Abstract
Objectives
This study assessed the efficacy, safety, pharmacokinetics (PK), and immunogenicity profiles of a denosumab biosimilar (LY06006) in Chinese postmenopausal osteoporotic women with a high risk of fracture.
Methods
In this multicenter, randomized, double-blind, placebo-controlled, phase 3 trial, 448 postmenopausal women aged 50–85 years with osteoporosis were enrolled at 49 centers in China and were randomly assigned (3:1) to receive 60 mg of the denosumab biosimilar (LY06006) or placebo subcutaneously every 6 months for 1 year. Lumbar spine bone mineral density (BMD) change was the primary endpoint.
Results
Of the 448 randomized patients, 409 (LY06006, n = 311; placebo, n = 98) completed the study. All 448 (100.0%) subjects were included in the intent-to-treat (ITT) trial, 427 (95.3%) were included in the full analysis set (FAS), 408 (91.1%) were included in the per protocol set (PPS), 446 (99.6%) were included in the safety set (SS), and 336 (75.0%) were included in the pharmacokinetics concentration set (PKCs). For the primary endpoint, a 4.71% (95% CI, 3.81%, 5.60%) treatment difference in percent change in lumbar spine BMD from baseline to month 12 was observed in the LY06006 group compared with the placebo group (P < 0.0001). For the secondary endpoints, LY06006 was associated with increased lumbar spine BMD levels measured at month 6, BMD levels at the femoral neck, total hip, and trochanter measured at months 6 and 12 and reduced serum C-terminal telopeptide of type 1 collagen (CTX) and procollagen type 1 N-peptide (P1NP) levels at months 1, 6, and 12. Safety analysis was based on the safety analysis set (SS), and 264 (78.6%) subjects in the LY06006 group and 83 (75.5%) in the placebo group experienced adverse events (AEs). Most events were mild or moderate and not related to the study drugs.
Conclusion
In postmenopausal women with a high risk of fracture, LY06006 increased the BMD and decreased bone resorption; thus, LY06006 might be an effective treatment for osteoporosis. LY06006 was generally safe and well tolerated without unexpected adverse reactions, similar to the reference drug Prolia®. The characteristics of effectiveness and safety were similar to those reported in previous studies.
The translational potential of this article
In this multi-center, randomized, double-blind, placebo-controlled phase 3 study, LY06006 showed substantially efficacy to increase BMD and well tolerance without unexpected adverse reactions, which is comparable to the reference drug Prolia ®. The presented results are encouraging and can offer some valuable evidence for the clinical practice.
Keywords: Biosimilar, Denosumab, Osteoporosis, Bone mineral density
1. Introduction
Osteoporosis is a common disease characterized by a systemic impairment of bone mass and microarchitecture that results in fragility fractures [1]. Estrogen deficiency increases tissue exposure to nuclear factor-kappa B ligand (RANKL), resulting in increased bone resorption and bone loss, which can lead to osteoporosis in postmenopausal women [2]. Denosumab can prevent RANKL from activating osteoclasts and inhibit the RANKL/receptor activator of nuclear factor kappa-B (RANK) interaction, thus reducing bone resorption and increasing the bone mass and strength of cortical bone and trabecular bone [3]. Denosumab can reduce hip, nonvertebral and vertebral fractures and is suitable for the initial treatment of most osteoporosis patients with a high fracture risk [4]. Treatment with denosumab general (Prolia®, Amgen Manufacturing Limited, Thousand Oaks, CA, USA), a fully human monoclonal antibody for RANKL [5], has been used in patients with osteoporosis for more than 10 years with proven efficacy and safety [6].
China and other global regions are actively developing denosumab biosimilars [[7], [8], [9]]. LY06006 possesses an identical primary structure to that of the denosumab reference product (Prolia®), and the posttranslational modifications, biochemical properties, and biological functions are also similar. The resemblance of this denosumab biosimilar (LY06006) to the denosumab reference product has also been evidenced in in-vivo studies, such as preclinical pharmacokinetics (PK), pharmacodynamics (PD), and pharmacological toxicological studies, that compared LY06006 and Prolia® (data not provided). Given the demonstrated highly similar analytical characterization and bioequivalence of LY06006 and Prolia® in PK and PD assessments, we conducted this multicenter, randomized, double-blind, placebo-controlled phase 3 study to explore the efficacy, safety, PK and immunogenicity of LY06006.
2. Materials and methods
This multicenter, randomized, double-blind, placebo-controlled phase 3 study was performed at 49 centers in China from June 2019 to August 2021 (ClinicalTrials.gov identifier: NCT05060406).
2.1. Study participants
2.1.1. Inclusion criteria
The subjects enrolled in this trial were ambulatory Chinese postmenopausal women aged 50–85 years old. The postmenopausal state was defined as the time of menopause ≥3 years, that is, spontaneous amenorrhea ≥3 years or ≥3 years after bilateral oophorectomy. For those aged <60 years with hysterectomy but ovarian retention, the menopausal state was confirmed by a level of follicle stimulating hormone (FSH) ≥ 40 U/L. All the enrolled subjects had osteoporosis based on a bone mineral density (BMD) T score of ≤ -2.5 and >-4.0 at either the lumbar spine or total hip. All the subjects had at least one of the following risk factors: a. History of previous fragility fractures, such as hip, distal ulna and radius or vertebral fractures; b. History of hip fracture of father or mother; c. Low body mass index (BMI ≤19 kg/m2); d. Old age (age ≥65 years); and e. Current smoking status. All the subjects volunteered to participate in the trial and signed informed consent forms.
