Abstract
Patients with diabetes mellitus (DM) have a higher prevalence of heart failure (HF) than those without it. Approximately 40% of HF patients have DM, having poorer outcomes than those without DM. Myocardial ischemia caused by endothelial dysfunction, renal dysfunction, obesity, and impaired myocardial energetics is pathophysiology of DM-induced HF (DM-HF). Also, patients with HF show an increased risk for the onset of DM due to several mechanisms including insulin resistance. This review is focused on the epidemiology, pathogenic mechanism and treatment strategy of DM-HF.
Keywords: Heart failure, Diabetes mellitus
INTRODUCTION
The global prevalence of diabetes mellitus (DM) in 20–79-year-olds in 2021 was estimated to be 10.5% (536.6 million people), rising to 12.2% (783.2 million) in 2045.1) It is also a common comorbid condition in heart failure (HF) patients, affecting approximately 26–43%.2,3,4,5,6,7) In a meta-analysis, DM patients were suggested to have a 2-fold increase in the risk of HF.8) Moreover, a high prevalence of asymptomatic cardiac dysfunction or structural abnormalities such as increased left ventricular mass, enlarged left atrium and reduced global longitudinal strain, was observed in patients with type 2 DM, suggesting that the risk of DM-induced HF (DM-HF) might be higher than reported.9) The rates of hospitalization due to HF in Korean populations with DM increased from 72 to 146 and 124 to 161 per 10,000 men and women respectively, based on data using the Korean National Health Insurance Service-National Sample Cohort from 2006 to 2015.10) Importantly, HF, itself, begets DM.11) Patients with DM-HF have a poorer outcome than those with HF alone,12,13) stressing on the need to establish an optimal treatment strategy in order to improve their prognosis.
DM IN HF PATIENTS
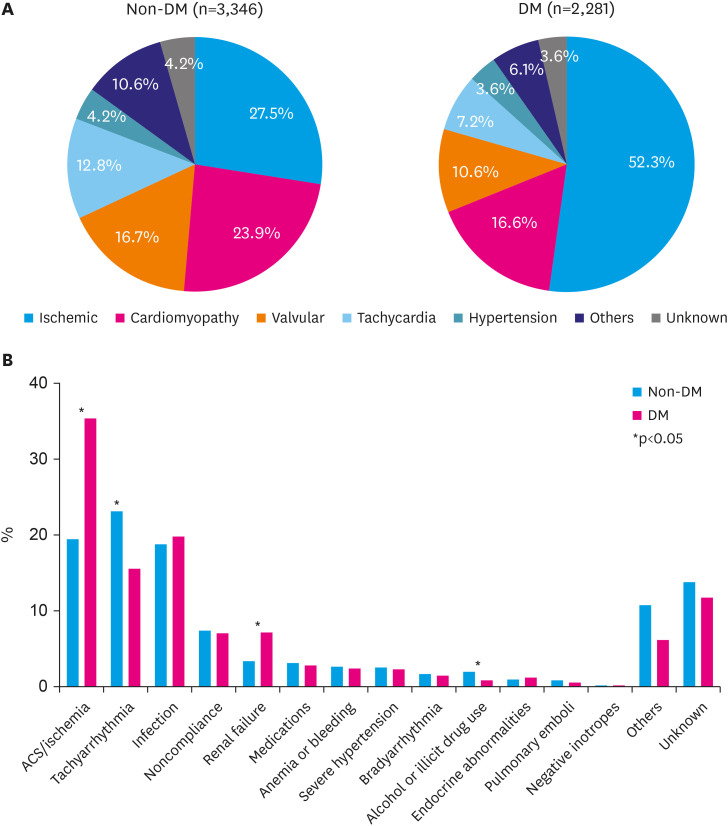
The Korean Acute Heart Failure (KorAHF) registry investigators evaluated the impact of DM on these endpoints according to HF subtypes and glycemic control.12) Among KorAHF registry patients, 40.0% had DM. DM-HF patients were older, more men, had more hypertension, chronic renal disease, whereas had less atrial fibrillation than non-DM-HF. Ischemia was both the leading cause and the most frequent aggravating factor in DM-HF patients, which were far more than in non-DM-HF patients (Figure 1). During a median follow-up of 3.5 years, DM was significantly associated with increased long-term mortality after adjusting for potential confounders (adjusted hazard ratio [HR], 1.11; 95% confidence interval [CI], 1.03–1.22). Conversely, KorAHF registry investigators reported that well-controlled diabetes (hemoglobin A1c [HbA1c] <7.0%) is associated with a lower risk of long-term mortality compared to uncontrolled DM.12)
Figure 1. Comparison of etiology and aggravating factors between acute heart failure with or without diabetes mellitus in Korean Acute Heart Failure registry. (A) Etiology of acute heart failure. (B) Aggravating factors of acute heart failure.
