Abstract
Introduction
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), formerly also called baboon syndrome, is characterized by symmetrical erythematous rash with typical localization in the gluteal and intertriginous areas. A type IV delayed hypersensitivity immune response is thought to be responsible for its development. CGRP monoclonal antibodies (CGRP mAbs) are a new class of drugs for the prevention of migraine. We present the first case of SDRIFE occurring in temporal relation to the use of erenumab for migraine prevention.
Case
A 48-year-old female patient with migraine received erenumab 140 mg subcutaneously in the thigh area for the prevention of migraine in repetitive cycles, each 1 month apart. Initially, the patient experienced no side effects. After the third cycle, a masseuse incidentally noticed a reddish, circular rash in the buttock area during a back massage. There were no other symptoms. The skin changes resolved spontaneously. Two years later, approximately 40 h after reapplication of erenumab 140 mg, the patient experienced a severe pain in the buttock area centered over the anal crease. The area of pain extended in a circular pattern with approximately 20 cm in diameter. The pain started abruptly and reached a severe intensity within about 30 min. Sitting on the buttocks was no longer possible for the patient. There was marked allodynia and hyperpathia in the entire buttocks region. A flat, broad-based blister-like skin swelling developed in this region. The blisters began opening up on the fourth day after the onset of the skin reaction. In addition, there was a pronounced redness in the entire buttock area. Here, the patient felt a strong burning pain, similar to a scald.
Results
The symptoms lasted for a period of 10 days. From this point on, they fully subsided under concomitant therapy with prednisolone.
Conclusion
SDRIFE as a rare dermatological side effect should be considered in the monitoring of skin lesions during migraine prophylaxis. In view of the high migraine prevalence, knowledge of this uncommon syndrome is important. It is crucial to recognize the relationship between the medication and the circumscribed exanthema occurring distant from the injection site.
Keywords: SDRIFE, Baboon syndrome, Erenumab, Migraine, Prevention, Dermatological side effect, Skin lesions, Exanthema
Key Summary Points
| Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), formerly also called baboon syndrome, is characterized by symmetrical erythematous rash with typical localization in the gluteal and intertriginous areas. |
| CGRP monoclonal antibodies (CGRP mAbs) are a new class of drugs for the prevention of migraine. |
| We present the first case of SDRIFE occurring in temporal relation to the use of erenumab for migraine prevention. |
| In view of the widespread nature of migraine, knowledge of this unusual dermatological rare syndrome is important. |
| It is crucial to recognize the relationship between the medication and the circumscribed exanthema occurring distant from the injection site. |
Introduction
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), formerly called baboon syndrome, is characterized by symmetrical erythematous rash with typical localization in the gluteal and intertriginous areas. It can be triggered by exposure to systemic drugs or contact allergens [1–6]. The name baboon syndrome was coined in 1984 because of the typical appearance of the skin lesions. The characteristic redness typically presents in the area of the buttocks and inner thighs and resembles the red rump of baboons [3].
In 2004, Häusermann proposed the term symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) for this condition [7]. The skin reaction can be triggered without prior sensitization. It shows a characteristic spatial distribution without systemic findings [3].
SDRIFE is a rare condition. Since its initial description in 1984, over 100 cases have been published ranging in age from 18 months to 84 years. It occurs in both women and men with a tendency to be more common in men [8, 9]. A type IV delayed hypersensitivity immune response is believed to be responsible for the development [10].
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide [11]. It is found in both the peripheral and central nervous system. The molecule causes vasodilation, neurogenic inflammation, and peripheral and central pain sensitization in migraine. CGRP monoclonal antibodies (CGRP mAbs) are a new class of drugs for the prevention of migraine [12]. CGRP mAbs block the physiological functions of CGRP in the area of the trigeminal nerve and cerebral vasculature and can thus have a preventive effect on the pathomechanisms of migraine [11, 12].
