Abstract
The disparity in the free radical generation and the production of antioxidants to counteract its effect is known as oxidative stress. Oxidative stress causes damage to the macromolecules such as lipids, carbohydrates, proteins, and DNA and RNA. The oxidative damage to the cellular components leads to a process of aging and various age-associated disorders. The literature survey for this review was done using PubMed, Google Scholar, and Science Direct. The papers showing the studies related to aging and age-associated disorders have been selected for reviewing this paper. Ellagic acid has been used as the keyword, and more emphasis has been put on papers from the last 10 years. However, some papers with significant studies prior to 10 years have also been considered. Almost 250 papers have been studied for reviewing this paper, and about 135 papers have been cited. Ellagic acid (EA) is present in high quantities in pomegranate and various types of berries. It is known to possess the antioxidant potential and protects from the harmful effects of free radicals. Various studies have shown its effect to protect cardiovascular, neurodegenerative, cancer, and diabetes. The present review focuses on the protective effect of ellagic acid in age-associated disorders. The effect of EA has been studied in various chronic disorders but the scope of this review is limited to cancer, diabetes, cardiovascular and neurodegenerative disorders. All the disease aspects have not been addressed in this particular review.
Keywords: Ellagic acid, Antioxidant, Cancer, Diabetes, Neurodegeneration, Cardiovascular
Introduction
The current advances in public health care and remarkable development in the field of medicinal chemistry have improved the expectancy of life. The elderly population is continuously increasing globally. Chronic diseases such as diabetes, heart attack, and strokes are more common and usually accompanied by advancing age (Jura and Kozak 2016). Aging is an intricate procedure that is characterized by the free radical species and mainly causes permanent oxidative injury at the cellular and molecular levels (Kharat et al. 2020; Maurya et al. 2016).
Free radicals are extremely reactive atoms or molecules which consist of an unpaired electron in their last orbit and are formed with the interaction of oxygen with some molecules (Losada-Barreiro and Bravo-Diaz 2017). These free radicals can be formed in cellular components with the loss or gain of a single electron and hence behaves as reductants or oxidants (Liguori et al. 2018). The reactive radicals and non-radicals derived from oxygen and nitrogen are described as reactive oxygen species (ROS) and reactive nitrogen species (RNS), respectively. ROS and RNS have a critical role in the process of aging and the diseases associated with it (Sun et al. 2018; Salehi et al. 2018).
Oxidation is a type of chemical reaction in which free radicals are produced and these free radicals can damage the cells of the organisms (Neha et al. 2019). ROS is responsible for the oxidative damage of the macromolecules such as lipids, carbohydrates, proteins, and nucleic acid. Oxidative stress is a definite state of cells in which the ROS and RNS are in higher numbers than the normal condition of the cells (Dong et al. 2021). The disparity between the oxidants and the antioxidant ratio is stated as oxidative stress (Trueb 2021; Gessner et al. 2017). Superoxide radical (O2−), hydrogen peroxide (H2O2), hydroxy radical (OH•), nitric oxide (NO), and peroxynitrite (ONOO−) are the reactive oxygen or nitrogen species that are naturally produced by all the aerobic organisms (Gulcin 2020; Rizvi and Maurya 2007). These are responsible for maintaining homeostasis along with the antioxidant molecules and enzymes (Unsal et al. 2021). Antioxidants are the molecules that negatively affect the oxidation reaction. The generation of ROS and RNS leads to increased oxidative stress in the body, which further is responsible for the occurrence of various chronic disorders.
Dietary antioxidants obtained through various fruits and vegetables balance the ratio of ROS and the antioxidants which help to reduce oxidative stress and further lower the occurrence of cancer, cardiovascular diseases, and aging (Zhou et al. 2021; Maurya and Rizvi 2009). Free radicals having unpaired electrons are extremely reactive. As the elderly population is increasing, aging has become an important area of research (Chen et al. 2018). Chronic diseases are mostly caused by the state of chronic inflammation. The occurrence of chronic diseases demands chronic treatment. The drugs used for the treatment of chronic diseases are highly priced and are often linked with several side effects when consumed for a longer period of time. Hence, there is an increasing demand for cheaper, multi-targeted, and easily available drugs. Natural sources are more affordable and safer for long-term use and hence have the potential to be used as drugs. Studies show that over the past 25 years, 70% of the drugs were obtained from natural sources (Gupta et al. 2018).
About 80% of the world’s population still relies on plant-derived medical formulations in the field of therapeutics. The phytochemicals include flavonoids, sesquiterpenes, alkaloids, and polyphenols. These phytochemicals exhibit hepato-protective, chemoprotective, antioxidant, anti-inflammatory, antimicrobial, and anti-diabetic properties (Gupta et al. 2019). Polyphenols are compounds that are widely present as plant secondary metabolites. The naturally occurring polyphenols can be classified into flavonoids, tannins, coumarins, stilbenes, and phenolic acids. These polyphenols contribute to the high antioxidant mechanism of the plants (Buljeta et al. 2021). These polyphenols exhibit their antioxidant behavior via the reducing potential and protect the components of the cell from oxidative damage (Okoro et al. 2021; Deepika and Maurya 2022). Besides having nutritional values, these also serve as potential therapeutic molecules. The biological activities of plant polyphenols include free radical scavenging, transferring electrons to free radicals, activating the antioxidant enzymes, and improving oxidative damage.
Natural substances have been used in medicines since ancient time. These compounds are easily available and come with minimum side effects. Plants such as Tinospora cordifolia, Withania somnifera, and Mucuna pruriens exhibit properties to treat neurodegenerative diseases. Similar to EA, another bioactive compound ursolic acid (UA) found in Mucuna pruriens shows protective effect against Parkinson’s disease. Another bioactive compound chlorogenic acid found in the plant Withania somnifera exhibits anti-Parkinsonian activity in mouse model (Rai et al. 2020). UA exhibits antioxidative, hepato-protective, anti-inflammatory and anticancerous properties. The study shows anti-Parkinsonian activity of UA against MPTP-induced mouse model of Parkinson’s disease (Rai et al. 2019).
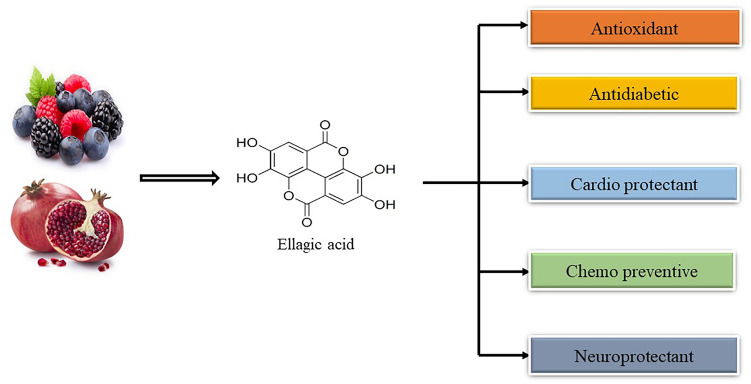
EA is a naturally occurring polyphenol having strong antioxidant property (Wang et al. 2019b). It contains four hydroxyl groups and two lactone groups, and the presence of four hydroxyl groups attributes to the antioxidant behavior of EA (Zeb 2018). EA falls under the category of plant polyphenol tannins. Hydrolyzable tannins, particularly ellagic acid, is an important constituent of certain fruits and berries including pomegranate, strawberry, blackberry, and raspberry (Shakeri et al. 2018) and grapes, persimmon, goji berries, and green tea (Iovine et al. 2021). Raspberries are known to contain the highest amount of EA (Aishwarya et al. 2021). EA is also present in nuts such as almonds and walnuts (Iflazoglu Mutlu et al. 2021). EA is present in compounds with antiglycation activities which include cumin, apples, guava, oranges, and grapes (Raghu et al. 2017). Medicinal plants such as the bark of eucalyptus (Mansouri et al. 2020) and cloves also contain EA (Maruszewska and Tarasiuk 2019). Strong antioxidant activity (Kilic et al. 2014) of EA attributes to the scavenging of free radicals. EA possesses antiviral and antibacterial properties and is also known to protect cell against apoptosis. It is also known to possess anticancer and anti-diabetic properties and therefore receives a great deal of attention. It protects the cell against lipid peroxidation and oxidative damage (Abdelkader et al. 2020).