DM = diabetes mellitus; ACS = acute coronary syndrome.
PATHOGENIC MECHANISM OF DIABETIC CARDIOMYOPATHY
Multiple factors such as ischemia, hypertension, and extracellular fluid volume expansion are involved in the pathogenesis of DM-HF.14) DM causes microangiopathy, myocardial fibrosis, and autonomic neuropathy and these lead to diabetic cardiomyopathy. Hyperglycemia leads to lipid accumulation in the heart, and this can cause cellular damage by lipotoxicity.15) Lipid accumulation, collagen deposition and fibrosis, and hyperinsulinemia induce myocardial hypertrophy.16,17)
Endothelial dysfunction is an initial critical factor in the pathogenesis of diabetic vascular complications. Hyperglycemia has been identified as an important contributor to endothelial dysfunction in both type 1 and type 2 DM.18,19) In DM, endothelial dysfunction is characterized by impaired availability of endothelium-derived vasodilating factors, mainly nitric oxide.20,21,22,23) We reported that a deregulated, chronic FOXO1 activation and resulting suppression of Krüppel-like factor-2 (KLF2) is responsible of diabetic endothelial dysfunction. KLF2 is a main KLF expressed in endothelial cells, playing an important role in the maintenance of endothelial function. KLF2 affects the expression of various factors, including endothelial nitric oxide synthase that confers anti-inflammatory, anti-thrombotic, and anti-proliferative effects.24) Our team have found KLF2 expression was suppressed by high glucose.25) The endothelial dysfunction is associated with an adverse lipid profile and vascular inflammation, which produce pro-atherogenic signals (P selectin, vascular grow factors, transforming grow factor β) which are responsible for systemic vascular damage. In therapeutic aspect, high glucose-induced, FOXO1-mediated KLF2 suppression was reversed by atorvastatin, suggesting the implication of statin treatment, if vascular dysfunction is ongoing problem in DM-HF.26)
Although ischemia is the main etiology of DM-HF, the considerable portion of DM-HF patients do not have the evidence of coronary artery disease, namely diabetic cardiomyopathy. Diabetic cardiomyopathy is defined by the existence of abnormal myocardial structure and performance in the absence of other cardiac risk factors, such as coronary artery disease, hypertension, and significant valvular disease, in individuals with DM.27) Multiple mechanisms have been suggested to explain the development of diabetic cardiomyopathy. They include mitochondrial fatty acid oxidation alterations, impaired mitochondrial calcium handling, impaired signaling of IRS, PI3K/Atk, and downstream pathways, cardiac autonomic neuropathy, and inflammatory pathways that result in myocardial fibrosis, stiffness, and hypertrophy.28)
Recently, hyperinsulinemia rather than hyperglycemia is suggested as main pathogenic mechanism of DM-HF.29) One important evidence is that the incidence of HF is low in type 1 DM patients, unless hypertension or coronary artery disease was developed.30) Differences in the cardiac effects of types 1 and 2 DM was also observed in animal models of the 2 diseases.31) Hyperinsulinemic condition promotes nutrient surplus signaling through Akt and mammalian target of rapamycin complex 1 and inhibits nutrient deprivation signaling through sirtuin-1 and its downstream effectors. As a result, ‘self-preserving’ autophagy process is suppressed, whereas ‘uncontrolled’ oxidative stress is activated. The hyperinsulinemia of DM may also activate sodium-hydrogen exchangers in cardiomyocytes and in the proximal renal tubules, leading to sodium retention.29) Skøtt et al.32) investigated the effects of insulin on kidney function and sodium excretion. They confirmed that the insulin infusion for 120-minute induced increase in sodium reabsorption, even within the physiological range. Insulin-induced sodium and water retention are likely to exacerbate cardiac congestion, leading to acute HF decompensation and increasing the need for loop diuretics.32,33) In addition, insulin use increases the risk of hypoglycemia, which can cause sympathetic over-activation. Laitinen et al.34) demonstrated that hyperinsulinemic hypoglycemia could cause a 12-fold increase in plasma epinephrine levels, which can induce a substantial increase in myocardial contractility, myocardial oxygen demand, heart rate, and cardiac output in short term. However, these hemodynamic changes, coupled with a shortage of glucose supply, can be deleterious for patients with HF for long term.
