Highlights
-
•
For patients with cervical cancer in Botswana, most presented with locally advanced cervical cancer (stages II-III disease).
-
•
Women living with HIV did not present with earlier stage cervical cancer despite routine screening encouragement in Botswana.
-
•
Among the cohort, 2-year OS by stage was 88.0% for stage I, 77.5% for stage II, 54% for stage III, and 40% for stage IV.
-
•
Overall survival was largely associated with patients’ disease stage.
Keywords: Cervical cancer, HIV, Stage, Outcomes, Chemoradiation
Abstract
Objective
To present the stage distribution, patterns of care, and outcomes of patients from Botswana with invasive cervical cancer, living with or without HIV.
Methods
Between 2013 and 2020, women with cervical cancer were prospectively enrolled in an observational cohort study.
Results
A total of 1,043 patients were enrolled; 69% were women living with HIV. The median age of the cohort was 47 years (interquartile range [IQR] 40–58 years), with women living with HIV presenting at a younger age compared to women without HIV (44 versus 61 years, p < 0.001). Among women living with HIV, the median CD4 count at the time of cancer diagnosis was 429.5 cells/μL (IQR 240–619.5 cells/μL), 13% had a detectable viral load, and 95% were on antiretroviral therapy. In regard to treatment, 6% (n = 58) underwent surgery, 33% (n = 341) received radiation therapy, 51% (n = 531) received chemoradiation, and 7% (n = 76) did not receive treatment. Stage distribution in the cohort was as follows: I 17% (n = 173), II 37% (n = 388), III 35% (n = 368), and IV 8% (n = 88). For all patients, 2-year OS was 67%. In multivariable Cox regression, worse OS was associated with stage: II (HR 1.91, p = 0.007), III (HR 3.99, p < 0.001), and IV (HR 5.06, p < 0.001) compared to stage I. Improved OS was associated with hemoglobin > 10 g/dL (HR 0.51, p < 0.001) compared to Hb ≤ 10 g/dL.
Conclusions
Among women in Botswana with cervical cancer, most patients presented with stage II or III disease warranting radiation therapy or chemoradiation. While two-thirds of cervical cancer patients were women living with HIV, HIV did not impact OS.
1. Introduction
Cervical cancer is the fourth most common cancer in women worldwide (Sung et al., 2021). In 2020, there were 604,000 new cases of cervical cancer and 342,000 deaths from the disease (Sung et al., 2021). Cervical cancer is disproportionately prevalent in low- and middle-income countries, where 84–90% of new cases occur (Sung et al., 2021).
Most cervical cancer cases are attributed to human papillomavirus (HPV) infection (Sung et al., 2021). Additionally, women infected with human immunodeficiency virus (HIV) have been found to exhibit high rates of chronic HPV infection, further increasing their risk of developing cervical cancer over time (Singh et al., 2009). Botswana is located at the center of Southern Africa, positioned between South Africa, Namibia, Zambia, and Zimbabwe. In 2021, Botswana’s population was estimated at 2,397,240. The economy of Botswana is currently one of the world’s fastest growing economies, with gross domestic product estimated at 17.61 billion USD in 2021. In regard to health, Botswana has a five tier health system comprising health post, clinic, primary hospital, district hospital and referral hospitals (World Bank, 2022). In Botswana, a low- and middle-income country in Sub-Saharan Africa with a high prevalence of HIV infection, cervical cancer is the most common cancer and the leading cause of cancer death in women (Sung et al., 2021). As of 2019, 25.1% of women in Botswana aged 15–49 years were living with HIV, and two-thirds of cervical cancer cases occurred among women living with HIV (Nilambur, 2019). Further, financial constraints and the resource-limited health system hindered appropriate cervical cancer screening among women with or without HIV who have cervical cancer (Cubie and Campbell, 2020).
To date, there exists extensive data on stage distribution and survival outcomes of cervical cancer patients in high-income regions such as the United States and European countries (Grigsby et al., 2020, John and Broggio, 2019). However, there remains a shortage of comprehensive stage distribution and outcomes data for cervical cancer cases in low- and middle-income countries, particularly in Sub-Saharan Africa (Sengayi-Muchengeti et al., 2020). Prior studies presenting the stage distribution for women diagnosed with cervical cancer in Botswana either included patients treated with chemoradiotherapy alone or assessed survival by HIV status and treatment only (Grover et al., 2018, Dryden-Peterson et al., 2016). Hence, there is a lack of comprehensive data on stage distribution and survival outcomes by stage of cervical cancer patients in Botswana. This article presents comprehensive stage distribution, patterns of care, and survival outcomes for cervical cancer patients in Botswana and evaluates the impact of stage at presentation on survival.
2. Methods
2.1. Study site and population
Patients with cervical cancer stages I-IV, between April 2013 and November 2020 were prospectively enrolled in the Botswana Prospective Cancer Cohort at Gaborone Private Hospital, the only radiation facility in Botswana, and gynecological multidisciplinary team clinic at Princess Marina Hospital, the only multidisciplinary clinic for management of cervical cancer in the country. Princess Marina Hospital is a tertiary public hospital in Gaborone that provides free care including oncology care to all the citizens of Botswana. The Botswana Prospective Cancer Cohort is an observational cohort study of patients receiving cancer treatment in Botswana (Grover et al., 2017). All patients enrolled and seen at the clinic have given consent to participate.
