Abstract
Iron plays a role in energy metabolism as a component of vital enzymes and electron transport chains (ETCs) for adenosine triphosphate (ATP) synthesis. The tricarboxylic acid (TCA) cycle and oxidative phosphorylation are crucial in generating ATP in mitochondria. At the mitochondria matrix, heme and iron-sulfur clusters are synthesized. Iron-sulfur cluster is a part of the aconitase in the TCA cycle and a functional or structural component of electron transfer proteins. Heme is the prosthetic group for cytochrome c, a principal component of the respiratory ETC. Regarding fat metabolism, iron regulates mitochondrial fat oxidation and affects the thermogenesis of brown adipose tissue (BAT). Thermogenesis is a process that increases energy expenditure, and BAT is a tissue that generates heat via mitochondrial fuel oxidation. Iron deficiency may impair mitochondrial fuel oxidation by inhibiting iron-containing molecules, leading to decreased energy expenditure. Although it is expected that impaired mitochondrial fuel oxidation may be restored by iron supplementation, its underlying mechanisms have not been clearly identified. Therefore, this review summarizes the current evidence on how iron regulates energy metabolism considering the TCA cycle, oxidative phosphorylation, and thermogenesis. Additionally, we relate iron-mediated metabolic regulation to obesity and obesity-related complications.
Keywords: Heme, Hepcidin, Iron-sulfur cluster, Obesity, Thermogenesis
INTRODUCTION
Iron is required for oxygen transfer, DNA repair, the activities of iron-dependent essential enzymes, and the function of iron-containing metalloproteins in the body [1,2]. The total body iron is maintained within a range of 3–5 g in adults to be used in a body without iron-mediated oxidative toxicity [2]. One of the main mechanisms maintaining systemic iron homeostasis is the hepcidin-ferroportin (FPN) axis, which regulates erythrophagocytosis, iron recycling from the mononuclear phagocyte system, and dietary iron absorption in the intestine [2]. The expression of hepcidin is affected by the bone morphogenetic protein (BMP)/small-mothers-against-decapentaplegic protein (SMAD) signaling pathway, hemojuvelin (HJV), and pro-inflammatory cytokines including interleukin-6 (IL-6) [3,4].
Iron homeostasis involves energy metabolism. A systematic meta-analysis of overweight/obese subjects and non-overweight subjects found a significant association between obesity and iron deficiency [5]. Iron deficiency reduced the protein expression of mitochondrial respiratory chain complexes I (reduced form of nicotinamide adenine dinucleotide [NADH]:ubiquinone oxidoreductase subunit A1; NDUFA1) and II (succinate dehydrogenase; SDH), which means damage to mitochondrial oxidative phosphorylation [6]. Also, iron deficiency decreased the activities of iron-dependent enzymes and mitochondrial function [7]. Decreased activities of iron-dependent enzymes are linked to reduced thermogenesis of brown adipose tissue (BAT), leading to lower energy expenditure levels [8]. According to a study confirming the associations between brown and beige adipocytes and iron regulatory proteins/iron-responsive elements (IRP/IRE), iron deficiency in BAT resulted in the inhibition of differentiation of brown adipose progenitor cells [6]. On the contrary, iron supplementation improved morphological abnormalities in mitochondria and up-regulated genes related to oxidative phosphorylation processes, including mitochondrial respiratory chain complexes in the liver and skeletal muscle [9]. In addition, iron supplementation also increased expression levels of genes involved in the synthesis pathway of heme and iron-sulfur clusters, which are iron-dependent molecules [9].
The oxidative phosphorylation of mitochondria and the thermogenic function of BAT play crucial roles in energy metabolism, especially energy expenditure. The decrease in the iron-sulfur cluster, an iron-dependent cofactor, led to an inhibition of oxidative phosphorylation, causing obesity [8]. In contrast, iron supplementation reduced body weight alongside the up-regulation of genes related to mitochondrial oxidative phosphorylation [9]. A study on the effects of iron in diabetic mice found that mice fed a high-iron diet (1,000 mg/kg chow) had lesser visceral fat mass than mice fed a low-iron diet (12 mg/kg chow) [10]. These anti-obesity effects of iron supplementation may be from increased energy expenditure through the activation of mitochondrial oxidative phosphorylation and thermogenic function, which reduces body weight and fat mass.
Likewise, although it has been suggested that iron deficiency may involve the development of obesity by regulating energy metabolism, the exact mechanisms have not been evidently identified. Therefore, this review summarizes current evidence regarding the effects of iron on energy metabolism and obesity, focusing on the key molecular functions and pathways.
RELATIONSHIP BETWEEN IRON AND ENERGY METABOLISM
Cells utilize energy from glucose and fatty acids, and iron involves the metabolism of these energy-yielding nutrients. Iron participates in the tricarboxylic acid (TCA) cycle and electron transport chain (ETC), regulating energy metabolism and affecting weight gain. Glucose is transported inside the cell and metabolized to pyruvate via glycolysis. Under the aerobic condition, pyruvates are transported to the mitochondrial matrix and are converted to acetyl coenzyme A (acetyl-CoA), which participates in the TCA cycle [11]. Fatty acids are transported to the mitochondrial matrix in the form of acyl-CoA and are converted to acetyl-CoA via beta-oxidation. Acetyl-CoA participates TCA cycle and oxidative phosphorylation [12]. Glucose and fatty acids convert to adenosine triphosphate (ATP), NADH, and reduced flavin adenine dinucleotide (FADH2) via glycolysis, beta-oxidation, and the TCA cycle [12]. NADH and FADH2 act as electron carriers in mitochondria, transporting electrons to ETC. ETC consists of complex I–V, coenzyme Q, and cytochrome c (Cyt c). ETC receives electrons from NADH and FADH2, and electrons are transferred through a series of complexes. Complexes use this energy to pump protons from the matrix into intermembrane space. When protons in intermembrane space are transported to the matrix through ATP synthase, ATP is generated [12]. Iron regulates these energy metabolic processes as an essential component of the regulatory molecules, mainly in the TCA cycle and ETC.