2.1.2. Exclusion criteria
The exclusion criteria included diseases affecting calcium or bone metabolism, such as osteogenesis imperfecta, osteomalacia, Paget's disease, Cushing syndrome, hyperprolactinemia, hypophysis disease, acromegaly, and parathyroid diseases, namely, a history of hyperparathyroidism or hypoparathyroidism. Those with a history of hyperthyroidism or hypothyroidism who were receiving stable thyroid hormone replacement therapy were enrolled if they met the following conditions: normal thyroid hormone level; 4.5 μ IU/mL < thyroid stimulating hormone (TSH) level ≤10.0 μ IU/mL; and normal serum thyroxine (T4) level. Subjects with malabsorption syndrome or various gastrointestinal diseases associated with malabsorption, such as Crohn's disease and chronic pancreatitis, were excluded. Those with a hypocalcemia, hypercalcemia, or serum albumin corrected blood calcium level outside the normal laboratory range were excluded. Vitamin D deficiency also precluded entry into the study. Subjects with vitamin D deficiency [defined as 25-hydroxy vitamin D (25OHD) < 20 ng/mL in this study] were allowed to inject vitamin D2 200,000 international units (IU) during the screening period, and the 25OHD concentration was rechecked once. Those with a 25OHD concentration ≥20 ng/mL were included. Subjects with other diseases, such as rheumatoid arthritis, gout, and multiple myeloma, were excluded. Patients were excluded if they had two or more vertebral fractures or malignant tumors in the past five years (patients with completely resected in situ skin basal cell or squamous cell carcinoma, cervical cancer or breast ductal cancer were excluded). Those with severe renal disease and a creatinine clearance rate <30 mL/min were excluded. Patients with the following were excluded: a. Cirrhosis; b. Biliary abnormalities (except asymptomatic gallstones); c. Hepatitis C virus (HCV) antibody positivity; d. Hepatitis B surface antigen (HBsAg) positivity and peripheral blood HBV DNA titer ≥1 × 103 copies/mL, although one exception was that if the subject exhibited HBsAg positivity and an HBV DNA titer in peripheral blood <1 × 103 copies/mL and if the researchers believed that the chronic hepatitis B of the subject was stable and did not increase the risk to the subject, that subject was eligible for enrollment; and e. Alkaline phosphatase < lower limit of normal value (LLN), alkaline phosphatase or total bilirubin ≥1.5 times the upper limit of normal value (ULN), serum aspartate aminotransferase (AST) ≥ 2.0 × ULN, and serum alanine aminotransferase (ALT) ≥ 2.0 × ULN. Patients with the following oral and dental diseases were excluded: a. Previous or current mandibular osteomyelitis or mandibular necrosis; b. Acute dental or mandibular disease requiring oral surgery; c. Invasive dental surgery planned during the trial; and d. Failure of dental or oral surgery. Conditions affecting dual-energy X-ray absorptiometry (DXA) BMD measurement were as follows: a. There were less than two lumbar vertebrae that could be measured with DXA, and b. Height, weight or waist circumference hindered accurate DXA measurement. Those who received anti-osteoporosis treatment or drugs affecting bone metabolism as follows were excluded: a. Received RANKL inhibitor, fluoride or strontium salt treatment or intravenous bisphosphonates in the past 5 years; b. Oral bisphosphonates, but patients with the following conditions could be included, cumulative use >3 months but <3 years, the distance from the last medication to the screening visit ≥6 months, and cumulative use ≤3 months; c. And application of parathyroid hormone (PTH) or parathyroid hormone analog (PTHA), such as teriparatide, within 6 weeks before screening, anabolic hormones or testosterone, glucocorticoids (equivalent to > 5 mg/day prednisone >10 days), systemic hormone replacement therapy, selective estrogen receptor modulators (SERMs), such as raloxifene, tibolone, calcitonin, active vitamin D and its analogs, other bone active drugs including anticonvulsant drugs (except benzodiazepines) and heparin, and long-term systemic use of ketoconazole, androgen, adrenocorticotropic hormone, cinacalcet, aluminum agent, lithium agent, protease inhibitor, methotrexate and GnRH agonist. Patients who were positive for human immunodeficiency virus (HIV) antibody were excluded. Patients who abused drugs or alcohol [defined as the average drinking of 14 units or more alcohol per week (1 unit = beer 350 mL, or Chinese spirits 45 mL, or wine 150 mL) during the first 3 months of screening were excluded. Those with known allergies to the therapeutic drugs used in the study protocol, including those allergic to the test drugs, were excluded. Those who had received any other trial drugs or participated in another intervention clinical trial within 3 months before screening were excluded. Patients were excluded for other serious acute or chronic diseases, mental diseases or abnormal laboratory tests or for being deemed not suitable to participate in this study according to the judgment of the researcher.
2.2. Study design
The eligible subjects were randomly assigned (3:1) to receive 60 mg of the denosumab biosimilar (LY06006) (produced by Shandong Luye Pharmaceutical Co., Ltd.) or placebo subcutaneously at baseline and month 6, and follow-up visits were scheduled at months 1, 3, 6, 9 and 12. All the subjects received 500 mg of daily oral calcium and 600 IU of vitamin D supplementation throughout the study.
DXA scans were performed using Hologic and GE Lunar DXA scanners to determine T scores and BMD equivalents at baseline and at months 6 and 12. All the measurements for an individual subject were performed using the same scanner. The DXA scan was recorded at investigational sites and analyzed by both sites and a central reading facility (Parexel International). The results from the central reading facility were used as the final DXA results. To diagnose vertebral fractures, anterior–posterior and lateral X-ray films of the thoracic and lumbar spine were taken at baseline and 12 months.
Whole-blood samples were collected in serum separator tubes (SSTs) from subjects who fasted overnight at baseline and months 1, 6 and 12. The SSTs were immediately and gently reversed 5 times after blood collection to ensure that they were mixed well. The samples were allowed to stand for 30–60 min to solidify and then were centrifuged at 1800 g–2200 g at 2–8 °C for 10 min. After centrifugation, 1.0 mL of supernatant was immediately transferred into a frozen tube via a disposable transfer pipette, and the sample was stored at −80 °C until assay. Bone turnover markers, serum C-terminal telopeptide of type 1 collagen (CTX) and procollagen type 1 N-peptide (P1NP), were measured using blood samples from fasted subjects at baseline and months 1, 6 and 12. Assays were performed at a central laboratory of WuXi PharmaTech on a Roche Cobas® 6000 System Version using electrochemiluminescence (ECL) immunoassays with the following kits from Roche Diagnostics: β-crosslaps kit for CTX and total P1NP kit for P1NP. The QCs (LOQ = 0.3511 ng/mL, HQC = 0.8110 ng/mL) of CTX's intraassay %CV were 1.91% and 0.70%, and the QCs (LOQ = 0.3591 ng/mL, HQC = 0.8244 ng/mL) of CTX's interassay %CV were 1.34% and 1.21%. The QCs (LOQ = 27.84 ng/mL, HQC = 175.60 ng/mL) of the total P1NP intraassay %CV were 1.15% and 1.31%, and the QCs (LOQ = 28.49 ng/mL, HQC = 180.36 ng/mL) of the total P1NP interassay %CV were 1.40% and 2.22%.
