Abstract
In contrast to the apparent paucity of Mycobacterium tuberculosis response to reactive oxygen intermediates, this organism has evolved a specific response to nitric oxide challenge. Exposure of M. tuberculosis to NO donors induces the synthesis of a set of polypeptides that have been collectively termed Nox. In this work, the most prominent Nox polypeptide, Nox16, was identified by immunoblotting and by N-terminal sequencing as the α-crystallin-related, 16-kDa small heat shock protein, sHsp16. A panel of chemically diverse donors of nitric oxide, with the exception of nitroprusside, induced sHsp16 (Nox16). Nitroprusside, a coordination complex of Fe2+ with a nitrosonium (NO+) ion, induced a 19-kDa polypeptide (Nox19) homologous to the nonheme bacterial ferritins. We conclude that the NO response in M. tuberculosis is dominated by increased synthesis of the α-crystallin homolog sHsp16, previously implicated in stationary-phase processes and found in this study to be a major M. tuberculosis protein induced upon exposure to reactive nitrogen intermediates.
Nitric oxide formation is believed to have originated in metazoan cells as an ancient first-line defense against intracellular parasites (46). The role of the high-Ca2+-independent, inducible NO synthase (iNOS) and reactive nitrogen intermediates (RNI) in the control of Mycobacterium tuberculosis by the mouse macrophage has been reasonably well established (9, 14, 22), albeit with some debate concerning the magnitude (3, 43) and apparent strain dependence (17, 43) of associated effects. Mice treated with iNOS inhibitors (aminoguanidine and l-NMMA) succumb to infection with M. tuberculosis (8). Other reports suggest that iNOS and NO may play a role in the control of stable infection in mice, since inhibition of NO production causes reactivation of M. tuberculosis growth (33). While the role of RNI in the control of M. tuberculosis in murine systems has been established with a relatively high level of confidence, the role of NO in human macrophages has been a contentious issue. The controversy associated with the inability to demonstrate induction of iNOS in human macrophages is additionally compounded by apparent differences in cytokine activation of human and murine macrophages (4, 12, 15, 18, 23). Nevertheless, iNOS has been recently detected in alveolar macrophages fixed immediately upon isolation from tuberculosis patients by using a monospecific antibody against the human isoform (37). Moreover, Nozaki et al. have recently reported a nitric oxide-dependent killing of Mycobacterium bovis BCG by alveolar macrophages from patients with idiopathic pulmonary fibrosis (38), albeit in the same study, NO-dependent killing could not be demonstrated in alveolar macrophages from two other classes of patients tested.
If NO and its metabolites contribute to the control of M. tuberculosis in the host, it is reasonable to assume that, in turn, this organism may have evolved ways to respond to RNI challenges. While M. tuberculosis appears to have only a limited response to peroxide stimulation, which can be attributed to the loss of oxyR function (16), we wondered whether this organism might have evolved systems to respond to RNI. There is currently little information about potential defense systems that may exist in M. tuberculosis for protection against nitric oxide and related metabolites, albeit some recent attempts to address such a possibility have been reported (11, 19, 25). We have previously initiated investigations of M. tuberculosis response to compounds releasing NO metabolites, and detected a number of newly synthesized polypeptides (termed Nox for nitric oxide response) differentially induced in response to such challenges (25). In the present work, we extend these investigations to the identification of a subset of Nox proteins.
Donors of nitric oxide induce differential gene expression in M. tuberculosis.