Iron metabolism
Iron is present as the form of heme-iron and non-heme-iron in foods. Non-heme-irons are absorbed by divalent metal transporter 1 (DMT1), a cell membrane transporter [13]. The heme-irons are presumed to be absorbed via heme carrier protein 1 [14,15]. Iron in intestinal cells is stored in the form of ferritin. When iron in the body is insufficient, iron is released into the blood by FPN [16]. Ferrous iron (Fe2+) is released into the blood and is re-oxidized into ferric iron (Fe3+) by ferroxidase hephaestin of the cell membrane and binds to iron-free apotransferrin (apo-Tf) to form diferric-transferrin (holo-Tf) [2]. Holo-Tf is transferred to the blood and binds to transferrin receptor 1 (TfR1) of the cell membrane. The conjugate of TfR1 and holo-Tf induces endocytosis using clathrin-coated pits. Endosome entering the cytoplasm is acidified by a proton pump, and holo-Tf is separated into iron and apo-Tf due to reduced affinity between iron and Tf [17,18]. Within the endosome, iron is converted from Fe3+ to Fe2+ by the 6-transmembrane epithelial antigen of prostate 3. Fe2+ is transported to the labile iron pool (LIP) by DMT1 of the endosome membrane [19]. Apo-Tf moves to the cell membrane and is released out of the cell, and irons in the cell move to mitochondria for heme synthesis and iron-sulfur cluster synthesis. Excess irons are stored in the cell as ferritin or move out of the cell by FPN [2,17]. Iron-sulfur cluster and heme are components in complexes I–III and Cyt c of the ETC, and are included in the aconitase of the TCA cycle [20,21].
Iron homeostasis
TfR1, ferritin, and FPN are regulated post-transcriptionally via IRP/IRE system [2]. There are 2 regulatory mechanisms of the IRP/IRE system at the post-transcriptional level. First, IRE is located at the 5'-untranslated region (UTR) portion of the mRNA of ferritin and FPN [22,23]. If IRE is bound with IRP, the translation of mRNA does not progress; however, if IRE is not bound with IRP, the translation of mRNA progresses to synthesize proteins [22]. If there is a large amount of iron in the cell, the iron binds to IRP. Thereby, the IRE is exposed, and then the translation of mRNA progresses to synthesize the protein [22]. Ferritin and FPN are translated when there are high concentrations of irons in the cell [2]. In iron deficiency, IRP is bound to IRE, which inhibits the translation of mRNA, and ferritin and FPN are not synthesized [2]. The second regulatory mechanism is when IRE is in the 3′-UTR site of the mRNA. The translation of mRNA progresses only when IRP is combined with IRE. The mRNA without IRP binding with IRE located in 3'-UTR is susceptible to degradation by endonuclease [22]. When there is a large amount of iron in the cell, iron binds to IRP. As a result, mRNAs that fail to bind to IRP are attacked by endonuclease and cannot be translated [22]. TfR1 corresponds to this case [2]. Conversely, in iron deficiency, IRP binds to IRE located at 3'-UTR. In this case, the translation of TfR1 mRNA progresses to synthesize the TfR1 [24].
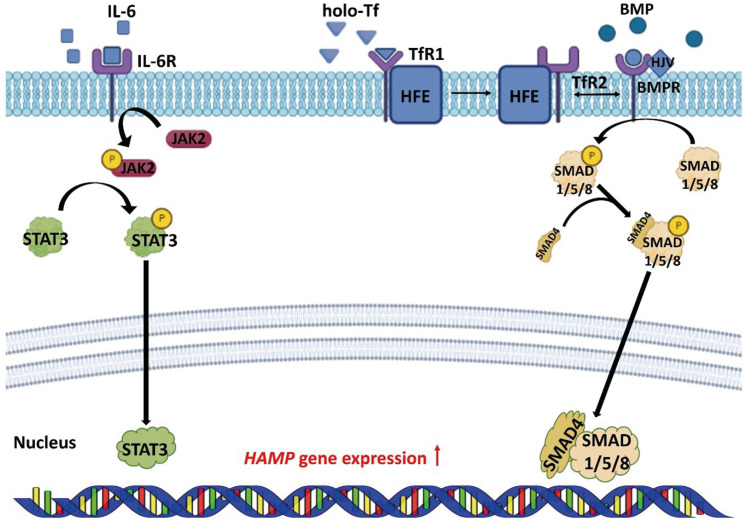
Hepcidin is a hormone secreted from hepatocytes and adipocytes, and degrades the FPN protein in cells. Degradation of FPN regulated by hepcidin plays the most important role, in controlling iron absorption [25]. Transcription and translation of the hepcidin are regulated via the BMP/SMAD pathway and pro-inflammatory cytokines [3,4]. BMP binds to BMP type I, II receptors to phosphorylate the SMAD 1/5/8 proteins. Phosphorylated SMAD 1/5/8 proteins bind to SMAD 4 and move to the nucleus, up-regulating the transcription of hepcidin. HJV acts as a BMP co-receptor to promote the expression of hepcidin [26,27]. When holo-Tf binds to TfR, the homeostatic iron regulator (HFE) recombines with transferrin receptor 2 (TfR2). HFE/TfR2 interacts with BMP type I/II receptors, and has a role in regulating hepcidin [28,29]. IL-6, a pro-inflammatory cytokine, activates Janus kinase 2 and phosphorylates signal transducer and activator of transcription 3 (STAT3). Phosphorylated STAT3 upregulates the transcription of the hepcidin in the nucleus [3,30,31] (Figure 1). Gluconeogenesis stimulates the cereblon-Kruppel-like factor 15 pathways, which induces to transcription of hepcidin [32]. In addition, the expression of hepcidin is downregulated in high-fat diet-induced obesity mice [33,34]. That energy status modulates the absorption, storage, recycling, and mobilization of irons suggests that iron homeostasis is related to energy metabolism.
Figure 1. Regulation of HAMP expression in the cell. BMP/SMAD and JAK2/STAT3 signaling pathways regulate the expression of HAMP.
HAMP, hepcidin; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; SMAD, small-mothers-against-decapentaplegic protein; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; HFE, homeostatic iron regulator; HJV, hemojuvelin; holo-Tf, diferric-transferrin; IL-6, interleukin-6; IL-6R, interleukin-6 receptor; TfR, transferrin receptor.
IRON HOMEOSTASIS AND ENERGY METABOLISM
Role of heme on energy metabolism
Most irons are present in the human body as a form of heme. 5'-aminolevulinic acid synthesized from succinyl-CoA and glycine is converted to protoporphyrin IX, and then combined with iron by ferrochelatase to form heme [17]. Heme is a component of cytochrome P450, Cyt c, hemoglobin, and myoglobin. Among these, Cyt c participates in the oxidative phosphorylation process as a component of the ETC in mitochondria [20]. Glucose and fatty acids in the body produce ATP via the TCA cycle and an oxidative phosphorylation process of the ETC. Therefore, heme synthesis induced by iron supplementation increases energy expenditure by activating the oxidative phosphorylation process. According to a study showing the relationship between iron supplementation and obesity, iron supplementation up-regulated enzymes of heme synthesis such as hydroxymethylbilane synthase and farnesyltransferase Cyt c oxidase assembly factor 10 [9]. In addition, heme synthesis-related genes showed a positive correlation with mitochondrial ETC-related genes [9]. Increased energy expenditure, along with the activation of the heme synthesis pathway and oxidative phosphorylation process in mitochondria, reduced body weight and liver lipid accumulation [9].