Blood samples were collected at baseline and at months 1, 6, and 12, and early withdrawal in the overnight fasting state (fasting without water) was performed to detect serum anti-drug antibody (ADA) and neutralizing antibodies (Nab). Blood (4.0 mL) was collected at each time point. If the drug was administered on the same day, blood samples were collected before administration. The baseline measured value was taken as the baseline value. ADA and Nab were detected by United-Power Pharma Tech Co., Ltd. ADA was detected by bridging ECL immunoassay. Nab was detected by the competitive ligand binding test (CLBA) method based on the Meso-Scale Discovery (MSD) platform verified by methodology.
PK analyses were performed by United-Power Pharma Tech Co., Ltd. Thirty-two subjects were intensively sampled, and the other subjects were sparsely sampled. Blood (4.0 mL) was collected at each time point. Specific sampling time points and time windows are shown in Table 1. The serum concentrations of LY06006 were analyzed with sensitive and specific ECL. The assay method used biotinylated HCA288 and ruthenium-labeled HCA280 as reagents and was validated in compliance with SOP at the United-Power Pharma Tech Co., LTD.
Table 1.
Blood sample collection form for pharmacokinetic study.
| Time for blood sampling | Intensively sampled | Sparsely sampled |
|---|---|---|
| 0 h (Before the first application) | X | X |
| 7±1 d | X | |
| 14±1 d | X | |
| 21±1 d | X | |
| 30±5 d | X | X |
| 60±5 d | X | |
| 90±5 d | X | X |
| 120±7 d | X | |
| 180±7 d (Before the second application) | X | X |
| 270 ± 14 d | X | X |
| 360 ± 14 d | X | X |
| early exit | X | X |
Abbreviation: D = day
Clinical efficacy and safety evaluations were conducted through vital sign assessment, physical examination, ECG analysis and laboratory examination of the subjects. The laboratory safety evaluation included routine blood tests, urinalysis, serum chemistry assessment, and measurements of parathyroid hormone and 25OHD. Adverse events (AEs) and drug combinations were also recorded and analyzed. The subjects, investigators, and sponsor were blinded to the results of BMD, bone turnover markers, and other laboratory analyses throughout the duration of the study.
2.3. Study endpoints
The primary endpoint was the mean percent change in lumbar spine BMD from baseline to month 12. Secondary endpoints included the mean percent change in lumbar spine BMD from baseline to month 6, the mean percent change in total hip, femoral neck, and trochanter BMD from baseline to months 1, 6 and 12, and the median percent change in CTX and P1NP from baseline to months 1, 6, and 12.
Safety was assessed by monitoring AEs, including serious AEs (SAEs), vital signs, laboratory tests, electrocardiogram and physical examination results at screening, and the denosumab biosimilar (LY06006) antibody assay results. The serum of the subjects was screened for anti-denosumab biosimilar (LY06006)-binding antibodies using an ECL bridging immunoassay.
2.4. Statistical analysis
SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) was used to analyze the significance level α = 0.05, and all statistical tests adopted a two-sided test. A P value < 0.05 was considered statistically significant. Continuous variables were grouped by study and analyzed by descriptive statistical methods. Categorical variables were grouped according to the study, and the number and percentage of patients were calculated.
The primary efficacy analysis population was the full-analysis-set (FAS) population. For the analyses of the primary endpoint, the percent change in BMD at the lumbar spine from pretreatment baseline to month 12 was compared between the denosumab biosimilar (LY06006) and placebo using the mixed model repeated measures (MMRM) approach with baseline as a covariate and treatment, visit, treatment-by-visit, and baseline-by-visit as fixed effects. An unstructured variance covariance matrix was used for the within-subject variation. In case there was a convergence problem in the MMRM model with the unstructured variance covariance matrix, the following variance covariance matrix structures were prespecified in the order of 1) heterogeneous Toeplitz, 2) heterogeneous autoregressive of order 1, and 3) heterogeneous compound symmetry. The first (co)variance structure that did not have a convergence problem was used for the analysis.
The analysis was based on the pharmacokinetics concentration set (PKCs). Descriptive statistics were made on the blood drug concentration of each group according to the grouping and planned blood collection time. The number of cases, > or below the quantization limit (BQL), arithmetic mean, standard deviation, coefficient of variation (based on arithmetic mean), median, minimum, maximum, geometric mean and geometric coefficient of variation were used for summary analysis. The time curve (linear and semilogarithmic) of the average blood drug concentration in each group was drawn according to the planned blood collection time.
Calculation of the sample size: The present study was designed as a phase III study (DPH114165) and the first to assess the efficacy and safety of denosumab (Prolia®) in a Chinese population. After 12 months of treatment, the difference in the change rate of the lumbar BMD between the denosumab group and placebo group was 4.42% (95% CI: 3.67–5.18%); assuming that the difference in the change rate of the lumbar BMD between the experimental group and placebo group after 12 months of treatment was 4% and the standard deviation (SD) was 6, when the grasp was 90%, α = 0.05 (bilateral), and the ratio of the experimental group to the placebo group was 3:1, so 99 patients were needed in the experimental group, and 33 patients were needed in the placebo group. According to the measures for the administration of drug registration (Order No. 28) and the written reply of the Center for Drug Evaluation (CDE) on the sample size, the minimum number of patients (experimental group) in the phase III clinical trial was required to be 300, which was designed according to a 3:1 ratio, so a total of 400 subjects were needed to be enrolled. Considering the loss of subjects, a total of 448 subjects were needed to be enrolled. Considering the statistics and regulatory requirements, 448 subjects were enrolled in this trial, including 336 in the experimental group and 112 in the control group.
