Abstract
Background
Intranasal corticosteroids (INCS) are frequently used to treat OSA syndrome (OSAS) in children. However, their efficacy has not been rigorously tested.
Research Question
Do INCS result in improved OSAS symptoms, polysomnography findings, behavior, and quality of life compared with placebo?
Study Design and Methods
In this randomized, double-blind, placebo-controlled trial, children with OSAS aged 5 to 12 years (N = 134) were randomized 2:1 to receive 3 months of INCS or placebo. Children in the INCS arm were then re-randomized to receive 9 months of INCS or placebo. Polysomnography, symptoms, and neurobehavioral findings were measured at baseline, 3 months, and 12 months. The primary outcome was change in obstructive apnea hypopnea index (OAHI) at 3 months, available for 122 children. The secondary outcome was OAHI change at 12 months, available for 70 children.
Results
Median (interquartile range) age and OAHI at baseline for the entire group were 7.9 (6.3 to 9.9) years and 5.8 (3.6 to 9.7) events per hour. OAHI changes at 3 months (–1.72 [–3.91 to 1.92] events per hour) and 12 months (–1.2 [–4.22 to 1.71] events per hour) were not different between the two groups (P = not significant). OSAS symptoms and neurobehavioral results did not differ between the INCS and placebo groups at 3 and 12 months. The 38 children who received INCS for 12 months reported a significant OAHI decrease from 7.2 (3.62 to 9.88) events per hour to 3.7 (1.56 to 6.4) events per hour (P = .039).
Interpretation
In children with OSAS, treatment with INCS did not result in significant polysomnography, neurobehavioral, or symptom changes at 3 and 12 months of treatment. Twelve months of INCS treatment resulted in a statistically significant but not clinically relevant OAHI reduction.
Clinical Trial Registration
ClinicalTrials.gov; No.: NCT02180672; URL: www.clinicaltrials.gov.
Key Words: double-blind method, fluticasone, intention-to-treat analysis, randomized controlled trial
Abbreviations: AOM, acute otitis media; CHOP, Children’s Hospital of Philadelphia; INCS, intranasal corticosteroids; NOSE, Nasal Obstruction Symptom Evaluation; OAHI, obstructive apnea hypopnea index; OSA-18, 18-item Obstructive Sleep Apnea assessment tool; OSAS, OSA syndrome; PSG, polysomnography; REM, rapid eye movement
Graphical Abstract
Take-home Points.
Study Question: Do INCS result in improved OSAS symptoms, PSG findings, behavior, and quality of life compared with placebo?
Results: INCS for the treatment of mild to severe childhood OSA did not result in significant OAHI or neurobehavioral, symptom, and PSG changes at 3 and 12 months of treatment. A 12-month INCS treatment resulted in symptomatic improvement and a statistically significant OAHI reduction but not resolution of OSA in most cases.
Interpretation: The use of INCS in children with mild to severe OSA should be limited to treat symptoms.
The childhood OSA syndrome (OSAS) is defined as a “disorder of breathing during sleep characterized by prolonged partial upper airway obstruction and/or intermittent complete obstruction (obstructive apnea) that disrupts normal ventilation during sleep and normal sleep patterns.”1 OSAS is common, occurring in 1% to 3% of otherwise healthy children.2 If untreated, it can result in significant morbidity, including cognitive deficits, behavioral abnormalities, poor growth, hypertension, cardiac hypertrophy, and metabolic derangements.2, 3, 4
Current treatment of childhood OSAS is primarily surgical (ie, adenotonsillectomy), with CPAP and other medical measures used much more rarely.5 Adenotonsillectomy is efficacious, but there is often residual OSAS.6,7 Furthermore, this invasive treatment is expensive, sometimes refused by families,8 and is associated with morbidity.9 CPAP use may be limited by poor adherence.10 Thus, efficacious alternative or adjunct medical treatments would be highly desirable. In vitro studies have reported increased glucocorticoid receptor expression in adenotonsillar tissue, with increased glucocorticoid receptor alpha messenger RNA expression and increased leukotriene cysteinyl receptors LT1-R and LT2-R in adenoid/tonsillar tissue from children with OSAS compared with those with recurrent pharyngitis.11,12 In vitro, corticosteroids have been shown to reduce tissue proliferation, increase apoptosis, and decrease the production of tumor necrosis factor-alpha and IL-6 and IL-8 in adenotonsillar tissue.13,14 These studies provide biologic plausibility for the use of intranasal corticosteroids (INCS) in the treatment of children with OSAS and adenotonsillar hypertrophy.
Studies have suggested that INCS may be effective in the treatment of childhood OSAS.15, 16, 17 However, these studies have been limited by factors such as small size, lack of randomization and blinding, short-term follow-up, involvement of children with only very mild OSAS, and/or lack of stratifying for the presence of atopy.17 We therefore conducted a randomized controlled trial evaluating the efficacy and safety of INCS vs placebo in children with a broad range of OSAS severity. We hypothesized that INCS would be efficacious in the treatment of childhood OSAS but would require ongoing maintenance therapy.
Study Design and Methods
Study Design and Patients
This study was a single-center, double-blind, parallel-group, randomized controlled trial performed at Children’s Hospital of Philadelphia (CHOP). Inclusion/exclusion criteria are available in the e-Appendix 1. Briefly, eligible subjects were typically developing children, 5 to 12 years of age, with OSAS defined as an obstructive apnea hypopnea index (OAHI) between 2 and 30 events per hour. Those with arterial oxyhemoglobin saturation of < 90% for ≥ 2% of the total sleep time were ineligible. Other exclusion criteria included history of adenotonsillectomy, any INCS use in the past 3 months or INCS use for > 2 weeks in the past year, recurrent tonsillitis, and a BMI z score ≥ 3.
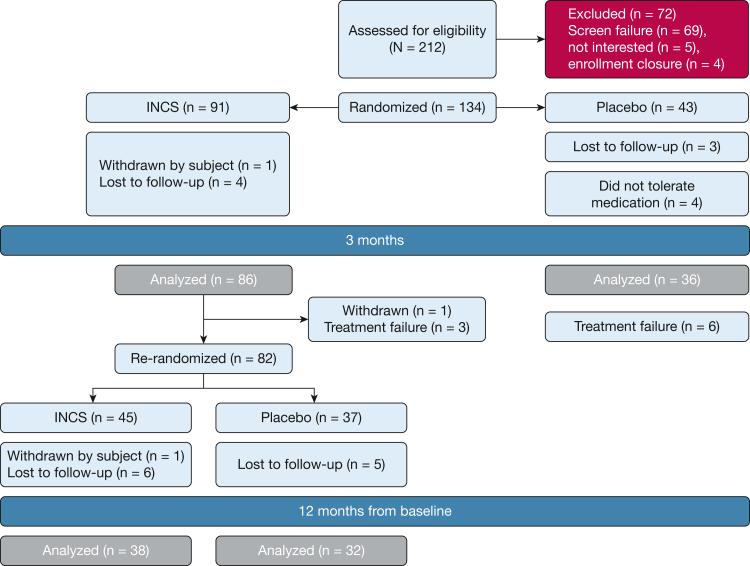
As shown in Figure 1, children were first randomly assigned in a 2:1 fashion to a 3-month course of INCS or placebo, at which time polysomnography (PSG) and baseline tests were repeated. At the end of this period, all subjects in the placebo group and subjects in the INCS group with OAHI > 30 events per hour on PSG and/or prolonged oxyhemoglobin desaturation as previously defined were then referred for standard clinical care. Children in the INCS group were further randomized in a 1:1 fashion to receive ongoing INCS vs placebo for an additional 9 months (a total of 12 months in the trial), at which point PSG and neurocognitive tests were repeated. Those with persistent PSG abnormalities at 12 months were referred for standard clinical care. The 12-month end point was chosen to enable assessment of long-term therapeutic and adverse effects in a time frame practical for subject retention and to help control for potential seasonal effects.
Figure 1.
Consolidated Standards of Reporting Trials diagram. Randomization to 3-month visit interval: One child in the INCS group was withdrawn by the family. Four children randomized to receive placebo withdrew from the study prior to the 3-month visit due to discomfort with the placebo nasal spray. At the 3- to 12-month visit interval, one child randomized to receive INCS was withdrawn at the 3-month visit because of the presence of coloboma. Three children receiving INCS exhibited worsening of the obstructive apnea hypopnea index at the 3-month polysomnography and therefore could not continue in the study. They were referred to clinical care. Six children randomized to receive placebo exhibited worsening of the obstructive apnea hypopnea index at the 3-month polysomnography and were referred to clinical care. Of the 45 children re-randomized to receive INCS, one refused to continue receiving INCS and withdrew from the study. INCS = intranasal corticosteroids.