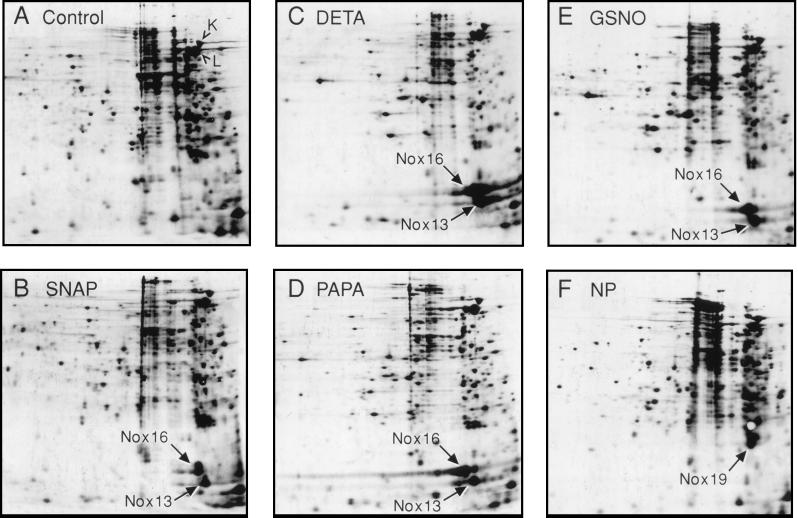
In this work, we used a panel of five structurally diverse NO donors which included S-nitrosothiols, nitric oxide adducts, and nitroprusside. The rationale for using chemically diverse NO donors was to ensure that the induction patterns observed can be attributed to NO or its metabolites rather than to other parts of the chemicals used. As previously discussed (25), we did not use NO gas, since it is rapidly consumed in reactions with oxygen. Furthermore, we did not test acidified nitrite, frequently used in experiments to investigate the effects of NO (9, 19). Acidification has been suspected to have intrinsic inhibitory effects independent of NO (43) and has been suggested as an effector mechanism acting directly or indirectly via downstream events in the elimination of mycobacteria by macrophages (3, 44, 49). Two virulent M. tuberculosis strains, I2646 (Fig. 1) and H37Rv (data not shown), were tested. The NO donors PAPA/NONOate [1-hydroxy-2-oxo-3-(3-aminopropyl)-3-propyl-1-triazene] and DETA/NO [N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino)-1,2-ethylene diamine] (which release NO with half-lives of 15 and >500 min, respectively), and two S-nitrosothiols, SNAP (S-nitroso-N-acetyl-d,l-penicillamine) and GSNO (S-nitroso glutathione), induced de novo synthesis of several polypeptides, as previously noted in strain H37Rv (25). The majority of NO donor compounds caused a strong induction of two major polypeptides (Fig. 1). These polypeptides, of 13 and 16 kDa, correspond to two of the previously reported SNAP-inducible polypeptides (previously assigned names corresponding to estimated relative molecular mass (Mr) values of 11 and 14 kDa [25]). Based on the refinements in Mr determinations, these polypeptides are designated now as Nox13 and Nox16.
FIG. 1.
Characterization of the M. tuberculosis response to a panel of nitric oxide donors. Autoradiograms of 2-D gels show newly synthesized polypeptides radiolabeled with [35S]Met and [35S]Cys in 1-ml aliquots of M. tuberculosis H37Rv cultured in Youmans medium for 8 days at 37°C and exposed to NO donors (Alexis Biochem., and Research Biochem.) as indicated. Positioning of autoradiograms is according to pH gradient, which ranged from 7.47 to 5.25 from left to right. Equal amounts of protein in cell homogenates were loaded. Metabolic labeling with [35S]methionine and [35S]cysteine (NEN protein labeling mix) (25), 2-D gel electrophoresis, and electroblotting were performed as previously described (6, 25). (A) Untreated control. (B) SNAP (500 μM). (C) DETA/NO (500 μM). (D) PAPA/NONOate (500 μM). (E) GSNO (500 μM). (F) Sodium nitroprusside dihydrate (NP [1 mM]). The high-virulence M. tuberculosis strain I2646 (from D. B. Young) was used. Similar results were obtained with H37Rv (data not shown).
However, not all NO donors tested caused induction of a typical Nox response. Exposure to the complex salt nitroprusside resulted in a different pattern (Fig. 1F) characterized by the absence of strong Nox13 and Nox16 induction, but caused increased synthesis of a novel polypeptide with an apparent Mr of 19 kDa (Nox19). Another compound, SIN-1 (3-morpholino-sydon-imine hydrochloride), a donor of both nitric oxide and superoxide believed to generate the highly reactive peroxynitrite (21), failed in five repeated experiments to induce a pattern consistent with differential gene expression in M. tuberculosis.
The degree of inhibition of protein synthesis by the NO donors was very low (Fig. 1 and 2A). Densitometric analyses of autoradiograms indicated that 0.5 mM DETA/NO or GSNO caused less than 50% inhibition, which was reached by DETA/NO during the first 4 h of exposure and by GSNO after 24 h. M. tuberculosis H37Rv exposed to 0.5 mM DETA/NO (under same conditions) for 4 h showed a 50% reduction in CFU, matching the magnitude of protein synthesis inhibition. The effects on overall protein synthesis, as judged by incorporation of 35S in polypeptides, were comparable to the degree seen with other NO donors. In comparison, the superoxide-generating compound menadione (Fig. 2A), was equally or more inhibitory but induced a different subset of polypeptides: the previously described Hsp15, Hsp20, and Hsp90 heat shock proteins (25) and another unidentified polypeptide of 27 kDa. The observed specific induction of Nox16 with NO donors, which was absent with menadione, is consistent with differential gene expression in response to NO challenge.