INCREASED RISK OF DM IN HF PATIENTS
Several trials suggested HF may increase the incidence of DM, whose onset is associated with a worse outcome. In a study of 58,056 non-diabetic adults aged ≥30 years with no evidence of DM from Kaiser Permanente Northwest database, HF was independently associated with an increase in DM incidence of 48% (95% CI, 27–73%).11) The actual incidence of new onset DM was 13.6/1,000 persons per year vs. 9.2/1,000 HF persons per year in non-HF patients. In the analysis of a Danish registry involving 104,522 HF subjects with a first HF hospitalization and a mean follow-up of 3.9 years, 10% of the patients were newly diagnosed with DM. What is worse, HF patients with new onset DM showed a higher risk of death (HR, 1.47; 95% CI, 1.42–1.52).35)
Insulin resistance is suggested as a main pathogenic mechanism underlying the greater incidence of DM in HF patients, even in the absence of noticeably elevated fasting glucose plasma levels.36) Insulin resistance is mainly driven by neuro-hormonal activation. In particular, sympathetic nervous system over-activation contributes the development of insulin resistance.37) This could be due to the stimulation of alpha-adrenergic receptors, causing vasoconstriction and hypoperfusion at the level of skeletal muscle and is responsible for reduced glucose uptake. Catecholamine-mediated insulin resistance could be also related to increased oxidative stress, lipolysis, as well as to reduced skeletal muscle glucose uptake, whereas increased hepatic gluconeogenesis.37,38) Renin-angiotensin-aldosterone system activation also contributes to the insulin resistance. Angiotensin II is responsible for skeletal muscle vasoconstriction, which is associated with reduced glucose delivery and insulin sensitivity.39) Also, angiotensin II promotes fibrosis, inflammation, apoptosis, and β-cell death in the pancreas, thus affecting insulin production.39) Aldosterone can alter insulin sensitivity at the level of peripheral tissue and induce fibrosis and inflammation in pancreatic islets.40)
Besides neurohormonal activation, limited physical activity of HF patients is also responsible for the insulin sensitivity. Lastly, HF medication can exert adverse effect in glucose metabolism. Although beta-blockers have been shown to improve clinical outcomes in patients with chronic HF but may be associated with the development of new-onset DM. Depending on the receptor specificity of the individual agent, beta-blockers have different effects on glucose and lipid metabolism as well as on the risk for developing new-onset DM.41) Thiazides and loop diuretic have unfavorable effect in insulin sensitivity mainly due to hypokalemic effects.42) The dose-dependent association of loop diuretic dosage with the risk of DM was observed in a Danish registry of 99,362 patients.43)
TREATMENT STRATEGY OF HF COMBINED WITH DM
Glucose lowering strategy
Although many studies confirmed well-controlled DM (HbA1c <7.0%) is associated with a lower risk of long-term mortality compared to uncontrolled DM in HF patients, several studies also suggested there is considerable difference in outcomes according to insulin-elevating or non-elevating natures of glucose-lowering agents.