Medication
The University of Pennsylvania’s Investigational Drug Service prepared blinded INCS and placebo bottles using the same metered pump for both INCS and placebo. Both INCS and placebo were solutions administered as a spray. Both placebo and INCS sprays smelled and looked the same. Each bottle contained a 30-day supply of INCS or placebo. Participants randomized to the INCS group received fluticasone propionate aqueous nasal spray at a dose of 55 μg (one spray) per nostril daily. The total daily dose was 110 μg. Children randomized to the placebo group received normal saline solution. Parents and children were instructed on the correct use of nasal sprays and demonstrated their proficiency to the research staff. Adherence was assessed by monthly parental report. Research staff called families monthly asking whether children had received the medication daily since the last telephone call (yes/no).
Randomization
A stratified blocked randomization scheme (with block sizes that vary randomly between two and four) written in Stata 13 (StataCorp) was used to initially randomize subjects to INCS or placebo at baseline. The randomization was stratified according to atopy/asthma, current use of inhaled corticosteroids for asthma (yes/no), obesity status, and seasonality (spring/summer vs autumn/winter). Assignments were stored in sealed envelopes in a locked cabinet accessible only to staff who were not involved with other aspects of the study.
Study Oversight
The study was approved by the CHOP Institutional Review Board (#14-010942). Written informed consent was obtained from caregivers and assent from children who were ≥ 7 years of age. An independent Data and Safety Monitoring Board reviewed interim data on safety and study quality. An external medical monitor adjudicated treatment failures, defined as changes in clinical status requiring a change in the assigned therapy.
Assessments
Children underwent standardized PSG, neurobehavioral testing, and other clinical and laboratory evaluations at baseline, 3 months, and 12 months following randomization. At these interval examinations, caregivers were asked to complete survey instruments.
Outcomes
The primary study outcome was the change in OAHI after 3 months of treatment, and the secondary outcome was the change in OAHI at 12 months of treatment. Other outcomes at both time intervals included PSG indexes, the Conners Continuous Performance Test-II,18 the Conners Kiddie Continuous Performance Test,19 the Purdue Pegboard test,20 the Conners-3 ADHD Index,21 the Child Behavior Checklist T score,22 and the Behavior Rating Inventory of Executive Function Global Executive Composite T score,23 comprising summary measures of behavioral regulation and metacognition (caregiver-rated scores range from 28-101, with higher scores indicating worse functioning); symptoms of OSAS, as assessed by means of the Pediatric Sleep Questionnaire sleep-related breathing disorder scale, in which scores range from 0 to 1, with higher scores indicating greater severity24; sleepiness, as assessed with the use of the Epworth Sleepiness Scale modified for children, in which scores range from 0 to 24, with higher scores indicating greater daytime sleepiness25; global quality of life (caregiver-rated total score from the Pediatric Quality of Life Inventory, in which scores range from 0-100, with higher scores indicating better quality of life)26; disease-specific quality of life (total score on the 18-item Obstructive Sleep Apnea assessment tool [OSA-18], in which scores range from 18-126, with higher scores indicating worse quality of life)27; and the Nasal Obstruction Symptom Evaluation (NOSE) scale, a validated scale of nasal obstructive symptoms,28 modified by deleting the question about sleep and written in child-friendly language.
Statistical Analysis and Power
Sample Size
This study was powered on a comparison of mean changes in OAHI between INCS vs control subjects at 3 months, based on the effect sizes observed in two prior studies.15,17 We had 81% power to detect an effect size of 0.50. This effect size corresponds to a mean reduction in OAHI of 4.2 events per hour in the INCS group and no change in the placebo group, assuming the SD of changes is 8.4 (as observed elsewhere15); and an OAHI reduction of 1 per hour with INCS (and no change in placebo) if the SD of change is 2 as observed elsewhere.17 Any smaller difference between groups with respect to changes in OAHI was not considered to be clinically meaningful.
Statistical Analysis
All analyses were performed with Stata 16.0, with two-sided tests of hypotheses and a P value < .05 as the criterion for statistical significance. Analyses were conducted according to the intention-to-treat principle, with subjects analyzed according to their initial treatment assignment. Descriptive analyses included computation of means (with 95% CIs) or medians (with interquartile ranges) for normally and nonnormally distributed variables, respectively. Graphical displays were used to explore relationships and distributional assumptions. The Shapiro-Wilk test was used to evaluate normality of outcomes and changes in outcomes. Box-Cox transformations were applied to nonnormally distributed outcomes for regression models in Stata 16.0.
Regression models were constructed with change in OAHI as the dependent variable and the following covariates: an indicator variable for the treatment group, baseline OAHI, and the stratification factors in the randomization: atopy/asthma, current use of inhaled corticosteroids for asthma (yes/no), obesity status, and seasonality (spring/summer vs autumn/winter). Secondary analysis was conducted to assess whether the impact of treatment depends on age by including age-by-treatment group interaction terms in the regression models. In addition, assessment of the use of inhaled corticosteroids prior to assessments at baseline according to treatment group interaction was conducted to assess whether corticosteroid-naive patients respond differently from children already on corticosteroids who may be resistant to the added effects of intranasal steroids. Similar approaches were implemented to analyze secondary variables; for example, from the neurocognitive and behavioral measures listed. The fit of the regression models was assessed via assessment of R, and the adequacy of model assumptions were assessed via standard tests and graphical checks (eg, quantile-quantile plots to assess the normality of residuals). Multiple comparisons were addressed following recommendations provided by Schulz and Grimes.29 The proposed analytic plan was described, and complete analyses were reported according to the Consolidated Standards of Reporting Trials statement. The primary end point (change in OAHI) was clearly specified, and secondary analyses are labeled as exploratory. All results were described, and their internal consistency was carefully assessed, as opposed to only reporting isolated significant findings. Similar subanalyses including only participants with a baseline OAHI ≤ 3 events per hour were also conducted.
Results
Study Overview
Figure 1 shows enrollment and randomization of participants. From November 2014 through January 2019, a total of 134 underwent randomization. A total of 82 underwent a second randomization.
Baseline characteristics are shown in Table 1. The median (interquartile range) OAHI of the group was 5.8 (3.6-9.7) events per hour. At baseline, there were no meaningful differences between the children randomized to receive INCS vs placebo.
Table 1.
Baseline Characteristics of the Study Group
| Characteristic | NCS Arm (n = 91) | Placebo Arm (n = 43) |
|---|---|---|
| Age, y | 8.2 ± 2.2 | 8.1 ± 2.0 |
| Female | 47 (52%) | 20 (47%) |
| Race | ||
| African American | 74 (81%) | 35 (81%) |
| White | 11 (12%) | 5 (12%) |
| Other | 6 (7%) | 3 (7%) |
| Hispanic ethnicity | 7 (8%) | 5 (12%) |
| Anthropometric measures | ||
| Height, cm | 131.9 ± 15.5 | 131.5 ± 13.5 |
| Height z score | 0.6 ± 0.9 | 0.6 ± 0.8 |
| Weight, kg | 37.5 ± 18.2 | 36.3 ± 15.8 |
| Weight z score | 1.2 ± 1.1 | 1.1 ± 1.1 |
| Weight class | ||
| Obese (BMI ≥ 95th percentile) | 33 (36%) | 15 (35%) |
| Overweight or obese (BMI ≥ 85th percentile) | 50 (55%) | 21 (49%) |
| Failure to thrive (weight < 5th percentile) | 2 (2%) | 0 |
| Upper airway measures | ||
| Friedman tongue position | 2.8 ± .9 | 2.9 ± .8 |
| Mallampati score | 2.3 ± .9 | 2.5 ± 1 |
| Right tonsil size | 2.5 ± .9 | 2.6 ± .8 |
| Left tonsil size | 2.5 ± .8 | 2.6 ± .8 |
| Maternal education less than high school | 6 (7%) | 2 (5%) |
| Household income less than $30,000/y | 44 (48%) | 24 (56%) |
| Fall/winter at time of randomization | 43 (47%) | 22 (51%) |
| Atopy/asthma at time of randomization | 34 (37%) | 17 (40%) |
| Current steroid use at time of randomization | 4 (4%) | 3 (7%) |
Data are presented as mean ± SD unless otherwise indicated.
Study Outcomes
First Randomization
After 3 months of treatment, there was no significant difference in OAHI change between the INCS and placebo groups as shown in Table 2. Similarly, there were no significant changes in neurobehavioral testing and questionnaires during this 3-month period. The percentage of time in rapid eye movement (REM) sleep decreased in the placebo group at the 3-month interval.
Table 2.