Erenumab is the first monoclonal antibody targeting the receptor of CGRP approved for the prevention of migraine [13–22]. The substance is injected subcutaneously in 4-week intervals by patients using an autoinjector. In phase 2, phase 3, open-label, and observational studies with erenumab in migraine, the treatment was well tolerated. The most common adverse events were local skin reactions at the injection site and constipation [13, 23].
We describe here, to our knowledge, the first report of an SDRIFE occurring in temporal relation to erenumab use. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Case Presentation
The 48-year-old Caucasian female patient (height 165 cm, weight 59 kg) had migraine without aura and migraine with aura according to ICHD-3 criteria on an average of 6–7 days per month since puberty. Since 2015, headache intensity, attack duration, and frequency of headaches per month increased, which is why she presented to our center. She now experienced 11 days with migraine per month. Attacks showed a very severe intensity, attack duration averaged 19 h in 2019, according to her reporting in the migraine app [24]. Acute medications were used an average of 10 days per month (naratriptan 2.5 mg, eletriptan 40 mg, or sumatriptan 6 mg subcutaneously). The patient did not report any other pre-existing conditions or medication use. The MIDAS score was grade IV, corresponding to a very severe impairment.
In December 2019, the patient was first prescribed erenumab 140 mg (Aimovig) subcutaneously after extensive patient information as other approved prophylactic migraine medication (amitriptyline, metoprolol, flunarizine, topiramate) had not been effective. This resulted in a reduction of migraine days per month to an average of 3 days. The attack duration shortened to an average of 10 h. The patient usually took acute medication (naratriptan 2.5 mg or sumatriptan 6 mg subcutaneously) on 2 days per month. Initially, the patient experienced no side effects. In February, a masseuse noticed a reddish, circular rash in the buttock area during back massage. The patient herself did not experience any subjective symptoms. In particular, there were no local skin changes at the injection site on the thigh. The patient therefore paid no further attention to the skin redness. She applied a cooling gel, and the redness resolved spontaneously after approximately 1 week. Treatment with erenumab 140 mg was continued in monthly intervals. Therapeutic efficacy remained continuously stable to the patient's satisfaction, with only about 1–2 mild migraine attacks per month. These responded very well to the acute medication.
On February 3, 2022, the patient experienced a migraine attack. She treated it with sumatriptan 6 mg subcutaneously. On February 4, a recurrent headache occurred. The patient treated this with naratriptan 2.5 mg with good effect. On February 5, 2022, the patient repeated the monthly injection of erenumab for the 27th time. On February 6, migraine headaches recurred. The patient initially treated these again with naratriptan 2.5 mg, and after 4 h with sumatriptan 6 mg subcutaneously because of lack of efficacy. The headache then subsided. To prevent recurrent headache, the patient used an additional 100 mg of prednisolone. She tapered off prednisolone because of continued headache with a gradual dose reduction of 20 mg every 2 days. On February 10, the migraine headache resolved. Approximately 40 h after the application of erenumab, the patient experienced very severe pain in the buttock area centered over the anal crease on February . The area of pain extended in a circular pattern over an area of approximately 20 cm (Fig. 1). The pain started abruptly and reached very severe intensity within about 30 min. Sitting on the buttocks was no longer possible for the patient. There was pronounced allodynia in the entire gluteal area. The patient was therefore no longer able to wear clothes in this body part and had to leave her buttocks undressed for 7 days and could not leave the house. A broad blister-like skin swelling formed in the area of pain. The blisters opened up on the fourth day after the onset of the skin reaction (Figs. 2, 3). In addition to the pain in this area, a pronounced redness appeared in the entire buttock area. Here, the patient experienced a burning pain similar to a severe sunburn or scald. The symptoms lasted for a period of 10 days. From this point on, they slowly subsided. In this time period, pain intensity fluctuated with intermittent intensifications of pain.
Fig. 1.