Insulin, sulfonylurea, and dipeptidyl peptidase 4 inhibitors increase insulin levels and clinical studies showed concerning results despite improved glycemic controls.29) Insulin has been associated with sodium retention and weight gain, potentially exerting harmful effects. Lawson et al.44) investigated the effect of DM on HF patients using the UK Clinical Practice Research Datalink, which covers 10% of the UK population. HF patients treated with oral hypoglycemia agents mainly sulfonylurea or insulin had an increased risk of all-cause hospitalization compared to HF patients without DM. KorAHF registry investigators evaluated the impact of insulin therapy on mortality due to acute HF analyzing the data of 1,740 patients with DM.45) Insulin therapy showed an increased mortality risk (HR, 1.29; 95% CI, 1.14–1.46) compared with the oral hypoglycemic agents group. Insulin therapy was consistently associated with increased mortality risk, regardless of the left ventricular ejection fraction (LVEF) or HF etiology. Meta-analysis, including the Valsartan Heart Failure Trial (Val-Heft), Controlled Rosuvastatin Multinational Trial in HF (CORONA), Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca-Heart Failure (GISSI-HF), and Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure (ATMOSPHERE), reported that the rate of all-cause mortality and HF hospitalization was higher in patients with DM than in those without, and the highest was for the patient group prescribed insulin.46,47,48,49,50) Another hypoglycemic agents with volume-retention effect have been shown to have adverse effects in HF patients. Thiazolidinedione, once the bestselling hypoglycemic agent, is no longer recommended for these patients due to its fluid retention effect.51,52)
In contrast, metformin and sodium-glucose cotransporter 2 (SGLT2) inhibitors exert antihyperglycemic effects without increasing insulin levels. Expectedly, metformin and SGLT2 inhibitors have reduced the risk of cardiovascular death and hospitalizations for HF.53,54)
Therefore, best hyperglycemic control strategy is tight glucose control with glucose-lowering agents without hyperinsulinemic effect.
HF medications
Four important components of the guideline-directed medical therapy including renin-angiotensin-aldosterone system inhibitors (angiotensin receptor-neprilysin inhibitor or angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker), beta-blockers, mineralocorticoid receptor antagonists, and SGLT-2 inhibitors should be treated in DM-HF patients with heart failure with reduced ejection fraction (HFrEF).55,56)
Pharmacological therapies for heart failure with preserved ejection fraction (HFpEF) and heart failure with midrange ejection fraction have been far less successful than those for HFrEF, and despite the large number of studies performed in patients with this condition, including a significant proportion with DM, no current therapies have been proven to reduce cardiovascular endpoints except for SGLT2 inhibitors, which are considered as class I recommendation in the most recently published by the Korean Society of Heart Failure. EMPEROR-Preserved trial (included 48.9% of patients with DM) showed that empagliflozin reduced the primary composite outcome event (cardiovascular death or hospitalization for HF) in patients with HFpEF, regardless of diabetic status.57) Recently, Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER) trial reported that the dapagliflozin significantly reduced the risk for CV death or worsening HF in HF with more than 40% of LVEF compared with placebo.58)
Management of concomitant cardiovascular risk factors
Among all DM-HF patients affected, 70% are dyslipidemic, and almost 70% suffer from hypertension.59) Therefore, effective management of accompanied cardiovascular risk factor might be even more important than the HF specific management.
First part is dyslipidemia control with statins. Undoubtedly, statin is key medication for DM patients, especially when considering endothelial cell protecting effect of statins.26) However, statins with less-diabetogenic effect may be preferred if target goal achievement is possible, considering that high risk of new-onset DM among HF patients.60) Although statin treatment might be associated with better outcome, current HF guideline do not recommend statins, especially high potency in HF. In Korean HF patients with ischemic origin, both low potency pravastatin and high potency pitavastatin had beneficial effect in cardiac reverse remodeling and improvement in systolic function. However, only pravastatin significantly improved exercise capacity. The findings of this study suggest that ‘too much’ lowering of cholesterol might not be beneficial to HF patients.61) Although it is still debated whether statins might cause fatigue, thus limiting exercise capacity, some studies actually reported the alarming signs. The post hoc analysis of Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA) study reported small but significant worsening of fatigue in older patients with HFrEF.62) A rare but well-reported adverse effects of statins, myopathy, might be responsible for the exercise limitation. Also, statins might increase the perception of fatigue through a central nervous system effect, and a lipophilic statins have higher possibility compared with hydrophilic agents such as rosuvastatin.63) Another hypotheses against statin treatment in HF are the endotoxin lipoprotein hypothesis, the coenzyme Q10 (ubiquinone) hypothesis, and the selenoprotein hypothesis.64) Especially, the endotoxin lipoprotein hypothesis is related to lower cholesterol levels. Whatever the mechanisms are, several studies have addressed the relation and relevance of serum cholesterol levels to outcome in HF patients, consistently suggesting that ‘too much’ low cholesterol is associated with increased mortality.65,66) Therefore, less-intensive statins with minimal diabetogenic effect might be best in DM-HF patients.