Outcome Measures Following Randomization for the INCS Arm Compared With the Placebo Group With Respect to Changes Between Baseline and Month 3 (Phase I of Study)
| Outcome | INCS |
Placebo |
Effect Size | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 3 | Change From Baseline to Month 3 | Baseline | Month 3 | Change From Baseline to Month 3 | |||
| Primary outcomea | ||||||||
| OAHI (N/h) | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.0 | .77 |
| Median | 5.0 | 3.7 | –1.7 | 5.3 | 4.3 | –1.3 | ||
| IQR | 3.6 to 9.7 | 1.6 to 9.4 | –3.9 to 1.9 | 3.3 to 8.2 | 2.3 to 8.4 | –4.2 to 1.7 | ||
| Secondary outcomesb | ||||||||
| Participant-performed behavior and executive function testing | ||||||||
| Conners Kiddie Continuous Performance Test | ||||||||
| No. | 82 | 82 | 82 | 36 | 36 | 36 | 0.1 | |
| Median | 55.7 | 54.5 | 0.2 | 60.2 | 57.1 | –0.4 | ||
| IQR | 48.6 to 61.8 | 47.5 to 60.3 | –5.9 to 4.8 | 51.6 to 63.5 | 51.5 to 63.3 | –4.8 to 4.0 | ||
| Purdue Pegboard dominant hand | ||||||||
| No. | 85 | 85 | 85 | 35 | 35 | 35 | 0.1 | |
| Median | –1.1 | –1.1 | 0.2 | –1.2 | –1.1 | 0.0 | ||
| IQR | –1.8 to –0.5 | –1.8 to –0.3 | –0.6 to 0.8 | –2.1 to –0.4 | –2.1 to –0.2 | –0.2 to 0.9 | ||
| Purdue Pegboard nondominant hand | ||||||||
| No. | 85 | 85 | 85 | 35 | 35 | 35 | 0.4 | |
| Median | –1.2 | –1.1 | 0.0 | –1.2 | –0.8 | 0.2 | ||
| IQR | –1.9 to –0.5 | –1.8 to –0.3 | –0.6 to 0.9 | –2.3 to –0.5 | –1.9 to –0.2 | –0.2 to 1.4 | ||
| Purdue Pegboard both hands | ||||||||
| No. | 85 | 85 | 85 | 36 | 36 | 36 | 0.2 | |
| Median | –1.1 | –1.1 | 0.0 | –1.5 | –1.2 | 0.0 | ||
| IQR | –2.2 to –0.2 | –1.7 to –0.2 | –0.6 to 0.9 | –2.0 to –0.9 | –1.9 to –0.5 | –0.2 to 0.9 | ||
| Caregiver-reported behavior and executive function | ||||||||
| CGI (caregiver) | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.1 | |
| Median | 5.0 | 5.5 | 0.0 | 4.5 | 3.0 | –0.5 | ||
| IQR | 2.0 to 14.0 | 2.0 to 12.0 | –2.0 to 2.0 | 1.0 to 10.5 | 0.0 to 8.0 | –2.0 to 1.0 | ||
| CBCL internalizing | ||||||||
| No. | 85 | 85 | 85 | 35 | 35 | 35 | 0.1 | |
| Median | 52.0 | 52.0 | 0.0 | 51.0 | 48.0 | 0.0 | ||
| IQR | 45.0 to 59.0 | 45.0 to 59.0 | –6.0 to 4.0 | 41.0 to 58.0 | 34.0 to 58.0 | –6.0 to 3.0 | ||
| CBCL externalizing (caregiver) | ||||||||
| No. | 85 | 85 | 85 | 35 | 35 | 35 | 0.0 | |
| Median | 53.0 | 50.0 | 0.0 | 44.0 | 44.0 | 0.0 | ||
| IQR | 46.0 to 59.0 | 46.0 to 59.0 | –6.0 to 4.0 | 34.0 to 56.0 | 35.0 to 54.0 | –4.0 to 3.0 | ||
| CBCL total problem (caregiver) | ||||||||
| No. | 85 | 85 | 85 | 35 | 35 | 35 | 0.1 | |
| Median | 52.0 | 51.0 | 0.0 | 49.0 | 48.0 | 0.0 | ||
| IQR | 45.0 to 62.0 | 45.0 to 60.0 | –7.0 to 3.0 | 39.0 to 56.0 | 38.0 to 55.0 | –4.0 to 1.0 | ||
| CBCL attention subscale (caregiver) | ||||||||
| No. | 85 | 85 | 85 | 35 | 35 | 35 | 0.1 | |
| Median | 55.0 | 53.0 | 0.0 | 52.0 | 51.0 | 0.0 | ||
| IQR | 51.0 to 62.0 | 52.0 to 59.0 | –2.0 to 2.0 | 50.0 to 57.0 | 50.0 to 59.0 | –1.0 to 0.0 | ||
| BRIEF GEC (caregiver) | ||||||||
| Mean | ||||||||
| No. | 85 | 85 | 85 | 36 | 36 | 36 | 0.1 | |
| Median | 48.0 | 47.0 | 0.0 | 45.5 | 41.5 | –1.0 | ||
| IQR | 40.0 to 59.0 | 41.0 to 58.0 | –3.0 to 3.0 | 35.0 to 52.0 | 36.0 to 53.5 | –4.0 to 2.0 | ||
| Symptoms and health-related quality of life | ||||||||
| Modified Epworth Sleepiness Scale | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.1 | |
| Median | 9.0 | 7.0 | 0.0 | 6.0 | 5.5 | 0.0 | ||
| IQR | 5.0 to 12.0 | 4.0 to 13.0 | –4.0 to 2.0 | 3.5 to 11 | 2.0 to 11.5 | –2.5 to 2.0 | ||
| PSQ-SRBD scale | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.0 | |
| Median | 0.5 | 0.4 | 0.0 | 0.4 | 0.4 | 0.0 | ||
| IQR | 0.3 to 0.6 | 0.3 to 0.6 | –0.1 to 0.1 | 0.3 to 0.6 | 0.2 to 0.6 | –0.1 to 0.1 | ||
| PedsQL (total score) | ||||||||
| No. | 85 | 85 | 85 | 36 | 36 | 36 | 0.1 | |
| Median | 75.0 | 77.2 | 0.4 | 78.4 | 79.6 | 1.2 | ||
| IQR | 58.7 to 91.3 | 58.7 to 91.3 | –9.8 to 12.0 | 68.5 to 94.0 | 66.3 to 97.8 | –6.5 to 7.1 | ||
| OSA-18 (total score) | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.2 | |
| Median | 50.0 | 40.5 | –6.0 | 43.0 | 36.0 | –5.5 | ||
| IQR | 37.0 to 60.0 | 30.0 to 56.0 | –18.0 to 4.0 | 31.0 to 54.5 | 25.0 to 53.5 | –12.0 to 4.0 | ||
| Nasal symptoms | ||||||||
| NOSE scale (total score) | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.4 | |
| Median | 5.0 | 3.0 | 0.0 | 3.5 | 3.0 | 0.0 | ||
| IQR | 2.0 to 8.0 | 1.0 to 7.0 | –3.0 to 1.0 | 1.0 to 7.0 | 1.0 to 7.5 | –2.0 to 2.0 | ||
| Polysomnography | ||||||||
| % TST end-tidal CO2 > 50 mm Hg | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.2 | |
| Median | 0.2 | 0.3 | 0.0 | 0.1 | 0.1 | 0.0 | ||
| IQR | 0.0 to 2.4 | 0.0 to 1.3 | –0.8 to 0.6 | 0.0 to 1.5 | 0.0 to 0.80 | –0.7 to 0.3 | ||
| Arousal index (N/h) | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.1 | |
| Median | 11.3 | 10.3 | –1.4 | 10.8 | 10.7 | –1.2 | ||
| IQR | 8.4 to 14.1 | 7.8 to 14.5 | –3.8 to 2.7 | 8.9 to 13.9 | 7.9 to 16.1 | –2.8 to 2.6 | ||
| % TST stage 1 | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.0 | |
| Median | 3.3 | 2.0 | –0.4 | 3.1 | 3.1 | 0.3 | ||
| IQR | 1.3 to 4.6 | 0.9 to 4.6 | –2.8 to 2.1 | 1.7 to 6.2 | 1.7 to 5.5 | –2.8 to 2.8 | ||
| % TST stage 2 | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.2 | |
| Median | 41.1 | 41.5 | 0.5 | 41.3 | 42.2 | 2.2 | ||
| IQR | 36.0 to 45.8 | 36.4 to 45.7 | –5.6 to 6.1 | 34.9 to 44.8 | 38.6 to 45.2 | –4.4 to 7.3 | ||
| % TST stage N3 | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.1 | |
| Mean | 34.8 | 33.5 | –1.3 | 34.8 | 34.3 | –0.5 | ||
| 95% CI | 33.3 to 36.3 | 31.9 to 35.1 | –3.1 to 0.4 | 32.4 to 37.1 | 31.9 to 36.5 | –3.7 to 2.7 | ||
| Time in stage REM (% TST) | ||||||||
| No. | 86 | 86 | 86 | 36 | 36 | 36 | 0.4 | |
| Mean | 20.6 | 22.0 | 1.4 | 20.6 | 19.6 | –1.0 | ||
| 95% CI | 19.5 to 21.7 | 20.9 to 23.1 | 0.1 to 2.7 | 18.9 to 22.3 | 17.7 to 21.5 | –3.3 to 1.2 | ||
Data are presented as mean ±SD or as medians with IQRs for nonnormally distributed data. The P value is provided for the primary outcome only and is adjusted for the stratification factors of atopy/asthma, current use of inhaled corticosteroids for asthma (yes/no, obesity status, and seasonality (spring/summer vs autumn/winter). Effect sizes were calculated with the use of Cohen’s d, the ratio of the absolute difference in means and the SD, and may be interpreted as: small, > 0.20 to 0.49; medium, 0.50 to 0.79; and large, ≥ 0.80. Note that the changes from 3 months to baseline are reported as median. Hence, displayed values do not necessarily match the 3-month and baseline cells, respectively. BRIEF GEC = Behavior Rating Inventory of 0Executive Function Global Executive Composite; CBCL = Child Behavior Checklist; CGI = Conners Global Index; INCS = intranasal corticosteroids; IQR = interquartile range; NOSE = Nasal Obstruction Symptom Evaluation; OAHI = obstructive apnea hypopnea index; OSA-18 = 18-item Obstructive Sleep Apnea assessment tool; PedsQL = Pediatric Quality of Life Inventory; PSQ-SRBD = Pediatric Sleep Questionnaire sleep-related breathing disorder scale; REM = rapid eye movement; TST = total sleep time.