3. Ethics statement
This study was approved by an ethics committee and conducted according to Good Clinical Practice, and each subject gave written informed consent prior to study entry. The subjects were free to withdraw at any time throughout the study.
4. Results
4.1. Subject disposition and demographics
Five hundred ninety-one (591) of the 1039 subjects failed the screening process (Fig. 1). The reasons for screening failures were failure to meet the inclusion/exclusion criteria (469 subjects), subject withdrawal of consent (14 subjects), AE (1 subject), and other reasons (107 subjects). The intent-to-treat (ITT) population (n = 448) included 337 and 111 subjects who were randomized to the denosumab biosimilar (LY06006) or placebo group, respectively. A total of 427 (95.3%) subjects were included in the FAS. Three hundred eleven (311) denosumab biosimilar (LY06006)-treated and 98 placebo-treated subjects completed the double-blind phase; the most common reason for dropping out of the double-blind phase was withdrawal of consent.
Fig. 1.
Subject disposition.
The baseline demographics were mostly similar between the treatment groups. The baseline T scores and bone turnover marker levels were also comparable between the treatment groups (Table 2). A history of previous bone fracture was noted in 118 (36.9%) subjects in the LY06006 group and 50 (46.7%) in the placebo group. The most common fracture was lumbar fracture (20 in the LY06006 group and 6 in the placebo group). A parent history of hip fracture was noted in 110 (34.4%) subjects in the LY06006 group and 31 (29.0%) in the placebo group.
Table 2.
Baseline characteristics (FAS population).
| Characteristic | LY06006 (N = 320) | Placebo (N = 107) |
|---|---|---|
| Age, mean ± SD (range) | 65.3 ± 6.04 (51,82) | 65.1 ± 6.13 (50,80) |
| Ethnicity | ||
| Han Chinese | 315 (98.4%) | 103 (96.3%) |
| Other | 5 (1.6%) | 4 (3.7%) |
| Height, cm, mean ± SD | 155.15 ± 5.848 | 155.93 ± 5.976 |
| Weight, kg, mean ± SD | 56.25 ± 7.126 | 55.58 ± 8.065 |
| BMI, kg/m2, mean ± SD (range) | 23.37 ± 2.701 (17.0, 33.0) | 22.842 ± 2.910 (16.9, 29.5) |
| Menopause time ≥3 years | 320 (100.0%) | 107 (100.0%) |
| Previous fracture, n (%) | 118 (36.9%) | 50 (46.7%) |
| Parent history of hip fracture, n (%) | 110 (34.4%) | 31 (29.0%) |
| BMD T-score mean ± SD | ||
| Lumbar spine | −3.14 ± 0.529 | −3.09 ± 0.538 |
| Femoral neck | −2.50 ± 0.688 | −2.57 ± 0.587 |
| Total hip | −2.03 ± 0.695 | −2.06 ± 0.637 |
| Trochanter | −1.92 ± 0.659 | −1.90 ± 0.638 |
| 25(OH)D, ng/mL, median (Q1,Q3) | 24.20 (21.40, 27.67) | 24.77 (20.79, 30.22) |
| Bone turnover markers, mean ± SD | ||
| s-CTX, ng/mL | 0.586 ± 0.2522 | 0.600 (0.2487) |
| s-PINP, ng/mL | 63.260 ± 22.9430 | 64.923 (24.6281) |
BMD, bone mineral density; BMI, body mass index; FAS, full analysis set; SD, standard deviation; s-CTX, serum C-terminal telopeptide of type I collagen; s-PINP, serum procollagen type I N-terminal propeptide.
4.2. Efficacy
The efficacy analysis was based on the FAS population.
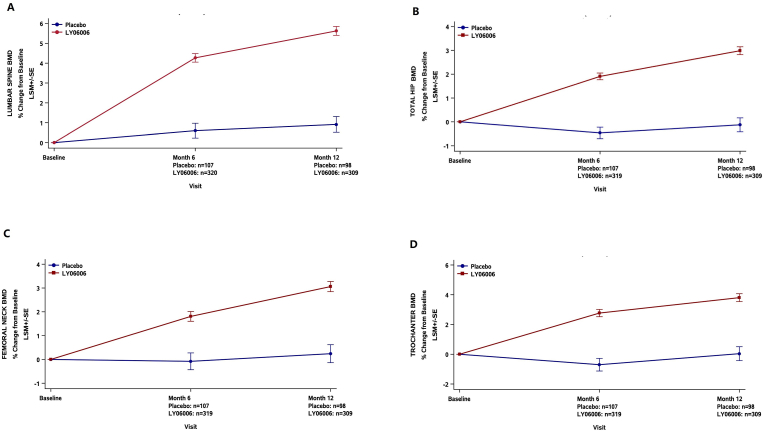
For the primary endpoint, a 4.71% (95% CI, 3.81%, 5.60%) treatment difference in the percent change in lumbar spine BMD from baseline to month 12 was observed in the LY06006 group compared with the placebo group (P < 0.0001) (Fig. 2A).
Fig. 2.
Mean percent change from baseline in BMD in lumbar spine (A), total hip (B), femoral neck (C), and trochanter (D). Least Squares Mean (±SE) of % Change from Baseline to months 6 and 12 (On-Treatment) (FAS, N = 427).