A very small part of EA is available in free form. Mostly, it is coupled with a glycoside moiety consisting of glucose, rhamnose, arabinose, or present in the form of ellagitannins. EA is a very thermostable molecule, and the lipophilic domain of the molecule is represented by the four rings, and four phenolic and two lactones represent the hydrophilic zone (Shakeri et al. 2018). This property of EA enables its low solubility in water but is sparingly soluble in alcohol (Garcia-Nino and Zazueta 2015). This further leads to the difficulty in designing pharmaceutical formulations using EA. EA has also been reported in distilled drinks such as scotch whisky cognac, and rum. EA represents the property to heal wounds by promoting blood coagulation by activating factor XII of the intrinsic cascade (Ceci et al. 2020). EA is therefore considered antioxidant, anti-inflammatory (Murphy et al. 2020), and chemoprotective and hence used in edible products, cosmetics, and pharmaceuticals (Xu et al. 2020). Some of the drawbacks of EA include its poor solubility in water, limitation of oral bioavailability, and a short plasma half-life (Li et al. 2021b). These limitations restrict EA clinical applications and its use as a potential systemic drug.
EA being a naturally occurring bioactive compound has been exploited by different researchers in different research areas. Its antioxidant and anti-inflammatory property lead to its use in various chronic disorders. The present review summarizes the role of EA in various age-related disorders. The physicochemical properties of EA have been elucidated. A further role of EA as an antioxidant and its preventive mechanism in age-related disorders have been highlighted. The protective effect of EA has been elucidated in the following sections.
Physio-chemical properties of ellagic acid
EA is present in both free forms and complex forms as ellagitannins (Cheshomi et al. 2021). The naturally occurring polyphenol EA is present in fruits and vegetables in the form of extremely soluble ellagitannins (ET). After the ingestion of ellagitannins by humans, EA is released by the process of hydrolysis (Hsu et al. 2019). Gut microbiota further metabolizes EA and ET to urolithin A (UA) and urolithin B (UB), and after consumption, these are distributed to plasma, feces, urine, and organs. A high level of UA and UB in plasma suggests its greater biological potential than the parent molecule, EA and ET (Wang et al. 2017). The chemoprotective and anti-inflammatory properties of urolithins have been derived as well (Ortenzi et al. 2021).
EA is a dimeric form of polyphenol gallic acid (Kim et al. 2021c). EA is known to possess antioxidant, anticancer, anti-inflammatory (Xu et al. 2021), antifibrosis (Zaazaa et al. 2018), antimicrobial, and antimutagenic properties (Sakurai et al. 2022). Due to its low solubility in water and low permeability, it has been classified in group IV under the biopharmaceutical system of classification (Savic et al. 2019). EA occurs naturally as a phenolic compound that possesses antioxidant properties. It widely occurs in a number of different fruits and vegetables. EA is commonly found conjugated with glycosidic moieties such as glucose and xylose. The melting point of ellagic acid is 350 ℃ accounting for its high thermostability. EA represents a weak acid, which has less solubility in water and alcohol but is easily soluble in caustic potash (Derosa et al. 2016). EA is an odorless compound. The four hydroxyl groups and two lactones present in EA owe to its hydrophilic moiety, and the two hydrocarbon rings owe to a lipophilic moiety (Kaur et al. 2021). This makes capable EA accept electrons from various substrates as well as participate in redox reactions making it a potent antioxidant (Rios et al. 2018). The low solubility of EA in water and its limited permeability interfere with its bioavailability. Cyclodextrins are used to enhance the solubility of EA in water and hence increase its bioavailability (Sampaio et al. 2021) (Fig. 1).
Fig. 1.
represents the chemical structure and the various activities of ellagic acid
The bioactivity of the compound is greatly altered by its structure. EA exhibits a planar symmetrical structure. The molecules of the phytochemical are bonded through hydrogen bonding. The low solubility of the compound restricts its antioxidant potential and various other activities (Li et al. 2021a).
Owing to its various therapeutic activities, EA has a wide range of applications in several chronic diseases. Studies have been performed to check the medicinal efficacy of EA. These studies suggest that EA can be considered for use in the treatment of various age-associated diseases.
Ellagic acid and oxidative stress
The metal chelating capability, ability to scavenge free radicals (Al-Ishaq et al. 2020), and the ability to induce antioxidant enzyme activity of the cells are attributed to the antioxidant properties of EA. EA has also been found to protect against the DNA damage of the cells caused by the free radicals (Gupta A et al. 2020). EA is found to decrease the level of Malondialdehyde (MDA), a lipid peroxidation byproduct, activates the activity of glutathione (GSH), and the activity of an antioxidant enzyme, catalase (CAT) (Aslan et al. 2020). The strong antioxidant property of EA prevents cell death, and the anti-inflammatory activity of EA helps to induce the proliferation of fibroblasts and increases the rate of wound healing process. Studies have shown that EA accelerates the process of wound healing in rats (Nirwana et al. 2021). Attributing to its antiproliferative properties EA exhibits various health-promoting benefits. It has a protective effect against nephrotoxicity induced by cyclophosphamide (Abdallah et al. 2019).
According to the studies performed by Mişe Yonar S et al. it was demonstrated that EA significantly lowered the level of tissue MDA in comparison with the control group (p < 0.05). After being fed with EA for about 8 weeks, the enzyme activity of superoxide dismutase (SOD), CAT, and glutathione peroxidase (GHS-Px) in the tissue was remarkably higher when compared to the control group (p < 0.05) (Mise Yonar 2019). Various animal studies show that EA prevents damage to testicles, helps restore sperm count, preserved the motility and morphology of sperm, and also attenuates sex hormone alterations. It is also found to prevent diabetic rats from erectile dysfunction. EA helps to stimulate the measure of testicular antioxidants which include SOD, CAT, GSH and attributes to the protective functions of reproductive health (ALTamimi et al. 2021). EA has been found to increase the level of expression of CAT, glutathione-S-transferase (GST), and glutathione peroxidase (GPx) (Rahimi et al. 2018).
Because of its antiproliferative property in some tumors, and owing to its anti-inflammatory and antioxidant properties, EA has extensively been studied. Studies show that the intake of EA lowers the severity of obesity and improves metabolic complexities such as resistance toward insulin and type 2 diabetes (Harakeh et al. 2020). EA is a significant bioactive molecule and finds its use in various industrial and pharmacological sectors. The synergistic relationship between EA and antimalarial drugs such as atovaquone, chloroquine, and mefloquine has been found. This combination can be used to reduce drug doses during the treatment process and to lower the side effects (Pavlova et al. 2018).
Various other properties such as anti-depressant, anti-ulcer, hepato-protective, anti-cataract, and antiviral of EA have been elucidated. Studies report that possibly EA exhibits medicinal properties because of the reduced expression of IL-1β, TNF-α, MCP-1, and IL-6, and it also downregulates the TNF-α and MCP-1 mRNA expression levels (Bhattacharjee et al. 2021). EA is known to inhibit the NADPH oxidase enzyme activity in the cell. EA protects from DNA alkylation, and its ability to scavenge ROS is comparable to two of the important antioxidants, i.e., vitamin E and vitamin C (Mehrzadi et al. 2019). Studies have reported that EA has a protective function against oxidative damage to the kidney and liver. EA has the capability to activate the antioxidants such as CAT, SOD, and GSH. The compound scavenges free radicals and prevents lipid peroxidation (Karimi et al. 2020). EA inhibits the mast cell activation, proliferation of transformed cells, and viral replication by increased antioxidant activity, induces apoptosis, downregulates the genes responsible for angiogenesis and cell cycle, and stimulates the cellular immune responses (Kim et al. 2021a). The antihepatotoxic activity of EA improves the function of hepatocytes against harmful and toxic conditions (Lin et al. 2020). EA protects the cell from DNA damage which is mediated by the free radicals (Gupta and Pandey 2020). Studies have proven that EA acts as an efficient compound for skin whitening and also suppresses pigmentation (Singh Hallan et al. 2020). In tumor cells, EA arrests the cell cycle in the G1 phase, inhibits the overall growth of the cell and induces apoptosis (Kumar et al. 2021). EA exhibits anti-inflammatory properties by the inhibition of cyclooxygenase enzyme and hence reducing the expression of these enzyme (Goudarzi et al. 2018). EA is widely used in the cosmetic industry due to its skin-whitening effects. EA inhibits the activity of the enzyme tyrosinase thus blocking the melanin formation (Wang et al. 2018). EA possesses the capability to improve the metabolism of cholesterol by increased expression of low-density lipoprotein receptor.