2.2. Data collection
Data were collected using pre-designed electronic forms hosted on the University of Pennsylvania’s Research Electronic Data Capture database management system. Patients were followed prospectively every three months until death or last follow-up and data were recorded for each patient via interviews, clinic visits, and medical records. Information was gathered about patient demographics, clinical history, initial presenting symptoms, and history of HIV and HIV-related clinical data including antiretroviral therapy, CD4 count (cells/μL) and viral load (cells/μL) at the time of cancer diagnosis. Additional patient baseline information was collected about performance status using the Karnofsky performance status score (KPS) and laboratory values including hemoglobin (g/dL), creatinine (μmol/L), absolute neutrophil count (×109/L), white blood cells (×109/L) and albumin (g/dL) (Schag et al., 1984). Disease characteristics recorded included histology, treatment prescribed and received, and time to treatment which was defined as the time from biopsy to treatment initiation. The treatment dates, regimen, and number of chemotherapy cycles received were recorded for patients who received chemoradiation. The total radiation dose received to point A was calculated using the radiobiological equivalent dose (EQD2) formula (American Brachytherapy Society).
The Institutional Review Boards of the Ministry of Health of Botswana, Princess Marina Hospital, and the University of Pennsylvania (820159) approved the study. Written consent was obtained from all participants prior to enrollment.
2.3. Work-up and treatment
Majority of patients were clinically staged according to the 2009 and then, starting in 2019, the 2018 International Federation of Gynecology and Obstetrics (FIGO) staging criteria (Grover et al., 2017, Bhatla et al., 2019). Diagnostic work-up included basic laboratory studies (i.e., complete blood count and renal function test), a chest X-ray, and an abdominal ultrasound before treatment (Grover et al., 2018).
Treatment for patients presenting with FIGO 2009 stages I-IV cervical cancer was prescribed according to National Comprehensive Cancer Network guidelines (Koh et al., 2019). For patients with early stage (FIGO stages IA-IB1) cervical cancer, the standard treatment was surgery and/or radiation therapy with or without cisplatin-based chemotherapy (Koh et al., 2019). For patients with locally advanced cervical cancer (FIGO stages IB2-IVA), the standard treatment was a combination of external beam radiotherapy with concurrent cisplatin-based chemotherapy and brachytherapy (Koh et al., 2019). Patients were treated with radiation therapy alone if they were observed to be a poor candidate for chemotherapy based on baseline laboratory values, renal failure, or Karnofsky performance status score per treating physician discretion. Patients requiring radiation therapy or chemoradiotherapy were referred to Gaborone Private Hospital from the gynecological multidisciplinary clinic at Princess Marina Hospital. All costs associated with radiation therapy or chemoradiation at Gaborone Private Hospital are covered through the publicly funded healthcare system (Grover et al., 2017).
All women living with HIV were started on antiretroviral therapy, if not already on HIV treatment. Patients accessed antiretroviral therapy through the Botswana National antiretroviral therapy program, which provides antiretroviral therapy free-of-charge to all (Nilambur, 2019). Participants with unknown HIV status or previous negative HIV test results were re-tested prior to initiation of cancer treatment.
2.4. Primary outcome and exposure
The primary reported exposures were stage of disease at presentation and patterns of care, and the primary outcome was overall survival (OS) by stage. Time-to-death was defined as the time from diagnosis until death, or censorship at the most recent follow-up. If a patient or their next of kin could not be reached by telephone, medical records were searched to determine vital status or the date of the patient’s last visit to a health care facility. The patient was censored, at that point, if vital status was not available.
Secondary objectives were to identify baseline demographic and clinical factors associated with OS. Main exposures of interest were age at cancer diagnosis, marital status, distance to clinic, HIV status, baseline (at time of treatment) creatinine, baseline hemoglobin, baseline absolute neutrophil count, baseline white blood cell count, baseline albumin, baseline Karnofsky performance status score, and stage.
2.5. Statistical analysis
Descriptive statistics were used to highlight patient, tumor, and treatment characteristics for the entire cohort and compare between patient subgroups divided by HIV status and treatment received.
Categorical variables (age, marital status, previous cervical cancer screening, distance from clinic, disease stage, hemoglobin, Karnofsky performance status score, HIV status, CD4, viral load, antiretroviral therapy received, and treatment type [surgery, chemoradiation, radiation therapy, chemotherapy, surgery + chemotherapy, surgery + radiation therapy, surgery + chemoradiation, no treatment]) were compared via χ2 tests and continuous variables (creatinine, absolute neutrophil count, white blood cell count, albumin) were compared with the t-test, as appropriate.
Descriptive statistics were used to describe stages of presentation and patterns of care defined as treatment type.