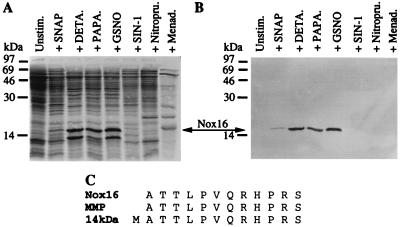
FIG. 2.
Identification of Nox16 as the 16-kDa α-crystallin homolog of M. tuberculosis. (A) SDS-PAGE analysis of metabolically labeled polypeptides in 1-ml aliquots from a 6-day M. tuberculosis I2646 culture grown in Youmans medium. Equal amounts of protein were loaded. Induction of Nox16 is detectable in M. tuberculosis treated with SNAP, DETA/NO, PAPA/NONOate, and GSNO (concentrations as in Fig. 1), but is not observed in the aliquots treated with SIN-1 or nitroprusside. Nox16 is also absent in the unstimulated control. (B) Western blot analysis of panel A with monoclonal antibody TB68, which is specific for sHsp16. (C) N-terminal sequence analysis of Nox16 and its alignment with the corresponding sequence of MMP (31) and that of the 14- or 16-kDa antigen (48) which is identical to that of the 16-kDa α-crystallin homolog in M. tuberculosis. Menad., menadione.
Nox16 is the previously characterized M. tuberculosis 16-kDa antigen homologous to α-crystallin.
Initial identification of Nox16 was performed by protein microsequence analysis. Nox16 was isolated from a DETA/NO-treated culture. Sixty percent of Nox16 was found in 150,000 × g supernatants from culture homogenates. The 16-kDa polypeptide band, visible on the blot by staining for total protein in the stimulated culture aliquot only (data not shown), was used for N-terminal amino acid sequence analysis. The sequence data (Fig. 2C) matched (except for the N-terminal methionine) the N-terminal amino acid sequence of the major membrane protein (MMP) of M. tuberculosis (31) (also referred to as the 14- or 16-kDa antigen, the small heat shock protein sHsp16, Hsp16.3, and Acr). The identification of Nox16 as the 16-kDa antigen was confirmed by Western blot analysis (Fig. 2B) with monoclonal antibody TB68 specific for the 16-kDa antigen (51). The immunoblotting signals in Fig. 2B matched the position and intensity of radioactive 16-kDa bands in Fig. 2A. Based on these results, it was possible to conclude that Nox16 is identical to the 16-kDa antigen, an M. tuberculosis protein which belongs to the α-crystallin superfamily of small heat shock proteins (26). This protein, initially identified as an M. tuberculosis antigen (31, 48), has been suggested to play a protective role as a chaperone based on its ability to prevent thermal denaturation of alcohol dehydrogenase (52) and aggregation of citrate synthase in vitro (10).
Nox19 is a homolog of the nonheme bacterial ferritins.