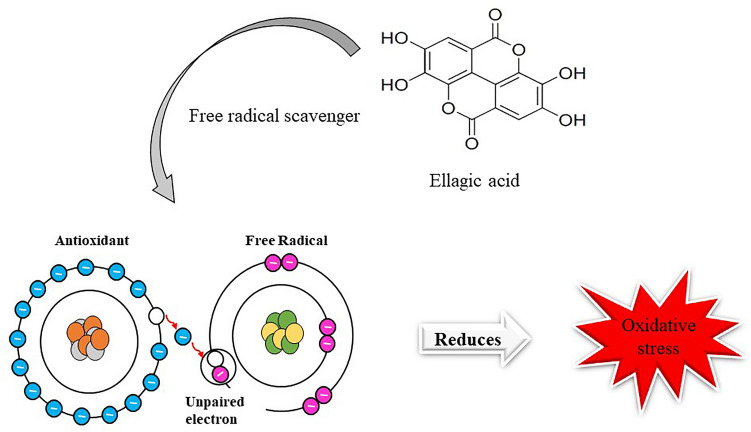
Due to its antioxidant properties and the ability to cure diseases, EA has been widely used in therapeutics. EA scavenges the free radicals and hence behaves as a potential antioxidant. Generation of free radicals in the body leads to the occurrence of several pathologies. Therefore, the use of EA in therapeutics might help to lower the free radicals and hence combat oxidative damage of the cells and can help in the process of healthy aging (Fig. 2).
Fig. 2.
represents the mechanism of action of ellagic acid. The hydroxyl group present in the compound is responsible for scavenging the free radicals. Ellagic acid scavenges the free electrons present in the last orbital shell and therefore acts as a potent antioxidant and hence reduces the oxidative stress of the cellular components
Ellagic acid in age-associated diseases
Naturally occurring antioxidants are commonly present in various fruits and vegetables. These antioxidants play an active role in protecting the body from the harmful effect of free radicals. EA is one such molecule that is present in the cell wall of various types of berries and other vegetables. The lactone and hydroxyl group of the molecule exhibits the antioxidant property (Baeeri et al. 2018). Various in vivo and in vitro studies on EA determined its role in different cellular and molecular functions showing its beneficial activities. The multi-target capabilities of EA include antithrombotic, anti-angiogenic, anti-inflammatory, anti-carcinogenic, and antioxidant properties (Yang et al. 2021; Gil et al. 2021). EA is often consumed as therapeutics as capsules, liquid, and powder for treating cancer and heart disorders. Due to low solubility in water and low bioavailability, efficient carriers have been designed for effectively using the plant molecule (Pirzadeh-Naeeni et al. 2020). EA is used as a dietary supplement to prevent or reduce the risk of various diseases (Cetin and Biltekin 2019).
The degenerative diseases which are related to the structural and functional decline of organs and tissues over a period of time are considered as age-associated diseases. These age-associated disorders include diabetes, cancers, cataracts, Alzheimer’s, Parkinson’s and cardiovascular diseases. These diseases are attributed to oxidative stress and the process of natural aging (Nandi et al. 2019). Various diseases such as cataract, osteoporosis, dementia, hypertension, Alzheimer’s, cancer, cardiovascular, diabetes and neurodegenerative disorders are linked with the progression of aging. This review focuses on the effect of EA on diabetes, cancer, cardiovascular and neurodegenerative as significant results have been obtained for these diseases.
Diabetes
Diabetes mellitus is a metabolic disorder and is mainly identified by hyperglycemic effects which are caused by improper insulin secretion or improper functioning of insulin or both (Ghadimi et al. 2021). ROS generation leads to the occurrence of hyperglycemia which further causes oxidative damage to the pancreas, liver, and kidney. Diabetes is known to affect the lives of millions of people across the world and increases mortality. Besides being costly insulin administration, other drugs are known to affect the eyes, kidneys, skin, nervous system, and cardiovascular system. According to World Health Organization, herbal treatment for diabetes has been suggested. The plant-based medicines are considered to be an excellent source with fewer side effects, and the compounds are non-toxic as well (Altindag et al. 2021).
EA is a bioactive compound used in the treatment of diabetes. The supplementation of EA helps to improve insulin resistance, metabolism of lipids, and abnormal glucose level. EA administration in the diabetic rat protects from the injuries to the kidney and heart caused due to hyperglycemia (Liu et al. 2021). Hyperglycemia is linked with the consequences of diabetes which include an increase in proinflammatory cytokines, such as IL-6 and TNF-α, and a decrease in the anti-inflammatory cytokines, such as IL-10 (Farbood et al. 2019). All these changes lead to neuronal degeneration and hence cause behavioral defects. Scientific studies suggest that the effect of EA on the central nervous system is the same as that of different drugs which are used in medical practices. According to Polce SA et al. EA is found to improve the vascular function of the blood vessels which are exposed to hyperglycaemic conditions by reducing oxidative stress. In the rodent models with ischemic reperfusion and aging, EA was found to have advantageous effects on the liver by reducing the effect of oxidative stress. The beneficial effects of EA on the liver with diabetes have been elucidated but its mechanism remains unclear (Polce et al. 2018).
As an antiglycation molecule, EA decreases the concentration of MDA and GSH in the plasma. EA has a protective role against diabetes induced by alloxan by decreasing lipid peroxidation and thus inhibits the formation of advanced glycation end products by the glycation process (Ahmad et al. 2022). Diabetes is a widely occurring disease in aging population. EA obtained from the natural sources comes with minimum to no side effects. Hence, EA can preferably be used as a treatment option to cure diabetes and related pathologies.
Cancer
Cancer leads in causing approximately 7.9 million deaths each year across the world. The death rate related to cancer is expected to reach 12 million by 2030 (Al-Ishaq et al. 2020). The anticancerous activity of EA includes the arrest of the cell cycle and induction of apoptosis and inhibiting the formation and growth of tumors (Spisso et al. 2018). EA is known to inhibit the proliferation of the cancerous cell by induction of apoptosis (Gulzar et al. 2019). EA inhibits the formation and growth of the tumor (Ali et al. 2020). Anticancerous property of the EA is exhibited by its property to inhibit the proliferation of tumor cells, induction of apoptosis, blockage of viral infection, disturbance of inflammation, angiogenesis, and metastasis (El-Sonbaty et al. 2022). EA arrests the cell cycle or is responsible for the induction of apoptosis and inhibits the proliferation of cancer cells in various types of cancer (Duan et al. 2020), (Cetin et al. 2019). Studies on cancer cells suggest that there is a disruption of DNA synthesizing machinery that stimulates the p21-regulated system, and this causes the arrest of the cycle at G1/S phase checkpoints. EA behaves as a potent chemoprotective compound as it exhibits promising antitumor activities against ovarian, breast, prostate, pancreas, colon, lymphoma, and bladder cancer. The mechanisms contributing to its antitumor property include its ability to inhibit the proliferation of cells, angiogenesis, invasion of extracellular matrix, and inducing caspase-dependent apoptosis (Ceci et al. 2020). EA was found to inhibit pancreatic cancer in human cell PANC-1 by blocking the Notch, shh and AKT signaling pathways in vivo. EA also targeted mitochondrial pathways in the case of B-lymphocytes, isolated from patients with chronic lymphocytic leukemia (Zhong et al. 2019). EA administration is known to decrease CDK6 expression, inhibit the proliferation of the cell and induction of apoptosis in the cells of breast cancer (Yousuf et al. 2020).
In vitro studies with EA are known to inhibit the proliferating activity of HCC1954 and SUM159 cell lines of breast cancer. EA is also found to increase the expression of poly ADP-ribose polymerase in HCC1954, SUM159, MDA-MB-231, and SKBr3 cancer cell lines. EA also reduces the AKT/Mtor signals which are in charge of controlling various cellular functions such as apoptosis, autophagy, proliferation, angiogenesis, and metabolism (Jaman and Sayeed 2018). In pancreatic cancer cells, EA inhibits growth by stimulating apoptosis. The apoptosis induced by EA is mediated by caspase-3 and caspase-9 activation. In pancreatic cell lines, EA is also known to suppress migration through the inhibition of epithelial-mesenchymal transition (Kim et al. 2021b). In vivo and in vitro studies show the antitumor activity of EA in endometrial cancer by the suppression of cell invasion and migration via targeting P13K signal pathways (Wang et al. 2019c).