Another part is blood pressure. Although target goal of high blood pressure level has been discussed in guidelines of DM patients,67,68) high blood pressure is not often a problem in the clinical field of actual HF patients. Rather, the main concern is whether the HF key medication could be initiated or should be reduced because of low blood pressure. Indeed, KorAHF registry investigators reported that low systolic and diastolic blood pressures <130/70 mmHg at discharge and during follow-up was associated with worse survival in HF patients.69) However, the dilemma in the clinical field is that blood pressure in most HF patients is usually much lower than 130/70 mmHg. Therefore individual approach balancing between instillation and titration of HF key medications and inevitable blood pressure lowering. One good point is that KorAHF registry investigators published subsequent report showing that although J-curve association between blood pressure and mortality was observed regardless of age the nadir value was lower and instillation of HF medications consistently effective in octogenarians.70)
Role of revascularization
Aggravating factors of HF differed significantly based on its etiology.7) Although the aggravating factors were predominantly non-ischemic in origin in HF patients with non-ischemic causes, in HF patients with ischemic causes, both ischemic and non-ischemic factors were involved in HF aggravation. Because ischemia is the main etiology of DM-HF, consideration for revascularization is frequently encountered situation in the care of DM-HF. The recent the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial showed no benefit of an invasive strategy with systematic myocardial revascularization in patients with stable angina and moderate-to-severe ischemia compared with a conservative strategy.71) However, large ischemic burden in DM and limited myocardial reserve in HF cause clinicians to consider revascularization procedures in initial evaluation as well as acute care of decompensated DM-HF patients. The subgroup analyses of the ISCHEMIA trial suggested the individualized approach in DM-HF patients. First subset is HF patients. Although LVEF <35% and symptoms NYHA III or IV at study entry were exclusion criteria, 398 patients in ISCHEMIA had mild-to-moderate LV dysfunction (LVEF of 35–45%) or HF hstory.72) In these patients, the invasive strategy was suggested to be beneficial than the conservative strategy with marginal statistical significance. These findings are consistent with earlier studies demonstrating a benefit of myocardial revascularization in patients with HF. However, the subgroup analysis of DM patients by merging ISCHEMIA and ISCHEMIA-CKD showed no heterogeneity of treatment between the invasive and the conservative strategy during a median follow-up of 3.1 years.73) Moreover, time-dependent analysis showed an overall treatment effect that favored the conservative strategy during the first year (HR, 1.34; 95% CI, 1.09–1.62), when the clinical decision might be critical in DM-HF patients. Small subgroup analysis of DM patients with LV dysfunction did not show any benefit of the invasive strategy. Thus the overall results of the ISCHEMIA trial also apply to patients with DM-HF, which does not address the prognostic indication for myocardial revascularization in patients with reduced LV function (EF <35%). Another concern is multi-vessel, diffuse atherosclerotic nature of DM patients. A patient-level meta-analysis that revealed a difference in mortality of 5 percentage points favoring coronary artery bypass graft (CABG) over percutaneous coronary intervention (PCI) during follow-up for up to 5 years.74) However, recommending CABG to DM-HF patients is not an easy decision, especially when the patients are in decompensating condition. Therefore, even though the etiology and the aggravating factors of DM-HF patients are ischemia, the conservative medical treatment might be more beneficial than the invasive strategy, unless the stenotic lesions are favoring PCI, for example single lesions including left main disease, pre-diagnosed by noninvasive imaging tests.