Role of the iron-sulfur cluster on energy metabolism
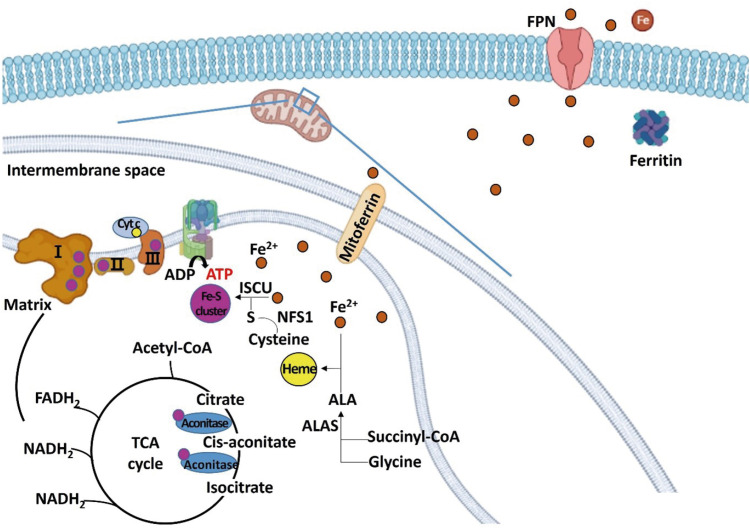
Iron-sulfur cluster and heme are synthesized in the mitochondrial matrix using the iron, and are components of complexes I, II, and III of mitochondrial ETC, aconitase, and Cyt c. Complexes I, II, III and aconitase are proteins that participate in the TCA cycle and the ETC. The increased synthesis of iron-sulfur cluster and heme up-regulated the expression of complex I (NDUFA1), complex II (SDH), and complex III (ubiquinol-Cyt c reductase complex subunits), aconitase, and Cyt c, increasing energy oxidation (Figure 2).
Figure 2. Schematic diagram regarding the role of iron in regulating energy metabolism in hepatocytes. Iron is transferred to the mitochondria for the synthesis of iron-sulfur cluster and heme. Excess irons are stored in the form of ferritin or exported through FPN. Iron-sulfur cluster is a component of mitochondrial complexes I, II, and III, and aconitase, and heme is a component of Cyt c.
FPN, ferroportin; Cyt c, cytochrome c; ALA, aminolevulinic acid; ALAS, aminolevulinic acid synthase; ISCU, iron-sulfur cluster assembly enzyme; NFS1, cysteine desulfurase; TCA, tricarboxylic acid; acetyl-CoA, acetyl coenzyme A; ATP, adenosine triphosphate; ADP, adenosine diphosphate; FADH2, flavin adenine dinucleotide; NADH, nicotinamide adenine dinucleotide.
Iron receives sulfur from cysteine, and the iron-sulfur cluster is synthesized via a series of processes [17]. Iron-sulfur cluster is a component of mitochondrial complexes I, II, and III of the ETC and aconitase, an enzyme acting on the intermediate process of the TCA cycle [21,35]. TCA cycle and ETC are essential for ATP synthesis, indicating the critical role of iron-sulfur clusters on energy metabolism. A recent study showed the reduction of the cysteine desulfurase, iron-sulfur cluster assembly 1 homolog mitochondrial, and bola-like 3 was involved in the iron-sulfur cluster synthesis pathway along with significant decreases in the mitochondrial fuel oxidation of BAT, causing obesity [8]. The knock-down of BOLA3 impaired the thermogenic function of beige cells [36], and the gene expression levels of BOLA3 were correlated with thermogenesis-related genes, including uncoupling protein 1 (UCP1), a regulator of thermogenesis via mitochondrial oxidative phosphorylation [36]. Moreover, a recent study showed that iron supplementation increased the gene expressions of ATP-binding cassette sub-family B member 7 (ABCB7), ISCA2, and BOLA3, the intermediate molecules of iron-sulfur clusters synthesis [9]. Also, up-regulation of genes associated with the mitochondrial ETC-related genes in the iron-supplemented group resulted in increased energy expenditure and lower weight gain than the HFD group [9].
Effects of iron on thermogenesis
There are 3 types of adipocytes in the body: white, brown, and beige adipocytes [37,38,39]. White adipose tissue (WAT) stores chemical energy in the form of triglyceride, and BAT generates heat using macronutrients. Beige adipocytes, found in WAT, also generate heat as brown adipocytes and are converted from white adipocytes in response to various stimuli [39]. UCP1 exists in addition to ATP synthase in brown and beige adipocytes, and generates heat instead of ATP. Therefore, an increase in heat generation also means an increase in energy expenditure. Recent studies have shown the effects of iron on the thermogenesis of brown and beige adipocytes [37,38]. Adipocytes related to thermogenesis convert carbohydrates, fatty acids, and proteins??chemical energy into heat to maintain body temperature in response to a cold environment. Adaptive thermogenesis accounts for a considerable portion of total energy expenditure [37,38]. Exposure to cold environments activates the sympathetic nervous system and releases norepinephrine. Norepinephrine binds to the β3-adrenergic receptor to induce the production of cyclic AMP (cAMP) in BAT. cAMP activates cAMP-dependent protein kinase, which promotes the expression of heat generation-related genes such as UCP1, peroxisome proliferator-activated receptor-γ coactivator 1-alpha (PGC1-alpha), and peroxisome proliferator-activated receptor (PPAR) in a p38 mitogen-activated protein kinase-dependent and -independent pathways [37,40,41].
Regarding the relationship between iron and thermogenesis, impairment in heat generation was accompanied by weight gain when inducing iron deficiency in mice [42]. Research using different adipocytes showed that a decrease in the LIP inhibited cell differentiation and mitochondrial biogenesis of brown and beige adipocytes [6]. Also, chelation of intracellular iron inhibited the differentiation of brown adipocytes and down-regulated the expression of thermogenic function-related genes PRD1-BF1-RIZ1 homologous domain containing 16, PPAR-alpha, PPAR-gamma, PGC1-alpha, and UCP1 in the in vivo study [6]. In studies examining the association between TfRs and thermogenesis, TfR1 deficiency of BAT transformed brown fat precursor cells into white adipocytes and muscle cells [43]. Results that iron deficiency impairs thermogenesis in both animal and clinical trials support that iron is necessary for thermogenesis [44,45]. Iron accumulates in adipocytes associated with thermogenesis when exposed to cold or stimulated heat [38,46]. In addition, IRP/IRE signaling pathway is activated when exposed to cold temperatures, suggesting that the IRP/IRE signaling pathway is required for heat generation. The expression of TfR1 increased when administered β3-adrenoreceptor agonist CL316,243 to activate beige adipocytes [38], and this result suggested the involvement of iron in producing BAT.