The depolarization of mitochondria in the bladder, neuroblastoma, pancreas, and B cell cancer is regulated by EA by indirect activation of apoptosis in the cancer cells. In the case of B cell chronic leukemia, EA directly affects mitochondria which result in ROS production and release of pro-apoptotic factors through mitochondria. EA is also known to downregulate the expression of sodium/hydrogen exchanger 1 which leads to the acidification of cells and glycolytic flux inhibition in the cancer cells of endometrial (Boehning et al. 2018). Reports suggest that in breast cancer cells, the treatment with EA significantly enhanced the cytotoxicity that is induced by radiation (Ahire et al. 2017). The effect of EA on melanoma cells has also been reported (Wang et al. 2020a).
The above studies suggest that EA can be used as a potent drug in treating various type of cancer. EA can be used in the treatment of cancer as other drugs come with several side effects. The use of natural substances is more affordable and comparatively easily available. EA can be preferred as a treatment option due to its easy availability and less or no cytotoxicity.
Cardiovascular disorders
According to WHO, every year about 17.9 million people die because of cardiovascular disorders. This accounts for about one-third of the deaths (Malinowski et al. 2019). It has been reported that EA acts to improve cardiovascular functions by behaving as an antioxidant molecule and restoring the dysfunction of endothelial in the heart and vessels as studied in the animal models with dyslipidemia, hypertension, and myocardial infarction (Jordao et al. 2017). EA as an antioxidant plays a role to reduce cardiotoxicity induced by chemicals and drugs and therefore acts as a cardio protectant (Ahangari et al. 2022). The antioxidant effect of EA inhibits free radical production and protects the tissues of the heart. Studies suggest the preventive function of EA for the myocardium. EA has a protective role on the injury of the myocardium after myocardial infarction as studied in rats (Wei et al. 2017). EA reduces the area of myocardial infarction and suppresses cardiac fibrosis by the regulation of the expression of anti-apoptotic genes and enhances mitochondrial respiratory enzyme activity. EA further decreases ventricular hypertrophy occurrence by suppressing lipid peroxidation (Lin et al. 2019). Scientific reports show that EA suppresses the injury of the mitochondria and necrotic cell death of the cardiac myocytes that are induced by hypoxia or doxorubicin (Dhingra et al. 2017). EA is known to protect against experimentally induced myocardial infarction (Kannan and Quine 2013).
Cardiovascular diseases are responsible for causing death to a large number of aging populations. With age, the prevalence of cardiovascular disorders increases. Therefore, it becomes necessary to look for options which are cheaper, easily available and are less cytotoxic. Various scientific studies have revealed the use the EA in cardiovascular diseases, and hence, it can be considered to be used as therapeutics to lower the occurrence or cure cardiovascular disorders leading to healthy aging.
Neurodegenerative disorders
Neurodegenerative disorders are primarily characterized by the continuous loss of the cells and tissues of the neural system and are also marked by the dysfunction of the nervous system as a consequence of aging. The well-known neuronal disorders include Parkinson’s disease, prion disease, Alzheimer’s disease, and Huntington’s disease. The symptoms of these disorders include anxiety, memory loss, impaired cognitive function, depression, and dysfunction of motor activities (Gupta et al. 2021).
The antioxidant property of EA and its ability to trap free radicals help to prevent oxidative stress and the occurrence of neurodegenerative disorders (Alfei et al. 2019). The phytochemical EA is used in the treatment to maintain the disorders associated with the central nervous system. EA regulates various molecular signaling pathways which normalize the mitochondrial dysfunctions resulting in free radical generations attenuating neurodegeneration. EA protects the inflammation of the brain by downregulating the expression of proinflammatory cytokines, such as TNF-α (Dornelles et al. 2020). The protective effect of EA in mice against anxiety and cognitive impairment induced due to sleep deprivation has been reported. EA exhibits its protective effect by inhibiting the activity of TLR4 and activating the activity of Nrf2 (Wang et al. 2020b).
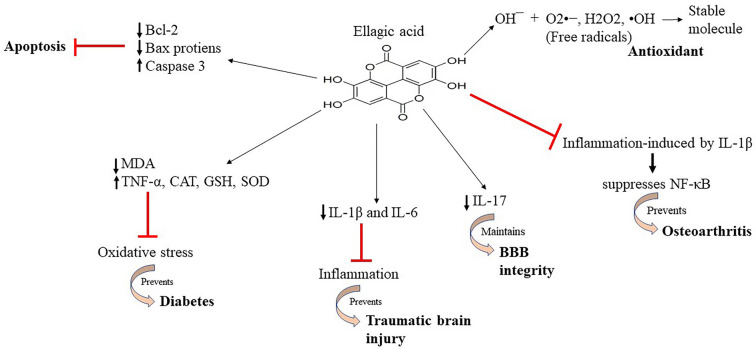
Neurodegenerative disorders lead to the process of accelerated aging. The study of EA in various pathways of neurodegeneration in various cells show that it can be used to cure this disorder. The study on various models suggests that EA can further be used in humans as well for the treatment neurodegenerative disorders (Fig. 3).
Table 1.
The following table summarizes the various activities of ellagic acid and its mechanism of action
| Sn. No | Activity | Mechanism of action | References |
|---|---|---|---|
| 1 | Anticancer |
EA exhibits a cytotoxic effect against the A549 cell lines by inhibiting the sphingosine kinase 1 (SphK1) In MCF-7 cell lines of breast cancer, EA inhibits integrin-linked kinase (IKL). It also regulates TGF-β/Smads signaling pathway to arrest the cycle in the G0/G1 phase |
(Yoganathan et al. 2021) |
| 2 | Anti-inflammation | EA reduces the effect of inflammation by regulating the inflammatory chemokines such as IL-6, LI-17, TNF-α, TGF-β, COX-2, and NO and promotes the anti-inflammatory biomarkers and receptors such as PPAR-γ, PPAR-α, IL-10, and Nrf2 |
(Baradaran Rahimi et al. 2020) (Liu et al. 2020) |
| 3 | Antidiabetic | In high glucose-induced type 2 diabetes mellitus HepG2 cells, EA is found to improve insulin resistance and oxidative damage by activating the keap1-Nrf2 signaling system | (Ding et al. 2019) |
| 4 | Antihypertensive | EA prevents the hypertensive effect developed by NOS inhibitors by increasing the expression of eNOS protein and reduces oxidative stress by the reduction in a subunit of NADPH oxidase |
(Berkban et al. 2015) (Olgar et al. 2014) |
| 5 | Neuroprotective |
Administration of EA helps in the proliferation of neural stem cells and improves injury of the brain by regulating the Wnt/β- catenin signaling pathway EA helps in the reconstruction of the blood–brain barrier by upregulating zonula occludens-1 (ZO-1) and downregulating aquaporin 4 (AQP-4) and metalloprotein 9 (MMP-9) damaged tissues of the brain |
(Liu et al. 2017). (Wang et al. 2019a, b, c, d) |
| 6 | Artheroprotective | The consumption of EA for the long term reduced the development of atherosclerotic lesions as studied in wild-type mice by improving the activation of Nrf2, nitric oxide, and oxidative stress | (Ding et al. 2014) |
| 7 | Renoprotective | EA acts to protect against renal ischemia injury by suppression of the NOX4/JAK/STAT signaling pathway | (Liu et al. 2020) |
| 8 | Antioxidant | Activity of antioxidant enzymes such as GSH, GPx, CAT, and SOD is increased in the tissues of the liver, heart, and kidney | (Goudarzi et al. 2018) |
| 9 | Cardioprotective | The administration of EA helps in preventing Ca2+ dysregulation which is associated with cardiac hypertrophy | (Yamasan et al. 2021) |
Fig. 3.
represents the therapeutic activities of ellagic acid and its effect on the signaling molecules in various diseases Table 1
Conclusion
Free radicals are unpaired electrons that are highly reactive. Antioxidants are the molecules that prevent cellular damage. The accumulations of ROS and RNS in the body damage the cellular components and hence lead to aging and various age-associated disorders.
Naturally occurring plant compounds are used as potent drug molecules. These plant compounds behave as natural antioxidants and are used to prevent and cure various age-associated disorders. These phytochemicals are safer to use with less toxic effects. EA is a plant polyphenol that is widely distributed in various fruits and vegetables. The antioxidant property of this polyphenol leads to free radical scavenging and protects from cellular damage. EA is known to protect the cell against oxidative damage and lipid peroxidation. Four hydroxyl groups in the compound owe to its antioxidant behavior. The dietary intake of EA is known to protect from various age-associated disorders.