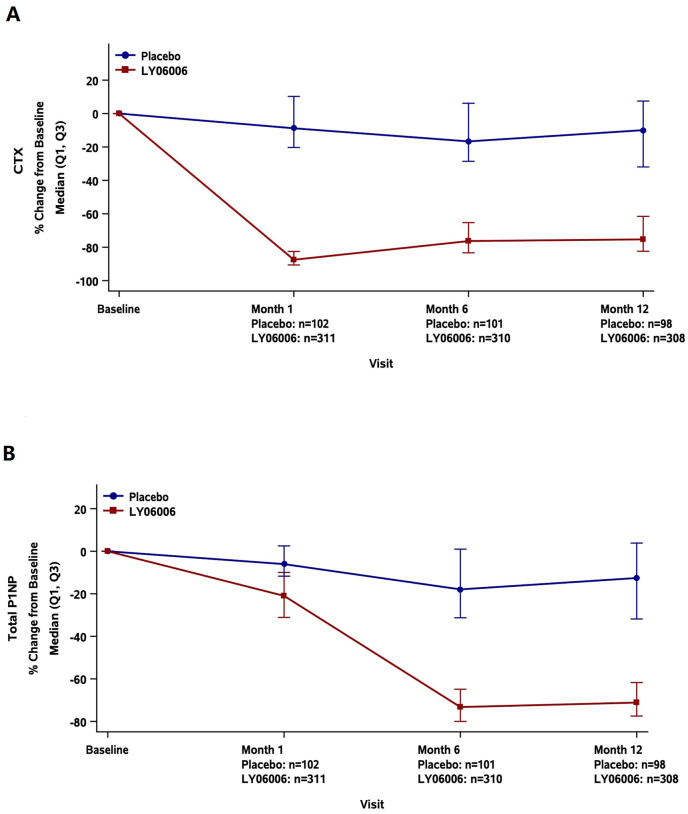
For the secondary endpoints, at month 6, LY06006 demonstrated a treatment difference compared with the placebo for the mean percent change in BMD for the lumbar spine [3.67% (95% CI, 2.82%, 4.51%; p < 0.0001)], femoral neck [1.89% (95% CI, 1.09%, 2.69%; p < 0.0001)], total hip [2.37% (95% CI, 1.82%, 2.92%; p < 0.0001)] and trochanter [3.47% (95% CI, 2.51%, 4.44%; p < 0.0001)]. At month 12, LY06006 demonstrated a treatment difference compared with the placebo for the mean percent change in BMD for the lumbar spine [3.67% (95% CI, 2.82%, 4.51%; p < 0.0001)], femoral neck [2.82% (95% CI, 1.97%, 3.68%; p < 0.0001)], total hip [3.11% (95% CI, 2.45%, 3.77%; p < 0.0001)] and trochanter [3.77% (95% CI, 2.71%, 4.82%; p < 0.0001)] (Fig. 2A-D). At months 1, 6 and 12, the change rate in CTX in the LY06006 group compared with the placebo group decreased by 78.42% (95% CI: 75.30%, 81.64%), 58.66% (95% CI: 54.07%, 63.41%) and 61.15% (95% CI: 54.97%, 66.68%), respectively (all P < 0.0001) (Fig. 3A). At months 1, 6 and 12, the change rates in P1NP in the LY06006 group compared with the placebo group decreased by 15.69% (95% CI: 12.58%, 18.71%), 53.89% (95% CI: 49.74%, 58.15%) and 55.31% (95% CI: 50.33%, 59.88%), respectively (all P < 0.0001) (Fig. 3B).
Fig. 3.
Median percent change from baseline in bone turnover markers, serum CTX(A) and P1NP(B). Median (Q1,Q3) of % Change from Baseline to months 1, 6 and 12 (On Treatment) (FAS, N = 427).
4.3. Pharmacokinetics results
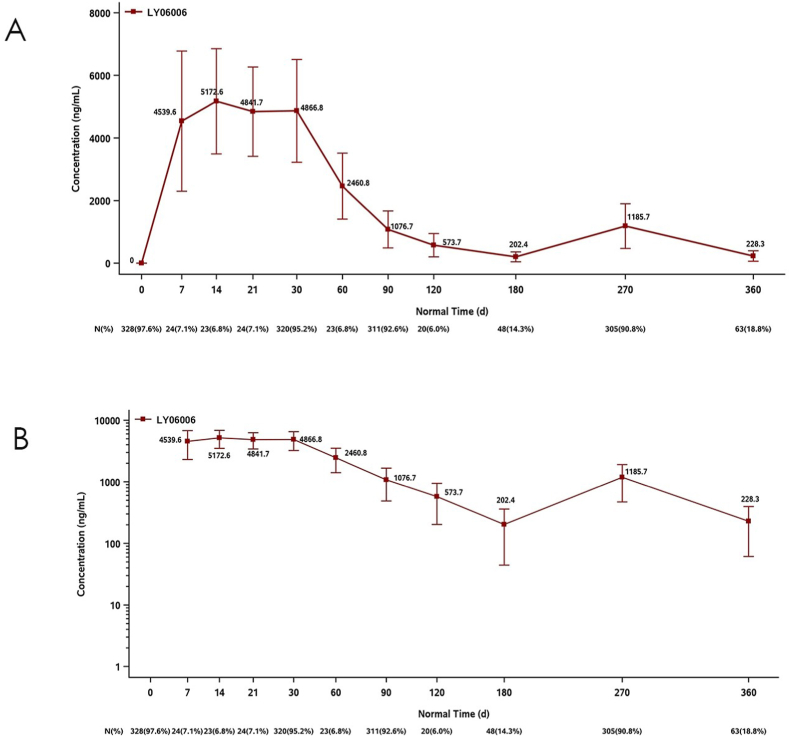
PK descriptive statistical analysis was based on the PKCs. A total of 336 (75.0%) subjects were included in the PKCs. In the PKCs, the subjects in the LY06006 group reached the peak at Day 14 after the first administration, and the serum drug peak concentration (CV%) was 5172.60 ng/mL (32.50%) and then decreased slowly (Fig. 4A). On the day of the second administration, the average serum drug concentration (CV%) of LY06006 before administration reached 202.40 ng/mL (78.10%), and then after the second administration, the serum drug concentration (CV%) on Day 90 (which was Day 270 after the first administration) rose to 1185.70 ng/mL (60.30%), which was close to 1076.70 ng/mL (54.80%) in Day 90 serum after the first administration (Fig. 4B).
Fig. 4.
Mean (±SD) plasma concentrations of LY06006 versus time by dose (linear scale) PKCS (A), and by dose (semilogarithmic scale) PKCS (B) (N = 336).