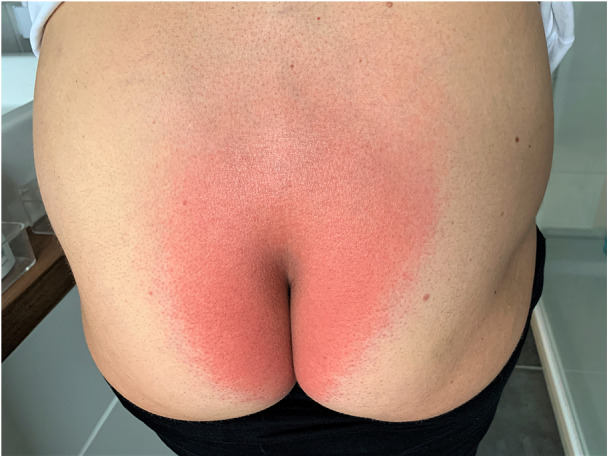
Sharply delineated red, large macula on the buttocks 40 h after application of erenumab 140 mg to the right thigh frontally
Fig. 2.
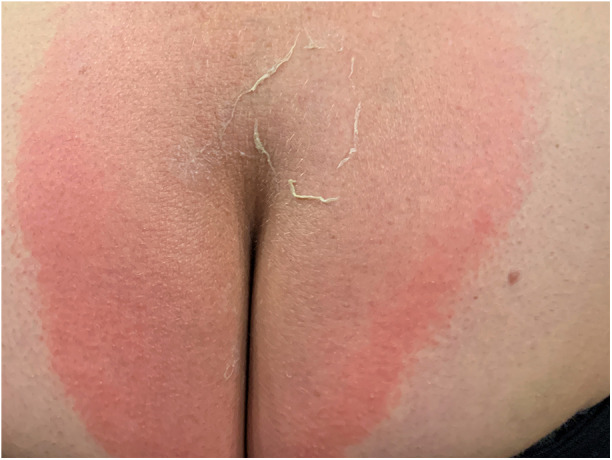
Day 4 after application of erenumab 140 mg. Opening of the blisters with lamellar scaling over the anal fold. In this area, the patient noticed a very strong burning pain with allodynia and hyperpathia, which radiated into the entire reddened area
Fig. 3.
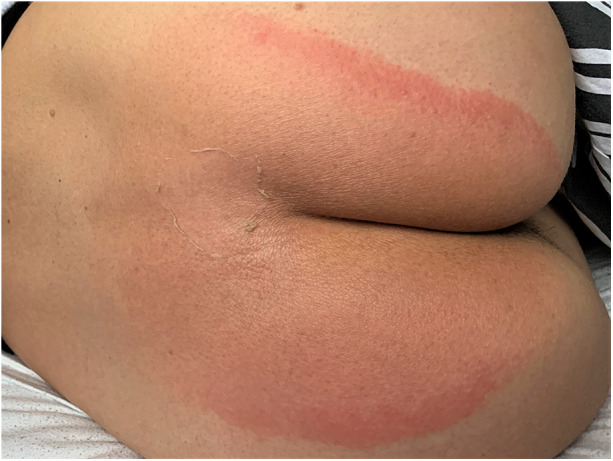
Day 5 after use of erenumab 140 mg with the exanthema at its peak
On February 12, i.e., 7 days after the use of erenumab, the patient noticed a painless circular redness on the inside of the right elbow (Fig. 4). Another area of redness appeared proximally on the flexor side of the right forearm. These areas did not cause any discomfort except for mild sensitivity to touch. The areas did not expand and resolved spontaneously after 3 weeks.
Fig. 4.