Scores on the OAHI scale ranged from 0.3 to 61.9, with higher scores indicating greater severity.
Scores on the Conners Kiddie Continuous Performance Test scale ranged from 28 to 72.4, with higher scores indicating greater severity. Scores on the Purdue Pegboard dominant hand scale ranged from –4.7 to 5, with higher scores indicating greater severity. Scores on the Purdue Pegboard nondominant hand scale ranged from –5 to 6.9, with higher scores indicating greater severity. Scores on the Purdue Pegboard both hands scale ranged from –4.4 to 2.4, with higher scores indicating greater severity. Scores on the CGI ranged from 0 to 30, with higher scores indicating greater severity. Scores on the CBCL internalizing scale ranged from 29 to 81, with higher scores indicating greater severity. Scores on the CBCL externalizing scale ranged from 28 to 82, with higher scores indicating greater severity. Scores on the CBCL total problem scale ranged from 24 to 83, with higher scores indicating greater severity. Scores on the CBCL attention subscale ranged from 50 to 93, with higher scores indicating greater severity. Scores on the BRIEF GEC (caregiver-rated) scale ranged from 30 to 90, with higher scores indicating greater severity. Scores on the Modified Epworth Sleepiness Scale ranged from 0 to 24, with higher scores indicating greater severity. Scores on the PSQ-SRBD Scale ranged from 0 to .9, with higher scores indicating greater severity. Scores on the PedsQL scale ranged from 35.9 to 100, with higher scores indicating greater severity. Scores on the OSA-18 (total score) scale ranged from 18 to 112, with higher scores indicating greater severity. Scores on the NOSE scale ranged from 0 to 20, with higher scores indicating greater severity. Scores on the time with end-tidal CO2 > 50 mm Hg (% TST) scale ranged from 0 to 87.9, with higher scores indicating greater severity. Scores on the Arousal index scale ranged from 2 to 48.2, with higher scores indicating greater severity. Scores on the Time in stage 1 (% TST) scale ranged from 0 to 17.7. Scores on the Time in stage 2 (% TST) scale ranged from 18.9 to 71. Scores on the Time in stage 3 (% TST) scale ranged from 13 to 58.7. Scores on the Time in REM (% TST) scale ranged from 9.20 to 34.9.
Treatment Failure
Four children in the INCS group and six assigned to the placebo group had worsening OAHI and hence, those in the INCS group were discontinued from the study and referred to clinical care.
Of the 86 participants randomized to receive INCS who were included in the analyses, 49 (57%) reported receiving INCS as instructed every single day of the trial.
Second Randomization
At 9 months after the second randomization (12 months of treatment from baseline), there were no significant differences in OAHI changes between the INCS and placebo groups. For both groups, neurobehavioral testing and questionnaires showed no significant changes. There were no treatment failures in either group at the end of the second randomization.
Of the 38 participants randomized to receive INCS who were included in the analyses, 12 (32%) reported perfect adherence. Regression analysis that adjusted for the stratification variables in the randomization and for fall/winter season and baseline OAHI index also failed to identify a significant difference between groups.
For participants who received 12 months of INCS, the OAHI significantly decreased from baseline but did not normalize in the 38 children who received 12 months of INCS (Fig 2, Table 3). Symptom questionnaires, such as the Pediatric Sleep Questionnaire sleep-related breathing disorder scale, the OSA-18, and NOSE, improved during the 12-month period. The arousal index, driven by changes in spontaneous arousals as shown in e-Tables 1 and 2, and percentage of total sleep time in stage N3 decreased significantly during this interval.
Figure 2.
The line represents the median, the box the interquartile range, the whiskers the 5th and 95th percentiles, and the dots, diamonds, and squares the outliers. End of phase 1 = end of the first randomization. End of phase 2 = end of the second randomization. Children who received intranasal corticosteroids for 12 months had significant reduction in the OAHI. OAHI = obstructive apnea hypopnea index.
Table 3.
Outcome Measures for Patients Who Received Intranasal Corticosteroids at Baseline, 3 Months, and 12 Months
| Outcome | Baseline | Month 3 | Month 12 | Change From Baseline to Month 12 | Effect Size | P Value∗ From Paired t test |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| OAHI (No./h) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.352 | .039 |
| Mean (95% CI) | 7.70 (5.82 to 9.57) | 5.63 (3.52 to 7.74) | 5.37 (3.51 to 7.23) | –2.33 (–4.54 to –.12) | ||
| Median (Q1 to Q3) | 7.20 (3.62 to 9.88) | 3.5 (1.55 to 7.10) | 3.70 (1.56 to 6.40) | –1.76 (–4.27 to 1.44) | ||
| Secondary outcomes | ||||||
| Participant-performed behavior and executive function testing | ||||||
| Conners Kiddie Continuous Performance Test | 36 | 36 | 36 | 36 | 0.219 | .204 |
| No. | ||||||
| Mean (95% CI) | 55.34 (52.17 to 58.51) | 53.95 (50.84 to 57.06) | 53.14 (49.39 to 56.89) | –2.2 (–5.65 to 1.25) | ||
| Median (Q1 to Q3) | 57.12 (48.57 to 62.65) | 53.88 (47.13 to 60.33) | 54.09 (43.72 to 64.13) | –1.64 (–5.07 to 3.96) | ||
| Purdue Pegboard dominant hand | ||||||
| No. | 38 | 38 | 38 | 38 | 0.049 | .769 |
| Mean (95% CI) | –1.03 (–1.36 to –0.70) | –1.07 (–1.5 to –0.65) | –1.12 (–1.71 to –0.52) | –0.09 (–0.71 to 0.53) | ||
| Median (Q1 to Q3) | –1 (–1.62 to –0.5) | –1.16 (–1.77 to –0.5) | –1.04 (–1.72 to 0.08) | –0.01 (–0.75 to 0.92) | ||
| Purdue Pegboard both hands | ||||||
| No. | 38 | 38 | 38 | 38 | 0.028 | .865 |
| Mean (95% CI) | 1.02 (–1.45 to –0.6) | –.950 (–1.34 to –0.57) | –.98 (–1.34 to –0.62 | 0.04 (–0.48 to 0.57) | ||
| Median (Q1 to Q3) | –1.05 (–1.96 to –0.29) | –.81 (–1.68 to –0.18) | –.88 (–1.5 to –0.29) | –0.15 (–0.91 to 0.83) | ||
| Purdue Pegboard nondominant hand | ||||||
| No. | 38 | 38 | 38 | 38 | 0.03 | .857 |
| Mean (95% CI) | –1.05 (–1.65 to –0.46) | –1.01 (–1.42 to –0.61) | –.99 (–1.36 to –0.62) | 0.06 (–0.59 to 0.70) | ||
| Median (Q1 to Q3) | –1.27 (–1.89 to –0.71) | –1.07 (–1.58 to –0.23) | –.86 (–1.45 to –0.31) | 0.43 (–0.56 to 1.09) | ||
| Caregiver-reported behavior and executive function | ||||||
| CGI | ||||||
| No. | 36 | 36 | 36 | 36 | 0.318 | .064 |
| Mean (95% CI) | 7.14 (4.55 to 9.73) | 7.47 (5.01 to 9.94) | 5.75 (3.82 to 7.68) | –1.39 (–2.87 to 0.09) | ||
| Median (Q1 to Q3) | 4 (1.5 to 11.5) | 5.5 (3 to 9.5) | 5 (1 to 8.5) | –1 (–5 to 1.5) | ||