Iron-sulfur clusters are associated with the thermogenic function of BAT. BAT contains a large number of mitochondria to generate heat via the mitochondrial fuel oxidation process [47]. The brown color of BAT corresponds to the number of mitochondria and UCP1 [48]. Iron-sulfur clusters are essential to mitochondria so that iron can affect the thermogenic function of BAT. BOLA3, a regulatory molecule for synthesizing iron-sulfur clusters, exists in high content in BAT compared to WAT [36]. In addition, this study showed that UCP1, cell death-inducing DFFA-like effector a, Cyt c oxidase subunit 7a1, and calsyntenin 3 associated with thermogenesis, were down-regulated when BOLA3 was deficient in beige adipocytes [36]. The expression of NDUFA1and SDH and glucose intake in BAT were lower with BOLA3 knock-down compared with the control group [8]. This study showed that impaired function of mitochondrial fuel oxidation in BAT led to obesity [8]. These results suggest that deficiency of iron-sulfur clusters exacerbates the mitochondrial fuel oxidation process and the thermogenic function of beige adipose tissue and BAT. Iron affects the thermogenic function of BAT because it is essential for the oxidative phosphorylation of mitochondria. A recent study showed that iron supplementation reduced the morphological abnormalities of mitochondria and raised the expression of genes involved in heme or iron-sulfur cluster biosynthesis [9]. Improved mitochondrial fuel oxidation and other mitochondrial functions by iron supplementation led to increased energy expenditure and subsequent weight loss [9].
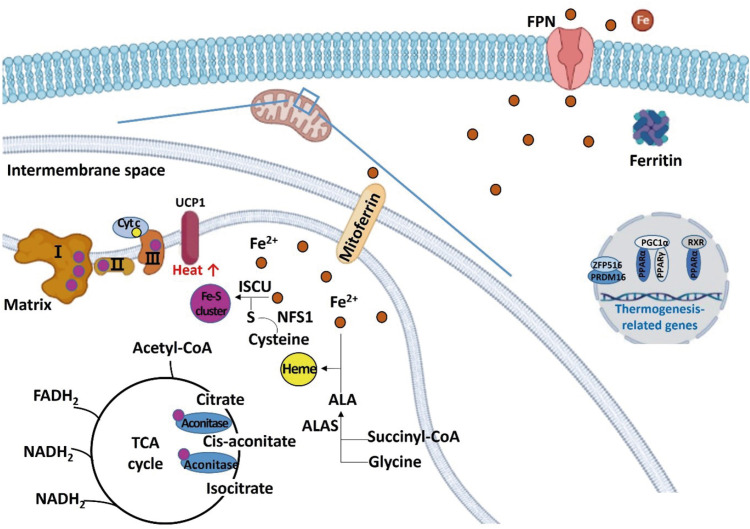
In the brown and beige adipocytes, iron also participates in energy metabolism as a component of the iron-sulfur cluster and heme. UCP1 generates heat instead of ATP in the BAT and beige adipose tissue. Iron deficiency, along with the reduced iron-sulfur cluster synthesis, results in impaired function of the ETC and impaired thermogenesis, leading to a decrease in energy expenditure (Figure 3).
Figure 3. Schematic diagram regarding the role of iron in regulating energy metabolism in beige and brown adipocytes. Irons are transferred to the mitochondria for synthesizing iron-sulfur clusters and heme. Excess irons are stored in the form of ferritin or exported through FPN. Iron-sulfur cluster is a component of mitochondrial complexes I, II, and III, and aconitase, and heme is a component of Cyt c. When iron is deficient, thermogenesis-related genes such as PRDM 16, PPAR, PGC1-alpha, and UCP1 are down-regulated.
FPN, ferroportin; Cyt c, cytochrome c; PRDM, PRD1-BF1-RIZ1 homologous domain containing; PPAR, peroxisome proliferator-activated receptor; PGC, peroxisome proliferator-activated receptor-gamma coactivator; UCP, uncoupling protein; ALA, aminolevulinic acid; ALAS, aminolevulinic acid synthase; ISCU, iron-sulfur cluster assembly enzyme; NFS1, cysteine desulfurase; RXR, retinoid X receptor; TCA, tricarboxylic acid; ZFP 516, zinc-finger protein 516; acetyl-CoA, acetyl coenzyme A; FADH2, flavin adenine dinucleotide; NADH, nicotinamide adenine dinucleotide.
BMPs are currently known to be involved in cell differentiation throughout the body, including the development of adipose tissue and adipocyte differentiation [49]. Among BMPs, BMP6 increases BAT mass in cold environments and induces fat precursor cells to play a similar role to BAT [49]. BMP/SMAD pathway is a mechanism to regulate hepcidin, and BMP6, participating in this process, is up-regulated as iron intake increases [50,51]. This finding suggests that sufficient iron intake induces the expression of BMP6 and consequently increases BAT [52]. A study examining the correlation between iron concentration in the liver and body fat mass demonstrated that the expressions of hepcidin and BMP6 were negatively correlated with the amount of WAT [34]. This mechanism needs further research in the future by linking BMP6 with weight loss effects.
IRON HOMEOSTASIS AND ENERGY METABOLISM IN OBESITY AND RELATED COMPLICATIONS
Iron deficiency and obesity
A systematic review of 13,393 obese subjects and 26,621 non-obese subjects showed that obese subjects had lower serum iron concentrations and a significantly higher risk of iron deficiency than non-obese subjects [5]. This study showed a negative correlation between obesity and serum iron concentrations [5]. In addition, in research about the correlation between high-fat diet-induced obesity and iron concentrations in the liver, the longer the HFD period, the lower the iron concentration in the liver and serum [53,54]. The above research confirms that there is a correlation between iron and obesity. According to a study investigating the relationship between body fat mass and iron concentrations, ferritin in the liver had a negative correlation with the amount of WAT [34]. In addition, mice with TfR1 deficiency in adipocytes exhibited heat generation disorder, insulin resistance, and mitochondrial dysfunction [43]. The results of this study suggest a decrease in energy expenditure in TfR1 deficiency in adipocytes. Reduced energy expenditure can lead to lipid accumulation. Transmembrane serine protease 6 (TMPRSS 6) is a repressor of hepcidin [55]. TMPRSS 6 has a negative correlation with hepcidin [55,56]. TMPRSS 6 deficiency in mice caused an iron deficiency in inguinal WAT and interscapular BAT. Iron deficiency impairs beige adipocyte differentiation and the heat generation function of BAT [43]. The current findings regarding iron deficiency and obesity are presented in Table 1. However, current evidence regarding changes in energy metabolism with TMPRSS 6 deficiency has been inconsistent. According to a paper that studied the effects of TMPRSS 6 deficiency on obesity, TMPRSS 6 deficiency resulted in excessive secretion of hepcidin, reduced fat mass, and improved insulin sensitivity [57]. Also, TMPRSS 6 deficient mice got less fat mass induced by the high-fat diet [57].