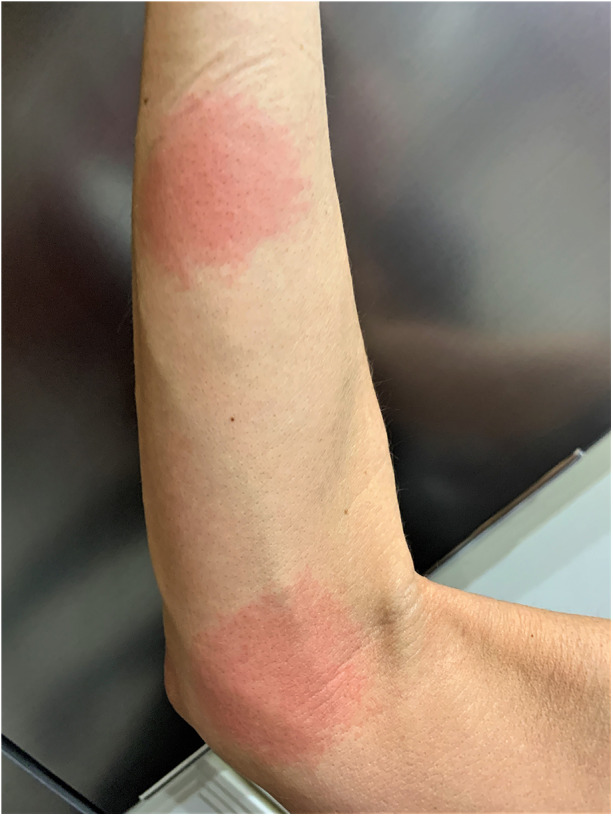
Painless circular redness 7 days after application of erenumab on the inner elbow of the right arm. Another area appeared on the flexor side of the proximal forearm
After 14 days, the redness in the buttock area was still present, but the marked allodynia had remitted (Fig. 5). After a period of 21 days, the symptoms resolved completely. The patient did not report any other sequelae.
Fig. 5.
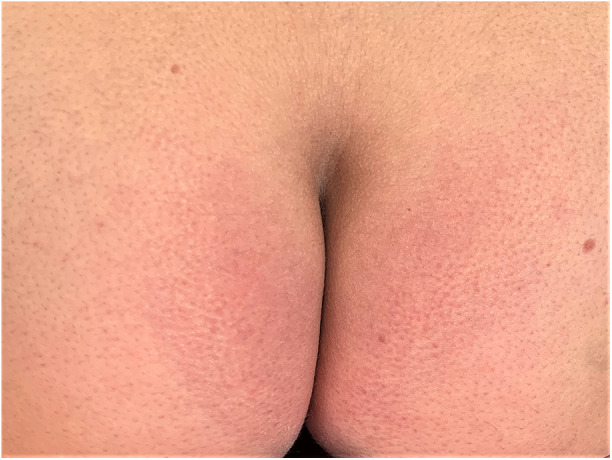
Day 14 after the application of erenumab 140 mg. The exanthema subsided and the pronounced pain, allodynia, and hyperpathia were remitted
The patient discontinued the use of erenumab thereafter. On March 7, 2022, she switched to fremanezumab 225 mg (Ajovy) subcutaneously, which also had a good effect on migraine. No skin reactions or other side effects occurred under regimen this so far. The prior acute medication was continued.
Discussion
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) or baboon syndrome has neither been described in triptan use nor after the use of erenumab or other CGRP monoclonal antibodies (CGRP mAbs). Häusermann et al. proposed the following criteria for SDRIFE: (1) exposure to a systemically administered drug either at the first or repeated dose (excluding contact allergens); (2) sharply demarcated erythema of the gluteal/perianal area and/or V-shaped erythema of the inguinal/perigenital area; (3) involvement of at least one other intertriginous/flexural localization; (4) symmetry of affected areas; and (5) absence of systemic symptoms and signs [7]. The skin lesions met these criteria. The diagnosis was confirmed clinically by dermatologists.
The diagnostic criteria [7] include that SDRIFE may occur at the first or after repeated administration. The latter is the case here. The fact that SDRIFE can only occur after a longer period of taking a specific substance is also known for other substances. For example, a patient developed SDRIFE only after taking tamoxifen for 8 years [25]. Likewise, SDRIFE may appear only with a longer time delay for weeks after ingestion [26]. The patient already had a skin change in the buttocks area 2 years ago after application of erenumab. It resolved spontaneously, and a diagnosis was not initially made. It is not known whether this skin lesion met the criteria for SDRIFE.