EA is known to exhibit antioxidant, anticancer, anti-inflammatory, antimicrobial, antiviral and antimutagenic properties. EA decreases the level of MDA and activates the activity of GSH and CAT in the cell. The antiproliferative activity of EA leads to health-promoting benefits. The dietary supplement of EA prevents and reduces the risk of various disorders. EA is known to have a protective role in metabolic disorders. This bioactive molecule is used in the treatment of diabetes. EA administration improves insulin resistance and the abnormal level of glucose. The anticancerous property of the EA includes cell cycle arrest and induction of apoptosis. EA inhibits the formation and growth of tumor cells. EA behaves as a chemo protectant and has antitumor activities against the cancer of the ovary, breast, prostate, pancreas, colon, lymphoma, and bladder. The inhibition of free radicals by EA protects the heart tissues and has a preventive function in the myocardium. EA is also used to treat the disorders associated with the central nervous system.
This review highlights the activity of EA as an antioxidant and its effect in various age-associated disorders; as the compound is naturally occurring with lower side effects, it can be used in the treatment of various malignancies. The low solubility and bioavailability of the molecule are still a problem but various formulations are being prepared to increase in efficacy as a potent drug molecule.
Acknowledgements
This study was supported by Fellowship from University Grant Commission to Deepika (Reference No: 201610000784). Agencies had no role in the interpretation or writing the manuscript.
Author contributions
D: contributed to conceptualization, writing–original draft preparation, PKM: contributed to supervision, reviewing and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical approval
This article does not contain any studies with human or animal subjects.
References
- Abdallah HMI, Abdel-Rahman RF, El Awdan SA, Allam RM, El-Mosallamy A, Selim MS, Mohamed SS, Arbid MS, Farrag ARH. Protective effect of some natural products against chemotherapy-induced toxicity in rats. Heliyon. 2019;5(5):e01590. doi: 10.1016/j.heliyon.2019.e01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelkader NF, Elyamany M, Gad AM, Assaf N, Fawzy HM, Elesawy WH. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J Pharmacol Sci. 2020;143(1):23–29. doi: 10.1016/j.jphs.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Ahangari R, Khezri S, Jahedsani A, Bakhshii S, Salimi A. Ellagic acid alleviates clozapineinduced oxidative stress and mitochondrial dysfunction in cardiomyocytes. Drug Chem Toxicol. 2022;45(4):1625–1633. doi: 10.1080/01480545.2020.1850758. [DOI] [PubMed] [Google Scholar]
- Ahire V, Kumar A, Mishra KP, Kulkarni G. Ellagic acid enhances apoptotic sensitivity of breast cancer cells to gamma-radiation. Nutr Cancer. 2017;69(6):904–910. doi: 10.1080/01635581.2017.1339811. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Alouffi S, Khan S, Khan M, Akasha R, Ashraf JM, Farhan M, Shahab U, Khan MY. Physicochemical characterization of in vitro LDL glycation and its inhibition by ellagic acid (EA): an in vivo approach to inhibit diabetes in experimental animals. Biomed Res Int. 2022;2022:5583298. doi: 10.1155/2022/5583298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aishwarya V, Solaipriya S, Sivaramakrishnan V. Role of ellagic acid for the prevention and treatment of liver diseases. Phytother Res. 2021;35(6):2925–2944. doi: 10.1002/ptr.7001. [DOI] [PubMed] [Google Scholar]
- Alfei S, Turrini F, Catena S, Zunin P, Grilli M, Pittaluga AM, Boggia R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur J Med Chem. 2019;183:111724. doi: 10.1016/j.ejmech.2019.111724. [DOI] [PubMed] [Google Scholar]
- Ali OM, Bekhit AA, Khattab SN, Helmy MW, Abdel-Ghany YS, Teleb M, Elzoghby AO. Synthesis of lactoferrin mesoporous silica nanoparticles for pemetrexed/ellagic acid synergistic breast cancer therapy. Colloids Surf B Biointerfaces. 2020;188:110824. doi: 10.1016/j.colsurfb.2020.110824. [DOI] [PubMed] [Google Scholar]
- ALTamimi JZ, AlFaris NA, Aljabryn DH, Alagal RI, Alshammari GM, Aldera H, Alqahtani S, Yahya MA (2021) Ellagic acid improved diabetes mellitus-induced testicular damage and sperm abnormalities by activation of Nrf2. Saudi J Biol Sci 28(8):4300–4310. 10.1016/j.sjbs.2021.04.005 [DOI] [PMC free article] [PubMed]
- Al-Ishaq RK, Overy AJ, Busselberg D. Phytochemicals and gastrointestinal cancer: cellular mechanisms and effects to change cancer progression. Biomolecules. 2020 doi: 10.3390/biom10010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altindag F, Ragbetli MC, Ozdek U, Koyun N, Ismael Alhalboosi JK, Elasan S. Combined treatment of sinapic acid and ellagic acid attenuates hyperglycemia in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2021;156:112443. doi: 10.1016/j.fct.2021.112443. [DOI] [PubMed] [Google Scholar]
- Aslan A, Hussein YT, Gok O, Beyaz S, Erman O, Baspinar S. Ellagic acid ameliorates lung damage in rats via modulating antioxidant activities, inhibitory effects on inflammatory mediators and apoptosis-inducing activities. Environ Sci Pollut Res Int. 2020;27(7):7526–7537. doi: 10.1007/s11356-019-07352-8. [DOI] [PubMed] [Google Scholar]
- Baeeri M, Mohammadi-Nejad S, Rahimifard M, Navaei-Nigjeh M, Moeini-Nodeh S, Khorasani R, Abdollahi M. Molecular and biochemical evidence on the protective role of ellagic acid and silybin against oxidative stress-induced cellular aging. Mol Cell Biochem. 2018;441(1–2):21–33. doi: 10.1007/s11010-017-3172-0. [DOI] [PubMed] [Google Scholar]
- Baradaran Rahimi V, Ghadiri M, Ramezani M, Askari VR. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother Res. 2020;34(4):685–720. doi: 10.1002/ptr.6565. [DOI] [PubMed] [Google Scholar]
- Berkban T, Boonprom P, Bunbupha S, Welbat JU, Kukongviriyapan U, Kukongviriyapan V, Pakdeechote P, Prachaney P. Ellagic acid prevents L-NAME-induced hypertension via restoration of eNOS and p47phox expression in rats. Nutrients. 2015;7(7):5265–5280. doi: 10.3390/nu7075222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Kulkarni VH, Chakraborty M, Habbu PV, Ray A. Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon. 2021;7(1):e05921. doi: 10.1016/j.heliyon.2021.e05921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning AL, Essien SA, Underwood EL, Dash PK, Boehning D. Cell type-dependent effects of ellagic acid on cellular metabolism. Biomed Pharmacother. 2018;106:411–418. doi: 10.1016/j.biopha.2018.06.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buljeta I, Pichler A, Simunovic J, Kopjar M. Polyphenols and antioxidant activity of citrus fiber/blackberry juice complexes. Molecules. 2021 doi: 10.3390/molecules26154400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci C, Graziani G, Faraoni I, Cacciotti I. Strategies to improve ellagic acid bioavailability: from natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology. 2020;31(38):382001. doi: 10.1088/1361-6528/ab912c. [DOI] [PubMed] [Google Scholar]
- Cetin A, Biltekin B. Combining ellagic acid with temozolomide mediates the cadherin switch and angiogenesis in a glioblastoma model. World Neurosurg. 2019;132:e178–e184. doi: 10.1016/j.wneu.2019.08.228. [DOI] [PubMed] [Google Scholar]
- Cetin A, Biltekin B, Degirmencioglu S. Ellagic acid enhances the antitumor efficacy of bevacizumab in an in vitro glioblastoma model. World Neurosurg. 2019;132:e59–e65. doi: 10.1016/j.wneu.2019.08.257. [DOI] [PubMed] [Google Scholar]
- Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018;8(1):1465. doi: 10.1038/s41598-018-19732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshomi H, Bahrami AR, Matin MM. Ellagic acid and human cancers: a systems pharmacology and docking study to identify principal hub genes and main mechanisms of action. Mol Divers. 2021;25(1):333–349. doi: 10.1007/s11030-020-10101-6. [DOI] [PubMed] [Google Scholar]