Survival by stage was investigated via the Kaplan-Meier method, with log-rank tests used to compare survival among subgroups. The Cox hazards regression model was used to evaluate factors associated with OS and survival by stage in all patients, in patients who received any treatment, and in radiation therapy and chemoradiation eligible patients (stages IB2 and above). Statistical analysis was performed using RStudio 2020 (RStudio Team, Boston, MA) and statistical significance was ascertained with the threshold of p < 0.05.
3. Results
3.1. Patient and clinical characteristics
A total of 1,043 patients were included with a median follow-up of 2.2 years (interquartile range [IQR] 0.03–9.13 years) and 3.4 years for living patients (IQR 0.2–9.13 years) (Table 1).
Table 1.
Baseline demographic characteristics of cervical cancer patients in Botswana 2013–2020.
| Characteristic | Total | All |
|---|---|---|
| N = 1043 (100%) | ||
| Age (years) | 1042 | 47 (40–58) |
| 21–39 | 227 (21.8%) | |
| 40–59 | 575 (55.1%) | |
| >60 | 240 (23.0%) | |
| HIV characteristics | ||
| HIV status | 1025 | |
| Seronegative | 311 (29.8%) | |
| Seropositive | 714 (68.5%) | |
| CD4 (cells/μL) | 544/714 | 429.5 (240.0–619.5) |
| <200 | 96 (13.4%) | |
| ≥200-<350 | 125 (17.5%) | |
| ≥350-<500 | 107 (15.0%) | |
| ≥500 | 216 (30.3%) | |
| Viral load (cells/μL) | 487/714 | |
| <400 (Undetectable) | 396 (55.5%) | |
| ≥400 (Detectable) | 91 (12.7%) | |
| On antiretroviral therapy | 704/714 | 676 (94.7%) |
| Treatment characteristics | 1043 | |
| Surgery | 58 (5.6%) | |
| Chemotherapy | 12 (1.2%) | |
| Radiation therapy | 341 (32.7%) | |
| Chemoradiation | 531 (50.9%) | |
| Surgery + Chemotherapy | 8 (0.8%) | |
| Surgery + Radiation therapy | 7 (0.7%) | |
| Surgery + Chemoradiation | 10 (1.0%) | |
| No treatment | 76 (7.3%) | |
| Disease stage | 1017 | |
| I (IA, IA1, IA2) | 45 (4.3%) | |
| I (IB, IB1, IB2, IB3) | 128 (12.3%) | |
| II (IIA, IIB) | 388 (37.2%) | |
| III (IIIA, IIIB, IIIC) | 368 (35.3%) | |
| IV (IVA, IVB) | 88 (8.4%) | |
| Marital status | 1042 | |
| Single | 669 (64.1%) | |
| Married/partnered | 240 (23.0%) | |
| Divorced/widowed | 133 (12.8%) | |
| Previously screened for cervical cancer | 1003 | 588 (56.4%) |
| Distance from treatment facility (km) | 1025 | |
| <100 | 478 (45.8%) | |
| 100–500 | 463 (44.4%) | |
| >500 | 84 (8.1%) | |
| Baseline laboratory values | ||
| Creatinine (μmol/L) | 962 | 60 (50–75) |
| Hemoglobin (g/dL) | 974 | 11.3 (9.5–12.6) |
| ≤10 | 313 (30%) | |
| >10 | 661 (63.4%) | |
| Absolute neutrophil count (×109/L) | 961 | 3.9 (2.4–6.2) |
| White blood cell count (×109/L) | 962 | 6.4 (4.7–8.6) |
| Albumin (g/L) | 656 | 39 (34.3–42.2) |
| Karnofsky performance status score | 1011 | |
| ≥90 | 757 (72.6%) | |
| <90 | 254 (24.4%) |
Values are presented as number (percentage) or median (interquartile range).
Abbreviations: HIV = human immunodeficiency virus.
Normal laboratory ranges: Creatinine (53–97 μmol/L); Hemoglobin (12.4–16.7 g/dL); Absolute neutrophil count (2.0–7.5 × 109/L); White blood cell count (4–12 × 109/L); Albumin (35–50 g/L).
Among the cohort, 714 (68.5%) were women living with HIV versus 311 (29.8%) women without HIV. Median age among women in the cohort was 47 years (IQR 40–58 years). Women living with HIV were significantly younger than women without HIV (44 versus 61 years respectively, p < 0.001) (Supplemental Table A). The median CD4 count for women living with HIV was 429.5 cells/μL (IQR 240–619.5 cells/μL). Of the 714 women living with HIV, 396 (55.5%) had a viral load < 400 cells/μL and the majority, 676 women (94.7%), were receiving antiretroviral therapy at the time of cervical cancer diagnosis.