4.4. Safety
The safety analysis was based on the safety set (SS). A total of 446 (99.6%) subjects were included in the SS. In the SS, the vast majority of the subjects in the experimental group and the control group (312 patients, 92.9% vs. 100 patients, 90.9%) completed two rounds of study drug treatment, with an average study time (SD) of 11.6 (2.14) months and 11.2 (2.50) months, respectively. A total of 264 (78.6%) subjects in the LY06006 group and 83 (75.5%) in the placebo group experienced 1053 and 312 AEs, respectively.
A total of 261 (77.7%) subjects in the LY06006 group and 82 (75.5%) in the placebo group experienced 1005 and 300 treatment-emergent adverse events (TEAEs), respectively. The most common TEAEs were urinary tract infections and upper respiratory tract infections. Fifty-six (16.7%) subjects in the LY06006 group and 11 (10.0%) in the placebo group experienced upper respiratory tract infections. Thirty-seven (11.0%) subjects in the LY06006 group and 8 (7.3%) in the placebo group experienced upper respiratory tract infections. A total of 105 (31.3%) subjects in the LY06006 group and 24 (21.8%) in the placebo group experienced 210 and 55 study drug-related TEAEs, respectively. The TEAEs related to the study drug with a high incidence in the two groups were urinary tract infections, arthralgia, back pain, decreased blood alkaline phosphatase levels, hypocalcemia and hypercalcemia (Table 3). The TEAEs related to the study drug that led to the withdrawal of the subjects from the study were back pain and eczema. The TEAE related to the study drug that led to the withdrawal of the study drug was back pain. The incidence of mild TEAEs was 56.0% and 60.0% in the experimental group and the control group, respectively, the incidence of moderate TEAEs was 18.5% and 11.8%, respectively, and the incidence of severe TEAEs was 3.3% and 2.7%, respectively. The incidence of mild TEAEs related to the study drug was 27.7% and 20.0%, respectively, and the incidence of moderate TEAEs related to the study drug was 3.6% and 1.8%, respectively. No severe TEAEs related to the study drug occurred in the LY06006 group or the placebo group. Five subjects withdrew due to TEAEs (four in the LY06006 group, one in the placebo group), and there were no unanticipated AEs or AEs of special interest, such as osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF) events, in the study. The incidence of TEAEs related to the study drug and leading to the withdrawal of the study drug in the LY06006 group and the control group were 0.6% and 0.0%, respectively, and the incidence of TEAEs related to the study drug and leading to the withdrawal of the study drug were 0.3% and 0.0%, respectively. The incidence of TEAEs causing subjects to withdraw from the trial was 1.2% and 0.9%, respectively. The incidence of TEAEs related to the study drug and causing subjects to withdraw from the trial was 0.6% and 0.0%, respectively. There was no TEAE leading to the death of subjects in the LY06006 group or the control group.
Table 3.
Adverse events.
| Adverse event, n (%) | LY06006 (n = 336) | Placebo (n = 110) |
|---|---|---|
| Any AE | 264 (78.6%) | 83 (75.5%) |
| SAEs | 35 (10.4%) | 9 (8.2%) |
| Death | 0 | 0 |
| TEAEs | 261 (77.7%) | 82 (74.5%) |
| Withdrawal due to TEAEs | 4 (1.2%) | 1 (0.9%) |
| Withdrawal due to TEAEs related to the study drug | 2 (0.6%) | 0 (0.0%) |
| TEAEs with an incidence ≥5% in either group | ||
| Urinary tract infection | 56 (16.7%) | 11 (10.0%) |
| Upper respiratory infection | 37 (11.0%) | 8 (7.3%) |
| Elevated serum creatine phosphokinase | 5 (1.5%) | 6 (5.5%) |
| Hyperglycemia | 5 (1.5%) | 6 (5.5%) |
| White blood cells urine positive | 5 (1.5%) | 6 (5.5%) |
| Backache | 19 (5.7%) | 5 (4.5%) |
| Arthralgia | 20 (6.0%) | 4 (3.6%) |
| Pain in limb | 9 (2.7%) | 6 (5.5%) |
| Hyperuricemia | 16 (4.8%) | 7 (6.4%) |
| Hyperlipidemia | 17 (5.1%) | 5 (4.5%) |
| TEAEs related to the study drug with an incidence ≥2% in either group | ||
| Urinary tract infection | 14 (4.2%) | 2 (1.8%) |
| Arthralgia | 9 (2.7%) | 1 (0.9%) |
| Hyperuricemia | 3 (0.9%) | 3 (2.7%) |
| Backache | 7 (2.1%) | 1 (0.9%) |
| Alkaline phosphatase decreased | 7 (2.1%) | 0 (0.0%) |
| Hypocalcemia | 7 (2.1%) | 0 (0.0%) |
| Hypercalcemia | 7 (2.1%) | 0 (0.0%) |
AE, adverse event; SAE, serious adverse event; TEAE Treatment-related adverse event
In the LY06006 group and the placebo group, 35 (10.4%) and 9 (8.2%) subjects had 41 cases and 11 cases of SAEs, respectively, which were determined to be unrelated to the study drug. In the SS, 2 (0.6%) subjects in the LY06006 group had SAEs that caused the subjects to withdraw from the test twice, and there was no SAE that caused the subjects to withdraw from the test in the control group. Neither the LY06006 group nor the control group had any SAEs related to the study drug that led to the subjects' withdrawal from the study. No unexpected laboratory or vital sign changes were observed.
4.5. Immunogenicity analysis results
Immunogenicity analysis was based on the SS. A total of 446 (99.6%) subjects were included in the SS. In the SS, before administration, 329 (98.5%) subjects in the test group were ADA negative, 5 (1.5%) subjects were ADA positive, the titer values were 1:50 (minimum dilution multiple of the sample), and 109 (100%) subjects in the control group were ADA negative. The total results for 1, 6 and 12 months of study drug treatment showed that there were no ADA-positive subjects in the control group. In the test group, 4 (1.2%) subjects were ADA positive at least once after administration, and one of them produced Nab.