| CBCL internalizing | ||||||
| No. | 38 | 38 | 38 | 38 | 0.233 | .164 |
| Mean (95% CI) | 51.76 (48.63 to 54.89) | 50.57 (47.83 to 53.31) | 49.7 (46.75 to 52.66) | –2.05 (–4.99 to 0.88) | ||
| Median (Q1 to Q3) | 52 (45 to 57) | 50 (46 to 58) | 50 (43 to 57) | –2 (–6 to 4) | ||
| CBCL externalizing | ||||||
| No. | 38 | 38 | 38 | 38 | 0.118 | .477 |
| Mean (95% CI) | 53.19 (49.4 to 56.97) | 54.08 (50.12 to 58.04) | 52.19 (48.7 to 55.67) | –1 (–3.82 to 1.82) | ||
| Median (Q1 to Q3) | 51 (46 to 59) | 50 (47 to 61) | 53 (44 to 58) | 0 (–7 to 5) | ||
| CBCL total problem | ||||||
| No. | 38 | 38 | 38 | 38 | 0.147 | .376 |
| Mean (95% CI) | 52.7 (49.27 to 56.14) | 52.51 (49.06 to 55.96) | 51.62 (48.58 to 54.67) | –1.08 (–3.53 to 1.38) | ||
| Median (Q1 to Q3) | 50 (45 to 57) | 51 (47 to 56) | 51 (45 to 58) | –1 (–5 to 4) | ||
| CBCL attention subscale | ||||||
| No. | 38 | 38 | 38 | 38 | 0.175 | . 295 |
| Mean (95% CI) | 56.32 (54.04 to 58.61) | 56.16 (54.28 to 58.05) | 55.27 (53.59 to 56.95) | –1.05 (–3.07 to 0.96) | ||
| Median (Q1 to Q3) | 53 (52 to 61) | 55 (52 to 57) | 53 (52 to 57) | 0 (–3 to 2) | ||
| BRIEF GEC | ||||||
| No. | 38 | 38 | 38 | 38 | 0.312 | . 066 |
| Mean (95% CI) | 50.03 (46.2 to 53.85) | 50.41 (46.03 to 54.78) | 47.7 (44.16 to 51.25) | –2.32 (–4.81 to 0.16) | ||
| Median (Q1 to Q3) | 46 (42 to 58) | 45 (42 to 58) | 42 (40 to 56) | –2 (–5 to 1) | ||
| Symptoms and health quality of life | ||||||
| Modified Epworth Sleepiness Scale | ||||||
| No. | 38 | 38 | 38 | 38 | 0.328 | .054 |
| Mean (95% CI) | 9.22 (7.64 to 10.79) | 8.38 (6.60 to 10.16) | 7.86 (6.43 to 9.30) | –1.35 (–2.73 to 0.02) | ||
| Median (Q1 to Q3) | 9 (6 to 12) | 9 (4 to 12) | 8 (4 to 11) | –2 (–3 to 2) | ||
| PSQ-SRBD scale | ||||||
| No. | 38 | 38 | 38 | 38 | 0.5 | .004 |
| Mean (95% CI) | 0.44 (0.39 to 0.49) | 0.38 (0.31 to 0.45) | 36 (0.3 to 0.42) | –0.08 (–0.14 to –0.03) | ||
| Median (Q1 to Q3) | 0.45 (0.32 to 0.570) | 0.36 (0.23 to 0.52) | 0.36 (0.23 to 0.5) | –0.05 (–0.21 to 0.03) | ||
| PedsQL | ||||||
| No. | 38 | 38 | 38 | 38 | 0.073 | .662 |
| Mean (95% CI) | 76.82 (71.36 to 82.29) | 75.85 (69.87 to 81.83) | 78.29 (72.43 to 84.15) | 1.47 (–5.29 to 8.22) | ||
| Median (Q1 to Q3) | 83.7 (63.04 to 92.39) | 81.52 (58.7 to 89.13) | 85.87 (66.3 to 92.39) | 0 (–6.52 to 8.70) | ||
| OSA-18 (total score) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.557 | .002 |
| Mean (95% CI) | 45.97 (41.27 to 50.67) | 40.95 (36.19 to 45.7) | 37.59 (33.07 to 42.12) | –8.38 (–13.4 to –3.36) | ||
| Median (Q1 to Q3) | 45 (36 to 55) | 40 (29 to 49) | 36 (29 to 47) | –8 (–15 to 3) | ||
| Nasal symptoms | ||||||
| NOSE scale | ||||||
| No. | 38 | 38 | 38 | 38 | ||
| Mean (95% CI) | 4.76 (3.61 to 5.9) | 4.03 (2.82 to 5.24) | 3.65 (2.5 to 4.79) | –1.11 (–2.2 to –0.01) | 0.338 | .047 |
| Median (Q1 to Q3) | 5 (2 to 6) | 2 (1 to 7) | 2 (1 to 6) | –1 (–3 to 1) | ||
| Polysomnography | ||||||
| % TST end-tidal CO2 > 50 mm Hg) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.326 | .055 |
| Mean (95% CI) | 6.2 (1.75 to 10.65) | 2.09 (–.37 to 4.55) | 1.92 (.47 to 3.37) | –4.28 (–8.66 to 0.09) | ||
| Median (Q1 to Q3) | 0.3 (0 to 4.4) | 0.2 (0 to 0.700) | 0.1 (0 to 1) | –0.1 (–3.5 to 0) | ||
| Arousal index (N/h) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.489 | .005 |
| Mean (95% CI) | 12.58 (11.2 to 13.96) | 11.69 (9.91 to 13.47) | 10.52 (8.93 to 12.1) | –2.06 (–3.46 to –0.66) | ||
| Median (Q1 to Q3) | 12.6 (9.78 to 15.51) | 10.54 (9.22 to 14.5) | 9.9 (7.07 to 13.6) | –2.66 (–4.66 to 1.1) | ||
| Time in stage 1 (% TST) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.031 | .85 |
| Mean (95% CI) | 4 (2.96 to 5.03) | 3.66 (2.49 to 4.83) | 3.86 (2.84 to 4.88) | –.13 (–1.53 to 1.26) | ||
| Median (Q1 to Q3) | 3.72 (1.27 to 5.08) | 2.3 (.9 to 6) | 3.38 (1.3 to 6) | –.560 (–3.35 to 2.07) | ||
| Time in stage 2 (% TST) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.156 | .348 |
| Mean (95% CI) | 40.86 (38.26 to 43.46) | 40.2 (37.21 to 43.19) | 42.45 (39.36 to 45.54) | 1.59 (–1.79 to 4.96) | ||
| Median (Q1 to Q3) | 41.1 (35.5 to 45.91) | 39.35 (36.41 to 46.8) | 41.79 (37.53 to 47.9 | 3.19 (–1.71 to 6.29) | ||
| Time in stage N3 (% TST) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.334 | .05 |
| Mean (95% CI) | 35.45 (33.05 to 37.84) | 34.16 (31.58 to 36.73) | 32.45 (29.54 to 35.36) | –3 (–5.99 to –0.01) | ||
| Median (Q1 to Q3) | 34.58 (30.12 to 39.77) | 34.1 (28.8 to 37.3) | 30.83 (28.01 to 38.6) | –4.7 (–8.80 to 0.59) | ||
| Time in stage REM (% TST) | ||||||
| No. | 38 | 38 | 38 | 38 | 0.216 | .197 |
| Mean (95% CI) | 19.69 (18.06 to 21.33) | 21.98 (20.21 to 23.76) | 21.24 (19.07 to 23.41) | 1.55 (–0.84 to 3.94) | ||
| Median (Q1 to Q3) | 19.53 (16.8 to 22.33) | 21.66 (18.68 to 26.19) | 21.6 (18.1 to 25.1) | 1.42 (–2.5 to 5.83) |
BRIEF GEC = Behavior Rating Inventory of Executive Function Global Executive Composite; CBCL = Child Behavior Checklist; CGI = Conners Global Index; NOSE = Nasal Obstruction Symptom Evaluation; OSA-18 = 18-item Obstructive Sleep Apnea assessment tool; PedsQL = Pediatric Quality of Life Inventory; PSQ-SRBD = Pediatric Sleep Questionnaire sleep-related breathing disorder scale; Q1 to Q3, quartile 1 to quartile 3; REM = rapid eye movement; TST = total sleep time.
Subanalyses including only participants with a baseline OAHI ≤ 3 events per hour revealed results similar to those of the entire cohort (e-Tables 3, 4).
Safety
One participant randomized to receive INCS was withdrawn at the 3-month visit due to a chorioretinal coloboma, as this entity has been associated with cataracts. Adverse events are reported in Table 4. Overall, the placebo group had more asthma exacerbation episodes, upper respiratory tract infections, and exacerbation of OSAS symptoms compared with the INCS group. Similarly, the placebo group had three serious asthma adverse events vs none in the INCS group.
Table 4.
Adverse Events and Serious Adverse Events
| Type of Event | INCS | Placebo |
|---|---|---|
| Adverse events, total | ||
| Asthma exacerbation | 3 | 18 |
| Lower respiratory tract | 8 | 6 |
| Upper respiratory tract (including ears) | 67 | 90 |
| Cough | 15 | 11 |
| GI tract | 11 | 12 |
| Dehydration | 3 | 1 |
| ADHD diagnosis | 3 | 4 |
| Other infections (not included in other listed categories) | 17 | 40 |
| Hypersomnolence | 1 | 2 |
| Sleep apnea symptom exacerbation | 0 | 6 |
| Other | 27 | 34 |
| Total | 155 | 224 |
| Serious adverse events | ||
| Asthma | 0 | 3 |
| Lower respiratory tract | 1 | 0 |
| Total | 1 | 4 |
ADHD = attention-deficit/hyperactivity disorder; INCS = intranasal corticosteroids.