SUMMARY AND CONCLUSION
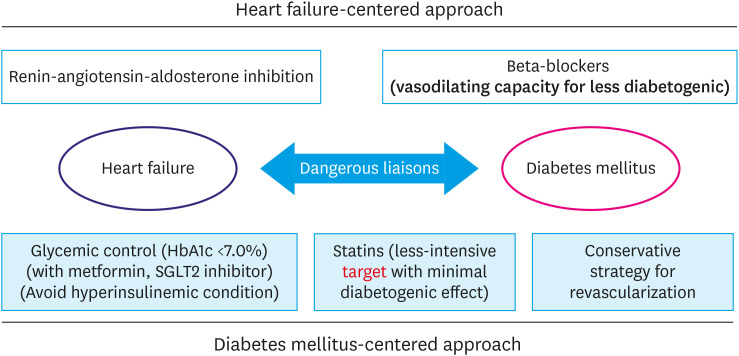
Among HF patients, near 40% have DM and patients with both HF and DM have a poorer outcome than those with HF alone. Although ischemia is the main etiology of DM-HF, the considerable portion of DM-HF patients do not have the evidence of coronary artery disease, namely diabetic cardiomyopathy. Recently, hyperinsulinemia rather than hyperglycemia is suggested as main pathogenic mechanism of DM-HF. Best hyperglycemic control strategy is tight glucose control with glucose-lowering agents without hyperinsulinemic effect. Four important components of the guideline-directed medical therapy including renin-angiotensin-aldosterone system inhibitors (angiotensin receptor-neprilysin inhibitor or angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker), beta-blockers, mineralocorticoid receptor antagonists, and SGLT-2 inhibitors are undoubtedly effective in DM-HF patients, especially in HFrEF. However, balanced management of combined cardiovascular risk factors is also important in DM-HF patients. Lastly, even though the etiology and the aggravating factors of DM-HF patients are ischemia, the conservative medical treatment might be more beneficial than the invasive strategy in coronary revascularization. The proposed optimal management for DM-HF patients is illustrated in Figure 2.
Figure 2. Optimal treatment strategy of heart failure patients combined with diabetes mellitus.
HbA1c = hemoglobin A1c; SGLT2 = sodium-glucose cotransporter 2.
Footnotes
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 7.Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aune D, Schlesinger S, Neuenschwander M, et al. Diabetes mellitus, blood glucose and the risk of heart failure: a systematic review and meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2018;28:1081–1091. doi: 10.1016/j.numecd.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Antakly-Hanon Y, Ben Hamou A, Garçon P, et al. Asymptomatic left ventricular dysfunction in patients with type 2 diabetes free of cardiovascular disease and its relationship with clinical characteristics: the DIACAR cohort study. Diabetes Obes Metab. 2021;23:434–443. doi: 10.1111/dom.14236. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Ha KH, Kim BY, Lee JH, Kim DJ. Trends in cardiovascular complications and mortality among patients with diabetes in South Korea. Diabetes Metab J. 2021;45:120–124. doi: 10.4093/dmj.2020.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols GA, Moler EJ. Cardiovascular disease, heart failure, chronic kidney disease and depression independently increase the risk of incident diabetes. Diabetologia. 2011;54:523–526. doi: 10.1007/s00125-010-1965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong MG, Jang SY, Jang J, et al. Impact of diabetes mellitus on mortality in patients with acute heart failure: a prospective cohort study. Cardiovasc Diabetol. 2020;19:49. doi: 10.1186/s12933-020-01026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart Coats AJ. Common co-morbidities in heart failure - diabetes, functional mitral regurgitation and sleep apnoea. Int J Heart Fail. 2019;1:25–41. doi: 10.36628/ijhf.2019.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcão-Pires I, Hamdani N, Borbély A, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1159. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu I, Minamino T, Toko H, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest. 2010;120:1506–1514. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkimattila S, Virkamäki A, Groop PH, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y, Vaziri ND, Coulson R, Kamanna VS, Roh DD. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab. 2000;279:E11–E17. doi: 10.1152/ajpendo.2000.279.1.E11. [DOI] [PubMed] [Google Scholar]
- 20.Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 21.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 24.Atkins GB, Jain MK. Role of Krüppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 25.Lee HY, Youn SW, Oh BH, Kim HS. Krüppel-like factor 2 suppression by high glucose as a possible mechanism of diabetic vasculopathy. Korean Circ J. 2012;42:239–245. doi: 10.4070/kcj.2012.42.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HY, Youn SW, Cho HJ, et al. FOXO1 impairs whereas statin protects endothelial function in diabetes through reciprocal regulation of Kruppel-like factor 2. Cardiovasc Res. 2013;97:143–152. doi: 10.1093/cvr/cvs283. [DOI] [PubMed] [Google Scholar]