Table 1. Evidence regarding the relationship between iron deficiency and obesity.
| Model | Design | Phenotype | Ref. |
|---|---|---|---|
| 8-wk-old male C57BL/6 mice | HFD feeding for 24 wk | • HFD feeding reduced hepatic iron stores with weight gain | [33] |
| 7-wk-old male C57BL/6 mice | Mice were fed control diet, HFD or calorie-restricted diet for 16 wk | • HFD group showed the lowest ferritin level compared to the other groups | [34] |
| • Negative correlation between hepatic iron concentrations and WAT mass | |||
| 6-8-wk-old C57BL/6, Tfr1fl/fl , and Tfr1Adp/Adp mice | HFD feeding for 12 wk | • Thermogenesis was impaired in mice with adipocyte-specific-deletion of Tfr (Tfr1Adp/Adp ) | [43] |
| • Amounts of both iBAT and iWAT were reduced in Tfr1Adp/Adp mice compared to control (Tfr1fl/fl ) littermates, despite no significant difference in total body weights | |||
| • Tfr1Adp/Adp mice showed dyslipidemia, insulin resistance, and inflammation | |||
| 6-wk-old male Sprague-Dawley rats | HFD feeding for 16 wk | • HFD feeding decreased iron concentrations in the liver along with weight gain | [53] |
| Weanling male Wistar rats | HFD feeding for 8 wk | • Body fat mass was negatively correlated to serum iron concentrations and positively correlated with liver iron storage | [54] |
| R26CreERT2Fthfl/fl mice | Fth deletion in germ-free R26CreERT2Fthfl/fl mice | • Fth deletion induced mitochondrial disorder, and impairments of energy metabolism and thermogenic functions | [68] |
Fth; ferritin heavy chain; HFD, high-fat diet; WAT, white adipose tissue; iBAT, interscapular brown adipose tissue; iWAT, inguinal white adipose tissue; Tfr1, transferrin receptor 1.
Iron supplementation and obesity
Recent evidence regarding the effects of iron supplementation on obesity is summarized in Table 2. The iron overload significantly reduced weight gain (15% less) and body fat mass in high-fat diet-fed mice [58]. In research studying the effects of iron supplementation on mitochondrial function, mice fed a high-fat diet with iron had lower body weight and liver lipid accumulation than mice fed a high-fat diet [9]. At the same time, iron supplementation reduced the morphological abnormalities of mitochondria and up-regulated the genes related to mitochondrial function and beta-oxidation [9]. In addition, mice fed a high-iron diet had lower visceral fat mass than the low-iron diet group. These results showed that iron supplementation could decrease weight gain [10]. A study regarding the decomposition effects of serum on lipids in mouse adipocytes found that adipocytes are decomposed by serum, which is partially mediated by iron in serum [59].
Table 2. Experimental studies regarding the effects of iron supplementation on obesity.
| Model | Design | Phenotype | Ref. |
|---|---|---|---|
| 6-wk-old male C57BL/6J mice | 0.023% (w/w) SFC supplementation in HFD or control diet for 15 wk | • Iron-supplemented HFD feeding lowered weight gain and liver lipid accumulation compared to the HFD group | [9] |
| • Iron supplementation reduced morphological abnormalities in mitochondria, and up-regulated gene expression related to mitochondrial function/beta-oxidation/the synthesis of heme or iron-sulfur clusters | |||
| 7-wk-old male db/db mice | High-iron diets (1,000 mg iron/kg chow) or low-iron diets (12 mg/kg chow) for 9 wk | • High-iron diets feeding reduced visceral fat mass and lipid accumulation in the liver | [10] |
| 6-wk-old female ApoE KO mice | 2% carbonyl iron in HFD for 16 wk | • Dietary iron overloading induced a significant decrease in serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol concentrations, along with a decrease in liver lipid accumulation | [69] |
| 3-wk-old male C57BL/6 mice | Control diet or HFD for 12 wk. The iron-treated group was injected 120 μg iron dextran/g body weight for 8 wk every other week | • Iron dextran-injected group had 15% weight loss and body fat loss compared with mice without iron supplementation | [58] |
| • Iron dextran injection prevented hepatic steatosis |
ApoE, apolipoprotein E; HFD, high-fat diet; KO, knockout; SFC, sodium ferrous citrate.
Iron and non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease is a complication of obesity, and hepatic steatosis is one of the manifestations of metabolic syndrome. High-fat diet feeding led to weight gain and liver lipid accumulation [33], and iron supplementation decreased lipogenesis and lipid accumulation in the liver, preventing the development of hepatic steatosis [9,58]. In contrast to in vivo experiments, in vitro studies utilizing mouse hepatocytes showed inconsistent results (Table 3). Iron treatment inhibited the expression of fatty acid synthase and acetyl-CoA carboxylase, enzymes involved in fatty acid synthesis in the liver [60]. On the contrary, 24 hour-iron treatment in AML12 hepatocytes increased the lipid accumulation in hepatocytes [61]. In addition, 48 or 72 hours of iron treatment also caused triglyceride accumulation in primary human hepatocytes [62]. Despite inconsistent results in in vitro studies, most showed an increase in lipid accumulation in iron-treated cells [61,62,63]. It can be presumed that inconsistent results between in vivo and in vitro studies are due to the presence or absence of interactions with other organs.