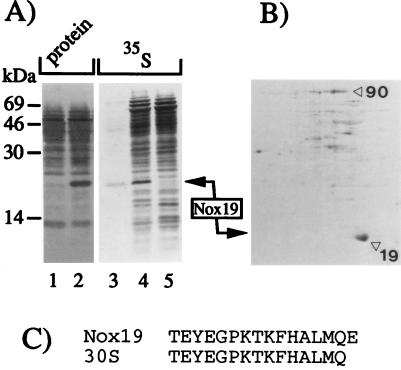
Enrichment of the nitroprusside-inducible Nox19 protein in M. tuberculosis was achieved by differential centrifugation of bacterial homogenates (Fig. 3). Analysis by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotting, and staining showed that the high-speed pellet from the nitroprusside-treated culture was enriched for the 19-kDa polypeptide (see the legend to Fig. 3). The position of this band corresponded to the 19-kDa polypeptide induced with nitroprusside (Fig. 3A, lanes 4 and 5 [35S-labeled proteins in crude extracts induced with nitroprusside and untreated control, respectively]). The final separation of the 19-kDa radioactive polypeptide from other polypeptides present in the high-speed pellet was achieved by two-dimensional (2-D) gel electrophoresis of the high-speed pellet (Fig. 3B). The protein spot corresponding to Nox19 was subjected to N-terminal sequence analysis, and the N terminus of Nox19 showed identity to the previously characterized M. bovis BCG polypeptide 24 copurified with the 30S ribosomal subunit in experiments carried out by Ohara et al. (40) (Fig. 3C). When the N-terminal sequence of Nox19 was further checked against the GenBank sequences, these global searches indicated a 100% match with a predicted open reading frame in the complete genome of M. tuberculosis H37Rv with no previously assigned function. Further analyses with the M. tuberculosis H37Rv genome showed that this gene was the only possible match with the N-terminal sequence of Nox19. When we performed a GenBank BLAST analysis by using the now-complete amino acid sequence of Nox19 derived from the corresponding open reading frame, a strong homology (Fig. 4) was observed with a subclass of bacterial ferritins containing nonheme iron previously described in Escherichia coli (28), Helicobacter pylori (24), and Campylobacter jejuni (50). In keeping with the nomenclature for nonheme ferritins, Nox19 was termed here Ftn. Ftn is different from the previously identified mycobacterial heme-containing bacterioferritins (7, 27, 42), including a bacterioferritin annotated in the genome of M. tuberculosis H37Rv.
FIG. 3.
N-terminal sequence determination of the nitroprusside-inducible Nox19. M. tuberculosis H37Rv culture (100 ml of Youmans medium) was divided into two aliquots, and 500 μl of a 100 mM nitroprusside solution in H2O was added to a final concentration of 1 mM. The culture was incubated with continuous stirring overnight at 37°C. The bacterial pellets were homogenized with glass beads in a Mini bead beater (Biospec Products, Bartlesville, Okla.) for 3 min at maximum speed. The nitroprusside-treated homogenate was mixed with a metabolically labeled homogenate (1-ml aliquot of the nitroprusside-treated culture radiolabeled with 35S) and clarified by a 5-min spin in an Eppendorf microcentrifuge. Next, the supernatant was centrifuged at 230,000 × g for 2 h at 4°C. The pellet was resuspended in 200 μl of H2O and spun at 3,000 × g for 1 h at 4°C, followed by 1 h at 230,000 × g, and the final supernatant was subjected to further analysis. (A) SDS-PAGE analysis of protein extracts from M. tuberculosis H37Rv. Lanes: 1 and 2, Ponceau red-stained proteins from a 230,000 × g pellet isolated from unstimulated and stimulated (1 mM nitroprusside) 50-ml culture aliquots of M. tuberculosis; 3, autoradiogram corresponding to lane 2; 4 and 5, a pair of radiolabeled extracts from nitroprusside-stimulated and unstimulated 1-ml culture aliquots. The major radioactive signal in lane 3 comigrates with the major protein band in lane 2 and with the inducible 19-kDa band in lane 4, indicating that Nox19 is enriched in the sample analyzed in lane 2. (B) 2-D gel electrophoresis of an aliquot from the 230,000 × g pellet from the nitroprusside-stimulated culture shown in panel A, lane 2. The major spot on the autoradiogram had an apparent molecular mass of 19 kDa and overlapped with the major protein spot on the blot visualized by Ponceau red. This protein spot was used for N-terminal sequence analysis. (C) N-terminal sequence of Nox19, aligned with the polypeptide 24 previously reported to copurify with the 30S ribosomal subunit from M. bovis BCG (40).
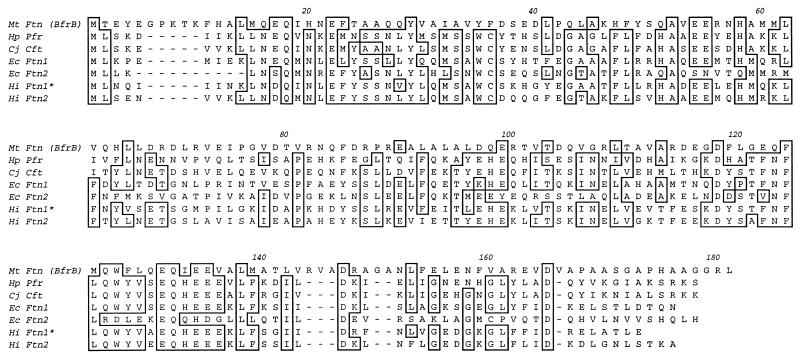
FIG. 4.