5. Discussion
This study assessed the efficacy, safety, PK, and immunogenicity profiles of a denosumab biosimilar (LY06006) in Chinese postmenopausal osteoporotic women with high risk of fracture. LY06006 showed a benefit over the placebo in increasing the BMD at the lumbar spine as well as at the total hip, femoral neck, and trochanter and decreasing the levels of the bone turnover markers CTX and P1NP.
Prolia® (denosumab injection) was approved by the National Medical Products Administration (NMPA) for the treatment of osteoporosis in postmenopausal women with a high risk of fracture in China in June 2020. It is the first and only anti-RANKL monoclonal antibody in China for the treatment of osteoporosis. Denosumab reduced the risk of vertebral, nonvertebral and hip fractures and increased the BMD across skeletal sites versus placebo in the FREEDOM trial, with these benefits maintained for up to 10 years of therapy [3,6]. It has broad prospects for clinical application and can be used in sequential or combination therapies [10]. The research and development of a denosumab biosimilar can benefit more patients, which improves drug accessibility to patients and decreases healthcare-associated costs.
LY06006 (recombinant anti-RANKL whole human monoclonal antibody injection) developed by Shandong Boan Biotechnology Co., Ltd. Shows high similarity with Prolia® in pharmaceutical and nonclinical studies, and its clinical localization is consistent with that of Prolia®.
The present study was designed as a twelve-month randomized, double-blind, placebo-controlled, parallel-group, multicenter, phase III study (DPH114165) to evaluate the efficacy and safety of LY06006 in Chinese postmenopausal women with osteoporosis at increased risk of fracture [11], which is the first study to assess the efficacy and safety of a denosumab biosimilar (Prolia®) in the Chinese population. In this study, the denosumab biosimilar LY06006 showed a benefit over placebo in increasing the BMD at the lumbar spine as well as at the total hip, femoral neck, and trochanter and decreasing the levels of the bone turnover markers CTX and P1NP. The results were similar to the data of the FREEDOM Trial [12].
In this study, 446 (99.6%) subjects were included in the SS. In the SS, 261 (77.7%) subjects in the LY06006 group and 82 (74.5%) subjects in the control group had 1005 and 300 TEAEs, respectively, and 105 (31.3%) subjects in the LY06006 group and 24 (21.8%) subjects in the control group had 210 and 55 TEAEs related to the study drug, respectively. The TEAEs related to the study drug with a high incidence in the two groups were urinary tract infections, arthralgia, hyperuricemia, back pain, decreased blood alkaline phosphatase levels, hypocalcemia and hypercalcemia. RANKL and RANK are members of the tumor necrosis factor superfamily that are expressed by a variety of lymphoid cells [13]. It has been theorized that the inhibition of RANKL might increase the risk of cancer or infection [14]. Among the TEAEs in our study, the infections were mainly common urinary tract infections and upper respiratory tract infections in elderly individuals, and no serious skin infections or interstitial pneumonia were seen. No cancer was reported in the LY06006 group or the placebo group. The TEAEs related to the study drug that led to the withdrawal of the subjects from the study were eczema and back pain. Eczema was reported in 3.0% of the subjects in the denosumab group in the FREEDOM Trial [12], and back pain was reported in a randomized placebo-controlled trial of the efficacy of denosumab in Indian postmenopausal women with osteoporosis [15]. The TEAE related to the study drug that led to the withdrawal of the study drug was back pain.
In the LY06006 group and the placebo group, 35 (10.4%) and 9 (8.2%) subjects had 41 cases and 11 cases of SAEs, respectively, which were determined to be unrelated to the study drug. The most common SAEs in the LY06006 group were various neurological diseases (8 cases, 2.4%), coronary artery diseases (4 cases, 1.2%), and musculoskeletal diseases (5 cases, 1.5%). There were no cases of ONJ or AFF events in the study. This may be related to our shorter study period and the use of only two medications.
LY06006 appeared to be well tolerated, and no significant safety issues were observed. The Prolia® label lists the most common AEs as hypocalcemia, musculoskeletal pain, arthralgia, back pain, and hypercholesterolemia, which are similar to the results of this study. The type and incidence of AEs in this study were also similar to the data and results of a phase III clinical study of Prolia® (DPH114165) among Chinese patients [11]. Because of the differences in osteoporosis management guidelines at the time of the study design [16], the minimum vitamin D entry criteria in the study (20 ng/mL) was higher than that in the FREEDOM study (12 ng/mL). The lower incidence of hypocalcemia (2.1% of the LY06006 group) in our study may be due to our higher baseline vitamin D levels, as shown in Table 2.
In the PKCs, LY06006 peaked on D14 in the denosumab biosimilar group after the first administration, with a concentration (CV%) of 5172.60 ng/mL (32.50%), and then decreased slowly. On the day of the second dosing, the average serum trough concentration (CV%) of LY06006 was 202.40 ng/mL (78.10%) before administration, and it increased to 1185.70 ng/mL (60.30%) on D90 after the second dosing (equivalent to D270 after the first dosing), which is similar to the serum concentration (CV%) of D90 after the first dosing, with a value of 1076.70 ng/mL (54.80%). The PK/PD model of LY06006 and serum CTX showed an exposure-effect (E-R) relationship using a simple Emax model connected with effect chambers. The concentration of CTX decreased with increasing dose. There was no E-R relationship between BMD and LY06006 exposure (Cmax and Cav) at month 12, which was consistent with the review documents of Prolia®, suggesting that the therapeutic effect had reached the “ceiling plateau”. In the PopPK model of LY06006, the exposure (Cmax and AUC) decreased significantly with increasing body weight by showing that the Cmax and AUC0∼6-month changes were 20.2% and −15.9%, 29.8% and −22.2%, respectively, in the comparison between the 5th and 95th weight and median weight percentiles of the study population. However, combined with the E-R analysis of efficacy and safety, the efficacy of 60 mg once every 6 months reached a plateau, while the safety risk did not increase, which supported a fixed-dose administration of LY06006.