Table 3. Experimental studies regarding the effects of iron supplementation on energy metabolism in cells.
| Model | Design | Phenotype | Ref. |
|---|---|---|---|
| Primary hepatocytes isolated from 5-wk-old male C57BL/6J mice | Treated with 100, 300, and 1,000 μM SFC for 24 hr | • SFC treatment up-regulated the genes related to mitochondrial function, heme, and iron-sulfur clusters | [9] |
| Primary hepatocytes isolated from male C57BL/6 mice | Treated with 0, 7.5, 75, or 750 μM FAC for 16 hr | • FAC treatment inhibited the expression of acetyl-CoA carboxylase and fatty acid synthase | [60] |
| AML12 hepatocytes | Treated with 30 μg/mL FAC for 12 (mild group) or 24 hr (moderate group) | • FAC treatment caused an increase in the accumulation of lipids in hepatocytes | [61] |
| • The 12 hr (mild group) iron treatment increased lipogenesis of hepatocytes | |||
| Primary human hepatocytes | Treated with 50 μM iron for 48 or 72 hr | • The iron treatment caused the accumulation of triglycerides in hepatocytes | [62] |
| Primary HUVECs | Treated with 100 μM of FAC, FAS, and ferric chloride, 2 mg/mL of apoferritin and holoferritin for 24 hr | • Cellular iron loading caused cholesterol biosynthesis | [63] |
| Human and mouse 3T3-L1 pre-adipocytes | Treated with 3 and 30 μg/mL FeSO4, 20 and 100 μmol/L deferoxamine for 7 or 14 day | • Transferrin was significantly elevated during adipocyte differentiation | [70] |
| • Iron deficiency in cells elevated gene expressions of inflammatory markers and disturbed adipocyte differentiation, which was restored by iron supplementation in a dose-dependent way | |||
| • Palmitic acid treatment induced iron deficiency during adipocyte differentiation, and led to a decrease in the transferrin gene expression, which was restored by the treatment of transferrin |
FAC, ferric ammonium citrate; FAS, ferrous ammonium sulfate; HUVEC, human umbilical vein endothelial cell; SFC, sodium ferrous citrate; acetyl-CoA, acetyl coenzyme A.
Iron and type 2 diabetes
Obesity is often accompanied by insulin resistance. Visceral fat pads release pro-inflammatory cytokines such as IL-6 and tumor necrosis factor-alpha, causing insulin resistance [64,65]. Previous studies reported that iron supplementation improved obesity without affecting insulin resistance [66]. One of the hypotheses that iron supplementation exacerbates insulin resistance is its regulation of leptin, an adipokine that inhibits appetite and stimulates the glucose transporter 4 in the liver and muscles to increase insulin sensitivity [66]. Expression levels of leptin and circulating leptin concentrations were low in mice fed an iron-supplemented diet [67]. Iron injection impaired glucose tolerance and insulin sensitivity in mice [10,58]. Accumulating evidence suggests that iron supplementation reduces weight gain but has the disadvantage of lowering insulin sensitivity.
CONCLUSION
The mechanisms by which iron affects energy homeostasis are summarized in 3 ways. The first is that iron regulates energy homeostasis by heme. Iron is a prosthetic group of heme, a component of Cyt c, and Cyt c is a component of the ETC [20]. Iron supplementation increased the synthesis of heme and up-regulated genes associated with mitochondrial oxidative phosphorylation, and the increased mitochondrial oxidative phosphorylation led to weight loss [9]. The second mechanism is that iron regulates energy homeostasis by iron-sulfur clusters. Iron-sulfur cluster is a component of mitochondrial complexes I, II, and III of the ETC, as well as aconitase required for the TCA cycle [21]. Proteins containing iron-sulfur clusters participate in the mitochondrial fuel oxidation process, suggesting that iron-sulfur clusters are critical for energy expenditure. Reduced synthesis of iron-sulfur clusters resulted in an impaired mitochondrial fuel oxidation process, leading to weight gain. Increased synthesis of iron-sulfur clusters improved mitochondrial fuel oxidation function, resulting in weight loss induced by increased energy expenditure [8,9]. The third mechanism is the iron-mediated regulation of thermogenesis for energy homeostasis. Brown and beige adipocytes contain a large number of mitochondria, and involves in thermogenesis [47]. Mitochondria require iron as a cofactor for various mitochondrial proteins, such as iron-sulfur clusters. Iron deficiency down-regulates thermogenesis-related genes and impairs BAT cell differentiation [6], reducing energy oxidation and weight gain.
Current evidence support that iron plays a role in regulating energy metabolism. Though it has been suggested that iron deficiency causes the down-regulation of thermogenesis-related markers and impaired thermogenesis, more research is needed on whether iron supplementation activates thermogenesis or restores impaired thermogenesis. In addition, because previous research on iron-mediated changes in energy metabolism and metabolic parameters often reported inconsistent results, well-controlled studies considering multi-organ/cell interactions are needed to prove the relation between iron and obesity/obesity-related metabolic complications, and to identify molecular mechanisms.
Footnotes
Funding: This work was supported by a sabbatical year (2021) and a research grant (2022-0268) from Seoul Women’s University. The funder had no role in study design, data collection, analysis and interpretation, the decision to publish, or manuscript preparation.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim SL, Yang SJ.
- Funding acquisition: Yang SJ.
- Writing - original draft: Kim SL.
- Writing - review & editing: Shin S, Yang SJ.
References
- 1.Altamura S, Marques O, Colucci S, Mertens C, Alikhanyan K, Muckenthaler MU. Regulation of iron homeostasis: lessons from mouse models. Mol Aspects Med. 2020;75:100872. doi: 10.1016/j.mam.2020.100872. [DOI] [PubMed] [Google Scholar]
- 2.Katsarou A, Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med. 2020;75:100866. doi: 10.1016/j.mam.2020.100866. [DOI] [PubMed] [Google Scholar]
- 3.Varga E, Pap R, Jánosa G, Sipos K, Pandur E. IL-6 regulates hepcidin expression via the BMP/SMAD pathway by altering BMP6, TMPRSS6 and TfR2 expressions at normal and inflammatory conditions in BV2 microglia. Neurochem Res. 2021;46:1224–1238. doi: 10.1007/s11064-021-03322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlebois E, Pantopoulos K. Iron overload inhibits BMP/SMAD and IL-6/STAT3 signaling to hepcidin in cultured hepatocytes. PLoS One. 2021;16:e0253475. doi: 10.1371/journal.pone.0253475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 2015;16:1081–1093. doi: 10.1111/obr.12323. [DOI] [PubMed] [Google Scholar]