3.2. Stage distribution
Among the 1,043 patients diagnosed with cervical cancer between 2013 and 2020, 821 (78.7%) were staged prior to 2018 using the 2009 FIGO staging criteria, and, starting in 2019, 215 (20.6%) were staged using the 2018 FIGO staging criteria. Stage distribution was: 4.3% (n = 45) stage IA (IA, IA1, IA2); 12.3% (n = 128) stage IB (IB, IB1, IB2, IB3); 37.2% (n = 388) stage II (IIA, IIB); 35.3% (n = 368) stage III (IIIA, IIIB, IIIC); 8.4% (n = 88) stage IV (IVA, IVB) (Table 1).
3.3. Patterns of care
A total of 967 patients (92.7%) received treatment. The distribution among those who were treated was: surgery alone (5.6%), radiation therapy alone (32.7%), chemotherapy alone (1.2%), chemoradiation (50.9%), surgery and radiation therapy (0.7%), surgery and chemotherapy (0.8%), and surgery and chemoradiation (1.0%). Over half of the patients who did not undergo treatment died before starting treatment or during treatment. Majority of patients who did not receive treatment were of advanced stages: 10.5% (n = 8) stage I; 6.6% (n = 5) stage II; 50% (n = 38) stage III; 15.8% (n = 12) stage IV. There were no significant differences in treatment characteristics between women living with HIV and women without HIV.
Of the 902 radiation and chemoradiation eligible cervical cancer patients (stages IB2 and above), 63.1% (n = 569) received brachytherapy; 52.1% (n = 470) received EQD2 ≥ 75 Gy; 57% (n = 514) received concurrent cisplatin ≥ 1 cycle. The median treatment duration, as defined as the time from treatment start date to treatment completion, was 44 days (IQR 36–51 days). Breakdown of stage and treatment is described (Supplemental Table B).
3.4. Overall survival by stage
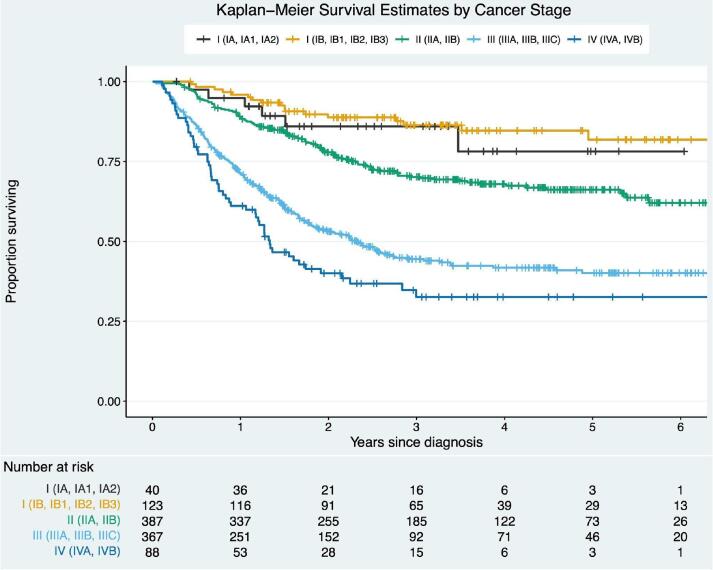
Survival based on stage is summarized (Fig. 1). The median survival time was not reached for stages IA, IB, and II, 2.32 years (95% CI 1.81–3.08 years) for stage III, and 1.33 years (95% CI 1.13–2.24 years) for stage IV. Stage was inversely proportional to patients’ 2-year OS rates among the entire cohort: stage I 88.0% (95% CI 83.0–93.4%), stage II 77.5% (95% CI 73.3–81.9%), stage III 54.3% (95% CI 49.2–59.9%), and stage IV 40% (95% CI 30.8–52.2%) (log rank, p < 0.001). Stage was also inversely proportional to patients’ 5-year OS rates among the entire cohort: stage I 80.9% (95% CI 73.2–89.4%), stage II 66.2% (95% CI 61.1–71.7%), stage III 40.1% (95% CI 34.6–46.5%), and stage IV 32.6% (95% CI 23.3–45.5%) (log rank, p < 0.001).
Fig. 1.
Survival outcomes by stage- Stage was inversely proportional to patients’ 2-year OS rates among the entire cohort: stage I 88.0% (95% CI 83.0–93.4%), stage II 77.5% (95% CI 73.3–81.9%), stage III 54.3% (95% CI 49.2–59.9%), and stage IV 40% (95% CI 30.8–52.2%) (log rank, p < 0.001).
