4 as a catalyst in deionized water (1.0 mL). The results showed that 30 mg of catalyst at room temperature provided the optimum yield (92%) for the corresponding trisindoline within 180 min. The reaction of isatins and different indoles containing electron-donating and electron-withdrawing group substituent on nitrogen proceeded smoothly with good to excellent yields in 2.5–4 h.
Figure 5.
The synthesis of trisindoline using ZnMo7O24/g-C3N4 as the catalyst.
Figure 6 shows the possible catalytic pathway for the ZnMo7O24/g-C3N4 catalyzed the synthesis of trisindoline. A Zn or Mo Lewis acid coordinates to carbonyl groups of Isatin 4, and the nucleophilic attack of indole 2 to activated carbonyls 4 creates the zwitterionic species 6. The resulting intermediate 6 undergoes dehydration to provide the coordinated intermediate 7, which can be captured by the second addition of indole 2 to furnish target product 5. We proposed porous graphitic carbon nitride (g-C3N4)-stabilized ZnMo7O24 materials as in protic solvents leading to highly organodispersible and colloidally stable carbon nitrides as bifunctional Lewis acid composite for condensation reaction.
Figure 6.
The possible reaction mechanism between isatin and indole for the synthesis of trisindoline catalyzed by ZnMo7O24/g-C3N4.
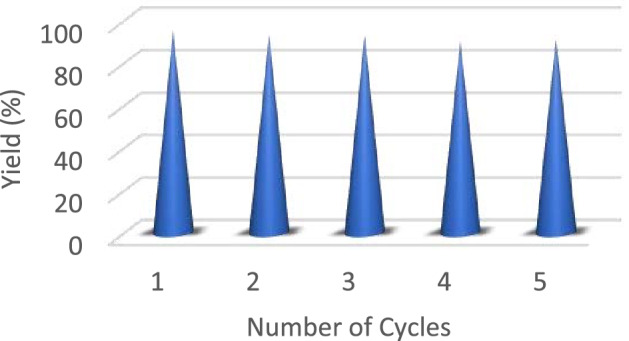
An imperative topic for implementing a heterogeneous composite is its recovery and reusability. The ZnMo7O24/g-C3N4 catalyst could be separated by centrifugation after each run. To show the recyclability of the ZnMo7O24/g-C3N4, the composite was recycled five times, and the results are shown in Fig. 7. Figure 4 shows the corresponding yields of the reused composite for a 5a, which demonstrates that the catalytic activity of ZnMo7O24/g-C3N4 did not significantly decrease after being used five times. After the reaction completion, ethyl acetate was added, and the reaction mixture was centrifuged and dried under a vacuum, and used for the next cycle. The SEM and FTIR images of the reused composite after 5 cycles did not change the nanocomposite morphology.
Figure 7.
Recycling results of the ZnMo7O24/g-C3N4 for the synthesis of trisindoline.
Conclusion
In this study, we have reported a simple and efficient method for the synthesis of bis (indolyl) methanes and trisindolines derivatives using a novel heterogeneous catalyst, Mo7O24(NH4)6·4H2O and ZnCl2 supported on graphitic carbon nitride (g-C3N4). The outstanding features of this catalyst were good to excellent yield, short reaction times, simple separation, and easy work-up. The g-C3N4 is considered an inexpensive and high surface area support for synthesizing bis (indolyl) methanes and trisindolines derivatives. Also, the ZnMo7O24/g-C3N4 showed high stability and reusability over several reaction sets without significant catalytic activity and selectivity loss.
Supplementary Information
Acknowledgements
Financial support for this work by Iran National Science Foundation: INSF (Grant number: 99005253) is gratefully appreciated.
Author contributions
N.A.: Supervision, Conceptualization, Methodology, Writing-review & editing. E.F.: Formal analysis, Writing-original draft. F.F.: Investigation, Methodology, Formal analysis. All authors reviewed the manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-23447-8.