- 6.Yook JS, You M, Kim Y, Zhou M, Liu Z, Kim YC, Lee J, Chung S. The thermogenic characteristics of adipocytes are dependent on the regulation of iron homeostasis. J Biol Chem. 2021;296:100452. doi: 10.1016/j.jbc.2021.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BN, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail. 2018;20:910–919. doi: 10.1002/ejhf.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tajima K, Ikeda K, Chang HY, Chang CH, Yoneshiro T, Oguri Y, Jun H, Wu J, Ishihama Y, Kajimura S. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat Metab. 2019;1:886–898. doi: 10.1038/s42255-019-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura N, Yokoyama Y, Taoka H, Nagano U, Hosoda S, Taworntawat T, Nakamura A, Ogawa Y, Tsubota K, Watanabe M. Iron supplementation regulates the progression of high fat diet induced obesity and hepatic steatosis via mitochondrial signaling pathways. Sci Rep. 2021;11:10753. doi: 10.1038/s41598-021-89673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma W, Feng Y, Jia L, Li S, Li J, Wang Z, Chen X, Du H. Dietary iron modulates glucose and lipid homeostasis in diabetic mice. Biol Trace Elem Res. 2019;189:194–200. doi: 10.1007/s12011-018-1446-3. [DOI] [PubMed] [Google Scholar]
- 11.Chandel NS. Carbohydrate metabolism. Cold Spring Harb Perspect Biol. 2021;13:a040568. doi: 10.1101/cshperspect.a040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judge A, Dodd MS. Metabolism. Essays Biochem. 2020;64:607–647. doi: 10.1042/EBC20190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galla R, Grisenti P, Farghali M, Saccuman L, Ferraboschi P, Uberti F. Ovotransferrin supplementation improves the iron absorption: an in vitro gastro-intestinal model. Biomedicines. 2021;9:1543. doi: 10.3390/biomedicines9111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito H, Kurokawa H, Matsui H. Mitochondrial reactive oxygen species and heme, non-heme iron metabolism. Arch Biochem Biophys. 2021;700:108695. doi: 10.1016/j.abb.2020.108695. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc S, Garrick MD, Arredondo M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am J Physiol Cell Physiol. 2012;302:C1780–C1785. doi: 10.1152/ajpcell.00080.2012. [DOI] [PubMed] [Google Scholar]
- 16.Waldvogel-Abramowski S, Waeber G, Gassner C, Buser A, Frey BM, Favrat B, Tissot JD. Physiology of iron metabolism. Transfus Med Hemother. 2014;41:213–221. doi: 10.1159/000362888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Zhou Q, Wu D, Chen L. Mitochondrial iron metabolism and its role in diseases. Clin Chim Acta. 2021;513:6–12. doi: 10.1016/j.cca.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 19.Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proc Natl Acad Sci U S A. 2008;105:7410–7415. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu T, Lengalova A, Martínek V, Martínková M. Heme: emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem Soc Rev. 2019;48:5624–5657. doi: 10.1039/c9cs00268e. [DOI] [PubMed] [Google Scholar]
- 21.Read AD, Bentley RE, Archer SL, Dunham-Snary KJ. Mitochondrial iron-sulfur clusters: structure, function, and an emerging role in vascular biology. Redox Biol. 2021;47:102164. doi: 10.1016/j.redox.2021.102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantopoulos K. Iron metabolism and the IRE/IRP regulatory system: an update. Ann N Y Acad Sci. 2004;1012:1–13. doi: 10.1196/annals.1306.001. [DOI] [PubMed] [Google Scholar]
- 23.Piccinelli P, Samuelsson T. Evolution of the iron-responsive element. RNA. 2007;13:952–966. doi: 10.1261/rna.464807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato J, Kobune M, Ohkubo S, Fujikawa K, Tanaka M, Takimoto R, Takada K, Takahari D, Kawano Y, Kohgo Y, Niitsu Y. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879–887. doi: 10.1016/j.exphem.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 25.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbicker AU, Bartnikas TB, Lohmeyer LK, Leyton P, Mayeur C, Kao SM, Pappas AE, Peterson RT, Bloch DB, Yu PB, Fleming MD, Bloch KD. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118:4224–4230. doi: 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayeur C, Leyton PA, Kolodziej SA, Yu B, Bloch KD. BMP type II receptors have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. Blood. 2014;124:2116–2123. doi: 10.1182/blood-2014-04-572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012;57:1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Vujić M. Molecular basis of HFE-hemochromatosis. Front Pharmacol. 2014;5:42. doi: 10.3389/fphar.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 31.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Jo JR, Lee SE, An S, Nedumaran B, Ghosh S, Park KG, Kim YD. Gluconeogenic signals regulate hepcidin gene expression via a CRBN-KLF15 axis. BMB Rep. 2021;54:221–226. doi: 10.5483/BMBRep.2021.54.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varghese J, James JV, Anand R, Narayanasamy M, Rebekah G, Ramakrishna B, Nellickal AJ, Jacob M. Development of insulin resistance preceded major changes in iron homeostasis in mice fed a high-fat diet. J Nutr Biochem. 2020;84:108441. doi: 10.1016/j.jnutbio.2020.108441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park CY, Chung J, Koo KO, Kim MS, Han SN. Hepatic iron storage is related to body adiposity and hepatic inflammation. Nutr Metab (Lond) 2017;14:14. doi: 10.1186/s12986-017-0169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy MC, Emptage MH, Dreyer JL, Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983;258:11098–11105. [PubMed] [Google Scholar]
- 36.Bai N, Ma J, Alimujiang M, Xu J, Hu F, Xu Y, Leng Q, Chen S, Li X, Han J, Jia W, Bao Y, Yang Y. Bola3 regulates beige adipocyte thermogenesis via maintaining mitochondrial homeostasis and lipolysis. Front Endocrinol (Lausanne) 2021;11:592154. doi: 10.3389/fendo.2020.592154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 38.Yook JS, You M, Kim J, Toney AM, Fan R, Puniya BL, Helikar T, Vaulont S, Deschemin JC, Okla M, Xie L, Ghosh MC, Rouault TA, Lee J, Chung S. Essential role of systemic iron mobilization and redistribution for adaptive thermogenesis through HIF2-α/hepcidin axis. Proc Natl Acad Sci U S A. 2021;118:e2109186118. doi: 10.1073/pnas.2109186118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G, Sun Q, Liu C. Influencing factors of thermogenic adipose tissue activity. Front Physiol. 2016;7:29. doi: 10.3389/fphys.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins S. β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front Endocrinol (Lausanne) 2012;2:102. doi: 10.3389/fendo.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yook JS, Thomas SS, Toney AM, You M, Kim YC, Liu Z, Lee J, Chung S. Dietary iron deficiency modulates adipocyte iron homeostasis, adaptive thermogenesis, and obesity in C57BL/6 mice. J Nutr. 2021;151:2967–2975. doi: 10.1093/jn/nxab222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Pan X, Pan G, Song Z, He Y, Zhang S, Ye X, Yang X, Xie E, Wang X, Mai X, Yin X, Tang B, Shu X, Chen P, Dai X, Tian Y, Yao L, Han M, Xu G, Zhang H, Sun J, Chen H, Wang F, Min J, Xie L. Transferrin receptor 1 regulates thermogenic capacity and cell fate in brown/beige adipocytes. Adv Sci (Weinh) 2020;7:1903366. doi: 10.1002/advs.201903366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillmann E, Johnson DG, Martin J, Mackler B, Finch C. Catecholamine elevation in iron deficiency. Am J Physiol. 1979;237:R297–R300. doi: 10.1152/ajpregu.1979.237.5.R297. [DOI] [PubMed] [Google Scholar]