3.5. Factors associated with overall survival
The median follow-up for the study population was 2.2 years (95% CI 2.1–2.4 years) and 3.4 years for those alive. Among the entire cohort, the 2-year OS rate was 67.2% (95% CI 64.3–70.3%) and the 5-year OS rate was 56.4% (95% CI 52.9–60%). On univariable Cox regression analysis, OS was associated with stage: stage II (HR 2.19, p < 0.001), stage III (HR 5.09, p < 0.001), and stage IV disease (HR 7.23, p < 0.001) versus stage I disease; previous cervical cancer screening (HR 0.72, p = 0.001); baseline creatinine (HR 1.001, p < 0.001); baseline hemoglobin > 10 g/dL (HR 0.37, p < 0.001) versus hemoglobin ≤ 10 g/dL; baseline albumin (HR 0.96, p < 0.001); and baseline Karnofsky performance status score < 90 (HR 1.89, p < 0.001) versus Karnofsky performance status score ≥ 90. On multivariable Cox regression analysis including all patients considering age, HIV status, disease stage, baseline creatinine, baseline hemoglobin, and baseline Karnofsky performance status score, OS was associated with: stage II (HR 1.91, p = 0.007), stage III (HR 3.99, p < 0.001), and stage IV disease (HR 5.06, p < 0.001) versus stage I disease; baseline creatinine (HR 1.001, p < 0.001); baseline hemoglobin > 10 g/dL (HR 0.51, p < 0.001) versus hemoglobin ≤ 10 g/dL; baseline Karnofsky performance status score < 90 (HR 1.53, p < 0.001) versus Karnofsky performance status score ≥ 90. HIV was not associated with OS on univariable or multivariable analysis (Table 2).
Table 2.
Factors associated with overall survival in cervical cancer patients in Botswana: univariable and multivariable analyses [N = 1043].
| Characteristic | Overall Survival, UVA | p value | Overall Survival, MVA | p value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (years) | ||||
| 21–39 | 1 (ref) | .. | 1 (ref) | .. |
| 40–59 | 0.81 (0.64–1.04) | 0.093 | 0.86 (0.66–1.11) | 0.24 |
| >60 | 0.91 (0.68–1.21) | 0.50 | 1.07 (0.75–1.52) | 0.71 |
| Marital status | ||||
| Single | 1 (ref) | .. | .. | .. |
| Married/partnered | 0.98 (0.77–1.24) | 0.84 | .. | .. |
| Divorced/widowed | 0.94 (0.69–1.27) | 0.68 | .. | .. |
| Distance (km) | 1 (ref) | |||
| <100 | 1 (ref) | .. | .. | .. |
| 100–500 | 1.06 (0.86–1.30) | 0.58 | .. | .. |
| >500 | 0.92 (0.62–1.37) | 0.68 | .. | .. |
| Previously screened for cervical cancer | ||||
| No | 1 (ref) | .. | .. | .. |
| Yes | 0.72 (0.59–11.77) | 0.0013 | .. | .. |
| HIV status | ||||
| Seronegative | 1 (ref) | .. | 1 (ref) | .. |
| Seropositive | 1.17 (0.94–1.46) | 0.16 | 1.28 (0.96–1.70) | 0.088 |
| Disease stage | ||||
| I | 1 (ref) | .. | 1 (ref) | .. |
| II | 2.19 (1.40–3.43) | 0.00058 | 1.91 (1.19–3.06) | 0.0074 |
| III | 5.09 (3.30–7.85) | <0.0001 | 3.99 (2.52–6.32) | <0.0001 |
| IV | 7.23 (4.44–11.77) | <0.0001 | 5.06 (2.98–8.59) | <0.0001 |
| Baseline laboratory values | ||||
| Creatinine (μmol/L) | 1.001 (1.001–1.001) | <0.0001 | 1.001 (1.000–1.001) | 0.00020 |
| Hemoglobin (g/dL) | ||||
| ≤10 | 1 (ref) | .. | 1 (ref) | .. |
| >10 | 0.37 (0.30–0.46) | <0.0001 | 0.51 (0.40–0.64) | <0.0001 |
| Absolute neutrophil count (×109/L) | 1.01 (1.00–1.02) | 0.084 | .. | .. |
| White blood cell count (×109/L) | 1.002 (0.999–1.006) | 0.17 | .. | .. |
| Albumin (g/L) | 0.96 (0.95–0.97) | <0.0001 | .. | .. |
| Baseline Karnofsky performance status score | ||||
| ≥90 | 1 (ref) | .. | 1 (ref) | .. |
| <90 | 1.89 (1.54–2.34) | <0.0001 | 1.53 (1.21–1.92) | 0.00033 |
Abbreviations: HIV = human immunodeficiency virus.
Normal laboratory ranges: Creatinine (53–97 μmol/L); Hemoglobin (12.4–16.7 g/dL); Absolute neutrophil count (2.0–7.5 × 109/L); White blood cell count (4–12 × 109/L); Albumin (35–50 g/L).
In a sub-group analysis of patients receiving treatment, the baseline factors associated with survival were similar to those above in the entire cohort of patients (Supplemental Table C).
3.6. Overall survival by HIV status
For women living with HIV, the median follow-up time was 2.2 years (95% CI 2.0–2.4 years) versus 2.3 years (95% CI 2.1–2.7 years) for women without HIV. Among women living with HIV, the 2-year OS rate was 65.9% (95% CI 62.4–69.6%) compared to 71.6% (95% CI 66.6–76.9%) for women without HIV. Further, the 5-year OS rates were 60% (95% CI 50.8–59.5%) and 60.5% (95 % CI 54.8–66.9%) for women living with HIV and women without HIV respectively. There was no significant difference in 2-year or 5-year OS rates between women without HIV and women living with HIV (log rank, p = 0.2) (Supplemental Figure A).