In this study, immunogenicity was analyzed along with safety. The subjects in the placebo group were ADA negative before and after administration. Before administration, 5 (1.5%) subjects in the LY06006 group were ADA positive. After receiving the study drug treatment, 4 (1.2%) subjects in the LY06006 group were ADA positive at least once after administration, of which 1 patient produced Nab. All ADA-positive subjects tolerated the drug safely. In this study, the serum of healthy subjects was used to determine the screening threshold (SCP), confirmation threshold (CCP) and titer threshold (TCP), which may fluctuate when used for the detection of phase III patients. It was reported that if the false-positive reporting rate (FPER) of clinical baseline samples in the study is in the range of 2–11% after excluding samples with previous ADA, the same SCP and CCP values determined by pre study validation can be used for clinical study sample evaluation [17]. In this study, the positive rate of ADA in patient samples before administration was only 1.5%, so the threshold was not redefined using patient samples before administration. Therefore, positive subjects with low titers may cause analysis error due to the difference in thresholds. Prolia® FDA instructions suggest that the incidence of ADA is highly dependent on the sensitivity and specificity of the analysis. In addition, the incidence of ADA (including Nab) observed in a certain analysis may be affected by several factors, including analytical methodology, sample processing, sampling time, drug combination and combined disease. According to the above analysis of ADA-positive subjects, transient ADA positivity may be affected by many factors and has no practical clinical significance. There was no significant change or abnormality in PK characteristics or safety events in 1 subject with persistent ADA positivity and Nab. In conclusion, compared with the placebo, LY06006 has similar safety in the treatment of osteoporosis in postmenopausal women with a high risk of fracture, and ADA has no clinical significance.
Among denosumab biosimilar studies searched in PubMed, we found only one phase 3 clinical study, published in India Journal of Pharmacology [9]. In their study, a total of 114 patients were randomly allocated to receive a denosumab biosimilar (n = 58) or denosumab reference (n = 56) at a subcutaneous dose of 60 mg every 6 months for a year. Although both studies assessed the efficacy, safety, PK and immunogenicity profiles, the sample size in our study was larger than that of the study conducted by Singh I et al. Therefore, our results may be more convincing to show the efficacy, safety, PK and immunogenicity of a denosumab biosimilar.
The limitation of this study was that Prolia® had not been approved in China at the beginning of this study, so this study did not carry out head-to-head research with Prolia®. After communication with CDE, it was suggested that a placebo control be used in this study.
6. Conclusion
This multicenter, randomized, double-blind, placebo-controlled phase 3 study demonstrated that compared with a placebo, subcutaneous injection of LY06006 every 6 months for one year significantly increased the BMD of the lumbar spine, total hip, femoral neck and femoral trochanter in Chinese postmenopausal osteoporosis women with a high risk of fracture and significantly reduced the levels of the bone turnover markers CTX and P1NP. The safety assessment indicated that LY06006 was well tolerated, and no unexpected adverse reactions occurred. Compared with previous studies of the reference drug Prolia®, the efficacy and safety characteristics were similar.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of all participating hospitals. Written informed consent to participate in this study was provided by the participants.
Funding
This study was supported by the sponsor, Shandong Boan Biotechnology Co., Ltd.
Trial Registration
The trial (NCT05060406) is registered with ClinicalTrials.gov.
Acknowledgments
We are grateful to the subjects who participated in this study. The study was supported by Shandong Boan Biotechnology Co., Ltd.
References
- 1.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofbauer L.C., Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 3.McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 4.Camacho P.M., Petak S.M., Binkley N., Diab D.L., Eldeiry L.S., Farooki A., et al. AMERICAN association of clinical endocrinologists/AMERICAN college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal OSTEOPOROSIS-2020 update. Endocr Pract. 2020;26:1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 5.Amgen. Amgen PROLIA (denosumab) prescribing information. Available online at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125320s202lbl.pdf.
- 6.Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Li C., Liu J., Wu M., Li X., Zhu X., et al. Safety and pharmacokinetics of a biosimilar of denosumab (KN012): phase 1 and bioequivalence study in healthy Chinese subjects. Expet Opin Invest Drugs. 2021;30:185–192. doi: 10.1080/13543784.2021.1863371. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., Wu M., Zhu X., Li C., Li X., Sun J., et al. A phase I, randomized, single-dose study to evaluate the biosimilarity of QL1206 to denosumab among Chinese healthy subjects. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh I., Jose V., Patel R., Arora S. Denosumab biosimilar in postmenopausal osteoporotic women: a randomized, assessor-blind, active-controlled clinical trial. Indian J Pharmacol. 2021;53:6–12. doi: 10.4103/ijp.IJP_346_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y.L., Huang Z.L., Wang Y., Xu W.C., Chen H.J., Xu J.K., et al. The efficacy and safety of denosumab in postmenopausal women with osteoporosis previously treated with bisphosphonates: a review. J Orthop Translat. 2019;9(22):7–13. doi: 10.1016/j.jot.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://s3.amazonaws.com/ctr-gsk-7381/114165/fae33418-e8da-41a5-9ff4-8f9a11d7df16/8a4034e8-aa04-4eda-b07a-16d742cafeec/gsk-114165-clinical-study-result-summary-v1.pdf, last accessed is 2022.10.27.
- 12.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 13.Martin T.J. Paracrine regulation of osteoclast formation and activity: milestones in discovery. J Musculoskelet Neuronal Interact. 2004;4:243–253. [PubMed] [Google Scholar]
- 14.Whyte M.P. The long and the short of bone therapy. N Engl J Med. 2006;354:860–863. doi: 10.1056/NEJMe068003. [DOI] [PubMed] [Google Scholar]
- 15.Pitale S., Thomas M., Rathi G., Deshmukh V., Kumar P., Reddy S., et al. A randomized placebo-controlled trial of the efficacy of denosumab in Indian postmenopausal women with osteoporosis. Indian J Endocrinol Metab. 2015;19:148–154. doi: 10.4103/2230-8210.146871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Devanarayan V., Smith W.C., Brunelle R.L., Seger M.E., Krug K., Bowsher R.R. Recommendations for systematic statistical computation of immunogenicity cut points. AAPS J. 2017;19:1487–1498. doi: 10.1208/s12248-017-0107-3. [DOI] [PubMed] [Google Scholar]