References
- 1.Chakraborti AK, Roy SR, Kumar D, Chopra P. Catalytic application of room temperature ionic liquids: [bmim][MeSO4] as a recyclable catalyst for synthesis of bis(indolyl)methanes. Ion-fishing by MALDI-TOF-TOF MS and MS/MS studies to probe the proposed mechanistic model of catalysis. Green Chem. 2008;10:1111–1118. [Google Scholar]
- 2.Chakraborti AK, Gopalakrishnan B, ElizabethSobhia M, Malde A. 3D-QSAR studies of indole derivatives as phosphodiesterase IV inhibitors. Eur. J. Med. Chem. 2013;38:975–982. doi: 10.1016/j.ejmech.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee B. Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrason Sonochem. 2017;35:15–35. doi: 10.1016/j.ultsonch.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Vintonyak VV, Warburg K, Kruse H, Grimme S, Hübel K, Rauh D, Waldmann H. Identification of thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of the mycobacterium tuberculosis protein tyrosine phosphatase B. Angew. Chem. Int. Ed. Engl. 2010;49:5902–5905. doi: 10.1002/anie.201002138. [DOI] [PubMed] [Google Scholar]
- 5.Kamal A, Khan MNA, Reddy KS, Srikanth YVV, Ahmed SK, Kumar KP, Murthy USN. An efficient synthesis of bis(indolyl)methanes and evaluation of their antimicrobial activities. J. Enzyme. Inhib. Med. Chem. 2009;24:559–565. doi: 10.1080/14756360802292974. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Ravichandiran P, El-Harairy A, Queneau Y, Li M, Gu Y. 4-Aminoindoles as 1,4-bisnucleophiles for diversity-oriented synthesis of tricyclic indoles bearing 3,4-fused seven-membered rings. Org. Biomol. Chem. 2019;17:5982–5989. doi: 10.1039/c9ob01045a. [DOI] [PubMed] [Google Scholar]
- 7.Babu G, Sridhar N, Perumal PT. A convenient method of synthesis of bis-indolylmethanes: indium trichloride catalyzed reactions of indole with aldehydes and schiff's bases. Synth. Commun. 2000;30:1609–1614. [Google Scholar]
- 8.Gao F, Ferlin F, Bai R, Li M, Vaccaro L, Gu Y. Replacing halogenated solvents by a butyl acetate solution of bisphenol S in the transformations of indoles. Green Chem. 2021;23:3588–3594. [Google Scholar]
- 9.Leitch JA, Bhonoah Y, Frost CG. Beyond C2 and C3: Transition-metal-catalyzed C−H functionalization of indole. ACS Catal. 2017;7:5618–5627. [Google Scholar]
- 10.Humphrey GR, Kuethe JT. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006;106(7):2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborti AK, Thilagavathi R. Computer-aided design of non sulphonyl COX-2 inhibitors: An improved comparative molecular field analysis incorporating additional descriptors and comparative molecular similarity indices analysis of 1,3-diarylisoindole derivatives. Bioorg. Med. Chem. 2003;11:3989–3996. doi: 10.1016/s0968-0896(03)00404-8. [DOI] [PubMed] [Google Scholar]
- 12.Khanna L, Shilp M, Misra YN, Khanna P. “In water” synthesis of bis(indolyl)methanes: A review. Synth. Commun. 2021;51:2892–2923. [Google Scholar]
- 13.Hagiwara H, Sekifuji M, Hoshi T, Qiao K, Yokoyama C. Synthesis of bis(indolyl)methanes catalyzed by acidic ionic liquid immobilized on silica (ILIS) Synlett. 2007;8:1320–1322. [Google Scholar]
- 14.Mehrazma S, Azizi N, Saidi MR. Clean and facile condensations reaction of indoles and carbonyl compounds under solvent-free conditions. Lett. Org. Chem. 2006;3:161–164. [Google Scholar]