In a sub-analysis evaluating survival outcomes by HIV status for each disease stage, only patients with stage III disease showed significant differences in survival by HIV status (log rank, p = 0.006).
3.7. Overall survival by treatment
Median survival time was not reached for patients who received any form of treatment as compared to 0.5 years (95% CI 0.3–1.07 years) for patients who did not receive treatment (log rank, p < 0.001). Among patients who received any form of treatment, the 2-year OS rate was 70.5% (95% CI 67.6–73.6%) compared to 23.4% (95% CI 15.1–36.1%) for patients who did not receive treatment (log rank, p < 0.001). Further, the 5-year OS rates were 59.4% (95% CI 55.9–63.2%) and 15.3% (95% CI 7.8–30.1%) for patients who received any form of treatment and patients who did not receive treatment respectively (log rank, p < 0.001). (Supplemental Figure B).
Among patients with stages IB2 disease and above who were eligible for radiation therapy and chemoradiation, multivariable analysis adjusting for age, HIV status, baseline creatinine, baseline hemoglobin, baseline Karnofsky performance status score, disease stage, response to treatment at the end of radiation therapy, dose of radiation, and number of chemotherapy cycles received, factors associated with improved survival included baseline hemoglobin > 10 g/dL (HR 0.57, p < 0.001) versus hemoglobin ≤ 10 g/dL. Worsened survival outcomes were associated with disease stage: stage II (HR 2.07, p = 0.005) and stage III (HR 2.59, p = 0.026) versus stage I, baseline Karnofsky performance status score (HR 1.39, p = 0.043), response to treatment at the end of radiation therapy: partial response (HR 1.58, p = 0.005) versus complete response, dose of radiation: EQD2 < 79 Gy (HR 1.47, p = 0.015) versus EQD2 ≥ 79 Gy, and number of chemotherapy cycles received: chemotherapy < 4 cycles (HR 1.63, p = 0.004) versus ≥ 4 cycles (Supplemental Table D).
4. Discussion
In this prospective cohort study of patients with cervical cancer in Botswana, most patients presented with locally advanced cervical cancer, with the majority of patients presenting with stages II-III disease. Women living with HIV did not present with earlier stage cervical cancer despite screening encouragement being incorporated into routine HIV care in Botswana (Barchi et al., 2019). Among the cohort, 2-year OS by stage was 88.0% for stage I, 77.5% for stage II, 54% for stage III, and 40% for stage IV. OS was largely associated with patients’ disease stage. Given that most women in our cohort were found to have locally advanced cervical cancer, this demonstrates the importance of cervical cancer screening and early, aggressive intervention to prevent progression of disease and improve OS.
Recent studies have presented FIGO data on the stage distribution of women diagnosed with cervical cancer in Ethiopia, Ghana, Kenya, Malawi, Nigeria, Sudan, and Tanzania (Wassie and Fentie, 2021, Kantelhardt et al., 2014, Vulpe et al., 2018, Asamoah et al., 2020, Maranga et al., 2013, Rudd et al., 2017, Awolude and Oyerinde, 2019, Musa et al., 2016, Ibrahim et al., 2011, Mlange et al., 2016). A 2019 registry study presented stage distribution and survival outcomes of cervical cancer patients across 11 countries in Sub-Saharan Africa, however the study did not include Botswana and provided less granular staging, classifying stage at diagnosis as stages I-II or stages III-IV (Sengayi-Muchengeti et al., 2020). Within the study’s findings, the 2-year survival outcomes varied by region and were inversely related to stage in the countries in Sub-Saharan Africa (Kantelhardt et al., 2014, Maranga et al., 2013, Musa et al., 2016). In an Ethiopian study, 2-year OS was found to be inversely related to stage: 84.8% for patients presenting with stages I–II and 55.8% for stages III–IV (Wassie and Fentie, 2021). A Nigerian study reported worse outcomes for 2-year OS by stage: about 50% for patients presenting with stages IIA and below and about 15% for IIB and above (Musa et al., 2016). Neither the Ethiopian nor the Nigerian study provide detailed staging and there still exist differences in survival outcomes in Botswana and other low- and middle-income countries compared to high-income regions. In comparison, a high-income region study reported a 3-year OS of 74% for stage IB, 79% for stage IIB, 45% for stage IIIB, and 33% for stage IVA (Pötter et al., 2011). The difference in survival between this high-income region study compared to the studies in low- and middle-income countries could be a result of higher overall EQD2 receipt due to the use of magnetic resonance imaging (MRI) guided adaptive brachytherapy or a result of overall smaller tumors, even for the same stage.
In evaluation of radiation therapy and chemoradiation eligible patients, baseline factors associated with survival such as hemoglobin > 10 g/dL were consistent with our prior studies in patients treated with curative intent (Grover et al., 2018, Grover et al., 2021, MacDuffie et al., 2021). This suggests the significance of hemoglobin in indicating overall performance status of the patient and ability to receive adequate treatment. Additionally, some studies have suggested that low hemoglobin may be associated with impaired tumor oxygenation, resulting in relative radioresistance (Grogan et al., 1999). Elevated creatinine level was previously found to be associated with radiation dose < 80 Gy and withholding of chemotherapy (Grover et al., 2021).