Discussion
This single-center, double-blind, randomized placebo-controlled trial of INCS for the treatment of childhood OSAS is the largest to date. This trial included objective assessments of sleep, neurobehavior, and symptoms at two time points following baseline. There were no significant differences in OAHI, neurobehavior, or symptoms following 3 months of treatment. To determine whether a longer treatment with INCS was required to observe reductions in OAHI, children initially randomized to receive INCS were re-randomized to an additional 9 months of INCS vs placebo. At the end of this second randomization, no significant differences in OAHI, neurobehavior, or symptoms were observed. Hence, this study has shown that INCS are not necessarily more effective than placebo for the treatment of pediatric OSAS.
INCS are widely used in clinical practice to treat OSAS in children. However, the most recent American Academy of Pediatrics Clinical Practice Guideline for the Diagnosis and Management of Childhood OSAS recommended that “Clinicians may prescribe topical intranasal corticosteroids for children with mild OSAS in whom adenotonsillectomy is contraindicated or for children with mild postoperative OSAS.”5 The strength of this recommendation was “option” (on a scale of option, recommendation, and strong recommendation), and the evidence quality was graded as B (on a scale of A-D). The low level of enthusiasm for INCS in these guidelines was based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data.15,30 The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS. The only difference between the two groups at the 3-month end point was the decreased percentage of REM sleep in the placebo group. One could assume that some children with untreated OSAS could have higher arousal indices, interrupted sleep, and, hence, decreased REM sleep. However, these characteristics were not present in the placebo group. The REM sleep difference in our data does not appear to be clinically relevant. Similarly, no clinically relevant differences between the two groups were observed at the end of the 12-month visit (Table 5).
Table 5.
Outcome Measures After Randomization for the INCS Arm∗ Compared With the Placebo Group With Respect to Changes Between Month 3 and Month 12 (Phase 2 of Study)
| Outcome | INCS |
Placebo |
Effect Size | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Month 3 | Month 12 | Change Month 3 to Month 12 | Month 3 | Month 12 | Change Month 3 to Month 12 | |||
| Primary outcomea | ||||||||
| OAHI (N/h) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.3 | .47 |
| Median | 3.4 | 3.6 | 0.1 | 3.1 | 4.3 | 1.0 | ||
| IQR | 1.5 to 7.1 | 1.3 to 6.4 | –3.3 to 2.5 | 1.5 to 9.2 | 1.7 to 8.9 | –1.3 to 2.9 | ||
| Secondary outcomesb | ||||||||
| Participant-performed behavior and executive function testing | ||||||||
| Conners Kiddie Continuous Performance Test | ||||||||
| No. | 37 | 37 | 37 | 29 | 29 | 29 | 0.2 | |
| Mean | 54.2 | 52.7 | –1.5 | 53.0 | 52.9 | –0.1 | ||
| 95% CI | 51.1 to 57.3 | 49.1 to 56.3 | –4.4 to 1.4 | 49.8 to 56.2 | 48.7 to 57.1 | –3.5 to 3.4 | ||
| Purdue Pegboard dominant hand | ||||||||
| No. | 38 | 38 | 38 | 31 | 31 | 31 | 0.1 | |
| Median | –1.2 | –1.0 | 0.1 | –1.2 | –1.3 | –0.3 | ||
| IQR | –1.8 to –0.5 | –1.7 to 0.1 | –0.5 to .8 | –1.7 to 0.1 | –2.3 to –0.3 | –1.3 to 0.8 | ||
| Purdue Pegboard both hands | ||||||||
| No. | 38 | 38 | 38 | 31 | 31 | 31 | 0.2 | |
| Median | –0.9 | –0.9 | 0.1 | –1.2 | –1.0 | –0.3 | ||
| IQR | –1.7 to –0.2 | –1.5 to –0.2 | –0.8 to 0.7 | –1.7 to –0.2 | –1.7 to –0.4 | –0.9 to 0.9 | ||
| Purdue Pegboard nondominant hands | ||||||||
| No. | 38 | 38 | 38 | 31 | 31 | 31 | 0.1 | |
| Median | –1.1 | –0.8 | 0.2 | –1.1 | –0.7 | 0.3 | ||
| IQR | –1.6 to –0.2 | –1.5 to –0.2 | –0.6 to 0.9 | –2.0 to –0.4 | –1.8 to –0.1 | –0.6 to 1.1 | ||
| Caregiver-reported behavior and executive function | ||||||||
| CGI (caregiver) | ||||||||
| No. | 37 | 37 | 37 | 32 | 32 | 32 | 0.2 | |
| Median | 5.0 | 5.0 | –1.0 | 4.0 | 3.0 | 0.0 | ||
| IQR | 3.0 to 9.0 | 1.0 to 8.0 | –4.0 to 0.0 | 0.5 to 14.5 | 1.0 to 11.5 | –1.5 to 1.0 | ||
| CBCL internalizing (caregiver) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.2 | |
| Mean | 50.7 | 49.9 | –0.8 | 54.2 | 51.9 | –2.2 | ||
| 95% CI | 48.0 to 53.4 | 47.0 to 52.8 | –3.7 to 2.1 | 50.4 to 57.9 | 47.5 to 56.4 | –5.4 to 0.9 | ||
| CBCL externalizing (caregiver) | ||||||||
| No. | 32 | 32 | 32 | 32 | 32 | 32 | 0.3 | |
| Mean | 53.8 | 51.9 | –1.9 | 51.3 | 52.0 | 0.7 | ||
| 95% CI | 49.9 to 57.7 | 48.5 to 55.3 | –4.3 to 0.5 | 46.5 to 56.1 | 47.3 to 56.7 | –2.5 to 3.9 | ||
| CBCL total problem (caregiver) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.0 | |
| Mean | 52.3 | 51.4 | –0.9 | 52.4 | 51.2 | –1.2 | ||
| 95% CI | 49.0 to 55.7 | 48.5 to 54.4 | –3.4 to 1.6 | 47.8 to 57.0 | 46.0 to 56.4 | –4.6 to 2.3 | ||
| CBCL attention subscale (caregiver) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.1 | |
| Median | 55.0 | 53.0 | –0.5 | 53.0 | 52.5 | 0.0 | ||
| IQR | 52.0 to 57.0 | 52.0 to 57.0 | –3.0 to 1.0 | 51.0 to 65.0 | 50.0 to 63.0 | –1.5 to 1.0 | ||
| BRIEF GEC (caregiver) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.1 | |
| Median | 44.5 | 42.0 | –2.5 | 48.5 | 44.0 | 0.0 | ||
| IQR | 42.0 to 58.0 | 39.0 to 56.0 | –6.0 to 2.0 | 39.5 to 64.5 | 37.5 to 62.5 | –7.5 to 3.0 | ||
| Symptoms and health quality of life | ||||||||
| Modified Epworth Sleepiness Scale | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.0 | |
| Median | 8.5 | 8.0 | 0.0 | 7.5 | 6.5 | –1.0 | ||
| IQR | 3.0 to 12.0 | 4.0 to 11.0 | –2.0 to 2.0 | 5.0 to 15.0 | 5.0 to 11.5 | –3.5 to 2.5 | ||
| PSQ-SRBD scale | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.2 | |
| Mean | 0.4 | 0.4 | 0.0 | 0.5 | 0.4 | –0.1 | ||
| 95% CI | 0.3 to 0.4 | 0.3 to 0.4 | –0.1 to 0.0 | 0.4 to 0.5 | 0.3 to 0.5 | –0.1 to 0.0 | ||
| PedsQL | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.1 | |
| Median | 81.5 | 86.4 | 2.2 | 74.5 | 77.2 | 1.1 | ||
| IQR | 58.7 to 91.3 | 66.3 to 93.5 | –4.3 to 12.0 | 57.6 to 93.5 | 56.5 to 90.2 | –6.5 to 7.6 | ||
| OSA-18 (total score) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.1 | |
| Median | 39.5 | 35.5 | –4.0 | 44.5 | 46.5 | –0.5 | ||
| IQR | 28.0 to 49.0 | 29.0 to 47.0 | –13.0 to 6.0 | 34.0 to 69.0 | 28.5 to 66.0 | –11.0.0 to 6.5 | ||
| Nasal symptoms | ||||||||
| NOSE scale | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.0 | |
| Median | 2.0 | 2.0 | –1.0 | 4.5 | 4.0 | 0.0 | ||
| IQR | 1.0 to 7.0 | 1.0 to 6.0 | –3.0 to 2.0 | 2.5 to 9.5 | 1.5 to 8.5 | –3.0 to 2.0 | ||
| Polysomnography | ||||||||
| % TST end-tidal CO2 > 50 mm Hg | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.0 | |
| Median | 0.2 | 0.1 | 0.0 | 0.6 | 0.7 | 0.0 | ||
| IQR | 0.0 to 0.7 | 0.0 to 1.0 | –0.1 to 0.2 | 0.1 to 1.6 | 0.0 to 3.7 | –0.6 to 0.8 | ||
| Arousal index (N/h) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.3 | |
| Median | 10.5 | 9.9 | –0.9 | 10.0 | 8.5 | –0.9 | ||
| IQR | 9.2 to 14.5 | 7.1 to 13.6 | –3.1 to 2.2 | 6.9 to 13.8 | 7.5 to 12.9 | –3.9 to 5.1 | ||
| (% TST) Stage 1 | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.1 | |
| Median | 2.3 | 3.3 | 0.0 | 2.0 | 1.7 | –0.1 | ||
| IQR | 0.9 to 6.0 | 1.2 to 6.0 | –2.1 to 3.0 | 0.6 to 3.9 | 1.2 to 2.9 | –1.4 to 1.3 | ||
| Time in stage 2 (% TST) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.1 | |
| Mean | 40.1 | 42.6 | 2.5 | 43.0 | 46.1 | 3.1 | ||
| 95% CI | 37.2 to 43.0 | 39.6 to 45.6 | –1.0 to 5.9 | 39.8 to 46.2 | 42.4 to 49.8 | –0.9 to 7.1 | ||
| Time in stage N3 (% TST) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.0 | |
| Median | 34.1 | 31.3 | –2.3 | 33.7 | 30.2 | –2.6 | ||
| IQR | 28.8 to 39.1 | 28.0 to 38.6 | –7.5 to 4.8 | 26.8 to 37.8 | 22.8 to 35.5 | –8.5 to 4.5 | ||
| Time in stage REM (% TST) | ||||||||
| No. | 38 | 38 | 38 | 32 | 32 | 32 | 0.0 | |
| Mean | 21.8 | 21.1 | –0.7 | 22.2 | 21.5 | –0.7 | ||
| 95% CI | 20.1 to 23.6 | 19.0 to 23.3 | –3.0 to 1.6 | 20.1 to 24.4 | 19.1 to 23.9 | –3.5 to 2.0 | ||