- 45.Beard JL, Borel MJ, Derr J. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am J Clin Nutr. 1990;52:813–819. doi: 10.1093/ajcn/52.5.813. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Liang X, Tao C, Yao X, Wang Y, Wang Y, Li K. Induction of copper and iron in acute cold-stimulated brown adipose tissues. Biochem Biophys Res Commun. 2017;488:496–500. doi: 10.1016/j.bbrc.2017.05.073. [DOI] [PubMed] [Google Scholar]
- 47.Oguri Y, Kajimura S. Cellular heterogeneity in brown adipose tissue. J Clin Invest. 2020;130:65–67. doi: 10.1172/JCI133786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, Kajimura S. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 2016;24:402–419. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blázquez-Medela AM, Jumabay M, Boström KI. Beyond the bone: bone morphogenetic protein signaling in adipose tissue. Obes Rev. 2019;20:648–658. doi: 10.1111/obr.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrow NL, Fleming RE. Bone morphogenetic proteins as regulators of iron metabolism. Annu Rev Nutr. 2014;34:77–94. doi: 10.1146/annurev-nutr-071813-105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 52.Kautz L, Besson-Fournier C, Meynard D, Latour C, Roth MP, Coppin H. Iron overload induces BMP6 expression in the liver but not in the duodenum. Haematologica. 2011;96:199–203. doi: 10.3324/haematol.2010.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang S, Yan K, Sun B, Gao S, Yang X, Ni Y, Ma W, Zhao R. Long-term high-fat diet decreases hepatic iron storage associated with suppressing TFR2 and ZIP14 expression in rats. J Agric Food Chem. 2018;66:11612–11621. doi: 10.1021/acs.jafc.8b02974. [DOI] [PubMed] [Google Scholar]
- 54.Lobo AR, Gaievski EH, de Mesquita CH, De Carli E, Teixeira PD, Pereira RM, Borelli P, de Sá LR, Colli C. Increased adiposity by feeding growing rats a high-fat diet results in iron decompartmentalisation. Br J Nutr. 2020;123:1094–1108. doi: 10.1017/S0007114519002320. [DOI] [PubMed] [Google Scholar]
- 55.Folgueras AR, de Lara FM, Pendás AM, Garabaya C, Rodríguez F, Astudillo A, Bernal T, Cabanillas R, López-Otín C, Velasco G. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 56.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folgueras AR, Freitas-Rodríguez S, Ramsay AJ, Garabaya C, Rodríguez F, Velasco G, López-Otín C. Matriptase-2 deficiency protects from obesity by modulating iron homeostasis. Nat Commun. 2018;9:1350. doi: 10.1038/s41467-018-03853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma W, Jia L, Xiong Q, Du H. Iron overload protects from obesity by ferroptosis. Foods. 2021;10:1787. doi: 10.3390/foods10081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rumberger JM, Peters T, Jr, Burrington C, Green A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes. 2004;53:2535–2541. doi: 10.2337/diabetes.53.10.2535. [DOI] [PubMed] [Google Scholar]
- 60.Varghese J, James J, Vaulont S, Mckie A, Jacob M. Increased intracellular iron in mouse primary hepatocytes in vitro causes activation of the Akt pathway but decreases its response to insulin. Biochim Biophys Acta, Gen Subj. 2018;1862:1870–1882. doi: 10.1016/j.bbagen.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kidman CJ, Mamotte CD, Eynaud MA, Reinhardt J, Vongsvivut J, Tobin MJ, Hackett MJ, Graham RM. Tracking biochemical changes induced by iron loading in AML12 cells with synchrotron live cell, time-lapse infrared microscopy. Biochem J. 2021;478:1227–1239. doi: 10.1042/BCJ20200653. [DOI] [PubMed] [Google Scholar]
- 62.Mayneris-Perxachs J, Cardellini M, Hoyles L, Latorre J, Davato F, Moreno-Navarrete JM, Arnoriaga-Rodríguez M, Serino M, Abbott J, Barton RH, Puig J, Fernández-Real X, Ricart W, Tomlinson C, Woodbridge M, Gentileschi P, Butcher SA, Holmes E, Nicholson JK, Pérez-Brocal V, Moya A, Clain DM, Burcelin R, Dumas ME, Federici M, Fernández-Real JM. Iron status influences non-alcoholic fatty liver disease in obesity through the gut microbiome. Microbiome. 2021;9:104. doi: 10.1186/s40168-021-01052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher AL, Srole DN, Palaskas NJ, Meriwether D, Reddy ST, Ganz T, Nemeth E. Iron loading induces cholesterol synthesis and sensitizes endothelial cells to TNFα-mediated apoptosis. J Biol Chem. 2021;297:101156. doi: 10.1016/j.jbc.2021.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li B, Leung JC, Chan LY, Yiu WH, Tang SC. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res. 2020;77:101020. doi: 10.1016/j.plipres.2019.101020. [DOI] [PubMed] [Google Scholar]
- 65.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miranda MA, Lawson HA. Ironing out the details: untangling dietary iron and genetic background in diabetes. Nutrients. 2018;10:1437. doi: 10.3390/nu10101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Y, Li Z, Gabrielsen JS, Simcox JA, Lee SH, Jones D, Cooksey B, Stoddard G, Cefalu WT, McClain DA. Adipocyte iron regulates leptin and food intake. J Clin Invest. 2015;125:3681–3691. doi: 10.1172/JCI81860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blankenhaus B, Braza F, Martins R, Bastos-Amador P, González-García I, Carlos AR, Mahu I, Faisca P, Nunes JM, Ventura P, Hoerr V, Weis S, Guerra J, Cardoso S, Domingos A, López M, Soares MP. Ferritin regulates organismal energy balance and thermogenesis. Mol Metab. 2019;24:64–79. doi: 10.1016/j.molmet.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao L, Luo G, Li H, Yao P, Tang Y. Dietary iron overload mitigates atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice: role of dysregulated hepatic fatty acid metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:159004. doi: 10.1016/j.bbalip.2021.159004. [DOI] [PubMed] [Google Scholar]
- 70.Moreno-Navarrete JM, Ortega F, Moreno M, Ricart W, Fernández-Real JM. Fine-tuned iron availability is essential to achieve optimal adipocyte differentiation and mitochondrial biogenesis. Diabetologia. 2014;57:1957–1967. doi: 10.1007/s00125-014-3298-5. [DOI] [PubMed] [Google Scholar]