In our patient, SDRIFE occurred in temporal relation with the use of triptans and erenumab. Although a causal relationship between the SDRIFE and therapy with erenumab has not been clearly proven, we consider it likely that erenumab induced the exanthema for the following reasons. At the time of occurrence of SDRIFE in February 2022, the plasma levels of the triptans used had largely decayed as a result of their short half-life [27]. In contrast, erenumab, with a half-life of approximately 28 days, has sustained plasma levels that could cause and maintain the response [28]. Triptans have been available for more than 30 years. Nevertheless, no similar skin reactions in the sense of SDRIFE after triptan administration have been described to date. On the contrary, local skin reactions at the injection site are among the most frequent side effects after use of erenumab [20–22, 29–34]. SDRIFE has also been described as a side effect after treatment with other monoclonal antibodies [35–38].
Clinical presentation, history, and exclusion of other causes of the rash establish the diagnosis of SDRIFE. Laboratory tests are not informative in establishing the diagnosis except to detect systemic involvement (e.g., cytopenia, hepatic or renal involvement) [9]. Patch tests, lymphocyte transformation tests, and drug provocation tests (DPT) can be useful for diagnosis, but the results of these tests are highly variable. Skin patch tests are usually the preferred testing procedure—they are applied to previously affected areas. Early reports indicate that patch testing only results in a positive response in up to 50% of patients [7, 8, 39]. The explanation for these results could be that the systemic agent is not fully absorbed when applied to the skin in the patch test [40]. The controlled DPT is considered the clinical gold standard and yields a positive result in most patients with SDRIFE [39]. DPTs have been reported for cases of SDRIFE with clindamycin, cimetidine, corticosteroids, terbinafine, and valacyclovir [3].
For the development of SDRIFE, a type IV delayed hypersensitivity immune response is assumed [10, 39]. Immunohistochemical studies showed CD4+ T cell infiltration. There is an increased endothelial and keratinocyte expression of CD26P-selectin. This activates type 1 helper T cells to the sites of inflammation [39]. It is also assumed that both a type IVa reaction involving CD4+ Th1 cells, macrophages, and a type of IVc reaction with cytotoxic CD4 and CD8 T cells are involved in the development of the skin reaction [41]. However, the pathomechanisms are largely unclear. In particular, it is unclear why SDRIFE can be triggered after the first exposure to an active substance without prior sensitization [7]. The reactivation of tissue toxicity at the intertriginous predilection sites is also discussed as a contributory cause, as is the direct activation of immunoreceptors and the particular distribution of eccrine sweat glands at the typical sites of occurrence [7, 42].
CGRP is involved in numerous physiological processes throughout the body. It has a pronounced vasodilatory effect. It also has a protective effect via inflammatory mechanisms in the cardiovascular and gastrointestinal systems. The expression of vascular endothelial growth factor (VEGF) is activated and thus revascularization is stimulated. Inflammatory mediators such as tumour necrosis factor alpha (TNFα) and macrophage infiltration are inhibited and the proliferation of keratinocytes is stimulated [43–46]. The blocking of these mechanisms by monoclonal antibodies against CGRP may be involved in the development of the skin reactions.
The patient did not develop a skin reaction when switching from erenumab to fremanezumab. This could suggest that it is not the blockade against CGRP that is responsible for the toxic skin reaction but an immunological response to the antibody molecule. However, it is also conceivable that antagonizing the CGRP receptor (erenumab) and antagonizing the CGRP molecule (fremanezumab) leads to different reactions. This has practical relevance, as in the case of an SDRIFE with one substance, a switch to another substance is possible. Like in this case, there may not be a skin reaction with the other substance. Fremanezumab, however, has been approved after erenumab, so it remains to be seen whether similar skin reactions will also be observed under this substance.