- 27.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murtaza G, Virk HU, Khalid M, et al. Diabetic cardiomyopathy - a comprehensive updated review. Prog Cardiovasc Dis. 2019;62:315–326. doi: 10.1016/j.pcad.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Packer M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail. 2021;9:535–549. doi: 10.1016/j.jchf.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Konduracka E, Cieslik G, Galicka-Latala D, et al. Myocardial dysfunction and chronic heart failure in patients with long-lasting type 1 diabetes: a 7-year prospective cohort study. Acta Diabetol. 2013;50:597–606. doi: 10.1007/s00592-013-0455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packer M. Potentiation of insulin signaling contributes to heart failure in type 2 diabetes: a hypothesis supported by both mechanistic studies and clinical trials. JACC Basic Transl Sci. 2018;3:415–419. doi: 10.1016/j.jacbts.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skøtt P, Hother-Nielsen O, Bruun NE, et al. Effects of insulin on kidney function and sodium excretion in healthy subjects. Diabetologia. 1989;32:694–699. doi: 10.1007/BF00274259. [DOI] [PubMed] [Google Scholar]
- 33.Cosmi F, Cosmi D, Savino K, Ambrosio G. Insulin therapy may hasten congestive heart failure in cardiac patients: case series and review of the literature. G Ital Cardiol (Rome) 2008;9:509–512. [PubMed] [Google Scholar]
- 34.Laitinen T, Lyyra-Laitinen T, Huopio H, et al. Electrocardiographic alterations during hyperinsulinemic hypoglycemia in healthy subjects. Ann Noninvasive Electrocardiol. 2008;13:97–105. doi: 10.1111/j.1542-474X.2008.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zareini B, Rørth R, Holt A, et al. Heart failure and the prognostic impact and incidence of new-onset of diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2019;18:79. doi: 10.1186/s12933-019-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arcopinto M, Schiavo A, Salzano A, et al. Metabolic syndrome in heart failure: friend or foe? Heart Fail Clin. 2019;15:349–358. doi: 10.1016/j.hfc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Hayden MR, Tyagi SC. Myocardial redox stress and remodeling in metabolic syndrome, type 2 diabetes mellitus, and congestive heart failure. Med Sci Monit. 2003;9:SR35–SR52. [PubMed] [Google Scholar]
- 38.Palazzuoli A, Iacoviello M. Diabetes leading to heart failure and heart failure leading to diabetes: epidemiological and clinical evidence. Heart Fail Rev. 2022 doi: 10.1007/s10741-022-10238-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens. 2005;23:463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]
- 40.Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54–60. doi: 10.1016/j.steroids.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torp-Pedersen C, Metra M, Charlesworth A, et al. Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the Carvedilol Or Metoprolol European Trial (COMET) Heart. 2007;93:968–973. doi: 10.1136/hrt.2006.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 43.Demant MN, Gislason GH, Køber L, Vaag A, Torp-Pedersen C, Andersson C. Association of heart failure severity with risk of diabetes: a Danish nationwide cohort study. Diabetologia. 2014;57:1595–1600. doi: 10.1007/s00125-014-3259-z. [DOI] [PubMed] [Google Scholar]
- 44.Lawson CA, Jones PW, Teece L, et al. Association between type 2 diabetes and all-cause hospitalization and mortality in the UK general heart failure population: stratification by diabetic glycemic control and medication intensification. JACC Heart Fail. 2018;6:18–26. doi: 10.1016/j.jchf.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Jang SY, Jang J, Yang DH, et al. Impact of insulin therapy on the mortality of acute heart failure patients with diabetes mellitus. Cardiovasc Diabetol. 2021;20:180. doi: 10.1186/s12933-021-01370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 47.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 48.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 49.McMurray JJ, Krum H, Abraham WT, et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016;374:1521–1532. doi: 10.1056/NEJMoa1514859. [DOI] [PubMed] [Google Scholar]