HIV was not found to be associated with OS in this cohort of cervical cancer patients receiving antiretroviral therapy. Although prior studies from this region have reported worse survival among women with HIV, our current data aligns with our findings from previous studies which found no difference in OS by HIV status (Grover et al., 2018, Grover et al., 2021, MacDuffie et al., 2021). We hypothesize that the women living with HIV in our cohort have well-managed HIV which likely contributes to both their ability to tolerate treatment and their OS. However, given that women living with HIV present with cervical cancer at significantly younger ages, it underlines the importance of aggressive cervical cancer screening implementation efforts at even earlier ages than women without HIV.
The present analysis yields the most extensive stage distribution and survival data to date for cervical cancer patients in Botswana and Sub-Saharan Africa and is the first to assess factors associated with OS at presentation in Botswana for all cervical cancer patients. The current findings remain subject to limitations. Patients among this cohort were primarily staged according to 2009 FIGO staging criteria due to limited diagnostic capacity in Botswana. As per 2018 FIGO staging criteria, it has been found that the inclusion of surgical pathologic and image findings resulted in upward stage migration for the majority of patients and improved survival discriminatory ability for stages I and IV patients (Grover et al., 2017). Additionally, OS evaluated limited baseline patient characteristics and did not assess the impact of treatment characteristics on survival in early stage patients treated with surgery. Future studies should assess whether treatment characteristics such as surgical quality or extent of surgery are associated with OS in early stage patients.
In summary, most women enrolled among the cohort presented with locally advanced cervical cancer and 2-year OS was found to be 67%, which was inversely proportional to the stage of disease at presentation. Within the cohort, OS was associated with disease stage, baseline creatinine, baseline hemoglobin, and Karnofsky performance status score regardless of HIV status. Given the prevalence of late-stage disease among this cohort in Botswana, it is imperative to optimize women’s access to screening to allow for early detection and treatment of cervical disease.
5. Funding statement
This work was supported by the Sub-Saharan African Collaborative HIV and Cancer Consortia-U54 (Grant No. 1U54 CA190158-01), the American Cancer Society International Fellowships for Beginning Investigators (ACSBI), Conquer Cancer Foundation Young Investigator Award, Mentored Patient Oriented Career Research Development Award (1-K08CA230170-01A1) and Penn Center for AIDS Research (Grant No. 5-P30-AI-045008-17).
CRediT authorship contribution statement
Surbhi Grover: Conceptualization, Data curation, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. Jessica George: Writing – review & editing. Shawna Tuli: Writing – review & editing. Katie Lichter: Writing – review & editing. Rohini Bhatia: Writing – review & editing. Barati Monare: Writing – review & editing. Ganen Chinniah: Writing – review & editing. Lisa Bazzett-Matabele: Writing – review & editing. Memory Bvochora-Nsingo: Writing – review & editing. Sebathu Chiyapo: Writing – review & editing. Dawn Balang: Writing – review & editing. Tlotlo Ralefala: Writing – review & editing. Peter Vuylsteke: Writing – review & editing. Rebecca Luckett: Writing – review & editing. Sanghyuk Shin: Methodology, Supervision, Writing – review & editing. Nicola Zetola: Methodology, Supervision, Writing – review & editing. Doreen Ramogola-Masire: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.101094.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- American Brachytherapy Society. Brachytherapy guidelines and consensus statements.