- 15.Zolfigol MA, Salehi P, Shiri M, Sayadi A, Abdoli A, Keypour H, Rezaeivala M, Niknam K, Kolvari E. A simple and efficient route for the synthesis of di and tri(bis(indolyl) methanes) as new triarylmethanes. Mol. Divers. 2008;12:203–207. doi: 10.1007/s11030-008-9091-y. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HY, Hao XP, Mo LP, Liu SS, Zhang WB, Zhang ZH. A magnetic metal–organic framework as a highly active heterogeneous catalyst for one-pot synthesis of 2-substituted alkyl and aryl(indolyl)kojic acid derivatives. New J. Chem. 2017;41:7108–7115. [Google Scholar]
- 17.Zhang M, Liu YH, Shang ZR, Hu HC, Zhang ZH. Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun. 2017;88:39–44. [Google Scholar]
- 18.Wu Z, Wang G, Yuan S, Wu D, Liu W, Ma B, Bi S, Zhan H, Chen X. Synthesis of bis(indolyl)methanes under dry grinding conditions, promoted by a Lewis acid–surfactant–SiO2-combined nanocatalyst. Green Chem. 2019;21:3542–3546. [Google Scholar]
- 19.Azizi N, Torkian L, Saidi MR. Highly efficient synthesis of bis(indolyl)methanes in water. J. Mol. Catal. A Chem. 2007;275:109–112. [Google Scholar]
- 20.Brahmachari G, Banerjee B. Facile and one-pot access of 3,3-bis(indol-3-yl)indolin-2-ones and 2,2-bis(indol-3-yl)acenaphthylen-1(2h)-one derivatives via an eco-friendly pseudo-multicomponent reaction at room temperature using sulfamic acid as an organo-catalyst. ACS Sustain. Chem. Eng. 2014;2:2802–2812. [Google Scholar]
- 21.Azizi N, Gholibeghlo E, Manocheri Z. Green procedure for the synthesis of bis(indolyl)methanes in water. Scientia Iranica. 2012;19:574–578. [Google Scholar]
- 22.Chen S, Chen Z, Zhang T, Zhao B, You B, Li M, Gu Y. Brønsted acid-catalyzed cascade cyclization: An efficient strategy for divergent synthesis of cyclohepta[b]indole derivatives. Green Chem. 2022;24:7376–7381. [Google Scholar]
- 23.Azizi N, Manocheri Z. Eutectic salts promote green synthesis of bis(indolyl) methanes. Res. Chem. Intermed. 2012;38:1495–1500. [Google Scholar]
- 24.Sun MX, He GY, Xu XY. Efficient and green synthesis of bis(indolyl)methanes catalyzed by ABS in aqueous media under ultrasound irradiation. Ultrasonics Sonochem. 2011;18:412–414. doi: 10.1016/j.ultsonch.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Chavan KA, Shukla M, Chauhan ANS, Maji S, Mali G, Bhattacharyya S, Erande RD. Effective synthesis and biological evaluation of natural and designed bis(indolyl)methanes via taurine-catalyzed green approach. ACS Omega. 2022;7(12):10438–10446. doi: 10.1021/acsomega.1c07258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Q, Chen N, Zhang J, Qu L. Graphene/graphitic carbon nitride hybrids for catalysis. Mater. Horiz. 2017;4:832–850. [Google Scholar]
- 27.Han Y, Zhang M, Zhang YQ, Zhang ZH. Copper immobilized at a covalent organic framework: An efficient and recyclable heterogeneous catalyst for the Chan-Lam coupling reaction of aryl boronic acids and amines. Green Chem. 2018;20:4891–4900. [Google Scholar]
- 28.Sun X, Zhu Q, Kang X, Liu H, Qian Q, Ma J, Zhang Z, Yang G, Han B. Design of a Cu(i)/C-doped boron nitride electrocatalyst for efficient conversion of CO2 into acetic acid. Green Chem. 2017;19:2086–2091. [Google Scholar]