M. tuberculosis Nox19 (Ftn [BfrB]) is a homolog of bacterial nonheme ferritins. Shown is a multiple sequence alignment of M. tuberculosis (Mt) Ftn (BfrB) (Nox19 [the sequence is based on the N-terminal amino acid sequence in Fig. 3 and the corresponding M. tuberculosis H37Rv genomic translated sequence, where it was termed BfrB subsequent to the completion of this work]) with the previously characterized eucaryotic-type nonheme ferritins from H. pylori (Hp Pfr), C. jejuni (Cj Cft), E. coli ferritin (Ec Ftn1 [also known as RsgA]), and additional homologs from the genomic E. coli (Ec Ftn2) and Haemophilus influenzae (Hi Ftn1 and Ftn2) databases. An asterisk indicates that the open reading frame corresponding to H. influenzae Ftn1 in the database has been truncated to match the start codon corresponding to other bacterial nonheme ferritins. Identical residues are boxed; additional similarities and conservative substitutions among bacterial nonheme ferritins are noticeable, but are not indicated.
Unlike other donors of NO, exposure of M. tuberculosis to sodium nitroprusside {Na2[Fe(CN)5NO]} did not induce Nox16 and instead increased the synthesis of Ftn, the newly recognized M. tuberculosis equivalent of nonheme ferritins. Since ferritins serve in iron storage, it is possible that excess iron caused by the addition of nitroprusside resulted in the induction of ferritin synthesis. However, exposure to SNAP, another NO donor used in our experiments, also induced Ftn, albeit less consistently compared to its effects on Nox16. Interestingly, some nonheme ferritins also display similarity (5) to the DNA-binding proteins Dps and MrgA which are inducible upon exposure to oxidative stress (1, 34). Thus, the expression of Ftn may reflect a more complex control that requires further study and clarification.
Nox response in M. tuberculosis.
Nox16 is immunologically active in individuals infected with M. tuberculosis (29, 31). Preliminary analyses suggest that Ftn (Nox19) shows reactivity with sera from healthy subjects with delayed-type hypersensitivity to tuberculin and patients with pulmonary tuberculosis (data not shown). Thus, both Ftn and the α-crystallin homolog Nox16 are expressed in vivo and may play a role in the physiology of the tubercle bacillus during infection. However, it is not possible at this stage to assign unequivocally a precise function (e.g., a direct protective role) to Nox proteins in the context of NO challenge. The roles of iNOS and NO in the progression of tuberculosis in human disease have yet to be firmly established. It is also becoming evident that iNOS and RNI may play a role in maintaining the latent state of various intracellular pathogens (45, 47) or persistent subacute infections, as in the case of M. tuberculosis (33). Under such circumstances, NO not only may suppress the growth of M. tuberculosis, but also could serve as a signal for induction of a potential physiological program leading to the development of dormant bacilli. While there is presently no firm information regarding the form that latent bacilli assume in the host (41), it is worth mentioning that studies with in vitro models based on stationary-phase or microaerophilic conditions also report induction of the 16-kDa α-crystallin homolog (52). Expression of the α-crystallin homolog prolongs the lag growth phase in Mycobacterium smegmatis, while in M. tuberculosis H37Rv it slows down the log phase and counteracts diminishing viability of the bacilli during the postexponential phase (52). Intriguingly, the monoclonal antibody TB68 against the α-crystallin homolog Nox16 has been used to document that the laminated calcified inclusions (Schaumann bodies) encountered in granulomas in sarcoidosis may contain remnants of tubercle bacilli (2). It is also worth noting that α-crystallin homologs have been implicated in biological states associated with low metabolic rates and dormant conditions, such as encystment in other organisms (32).
In addition to their chaperone properties as members of a diverse group of small (15 to 30 kDa) heat shock proteins, α-crystallins participate in a variety of processes in eucaryotic cells (26), including inhibition of actin polymerization (30, 35) and participation in intermediate filament assembly (36). Inasmuch as the effects of M. tuberculosis on the intracellular trafficking in infected macrophages are not well understood, it is perhaps of interest that sHsp16 (Nox16) has been suggested to associate with the bacillus cell wall (31) and has been found within the outermost capsular structure (13). This localization, or, alternatively, a release from the bacilli entering the stationary phase, could potentially have implications on the cell biology of the intracellular growth and persistence of M. tuberculosis.