A limiting factor is that a biopsy is not available for our patient. The possibility of a purely coincidental occurrence of the skin reaction is also conceivable. Furthermore, the effect of drug–drug interaction of CGRP-inhibiting therapies is not yet known in detail. This may be particularly relevant in the treatment of migraine, as multiple agents targeting CGRP mechanisms are often applied. However, given the temporal association of the skin reaction with the use of erenumab and the known extensive immune effects of CGRP inhibition, a likely association can be assumed. In the care of patients with migraine using monoclonal CGRP antibodies, close attention should therefore be paid to whether there is a subgroup of patients who are at risk of immunological inflammatory complications of CGRP inhibition [47, 48].
In conclusion, SDRIFE can occur in temporal relationship with the administration of erenumab in migraine prophylaxis. The rare dermatological side effect should be considered in the monitoring of skin lesions during migraine care. In view of the high migraine prevalence, knowledge of this uncommon syndrome is important. It is crucial to recognize the relationship between the medication and the circumscribed exanthema occurring distant from the injection site.
Acknowledgements
We thank the participating patient of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
Carl H. Göbel and Hartmut Göbel drafted the manuscript and edited and approved the final version. Axel Heinze, Sarah Karstedt, Anna Cirkel and Thomas Münte revised the first draft for important intellectual content and contributed to the final manuscript.
Disclosures
Hartmut Göbel has received grants, non-financial support, personal fees, and fees to the institution from Amgen, Abbvie, Allergan, Eli Lilly, Lundbeck, Novartis and Teva Pharmaceutical. Axel Heinze reports personal fees from Eli Lilly, Novartis, and Teva Pharmaceutical. Carl H. Göbel, Sarah Karstedt, Anna Cirkel and Thomas Münte have nothing to disclose.
Compliance with Ethics Guidelines
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Institutional review board approval is not required for a case report.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Bulur I, Keseroglu HO, Saracoglu ZN, et al. Symmetrical drug-related intertriginous and flexural exanthema (baboon syndrome) associated with infliximab. J Dermatol Case Rep. 2015;9:12–14. doi: 10.3315/jdcr.2015.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Risi-Pugliese T, Barailler H, Hamelin A, et al. Symmetrical drug-related intertriginous and flexural exanthema: a little-known drug allergy. J Allergy Clin Immunol Pract. 2020;8(3185–3189):e3184. doi: 10.1016/j.jaip.2020.04.052. [DOI] [PubMed] [Google Scholar]
- 3.Harbaoui S, Litaiem N. Symmetrical drug-related intertriginous and flexural exanthema. In: StatPearls. Treasure Island; 2022. [PubMed]
- 4.Lima Miranda O, Martins J, Almeida A, et al. Symmetrical drug-related intertriginous and flexural exanthema (baboon syndrome) Eur J Case Rep Intern Med. 2021;8:003029. doi: 10.12890/2021_003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neema S, Shaw SC, Gopalakrishnan S. Symmetrical drug-related intertriginous and flexural exanthema. Indian Pediatr. 2020;57:1093. [PubMed] [Google Scholar]
- 6.Seok J, Kim JM, Park KY, et al. Symmetrical drug-related intertriginous and flexural exanthema: two cases and brief literature review. Ann Dermatol. 2018;30:606–609. doi: 10.5021/ad.2018.30.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297–310. doi: 10.1111/j.0105-1873.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- 8.Karadag AS, Ozlu E, Akdeniz N, et al. Oral mucosal involvement and petechial lesions: a SDRIFE case with unusual findings. Cutan Ocul Toxicol. 2016;35:157–159. doi: 10.3109/15569527.2015.1067227. [DOI] [PubMed] [Google Scholar]