- 29.Ong WJ, Tan LL, Ng YH, Yong ST, Chai SP. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016;116:7159–7329. doi: 10.1021/acs.chemrev.6b00075. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Wang W, Wang H, He ZH, Yang Y, Wang K, Liu ZT, Han B. Solvent-free aerobic photocatalytic oxidation of alcohols to aldehydes over ZnO/C3N4. Green Chem. 2022;24:7652–7660. [Google Scholar]
- 31.Di JQ, Zhang M, Chen YX, Wang JX, Geng SS, Tang JQ, Zhang ZH. Copper anchored on phosphorus g-C3N4 as a highly efficient photocatalyst for the synthesis of N-arylpyridin-2-amines. Green Chem. 2021;23:1041–1049. [Google Scholar]
- 32.Ding G, Wang W, Jiang T, Han B, Fan H, Yang G. Highly selective synthesis of phenol from benzene over a vanadium-doped graphitic carbon nitride catalyst. ChemCatChem. 2013;5:192–200. [Google Scholar]
- 33.Yang G, Li K, Lin X, Li Y, Cui C, Li S, Cheng Y, Liu Y. Regio- and stereoselective synthesis of (Z)-3-ylidenephthalides via H3PMo12O40-catalyzed cyclization of 2-acylbenzoic acids with benzylic alcohols. Chin. J. Chem. 2021;39:3017–3022. [Google Scholar]
- 34.Huang D, Yan X, Yan M, Zeng G, Zhou C, Wan J, Cheng M, Xue W. g-C3N4/NiAl-LDH 2D/2D hybrid heterojunction for high-performance photocatalytic reduction of CO2 into renewable fuels. ACS Appl. Mater. Interfaces. 2018;10:21035–21055. doi: 10.1021/acsami.7b18835. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Wang X, Antonietti M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012;51:68–89. doi: 10.1002/anie.201101182. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Xiao P, Li H, Carabineiro SAC. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Inter. 2014;6:16449–16465. doi: 10.1021/am502925j. [DOI] [PubMed] [Google Scholar]
- 37.Yang GP, Zhang XL, Liu YF, Zhang DD, Li K, Hu CW. Self-assembly of Keggin-type U(vi)-containing tungstophosphates with a sandwich structure: An efficient catalyst for the synthesis of sulfonyl pyrazoles. Inorg. Chem. Front. 2021;8:4650–4656. [Google Scholar]
- 38.Li XH, Wang X, Antonietti M. Solvent-free and metal-free oxidation of toluene using O2 and g-C3N4 with nanopores: Nanostructure boosts the catalytic selectivity. Acs Catal. 2012;2:2082–2086. [Google Scholar]
- 39.Liu Q, Zhang J. Graphene supported Co-g-C3N4 as a novel metal-macrocyclic electrocatalyst for the oxygen reduction reaction in fuel cells. Langmuir. 2013;29:3821–3828. doi: 10.1021/la400003h. [DOI] [PubMed] [Google Scholar]
- 40.Yan L, Jiang H, Xing Y, Wang Y, Liu D, Gu X, Dai P, Li L, Zhao X. Nickel metal–organic framework implanted on graphene and incubated to be ultrasmall nickel phosphide nanocrystals acts as a highly efficient water splitting electrocatalyst. J. Mater. Chem. A. 2018;6:1682–1691. [Google Scholar]
- 41.Azizi N, Ahooie TS, Hashemi MM. Multicomponent domino reactions in deep eutectic solvent: An efficient strategy to synthesize multisubstituted cyclohexa-1,3-dienamines. J. Mol. Liq. 2017;246:221–224. [Google Scholar]
- 42.Azizi N, Amiri AK, Baghi R, Bolourtchian M, Hashemi MM. PTSA catalyzed simple and green synthesis of benzothiazole derivatives in water. Monatsh. Chem. 2009;140:1471–1473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.