- Deepika MPK. Health benefits of quercetin in age-related diseases. Molecules. 2022 doi: 10.3390/molecules27082498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa G, Maffioli P, Sahebkar A. Ellagic acid and its role in chronic diseases. Adv Exp Med Biol. 2016;928:473–479. doi: 10.1007/978-3-319-41334-1_20. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Jayas R, Afshar P, Guberman M, Maddaford G, Gerstein J, Lieberman B, Nepon H, Margulets V, Dhingra R, Kirshenbaum LA. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic Biol Med. 2017;112:411–422. doi: 10.1016/j.freeradbiomed.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y, Song Y, Li Y, Wen A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. Int J Cardiol. 2014;175(3):508–514. doi: 10.1016/j.ijcard.2014.06.045. [DOI] [PubMed] [Google Scholar]
- Ding X, Jian T, Wu Y, Zuo Y, Li J, Lv H, Ma L, Ren B, Zhao L, Li W, Chen J. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed Pharmacother. 2019;110:85–94. doi: 10.1016/j.biopha.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhang NJ, Zhang LJ. Oxidative stress in leukemia and antioxidant treatment. Chin Med J (engl) 2021;134(16):1897–1907. doi: 10.1097/CM9.0000000000001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornelles GL, de Oliveira JS, de Almeida EJR, Mello CBE, B R ER, da Silva CB, Petry LDS, Pillat MM, Palma TV, de Andrade CM. Ellagic acid inhibits neuroinflammation and cognitive impairment induced by lipopolysaccharides. Neurochem Res. 2020;45(10):2456–2473. doi: 10.1007/s11064-020-03105-z. [DOI] [PubMed] [Google Scholar]
- Duan J, Li Y, Gao H, Yang D, He X, Fang Y, Zhou G. Phenolic compound ellagic acid inhibits mitochondrial respiration and tumor growth in lung cancer. Food Funct. 2020;11(7):6332–6339. doi: 10.1039/d0fo01177k. [DOI] [PubMed] [Google Scholar]
- El-Sonbaty SM, Moawed FS, Kandil EI, A MT Antitumor and antibacterial efficacy of gallium nanoparticles coated by ellagic acid. Dose Response. 2022;20(1):15593258211068998. doi: 10.1177/15593258211068998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbood Y, Rashno M, Ghaderi S, Khoshnam SE, Sarkaki A, Rashidi K, Rashno M, Badavi M. Ellagic acid protects against diabetes-associated behavioral deficits in rats: possible involved mechanisms. Life Sci. 2019;225:8–19. doi: 10.1016/j.lfs.2019.03.078. [DOI] [PubMed] [Google Scholar]
- Garcia-Nino WR, Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Gessner DK, Ringseis R, Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr (berl) 2017;101(4):605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Ghadimi M, Foroughi F, Hashemipour S, Rashidi Nooshabadi M, Ahmadi MH, Ahadi Nezhad B, Khadem Haghighian H. Randomized double-blind clinical trial examining the Ellagic acid effects on glycemic status, insulin resistance, antioxidant, and inflammatory factors in patients with type 2 diabetes. Phytother Res. 2021;35(2):1023–1032. doi: 10.1002/ptr.6867. [DOI] [PubMed] [Google Scholar]
- Gil TY, Hong CH, An HJ. Anti-inflammatory effects of ellagic acid on Keratinocytes via MAPK and STAT pathways. Int J Mol Sci. 2021 doi: 10.3390/ijms22031277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Fatemi I, Siahpoosh A, Sezavar SH, Mansouri E, Mehrzadi S. Protective effect of ellagic acid against sodium arsenite-induced cardio- and hematotoxicity in rats. Cardiovasc Toxicol. 2018;18(4):337–345. doi: 10.1007/s12012-018-9446-2. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94(3):651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- Gulzar M, Syed SB, Khan FI, Khan P, Ali S, Hasan GM, Taneja P, Hassan MI. Elucidation of interaction mechanism of ellagic acid to the integrin linked kinase. Int J Biol Macromol. 2019;122:1297–1304. doi: 10.1016/j.ijbiomac.2018.09.089. [DOI] [PubMed] [Google Scholar]
- Gupta A, Pandey AK. Aceclofenac-induced hepatotoxicity: An ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol. 2020;12(11):949–964. doi: 10.4254/wjh.v12.i11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kunnumakkara AB, Aggarwal S, Aggarwal BB. Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol. 2018;9:2160. doi: 10.3389/fimmu.2018.02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Mohammad T, Khan P, Alajmi MF, Hussain A, Rehman MT, Hassan MI. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: a targeted approach towards anticancer therapy. Biomed Pharmacother. 2019;118:109245. doi: 10.1016/j.biopha.2019.109245. [DOI] [PubMed] [Google Scholar]
- Gupta A, Singh AK, Kumar R, Jamieson S, Pandey AK, Bishayee A. Neuroprotective potential of ellagic acid: a critical review. Adv Nutr. 2021;12(4):1211–1238. doi: 10.1093/advances/nmab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh S, Almuhayawi M, Jaouni SA, Almasaudi S, Hassan S, Amri TA, Azhar N, Abd-Allah E, Ali S, El-Shitany N, Mousa SA. Antidiabetic effects of novel ellagic acid nanoformulation: insulin-secreting and anti-apoptosis effects. Saudi J Biol Sci. 2020;27(12):3474–3480. doi: 10.1016/j.sjbs.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Chao YY, Wang SW, Chen YL. Polyethylenimine-capped silver nanoclusters as fluorescent sensors for the rapid detection of ellagic acid in cosmetics. Talanta. 2019;204:484–490. doi: 10.1016/j.talanta.2019.06.047. [DOI] [PubMed] [Google Scholar]
- Iflazoglu Mutlu S, Seven I, Arkali G, Birben N, Sur Arslan A, Aksakal M, Tatli Seven P. Ellagic acid plays an important role in enhancing productive performance and alleviating oxidative stress, apoptosis in laying quail exposed to lead toxicity. Ecotoxicol Environ Saf. 2021;208:111608. doi: 10.1016/j.ecoenv.2020.111608. [DOI] [PubMed] [Google Scholar]
- Iovine C, Mottola F, Santonastaso M, Finelli R, Agarwal A, Rocco L. In vitro ameliorative effects of ellagic acid on vitality, motility and DNA quality in human spermatozoa. Mol Reprod Dev. 2021;88(2):167–174. doi: 10.1002/mrd.23455. [DOI] [PubMed] [Google Scholar]
- Jaman MS, Sayeed MA. Ellagic acid, sulforaphane, and ursolic acid in the prevention and therapy of breast cancer: current evidence and future perspectives. Breast Cancer. 2018;25(5):517–528. doi: 10.1007/s12282-018-0866-4. [DOI] [PubMed] [Google Scholar]
- Jordao JBR, Porto HKP, Lopes FM, Batista AC, Rocha ML. Protective effects of ellagic acid on cardiovascular injuries caused by hypertension in rats. Planta Med. 2017;83(10):830–836. doi: 10.1055/s-0043-103281. [DOI] [PubMed] [Google Scholar]
- Jura M, Kozak LP. Obesity and related consequences to ageing. Age (dordr) 2016;38(1):23. doi: 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan MM, Quine SD. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism. 2013;62(1):52–61. doi: 10.1016/j.metabol.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Karimi MY, Fatemi I, Kalantari H, Mombeini MA, Mehrzadi S, Goudarzi M. Ellagic acid prevents oxidative stress, inflammation, and histopathological alterations in acrylamide-induced hepatotoxicity in wistar rats. J Diet Suppl. 2020;17(6):651–662. doi: 10.1080/19390211.2019.1634175. [DOI] [PubMed] [Google Scholar]
- Kaur H, Ghosh S, Kumar P, Basu B, Nagpal K. Ellagic acid-loaded, tween 80-coated, chitosan nanoparticles as a promising therapeutic approach against breast cancer: In-vitro and in-vivo study. Life Sci. 2021;284:119927. doi: 10.1016/j.lfs.2021.119927. [DOI] [PubMed] [Google Scholar]