All placebo participants in Phase 2 received INCS intervention during Phase 1 of the study (see Fig 1). Data are presented as mean ±SD, and as medians with IQRs for nonnormally distributed data. The P value is provided for the primary outcome only and is adjusted for the stratification factors of adjusted for the stratification factors of atopy/asthma, current use of inhaled corticosteroids for asthma (yes/no), obesity status, and seasonality (spring/summer vs autumn/winter). Effect sizes were calculated with the use of Cohen’s d, the ratio of the absolute difference in means, and the SD, and may be interpreted as follows: small, > 0.20 to 0.49; medium, 0.50 to 0.79; and large, ≥ 0.80. BRIEF GEC = Behavior Rating Inventory of Executive Function Global Executive Composite; CBCL = Child Behavior Checklist; CGI = Conners Global Index; INCS = intranasal corticosteroids; IQR = interquartile range; NOSE = Nasal Obstruction Symptom Evaluation; OAHI = obstructive apnea hypopnea index; OSA-18 = 18-item Obstructive Sleep Apnea assessment tool; PSQ-SRBD = Pediatric Sleep Questionnaire sleep-related breathing disorder scale; REM = rapid eye movement; TST = total sleep time.
Scores on the OAHI scale ranged from 0 to 29, with higher scores indicating greater severity.
Scores on the Conners Kiddie Continuous Performance Test scale ranged from 33 to 72, with higher scores indicating greater severity. Scores on the Purdue Pegboard dominant hand scale ranged from –4 to 4, with higher scores indicating greater severity. Scores on the Purdue Pegboard both hands scale ranged from –4 to 2, with higher scores indicating greater severity. Scores on the Purdue Pegboard nondominant hand scale ranged from –4 to 6, with higher scores indicating greater severity. Scores on the CGI scale ranged from 0 to 30, with higher scores indicating greater severity. Scores on the CBCL internalizing scale ranged from 29 to 81, with higher scores indicating greater severity. Scores on the CBCL externalizing scale ranged from 28 to 82, with higher scores indicating greater severity. Scores on the CBCL total problem scale ranged from 24 to 83, with higher scores indicating greater severity. Scores on the CBCL attention subscale scale ranged from 50 to 83, with higher scores indicating greater severity. Scores on the BRIEF GEC (caregiver) scale ranged from 31 to 90, with higher scores indicating greater severity. Scores on the Modified Epworth Sleepiness Scale ranged from 0 to 24, with higher scores indicating greater severity. Scores on the PSQ-SRBD scale ranged from 0 to 0, with higher scores indicating greater severity. Scores on the PedsQL scale ranged from 35 to 100, with higher scores indicating greater severity. Scores on the OSA-18 (total score) scale ranged from 18 to 97, with higher scores indicating greater severity. Scores on the NOSE scale ranged from 0 to 18, with higher scores indicating greater severity. Scores on the Time with end-tidal CO2 > 50 mm Hg (% TST) scale ranged from 0 to 81, with higher scores indicating greater severity. Scores on the Arousal index scale ranged from 2 to 33, with higher scores indicating greater severity. Scores on the Time in stage 1 (% TST) scale ranged from 0 to 11. Scores on the Time in stage 2 (% TST) scale ranged from 18 to 71. Scores on the Time in stage 3 (% TST) scale ranged from 13 to 58. Scores on the Time in REM (% TST) scale ranged from 9 to 34.
Children who received INCS for a full year displayed a significant reduction in OAHI, spontaneous arousal index, and improvement of symptoms. The OAHI reduction was similar to that reported by Brouillette et al15 and Kheirandish-Gozal et al17 in children with milder OSAS. Despite the statistically significant OAHI reduction in this group, most children treated with INCS for 12 months still qualified as having mild to moderate OSAS and, therefore, additional treatment could be needed. Furthermore, the potential benefit of INCS in OAHI plateaued after 3 months. Sleep architecture and the arousal index were within normal limits at baseline, as previously reported in other larger studies of childhood OSAS.7 The decrease in spontaneous arousals could be related to improvement in symptoms. Similarly, the reduction in percent sleep time in stage N3 could be explained by increased age31 and is likely unrelated to the effect of INCS.
Importantly, prolonged treatment with INCS had a significant impact on sleep-disordered breathing symptoms. The PSQ-SRDB, NOSE, and OSA-18 scores progressively improved with INCS use and highlight the importance of including patient- and family-reported outcomes on OSAS clinical trials. Based on these findings, providers may consider prescribing INCS in children with very mild OSAS to improve their symptoms.
The neurobehavioral battery and symptoms questionnaires selected for this study were based on the Childhood Adenotonsillectomy Trial (CHAT),7 and baseline results were similar to those of CHAT. Unlike CHAT, there were no significant changes in neurobehavioral outcomes, such as in parent-reported executive function or behavior or indirectly tested fine motor coordination. These findings suggest that INCS for the treatment of OSAS do not have a significant effect in children’s neurobehavioral function.
Adverse events were more common in the placebo group. Interestingly, this group had more asthma episodes, three of which were catalogued as serious. It is possible that children in the INCS group received certain degree of protection for asthma exacerbation.32 Children receiving placebo reported more upper respiratory tract infections, including acute otitis media (AOM). Research has shown that INCS administered within 48 h of a viral respiratory infection does not prevent the development of AOM.33 However, it is still unknown whether chronic use of INCS may prevent some AOM symptoms. A large retrospective review showed that INCS significantly reduces the number of children requiring tympanostomy tube placement for Eustachian tube dysfunction.34 Because the latter has been associated with AOM,35 it is therefore possible that some of the children in the placebo group may have more AOM due to Eustachian tube dysfunction. One child in the INCS group was withdrawn at the 3-month visit due to bilateral coloboma, which has been associated with early cataracts.36 Cataracts have been described in the literature as an infrequent complication in adults with chronic asthma treated with inhaled corticosteroids for several years.37,38 Pediatric cases are rare and reversible following discontinuation of inhaled corticosteroids.39 This finding, nonetheless, emphasizes the importance of weighing risks and benefits when prescribing INCS and closely monitoring children receiving INCS to avoid harm.
The strengths of the current study include a large sample, a randomized design, standardization of state-of-the-art measurements, blinding of key personnel, and moderate attrition. Limitations include that participants who were initially randomized to receive placebo did not continue participation after 3 months; those who were randomized to receive placebo for the final 9 months of the trial were initially treated with INCS. Hence, an untreated placebo control group was available only during the first 3 months of this trial. The lack of a group exclusively receiving placebo for 12 months prevented us from ascertaining whether the improvement in symptoms observed at 12 months was mostly due to INCS. However, considering that the half-life of fluticasone in adults is 7.8 h and possibly shorter in children,40 the results are likely unaffected by this issue. Adherence was assessed per parental report only because electronic tracking systems were not affordable for this study. To account for adherence limitations, analyses were based on the intention-to-treat principle. Participants did not report perfect adherence, which is similar to other studies of INCS,41, 42, 43 and also highlights the difficulties that children and families may encounter when using intranasal sprays. The participants’ racial and ethnic distribution is representative of the Philadelphia area and may not be applicable to other racial and ethnic groups. However, no difference in response based on race was found.