Recent studies in heterologous hosts (e.g., M. smegmatis, Salmonella sp., and E. coli) have implicated two proteins, AhpC and NoxR1, in resistance to oxidants, including acidified nitrite (11, 19). Based on the studies presented here, NoxR1 and AhpC appear not to be induced in response to NO challenge. Nevertheless, RNI encompass a wide variety of products, including those that result from interactions with reactive oxygen intermediates (ROI), and crossovers between detoxification of ROI and NO metabolites can be expected. It is also important to consider differential sensitivities of various microbes to RNI (20). The elegant studies of Ehrt and colleagues (19) indicate that M. smegmatis and M. tuberculosis differ fundamentally by displaying inverse sensitivity patterns to RNI and ROI. The differences can be even more profound when enterics are used as hosts, as illustrated by our previous demonstration that superoxide and NO response in M. tuberculosis have no significant overlaps (25), in contrast to what has been reported for E. coli (39). Thus, the roles of NoxR1, AhpC, Nox16, and Ftn in M. tuberculosis in the context of RNI protection remain to be established by further direct analyses of the tubercle bacillus.
Acknowledgments
We thank M. Marletta for discussions regarding NO compounds, M. Vordermeier and J. Ivanyi for providing monoclonal antibody TB68, and M. Mudd for help with completing the manuscript.
This work was supported by grant AI42999 from the National Institute of Allergy and Infectious Diseases.
ADDENDUM
Subsequent to the completion and submission of this work, the gene corresponding to the Nox19 polypeptide, identified here as the nitroprusside-inducible nonheme ferritin homolog and termed throughout the manuscript M. tuberculosis Ftn (in accordance with the nomenclature adopted for Haemophilus influenzae and Escherichia coli), was annotated bfrB within the published complete sequence of the M. tuberculosis H37Rv genome (GenBank accession no. AL123456) (11a).
REFERENCES
- 1.Altuvia S, Almirom M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςS in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 2.Ang S C, Moscovic E A. Cross-reactive and species specific Mycobacterium tuberculosis antigens in the immunoprofile of Schaumann bodies: a major clue to the etiology of sarcoidosis. Histol Histopathol. 1996;11:125–134. [PubMed] [Google Scholar]
- 3.Appleberg R, Orme I M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993;80:352–359. [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez E L, Young L S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–3013. [PubMed] [Google Scholar]
- 5.Bozzi M, Mignogna G, Stefanini S, Barr D, Longhi C, Valenti P, Chiancone E. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. doi: 10.1074/jbc.272.6.3259. [DOI] [PubMed] [Google Scholar]
- 6.Bravo R. Two-dimensional gel electrophoresis: a guide for the beginner. In: Celis J E, Bravo R, editors. Two-dimensional gel electrophoresis of proteins. Orlando, Fla: Academic Press; 1984. pp. 3–36. [Google Scholar]
- 7.Brooks B W, Young N M, Watson D C, Robertson R H, Sugden E, Nielsen K H, Becker S A W E. Mycobacterium paratuberculosis antigen D: characterization and evidence that it is a bacterioferritin. J Clin Microbiol. 1991;29:1652–1659. doi: 10.1128/jcm.29.8.1652-1658.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J, Tanaka K, Carroll D, Flynn J, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chiu W, Gilbert H F, Quiocho F A. Mycobacterium tuberculosis 16-kDa antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- 11.Chen L, Xie Q-W, Nathan C. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol Cell. 1998;1:795–805. doi: 10.1016/s1097-2765(00)80079-9. [DOI] [PubMed] [Google Scholar]
- 11a.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLeam J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Tayler K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med. 1993;178:2242–2248. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham A F, Spreadbury C L. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 15.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991;49:380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 16.Deretic V, Song J, Pagan-Ramos E. Loss of oxyR in Mycobacterium tuberculosis. Trends Microbiol. 1997;5:367–372. doi: 10.1016/S0966-842X(97)01112-8. [DOI] [PubMed] [Google Scholar]
- 17.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–1989. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douvas G S, Looker D L, Vatter A E, Crowle A J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrt S, Shiloh M U, Ruan J, Choi M, Gunzburg S, Nathan C, Riley L W. A novel antioxidant gene from Mycobacterium tuberculosis. J Exp Med. 1997;186:1885–1896. doi: 10.1084/jem.186.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang, F. C. Mechanism of nitric oxide-related antimicrobial activity. J. Clin. Investig., in press. [DOI] [PMC free article] [PubMed]