- Kharat P, Sarkar P, Mouliganesh S, Tiwary V, Priya VBR, Sree NY, Annapoorna HV, Saikia DK, Mahanta K, Thirumurugan K. Ellagic acid prolongs the lifespan of Drosophila melanogaster. Geroscience. 2020;42(1):271–285. doi: 10.1007/s11357-019-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic I, Yesiloglu Y, Bayrak Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim Acta A Mol Biomol Spectrosc. 2014;130:447–452. doi: 10.1016/j.saa.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sim Y, Hwang JH, Kwun IS, Lim JH, Kim J, Kim JI, Baek MC, Akbar M, Seo W, Kim DK, Song BJ, Cho YE. Ellagic acid prevents binge alcohol-induced leaky gut and liver injury through inhibiting gut dysbiosis and oxidative stress. Antioxidants (Basel) 2021 doi: 10.3390/antiox10091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Choi YJ, Kim HJ. Determining the effect of ellagic acid on the proliferation and migration of pancreatic cancer cell lines. Transl Cancer Res. 2021;10(1):424–433. doi: 10.21037/tcr-20-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Shin HY, Park SY, Kim H, Chung DK. Development and validation of a method for determining the quercetin-3-O-glucuronide and ellagic acid content of common evening primrose (Oenothera biennis) by HPLC-UVD. Molecules. 2021 doi: 10.3390/molecules26020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kaushik P, Incerpi S, Pedersen JZ, Goel S, Prasad AK, Rohil V, Parmar VS, Saso L, Len C. Evaluation of the free radical scavenging activities of ellagic acid and ellagic acid peracetate by EPR spectrometry. Molecules. 2021 doi: 10.3390/molecules26164800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mei L, Guan X, Hu Y. Ellagic acid solid dispersion: Characterization and bioactivity in the hydroxyl radical oxidation system. Food Res Int. 2021;142:110184. doi: 10.1016/j.foodres.2021.110184. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Dai W, Zhang Q. Enhanced oral absorption and anti-inflammatory activity of ellagic acid via a novel type of case in nanosheets constructed by simple coacervation. Int J Pharm. 2021;594:120131. doi: 10.1016/j.ijpharm.2020.120131. [DOI] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Wei D, Xin D, Pan J, Huang M. Ellagic acid inhibits proliferation and migration of cardiac fibroblasts by down-regulating expression of HDAC1. J Toxicol Sci. 2019;44(6):425–433. doi: 10.2131/jts.44.425. [DOI] [PubMed] [Google Scholar]
- Lin X, Yuan G, Li Z, Zhou M, Hu X, Song F, Shao S, Fu F, Zhao J, Xu J, Liu Q, Feng H. Ellagic acid protects ovariectomy-induced bone loss in mice by inhibiting osteoclast differentiation and bone resorption. J Cell Physiol. 2020;235(9):5951–5961. doi: 10.1002/jcp.29520. [DOI] [PubMed] [Google Scholar]
- Liu QS, Li SR, Li K, Li X, Yin X, Pang Z. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/beta-catenin signaling in vivo and in vitro. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600587. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liang X, Liang M, Qin R, Qin F, Wang X. Ellagic acid ameliorates renal ischemic-reperfusion injury through NOX4/JAK/STAT signaling pathway. Inflammation. 2020;43(1):298–309. doi: 10.1007/s10753-019-01120-z. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li X, Zhu Y, Liu J, Liu S. Subclinical hypothyroidism contributes to poor glycemic control in patients with type 2 diabetes mellitus, and ellagic acid attenuates methimazole-induced abnormal glucose metabolism in mice model. J Food Biochem. 2021;45(6):e13753. doi: 10.1111/jfbc.13753. [DOI] [PubMed] [Google Scholar]
- Losada-Barreiro S, Bravo-Diaz C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur J Med Chem. 2017;133:379–402. doi: 10.1016/j.ejmech.2017.03.061. [DOI] [PubMed] [Google Scholar]
- Malinowski B, Zalewska K, Wesierska A, Sokolowska MM, Socha M, Liczner G, Pawlak-Osinska K, Wicinski M. Intermittent fasting in cardiovascular disorders-an overview. Nutrients. 2019 doi: 10.3390/nu11030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri Z, Dianat M, Radan M, Badavi M. Ellagic acid ameliorates lung inflammation and heart oxidative stress in elastase-induced emphysema model in rat. Inflammation. 2020;43(3):1143–1156. doi: 10.1007/s10753-020-01201-4. [DOI] [PubMed] [Google Scholar]
- Maruszewska A, Tarasiuk J. Antitumour effects of selected plant polyphenols, gallic acid and ellagic acid, on sensitive and multidrug-resistant leukaemia HL60 cells. Phytother Res. 2019;33(4):1208–1221. doi: 10.1002/ptr.6317. [DOI] [PubMed] [Google Scholar]
- Maurya PK, Rizvi SI. Protective role of tea catechins on erythrocytes subjected to oxidative stress during human aging. Nat Prod Res. 2009;23(12):1072–1079. doi: 10.1080/14786410802267643. [DOI] [PubMed] [Google Scholar]
- Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SO, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M, Brietzke E. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:134–144. doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Mehrzadi S, Mehrabani M, Malayeri AR, Bakhshayesh M, Kalantari H, Goudarzi M. Ellagic acid as a potential antioxidant, alleviates methotrexate-induced hepatotoxicity in male rats. Acta Chir Belg. 2019;119(2):69–77. doi: 10.1080/00015458.2018.1455419. [DOI] [PubMed] [Google Scholar]
- Mise Yonar S. Growth performance, haematological changes, immune response, antioxidant activity and disease resistance in rainbow trout (Oncorhynchus mykiss) fed diet supplemented with ellagic acid. Fish Shellfish Immunol. 2019;95:391–398. doi: 10.1016/j.fsi.2019.10.056. [DOI] [PubMed] [Google Scholar]
- Murphy MT, Qin X, Kaul S, Barrientos G, Zou Z, Mathias CB, Thomas D, Bose DD. The polyphenol ellagic acid exerts anti-inflammatory actions via disruption of store-operated calcium entry (SOCE) pathway activators and coupling mediators. Eur J Pharmacol. 2020;875:173036. doi: 10.1016/j.ejphar.2020.173036. [DOI] [PubMed] [Google Scholar]
- Nandi A, Yan LJ, Jana CK, Das N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med Cell Longev. 2019;2019:9613090. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: a review. Eur J Med Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Nirwana I, Munadziroh E, Yogiartono RM, Thiyagu C, Ying CS, Dinaryanti A. Cytotoxicity and proliferation evaluation on fibroblast after combining calcium hydroxide and ellagic acid. J Adv Pharm Technol Res. 2021;12(1):27–31. doi: 10.4103/japtr.JAPTR_154_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro NO, Odiba AS, Osadebe PO, Omeje EO, Liao G, Fang W, Jin C, Wang B. Bioactive phytochemicals with anti-aging and lifespan extending potentials in caenorhabditis elegans. Molecules. 2021 doi: 10.3390/molecules26237323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgar Y, Ozturk N, Usta C, Puddu PE, Ozdemir S. Ellagic acid reduces L-type Ca2+ current and contractility through modulation of NO-GC-cGMP pathways in rat ventricular myocytes. J Cardiovasc Pharmacol. 2014;64(6):567–573. doi: 10.1097/FJC.0000000000000153. [DOI] [PubMed] [Google Scholar]
- Ortenzi MA, Antenucci S, Marzorati S, Panzella L, Molino S, Rufian-Henares JA, Napolitano A, Verotta L. Pectin-based formulations for controlled release of an ellagic acid salt with high solubility profile in physiological media. Molecules. 2021 doi: 10.3390/molecules26020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova EL, Simeonova LS, Gegova GA. Combined efficacy of oseltamivir, isoprinosine and ellagic acid in influenza A(H3N2)-infected mice. Biomed Pharmacother. 2018;98:29–35. doi: 10.1016/j.biopha.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Pirzadeh-Naeeni S, Mozdianfard MR, Shojaosadati SA, Khorasani AC, Saleh T. A comparative study on schizophyllan and chitin nanoparticles for ellagic acid delivery in treating breast cancer. Int J Biol Macromol. 2020;144:380–388. doi: 10.1016/j.ijbiomac.2019.12.079. [DOI] [PubMed] [Google Scholar]
- Polce SA, Burke C, Franca LM, Kramer B, de Andrade Paes AM, Carrillo-Sepulveda MA. Ellagic acid alleviates hepatic oxidative stress and insulin resistance in diabetic female rats. Nutrients. 2018 doi: 10.3390/nu10050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Akileshwari C, Reddy VS, Reddy GB. Attenuation of diabetic retinopathy in rats by ellagic acid through inhibition of AGE formation. J Food Sci Technol. 2017;54(8):2411–2421. doi: 10.1007/s13197-017-2683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi VB, Askari VR, Mousavi SH. Ellagic acid reveals promising anti-aging effects against d-galactose-induced aging on human neuroblastoma cell line, SH-SY5Y: a mechanistic study. Biomed Pharmacother. 2018;108:1712–1724. doi: 10.1016/j.biopha.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Rai SN, Zahra W, Singh SS, Birla H, Keswani C, Dilnashin H, Rathore AS, Singh R, Singh RK, Singh SP. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotox Res. 2019;36(3):452–462. doi: 10.1007/s12640-019-00038-6. [DOI] [PubMed] [Google Scholar]