- 9.Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313–318. doi: 10.1097/ACI.0b013e3283489d5f. [DOI] [PubMed] [Google Scholar]
- 10.Li DG, Thomas C, Weintraub GS, et al. Symmetrical drug-related intertriginous and flexural exanthema induced by doxycycline. Cureus. 2017;9:e1836. doi: 10.7759/cureus.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deligianni CI, Mitsikostas DD, Ashina M. Safety and tolerability evaluation of erenumab for the preventive treatment of migraine. Expert Opin Drug Saf. 2021;20:867–876. doi: 10.1080/14740338.2021.1933941. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Prim. 2022;8:2. doi: 10.1038/s41572-021-00328-4. [DOI] [PubMed] [Google Scholar]
- 13.Ashina M, Goadsby PJ, Dodick DW, et al. Assessment of erenumab safety and efficacy in patients with migraine with and without aura: a secondary analysis of randomized clinical trials. JAMA Neurol. 2022;79:159–168. doi: 10.1001/jamaneurol.2021.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vries Lentsch S, Al-Hassany L, Ferrari MD, et al. CGRP-mediated trigeminovascular reactivity in migraine patients treated with erenumab. J Neurol Neurosurg Psychiatry. 202;93:911–12. [DOI] [PubMed]
- 15.Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026–1037. doi: 10.1177/0333102418759786. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 17.Goadsby PJ, Reuter U, Hallstrom Y, et al. One-year sustained efficacy of erenumab in episodic migraine: results of the STRIVE study. Neurology. 2020;95:e469–e479. doi: 10.1212/WNL.0000000000010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang D, Sessa M. Post-marketing safety surveillance of erenumab: new insight from Eudravigilance. Expert Opin Drug Saf. 2022;1–6. [DOI] [PubMed]
- 19.Noseda R, Bedussi F, Gobbi C, et al. Safety profile of erenumab, galcanezumab and fremanezumab in pregnancy and lactation: analysis of the WHO pharmacovigilance database. Cephalalgia. 2021;41:789–798. doi: 10.1177/0333102420983292. [DOI] [PubMed] [Google Scholar]
- 20.Schenk H, Holle D, Nsaka M, et al. Twelve-month safety, tolerability and susceptibility to adverse events of prophylactic migraine therapy with erenumab: a retrospective real-world study. J Headache Pain. 2022;23:55. doi: 10.1186/s10194-022-01426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sessa M, Andersen M. New insight on the safety of erenumab: an analysis of spontaneous reports of adverse events recorded in the US Food and Drug Administration Adverse Event Reporting System Database. BioDrugs. 2021;35:215–227. doi: 10.1007/s40259-021-00469-8. [DOI] [PubMed] [Google Scholar]
- 22.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–434. doi: 10.1016/S1474-4422(17)30083-2. [DOI] [PubMed] [Google Scholar]
- 23.Ashina M, Goadsby PJ, Reuter U, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39:1455–1464. doi: 10.1177/0333102419854082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Göbel H, Frank B, Heinze A, et al. Healthcare behavior of migraine and headache patients when treatment is accompanied by the digital migraine app. Schmerz. 2019;33:147–155. doi: 10.1007/s00482-018-0355-x. [DOI] [PubMed] [Google Scholar]
- 25.Mofarrah R, Mofarrah R, Kranke B, et al. First report of tamoxifen-induced baboon syndrome. J Cosmet Dermatol. 2021;20:2574–2578. doi: 10.1111/jocd.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hai J, Shawa H, Kim-Lim P, et al. Systemic drug-related intertriginous and flexural exanthema induced by the Pfizer-BioNTech COVID-19 vaccine: a report of 2 cases. JAAD Case Rep. 2021;18:57–60. doi: 10.1016/j.jdcr.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolas S, Nicolas D. Triptans. In: StatPearls. StatPearls Publishing, Treasure Island; 2022.