- 21.Feelisch M. The biological pathways of nitric oxide formation from nitrovasodilators: appropriate choice of exogenous NO donors and aspects of preparation and handling of aqueous NO solutions. J Cardiovasc Pharmacol. 1991;17:S25–S33. [Google Scholar]
- 22.Flesch I E A, Kaufmann S H E. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn J L, Chan J, Triebold K J, Dalton D K, Steward T A, Bloom B R. An essential role for IFN-gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazier B A, Pfeifer J D, Russell D G, Falk P, Olsén A N, Hammar M, Westblom T U, Normark S J. Paracrystalline inclusions of a novel ferritin containing nonheme iron, produced by the human gastric pathogen Helicobacter pylori: evidence for a third class of ferritins. J Bacteriol. 1993;175:966–972. doi: 10.1128/jb.175.4.966-972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garbe T R, Hibler N S, Deretic V. Response of Mycobacterium tuberculosis to reactive oxygen and nitrogen intermediates. Mol Med. 1996;2:134–142. [PMC free article] [PubMed] [Google Scholar]
- 26.Groenen J T A, Merck K B, De Jong W W, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. Eur J Biochem. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- 27.Inglis N F, Stevenson K, Hosie A H, Sharp J M. Complete sequence of the gene encoding the bacterioferritin subunit of Mycobacterium avium subspecies silvaticum. Gene. 1994;150:205–206. doi: 10.1016/0378-1119(94)90889-3. [DOI] [PubMed] [Google Scholar]
- 28.Izuhara M, Takanabe K, Takaka R. Cloning and sequencing of an Escherichia coli K12 gene which encodes a polypeptide having similarity to the human ferritin H subunit. Mol Gen Genet. 1991;225:510–513. doi: 10.1007/BF00261694. [DOI] [PubMed] [Google Scholar]
- 29.Jackett P S, Bothamley G H, Bathra H V, Mistry A, Young D B, Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988;26:2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavoie J N, Hickey E, Weber L A, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat-shock protein-27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 31.Lee B-Y, Hefta S A, Brennan P J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang P, Amons R, Clegg J S, MacRae T H. Molecular characterization of small heat shock/alpha-crystallin protein in encysted Artemia embryos. J Biol Chem. 1997;272:19051–19058. doi: 10.1074/jbc.272.30.19051. [DOI] [PubMed] [Google Scholar]
- 33.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of NOS2 as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miron T, Vancompernolle K, Vandekerckhove J, Wichek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat-shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholl I D, Quinlan R A. Chaperone activity of alpha-crystallins modulates intermediate filaments assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson S, da Gloria Bonecini-Almeida M, Lapa e Silva J R, Nathan C, Xie Q-W, Mumford R, Widner J R, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho J L. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozaki Y, Hasegava Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–3647. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunoshiba T, deRojas-Walker T, Wishnok J S, Tannenbaum S R, Demple B. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc Natl Acad Sci USA. 1993;90:9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohara N, Kimura M, Higashi Y, Yamada T. Isolation and amino acid sequence of the 30S ribosomal protein S19 from Mycobacterium bovis BCG. FEBS Lett. 1993;331:9–12. doi: 10.1016/0014-5793(93)80287-5. [DOI] [PubMed] [Google Scholar]
- 41.Parrish N M, Dick J D, Bishai W R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 42.Pessolani M C, Smith D R, Rivoire B, McCormick J, Hefta S A, Cole S T, Brennan P J. Purification, characterization, gene sequence, and significance of bacterioferritin from Mycobacterium leprae. J Exp Med. 1994;180:319–327. doi: 10.1084/jem.180.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaible U E, Strugill-Koszycki S, Schlesinger P H, Russell D G. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 45.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1274. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt H H H W, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 47.Stenger S, Donhauser N, Thuring H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verbon A, Hartskeerl R A, Schuitema A, Kolk A H J, Young D B, Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992;174:1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Via L E, Fratti R A, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 50.Wai S N, Nakayama K, Umene K, Moriya T, Amako K. Construction of a ferritin-deficient mutant of Campylobacter jejuni: contribution of ferritin to iron storage and protection against oxidative stress. Mol Microbiol. 1996;20:1127–1134. doi: 10.1111/j.1365-2958.1996.tb02633.x. [DOI] [PubMed] [Google Scholar]
- 51.Young D B, Kaufmann S H E, Hermans P W M, Thole J E R. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- 52.Yuan Y, Crane D D, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]