- Rai SN, Chaturvedi VK, Singh P, Singh BK, Singh MP. Mucuna pruriens in Parkinson's and in some other diseases: recent advancement and future prospective. 3 Biotech. 2020;10(12):522. doi: 10.1007/s13205-020-02532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JL, Giner RM, Marin M, Recio MC. A pharmacological update of ellagic acid. Planta Med. 2018;84(15):1068–1093. doi: 10.1055/a-0633-9492. [DOI] [PubMed] [Google Scholar]
- Rizvi SI, Maurya PK. Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci. 2007;1100:373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Suzuki M, Itakura S, Todo H, Arce F, Jr, See GL, Tanikawa T, Inoue Y. Preparation, characterization, solubility, and antioxidant capacity of ellagic acid-urea complex. Materials (Basel) 2022 doi: 10.3390/ma15082836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Martorell M, Arbiser JL, Sureda A, Martins N, Maurya PK, Sharifi-Rad M, Kumar P, Sharifi-Rad J. Antioxidants: positive or negative actors? Biomolecules. 2018 doi: 10.3390/biom8040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio ADG, Gontijo AVL, Lima GMG, de Oliveira MAC, Lepesqueur LSS, Koga-Ito CY. Ellagic acid-cyclodextrin complexes for the treatment of oral candidiasis. Molecules. 2021 doi: 10.3390/molecules26020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic IM, Jocic E, Nikolic VD, Popsavin MM, Rakic SJ, Savic-Gajic IM. The effect of complexation with cyclodextrins on the antioxidant and antimicrobial activity of ellagic acid. Pharm Dev Technol. 2019;24(4):410–418. doi: 10.1080/10837450.2018.1502318. [DOI] [PubMed] [Google Scholar]
- Shakeri A, Zirak MR, Sahebkar A. Ellagic acid: a logical lead for drug development? Curr Pharm Des. 2018;24(2):106–122. doi: 10.2174/1381612823666171115094557. [DOI] [PubMed] [Google Scholar]
- Singh Hallan S, Sguizzato M, Pavoni G, Baldisserotto A, Drechsler M, Mariani P, Esposito E, Cortesi R. Ellagic acid containing nanostructured lipid carriers for topical application: a preliminary study. Molecules. 2020 doi: 10.3390/molecules25061449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spisso A, Gomez FJV, Fernanda Silva M. Determination of ellagic acid by capillary electrophoresis in Argentinian wines. Electrophoresis. 2018;39(13):1621–1627. doi: 10.1002/elps.201700487. [DOI] [PubMed] [Google Scholar]
- Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, Yang Y. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueb RM. Oxidative stress and its impact on skin, scalp and hair. Int J Cosmet Sci. 2021;43(Suppl 1):S9–S13. doi: 10.1111/ics.12736. [DOI] [PubMed] [Google Scholar]
- Unsal V, Cicek M, Sabancilar I. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev Environ Health. 2021;36(2):279–295. doi: 10.1515/reveh-2020-0048. [DOI] [PubMed] [Google Scholar]
- Wang ST, Chang WC, Hsu C, Su NW. Antimelanogenic effect of Urolithin A and Urolithin B, the colonic metabolites of ellagic acid, in B16 melanoma cells. J Agric Food Chem. 2017;65(32):6870–6876. doi: 10.1021/acs.jafc.7b02442. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zeng Y, Fu W, Zhang P, Li L, Ye C, Yu L, Zhu X, Zhao S. Seed-mediated growth of Au@Ag core-shell nanorods for the detection of ellagic acid in whitening cosmetics. Anal Chim Acta. 2018;1002:97–104. doi: 10.1016/j.aca.2017.11.067. [DOI] [PubMed] [Google Scholar]
- Wang GQ, He XM, Zhu GF, Li DD, Shi JS, Zhang F. Ellagic acid supports neuron by regulating astroglia Nrf2. Biotechnol Appl Biochem. 2019;66(5):738–743. doi: 10.1002/bab.1791. [DOI] [PubMed] [Google Scholar]
- Wang HR, Sui HC, Zhu BT. Ellagic acid, a plant phenolic compound, activates cyclooxygenase-mediated prostaglandin production. Exp Ther Med. 2019;18(2):987–996. doi: 10.3892/etm.2019.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ren F, Li B, Song Z, Chen P, Ouyang L. Ellagic acid exerts antitumor effects via the PI3K signaling pathway in endometrial cancer. J Cancer. 2019;10(15):3303–3314. doi: 10.7150/jca.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Y, Liang C, Tan R, Tan L, Tan R. Pharmacodynamic effect of ellagic acid on ameliorating cerebral ischemia/reperfusion injury. Pharmacology. 2019;104(5–6):320–331. doi: 10.1159/000502401. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen J, Xiang D, Lian X, Wu C, Quan J. Ellagic acid inhibits cell proliferation, migration, and invasion in melanoma via EGFR pathway. Am J Transl Res. 2020;12(5):2295–2304. [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang L, Liu T, Wang J, Wen A, Ding Y. Ellagic acid protects mice against sleep deprivation-induced memory impairment and anxiety by inhibiting TLR4 and activating Nrf2. Aging (albany NY) 2020;12(11):10457–10472. doi: 10.18632/aging.103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei DZ, Lin C, Huang YQ, Wu LP, Huang MY. Ellagic acid promotes ventricular remodeling after acute myocardial infarction by up-regulating miR-140-3p. Biomed Pharmacother. 2017;95:983–989. doi: 10.1016/j.biopha.2017.07.106. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen F, Liu T, Xu J, Li J, Jiang L, Wang X, Sheng J. Ellagic acid blocks RANKL-RANK interaction and suppresses RANKL-induced osteoclastogenesis by inhibiting RANK signaling pathways. Chem Biol Interact. 2020;331:109235. doi: 10.1016/j.cbi.2020.109235. [DOI] [PubMed] [Google Scholar]
- Xu Q, Shen M, Han Y, Diao H. Effects of ellagic acid supplementation on jejunal morphology, digestive enzyme activities, antioxidant capacity, and microbiota in mice. Front Microbiol. 2021;12:793576. doi: 10.3389/fmicb.2021.793576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasan BE, Mercan T, Erkan O, Ozdemir S (2021) Ellagic Acid Prevents Ca(2+) Dysregulation and improves functional abnormalities of ventricular myocytes via attenuation of oxidative stress in pathological cardiac hypertrophy. Cardiovasc Toxicol 21(8):630–641. 10.1007/s12012-021-09654-1 [DOI] [PubMed]
- Yang HL, Lin CP, Vudhya Gowrisankar Y, Huang PJ, Chang WL, Shrestha S, Hseu YC. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated alpha-MSH pathways via Nrf2 activation in keratinocytes. Biochem Pharmacol. 2021;185:114454. doi: 10.1016/j.bcp.2021.114454. [DOI] [PubMed] [Google Scholar]
- Yoganathan S, Alagaratnam A, Acharekar N, Kong J. Ellagic acid and schisandrins: natural biaryl polyphenols with therapeutic potential to overcome multidrug resistance in cancer. Cells. 2021 doi: 10.3390/cells10020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf M, Shamsi A, Khan P, Shahbaaz M, AlAjmi MF, Hussain A, Hassan GM, Islam A, Rizwanul Haque QM, Hassan MI. Ellagic acid controls cell proliferation and induces apoptosis in breast cancer cells via inhibition of cyclin-dependent kinase 6. Int J Mol Sci. 2020 doi: 10.3390/ijms21103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaazaa AM, Lokman MS, Shalby AB, Ahmed HH, El-Toumy SA. Ellagic acid holds promise against hepatocellular carcinoma in an experimental model: mechanisms of action. Asian Pac J Cancer Prev. 2018;19(2):387–393. doi: 10.22034/APJCP.2018.19.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeb A. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Mol Cell Biochem. 2018;448(1–2):27–41. doi: 10.1007/s11010-018-3310-3. [DOI] [PubMed] [Google Scholar]
- Zhong C, Qiu S, Li J, Shen J, Zu Y, Shi J, Sui G. Ellagic acid synergistically potentiates inhibitory activities of chemotherapeutic agents to human hepatocellular carcinoma. Phytomedicine. 2019;59:152921. doi: 10.1016/j.phymed.2019.152921. [DOI] [PubMed] [Google Scholar]
- Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, Li HB. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid Med Cell Longev. 2021;2021:9932218. doi: 10.1155/2021/9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]