- 50.Cosmi F, Shen L, Magnoli M, et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. 2018;20:888–895. doi: 10.1002/ejhf.1146. [DOI] [PubMed] [Google Scholar]
- 51.Dargie HJ, Hildebrandt PR, Riegger GA, et al. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J Am Coll Cardiol. 2007;49:1696–1704. doi: 10.1016/j.jacc.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 52.Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08) Diabetes Care. 2007;30:2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 53.Packer M. Is metformin beneficial for heart failure in patients with type 2 diabetes? Diabetes Res Clin Pract. 2018;136:168–170. doi: 10.1016/j.diabres.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Liang B, Gu N. Sodium-glucose co-transporter-2 inhibitors in the treatment of diabetes with heart failure. Cardiovasc Diabetol. 2022;21:84. doi: 10.1186/s12933-022-01526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 56.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham JW, Vaduganathan M, Claggett BL, et al. Dapagliflozin in patients recently hospitalized with heart failure and mildly reduced or preserved ejection fraction. J Am Coll Cardiol. 2022;80:1302–1310. doi: 10.1016/j.jacc.2022.07.021. [DOI] [PubMed] [Google Scholar]
- 59.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 60.Lee HY, Han KH, Chung WB, et al. Safety and efficacy of pitavastatin in patients with impaired fasting glucose and hyperlipidemia: a randomized, open-labeled, multicentered, phase IV study. Clin Ther. 2020;42:2036–2048. doi: 10.1016/j.clinthera.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Lee HY, Cho HJ, Kim HY, et al. Effects of intensive versus mild lipid lowering by statins in patients with ischemic congestive heart failure: Korean Pitavastatin Heart Failure (SAPHIRE) study. Korean J Intern Med. 2014;29:754–763. doi: 10.3904/kjim.2014.29.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez AC, Jhund P, Preiss D, Kjekshus J, McMurray JJ. Effect of rosuvastatin on fatigue in patients with heart failure. J Am Coll Cardiol. 2013;61:1121–1122. doi: 10.1016/j.jacc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 64.Kihara Y. Statin therapy in chronic heart failure: frog prince or bare frog? Circ J. 2013;77:895–897. doi: 10.1253/circj.cj-13-0314. [DOI] [PubMed] [Google Scholar]
- 65.Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–224. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 66.Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 67.Choi JH, Cho YJ, Kim HJ, et al. Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: consensus statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hypertension. Clin Hypertens. 2022;28:26. doi: 10.1186/s40885-022-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee HY, Lee JY, Shin HG, et al. The Korean Hypertension Cohort study: design and baseline characteristics. Korean J Intern Med. 2021;36:1115–1125. doi: 10.3904/kjim.2020.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee SE, Lee HY, Cho HJ, et al. Reverse J-curve relationship between on-treatment blood pressure and mortality in patients with heart failure. JACC Heart Fail. 2017;5:810–819. doi: 10.1016/j.jchf.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 70.Oh GC, Cho HJ, Lee SE, et al. Management and prognosis of heart failure in octogenarians: final report from the KorAHF registry. J Clin Med. 2020;9:501. doi: 10.3390/jcm9020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopes RD, Alexander KP, Stevens SR, et al. Initial invasive versus conservative management of stable ischemic heart disease in patients with a history of heart failure or left ventricular dysfunction: insights from the ISCHEMIA trial. Circulation. 2020;142:1725–1735. doi: 10.1161/CIRCULATIONAHA.120.050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newman JD, Anthopolos R, Mancini GB, et al. Outcomes of participants with diabetes in the ISCHEMIA trials. Circulation. 2021;144:1380–1395. doi: 10.1161/CIRCULATIONAHA.121.054439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391:939–948. doi: 10.1016/S0140-6736(18)30423-9. [DOI] [PubMed] [Google Scholar]