Interpretation
This single-center, double-blind, randomized controlled trial of INCS for the treatment of mild to severe childhood OSA did not result in significant OAHI or neurobehavioral, symptom, and PSG changes at 3 and 12 months of treatment. These findings do not support the use of INCS in most children with OSA. A 12 months, INCS treatment resulted in a statistically significant OAHI reduction but not resolution of OSA in most cases. Further targeted trials are needed in children most likely to benefit from INCS, including those with OSAS who have comorbid allergic rhinitis, to better target therapy.
Acknowledgments
Author contributions: I. E. T. is the guarantor of the manuscript and was the principal investigator of the project following the death of Carole L. Marcus, MBBCh. I. E. T. was responsible for study design, analysis, interpretation of the data, and drafting of the manuscript; J. S. contributed to the study design, analysis, interpretation of the data, drafting of the manuscript, and critical revisions of the manuscript; C. M. C., J. R., R. M. B., and M. A. C. contributed to acquisition and interpretation of the data, analysis, and critical revision of the manuscript; A. B. K., L. M. E., J. M. S., and L. M. S. contributed to the study design, interpretation of the data, and critical revision of the manuscript; I. E. T., J. S., and J. R. had full access to the data; and I. E. T. and J. R. had the responsibility for the decision to submit the publication.
Funding/support: This study was supported by the National Institutes of Health [R01HL120909 and UL1TR001878]. There was no commercial support for this study.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors acknowledge the invaluable contributions from their deceased colleague and original principal investigator of this project, Carole Marcus, MBBCh. They also thank the network of primary care clinicians, their patients, and families for their contribution to this project and clinical research facilitated through the Pediatric Research Consortium at CHOP as well as the CHOP Center for Human Phenomic Science.
Additional information: The e-Appendix and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.American Thoracic Soceity Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153(2):866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 2.Marcus C.L., Brooks L.J., Draper K.A., et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 3.Amin R.S., Carroll J.L., Jeffries J.L., et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169(8):950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 4.Amin R.S., Kimball T.R., Bean J.A., et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(10):1395–1399. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 5.Marcus C.L., Brooks L.J., Draper K.A., et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee R., Kheirandish-Gozal L., Spruyt K., et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 7.Marcus C.L., Moore R.H., Rosen C.L., et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 pt 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 9.Baugh R.F., Archer S.M., Mitchell R.B., et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30. doi: 10.1177/0194599810389949. [DOI] [PubMed] [Google Scholar]
- 10.DiFeo N., Meltzer L.J., Beck S.E., et al. Predictors of positive airway pressure therapy adherence in children: a prospective study. J Clin Sleep Med. 2012;8(3):279–286. doi: 10.5664/jcsm.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldbart A.D., Veling M.C., Goldman J.L., Li R.C., Brittian K.R., Gozal D. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea. Pediatr Res. 2005;57(2):232–236. doi: 10.1203/01.PDR.0000150722.34561.E6. [DOI] [PubMed] [Google Scholar]
- 12.Goldbart A.D., Goldman J.L., Veling M.C., Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172(3):364–370. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheirandish-Gozal L., Serpero L.D., Dayyat E., et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33(5):1077–1084. doi: 10.1183/09031936.00130608. [DOI] [PubMed] [Google Scholar]
- 14.Esteitie R., Emani J., Sharma S., Suskind D.L., Baroody F.M. Effect of fluticasone furoate on interleukin 6 secretion from adenoid tissues in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2011 doi: 10.1001/archoto.2011.86. [DOI] [PubMed] [Google Scholar]
- 15.Brouillette R.T., Manoukian J.J., Ducharme F.M., Oudjhane K., Earle L.G., Ladan S., Morielli A. 2001. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. [DOI] [PubMed] [Google Scholar]
- 16.Kheirandish L., Goldbart A.D., Gozal D. Intranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing after tonsillectomy and adenoidectomy in children. Pediatrics. 2006;117(1):e61–e66. doi: 10.1542/peds.2005-0795. [DOI] [PubMed] [Google Scholar]
- 17.Kheirandish-Gozal L., Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122(1):e149–e155. doi: 10.1542/peds.2007-3398. [DOI] [PubMed] [Google Scholar]
- 18.Conners C.K., Staff M. Multi-Health Systems Inc; Toronto, ON, Canada: 2010. Conners' Continuous Performance Test (CPT II) computer programs for Windows. Technical Guide and Software Manual. [Google Scholar]
- 19.Conners C.K., Staff M. Multi-Health Systems Inc; Toronto, ON, Canada: 2010. Conners' Kiddie Continuous Performance Test -5 (K-CPT): Computer Program for Windows Technical Guide and Software Manual. [Google Scholar]
- 20.Tiffin J., Asher E.J. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 21.Conners C.K. Multi-Health Systems Inc; Tonawanda, NY: 2008. Conners 3rd Edition Manual. [Google Scholar]
- 22.Achenbach T.M., Rescorla L.A. University of Vermont , Research Center for Children, Youth & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- 23.Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L. Second edition. Professional Manual. Psychological Assessment Resources; 2015. Behavior Rating Inventory of Executive Function. [Google Scholar]
- 24.Chervin R.D., Hedger K., Dillon J.E., Pituch K.J. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 25.Melendres M.C., Lutz J.M., Rubin E.D., Marcus C.L. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114(3):768–775. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 26.Varni J.W., Seid M., Kurtin P.S. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Franco R.A., Jr., Rosenfeld R.M., Rao M. First place—resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123(1):9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 28.Stewart M.G., Witsell D.L., Smith T.L., Weaver E.M., Yueh B., Hannley M.T. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157–163. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Schulz K.F., Grimes D.A. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365(9470):1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos E.I., Kaditis A.G., Kalampouka E., et al. Nasal corticosteroids for children with snoring. Pediatr Pulmonol. 2004;38(2):161–167. doi: 10.1002/ppul.20079. [DOI] [PubMed] [Google Scholar]
- 31.Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro de Andrade C., Chatkin J.M., Fiterman J., Scaglia N., Camargos P.A. Unified disease, unified management: treating allergic rhinitis and asthma with nasally inhaled corticosteroid. Respir Med. 2010;104(10):1577–1580. doi: 10.1016/j.rmed.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Ruohola A., Heikkinen T., Waris M., Puhakka T., Ruuskanen O. Intranasal fluticasone propionate does not prevent acute otitis media during viral upper respiratory infection in children. J Allergy Clin Immunol. 2000;106(3):467–471. doi: 10.1067/mai.2000.108912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowson M.G., Ryan M.A., Ramprasad V.H., Choi K.J., Raynor E. Intranasal fluticasone associated with delayed tympanostomy tube placement in children with eustachian tube dysfunction. Int J Pediatr Otorhinolaryngol. 2017;94:121–126. doi: 10.1016/j.ijporl.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Stenström C., Bylander-Groth A., Ingvarsson L. Eustachian tube function in otitis-prone and healthy children. Int J Pediatr Otorhinolaryngol. 1991;21(2):127–138. doi: 10.1016/0165-5876(91)90143-y. [DOI] [PubMed] [Google Scholar]
- 36.Daich Varela M., Huryn L.A., Hufnagel R.B., Zein W.M., Blain D., Brooks B.P. Ocular and systemic findings in adults with uveal coloboma. Ophthalmology. 2020;127(12):1772–1774. doi: 10.1016/j.ophtha.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cumming R.G., Mitchell P., Leeder S.R. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337(1):8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 38.Jick S.S., Vasilakis-Scaramozza C., Maier W.C. The risk of cataract among users of inhaled steroids. Epidemiology. 2001;12(2):229–234. doi: 10.1097/00001648-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Bisgaard H., Allen D.B., Milanowski J., Kalev I., Willits L., Davies P. Twelve-month safety and efficacy of inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics. 2004;113(2):e87–e94. doi: 10.1542/peds.113.2.e87. [DOI] [PubMed] [Google Scholar]
- 40.Szefler S.J. Pharmacokinetics of intranasal corticosteroids. J Allergy Clin Immunol. 2001;108(1 suppl):S26–S31. doi: 10.1067/mai.2001.115563. [DOI] [PubMed] [Google Scholar]
- 41.Bousquet J., Chanal I., Alquié M.C., et al. Prevention of pollen rhinitis symptoms:comparison of fluticasone propionate aqueous nasal spray and disodium cromoglycate aqueous nasal spray. A multicenter, double-blind, double-dummy, parallel-group study. Allergy. 1993;48(5):327–333. doi: 10.1111/j.1398-9995.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 42.Thio B.J., Slingerland G.L., Fredriks A.M., et al. Influence of intranasal steroids during the grass pollen season on bronchial responsiveness in children and young adults with asthma and hay fever. Thorax. 2000;55(10):826–832. doi: 10.1136/thorax.55.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wartna J.B., Bohnen A.M., Elshout G., et al. Symptomatic treatment of pollen-related allergic rhinoconjunctivitis in children: randomized controlled trial. Allergy. 2017;72(4):636–644. doi: 10.1111/all.13056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.