- 28.Szkutnik-Fiedler D. Pharmacokinetics, pharmacodynamics and drug–drug interactions of new anti-migraine drugs-lasmiditan, gepants, and calcitonin-gene-related peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics. 2020;12:1180. [DOI] [PMC free article] [PubMed]
- 29.Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280–2287. doi: 10.1016/S0140-6736(18)32534-0. [DOI] [PubMed] [Google Scholar]
- 30.Sakai F, Takeshima T, Tatsuoka Y, et al. Long-term efficacy and safety during open-label erenumab treatment in Japanese patients with episodic migraine. Headache. 2021;61:653–661. doi: 10.1111/head.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia. 2020;40:543–553. doi: 10.1177/0333102420912726. [DOI] [PubMed] [Google Scholar]
- 32.Viudez-Martinez A, Pascual-Carrasco A, Beltran-Blasco I, et al. Effectiveness and safety of erenumab and galcanezumab in the prevention of chronic and episodic migraine: a retrospective cohort study. J Clin Pharm Ther. 2022;47(6):814–23. [DOI] [PubMed]
- 33.Zhou Y, Zhang F, Starcevic Manning M et al. Immunogenicity of erenumab: a pooled analysis of six placebo-controlled trials with long-term extensions. Cephalalgia. 2022;3331024221075621. [DOI] [PubMed]
- 34.Zhu C, Guan J, Xiao H, et al. Erenumab safety and efficacy in migraine: a systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore) 2019;98:e18483. doi: 10.1097/MD.0000000000018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE) Dermatol Online J. 2009;15:3. [PubMed] [Google Scholar]
- 36.Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171–180. doi: 10.2165/11539080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261–266. doi: 10.1080/15569527.2019.1594874. [DOI] [PubMed] [Google Scholar]
- 38.Yang SY, Lan CC, Hu SC. Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) induced by golimumab. Int J Dermatol. 2017;56:571–572. doi: 10.1111/ijd.13565. [DOI] [PubMed] [Google Scholar]
- 39.Nespoulous L, Matei I, Charissoux A, et al. Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) associated with pristinamycin, secnidazole, and nefopam, with a review of the literature. Contact Dermatitis. 2018;79:378–380. doi: 10.1111/cod.13084. [DOI] [PubMed] [Google Scholar]
- 40.Labadie JG, Florek AG, Croitoru A, et al. First case of symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) due to Berberine, an over-the-counter herbal glycemic control agent. Int J Dermatol. 2018;57:e68–e70. doi: 10.1111/ijd.14059. [DOI] [PubMed] [Google Scholar]
- 41.Huynh T, Hughey LC, Mckay K, et al. Systemic drug-related intertriginous and flexural exanthema from radio contrast media: a series of 3 cases. JAAD Case Rep. 2015;1:147–149. doi: 10.1016/j.jdcr.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magnolo N, Metze D, Stander S. Pustulobullose Variante eines SDRIFE (symmetrical drug-related intertriginous and flexural exanthema) J Dtsch Dermatol Ges. 2017;15:657–659. doi: 10.1111/ddg.13031_g. [DOI] [PubMed] [Google Scholar]
- 43.Granstein RD, Wagner JA, Stohl LL, et al. Calcitonin gene-related peptide: key regulator of cutaneous immunity. Acta Physiol (Oxf) 2015;213:586–594. doi: 10.1111/apha.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roggenkamp D, Kopnick S, Stab F, et al. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Investig Dermatol. 2013;133:1620–1628. doi: 10.1038/jid.2012.464. [DOI] [PubMed] [Google Scholar]
- 45.Toda M, Suzuki T, Hosono K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62:352–359. doi: 10.1016/j.biopha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Wurthmann S, Nagel S, Hadaschik E, et al. Impaired wound healing in a migraine patient as a possible side effect of calcitonin gene-related peptide receptor antibody treatment: a case report. Cephalalgia. 2020;40:1255–1260. doi: 10.1177/0333102420933571. [DOI] [PubMed] [Google Scholar]
- 47.Ray JC, Allen P, Bacsi A, et al. Inflammatory complications of CGRP monoclonal antibodies: a case series. J Headache Pain. 2021;22:121. doi: 10.1186/s10194-021-01330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray JC, Kapoor M, Stark RJ, et al. Calcitonin gene related peptide in migraine: current therapeutics, future implications and potential off-target effects. J Neurol Neurosurg Psychiatry. 2021;92:1325–1334. doi: 10.1136/jnnp-2020-324674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