- Asamoah F.A., Yarney J., Scott A., et al. Comparison of Definitive Cervical Cancer Management With Chemotherapy and Radiation Between Two Centers With Variable Resources and Opportunities for Improved Treatment. JCO Glob Oncol. 2020;6:1510–2158. doi: 10.1200/GO.20.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolude O.A., Oyerinde S.O. Invasive cervical cancer in ibadan: Socio-sexual characteristics, clinical stage at presentation, histopathology distributions and hiv status. Afr J Infect Dis. 2019;13:32–38. doi: 10.21010/ajid.v13i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi F., Winter S.C., Ketshogile F.M., Ramogola-Masire D. Adherence to screening appointments in a cervical cancer clinic serving HIV-positive women in Botswana. BMC Public Health. 2019;19:1–13. doi: 10.1186/s12889-019-6638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla N., Berek J.S., Fredes M.C., et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- Cubie H.A., Campbell C. Cervical cancer screening - The challenges of complete pathways of care in low-income countries: Focus on Malawi. Women’s Health (Lond) 2020;16:1–10. doi: 10.1177/1745506520914804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden-Peterson S., Bvochora-Nsingo M., Suneja G., et al. HIV Infection and Survival Among Women With Cervical Cancer. J. Clin. Oncol. 2016;34:3749–3757. doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby P.W., Massad L.S., Mutch D.G., et al. FIGO 2018 staging criteria for cervical cancer: Impact on stage migration and survival. Gynecol. Oncol. 2020;157:639–643. doi: 10.1016/j.ygyno.2020.03.027. [DOI] [PubMed] [Google Scholar]
- Grogan M., Thomas G.M., Melamed I., et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528–1536. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Grover S., Chiyapo S.P., Puri P., et al. Multidisciplinary Gynecologic Oncology Clinic in Botswana: A Model for Multidisciplinary Oncology Care in Low- and Middle-Income Settings. J Glob Oncol. 2017;3:666–670. doi: 10.1200/JGO.2016.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Bvochora-Nsingo M., Yeager A., et al. Impact of Human Immunodeficiency Virus Infection on Survival and Acute Toxicities From Chemoradiation Therapy for Cervical Cancer Patients in a Limited-Resource Setting. Int. J. Radiat. Oncol. Biol. Phys. 2018;101:201–210. doi: 10.1016/j.ijrobp.2018.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S., Ning M.S., Bale M., et al. Chemoradiation versus radiation alone in stage IIIB cervical cancer patients with or without human immunodeficiency virus. Int J Gynecol Cancer. 2021;31:1220–2127. doi: 10.1136/ijgc-2021-002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A., Rasch V., Pukkala E., Aro A.R. Predictors of cervical cancer being at an advanced stage at diagnosis in Sudan. Int J Womens Health. 2011;3:385–439. doi: 10.2147/IJWH.S21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, S., Broggio, J. 2019. Cancer survival in England - adults diagnosed. Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed (accessed Jan 6, 2022).
- Kantelhardt E.J., Moelle U., Begoihn M., et al. Cervical Cancer in Ethiopia: Survival of 1,059 Patients Who Received Oncologic Therapy. Oncologist. 2014;19:727–734. doi: 10.1634/theoncologist.2013-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W.J., Abu-Rustum N.R., Bean S., et al. NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019;17:64–84. doi: 10.6004/jnccn.2019.0001. [DOI] [PubMed] [Google Scholar]
- MacDuffie E., Bvochora-Nsingo M., Chiyapo S., et al. Five-year overall survival following chemoradiation therapy for locally advanced cervical carcinoma in women living with and without HIV infection in Botswana. BMC Infect Agents and Cancer. 2021;16:55. doi: 10.1186/s13027-021-00389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranga I.O., Hampson L., Oliver A.W., et al. Analysis of Factors Contributing to the Low Survival of Cervical Cancer Patients Undergoing Radiotherapy in Kenya. PLoS ONE. 2013;8:e78411. doi: 10.1371/journal.pone.0078411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlange R., Matovelo D., Rambau P., Kidenya B. Patient and disease characteristics associated with late tumour stage at presentation of cervical cancer in northwestern Tanzania. BMC Womens Health. 2016;16:1–6. doi: 10.1186/s12905-016-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa J., Nankat J., Achenbach C.J., et al. Cervical cancer survival in a resource-limited setting-North Central Nigeria. BMC Infect Agents and Cancer. 2016;11:1–7. doi: 10.1186/s13027-016-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilambur, J. Country Factsheets: Botswana 2019. UNAIDS. 2020. https://www.unaids.org/en/regionscountries/countries/botswana (accessed Jan 5, 2022).
- Pötter R., Georg P., Dimopoulos J.C., et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd P., Gorman D., Meja S., et al. Cervical cancer in southern Malawi: A prospective analysis of presentation, management, and outcomes. Malawi Med J. 2017;29:124–219. doi: 10.4314/mmj.v29i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schag C.C., Heinrich R.L., Ganz P.A. Karnofsky performance status revisited: reliability, validity, and guidelines. J. Clin. Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- Sengayi-Muchengeti M., Joko-Fru W.Y., Miranda-Filho A., et al. Cervical cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: A population-based registry study. Int. J. Cancer. 2020;147:3037–3048. doi: 10.1002/ijc.33120. [DOI] [PubMed] [Google Scholar]
- Singh D.K., Anastos K., Hoover D.R., et al. Human Papillomavirus Infection and Cervical Cytology in HIV-Infected and HIV-Uninfected Rwandan Women. J. Infect. Dis. 2009;199:1851–1861. doi: 10.1086/599123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Vulpe H., Asamoah F.A., Maganti M., Vanderpuye V., Fyles A., Yarney J. External Beam Radiation Therapy and Brachytherapy for Cervical Cancer: The Experience of the National Centre for Radiotherapy in Accra, Ghana. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:1246–1253. doi: 10.1016/j.ijrobp.2017.12.270. [DOI] [PubMed] [Google Scholar]
- Wassie M., Fentie B. Prevalence of late-stage presentation and associated factors of cervical cancer patients in Tikur Anbesa Specialized Hospital, Ethiopia: institutional based cross-sectional study. BMC Infect Agents and Cancer. 2021;16:1–6. doi: 10.1186/s13027-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. Botswana Overview: Development news, research, data. World Development Indicators. 2022. https://www.worldbank.org/en/country/botswana/overview (accessed Oct 9, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.