Abstract
Background and Objectives
Inhibitors of sodium–glucose cotransporter 2 (SGLT2i) reduce the risk of hospitalization for heart failure (HF). We aimed to examine the effect of empagliflozin on change of diuretics dose in outpatient HF patients.
Methods
We retrospectively reviewed the medical records of 612 patients who were treated using both empagliflozin and diuretics. We excluded patients who did not meet the criteria for HF. Dose and duration of empagliflozin and diuretics were measured.
Results
Of 612 patients, a total of 251 was analyzed and followed for a mean 430.0±175.4 days. The mean age was 69.3, 51.8% were female, and 93.2% had type 2 diabetes. The distribution of initial diuretics type when starting empagliflozin showed that furosemide comprised 24.7%, spironolactone 20.7%, thiazide 36.9%, and others. Total 23.1% of patients reduced diuretic dose, 13.1% increased diuretic dose, 41.4% continued at the same diuretic dose, and 22.3% switched to different diuretics. Among patients who were using furosemide, 36.0% reduced diuretics dose. There was a diuretic reduction in 22.6% of HF preserved ejection fraction (HFpEF, left ventricular ejection fraction [LVEF] ≥50%) and in 26.5% of HF reduced EF (HFrEF, LVEF <50%). The average doses furosemide at the start of empagliflozin decreased from 16.3mg/day to 8.5mg/day at the time of follow-up.
Conclusions
Among outpatient clinic HF patients treated with both diuretics and empagliflozin, 23.1% of patients had their diuretics reduced, and the mean dose of furosemide was reduced by about half. This suggests that empagliflozin has clinical advantages in managing outpatient HF patients.
Keywords: Sodium-glucose transporter 3 inhibitors, Diuretics, Outpatient, Heart failure
INTRODUCTION
Two major trials, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) and Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced), showed that inhibitors of sodium–glucose cotransporter 2 (SGLT2i) reduce the risk of hospitalization for heart failure (HF) regardless of the presence or absence of diabetes.1,2) According to a meta-analysis of both trials, SGLT2i was accompanied by a 26% relative reduction in the combined risk of cardiovascular (CV) death or first hospitalization for HF.3) Based on these trials, in the recently updated European Society of Cardiology (ESC) guidelines for HF, SGLT2i is recommended as class I, level of evidence A for the treatment of HF reduced ejection fraction (HFrEF).4) A recent study, the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR preserved trial), demonstrated that empagliflozin reduced the CV death or hospitalization for HF, even in patients with HF preserved ejection fraction (HFpEF), regardless of the presence or absence of diabetes.5) In the subgroup analysis of this study, a significant outcome improvement was demonstrated in patients with an left ventricular ejection fraction (LVEF) of 50–60%, which is a very encouraging result in treatment of HFpEF patients.
There are many potential mechanisms of SGLT2i that lead to a beneficial effect on the heart and kidneys, such as decreasing intraglomerular pressure, increasing natriuresis.6) In addition to intraglomerular hemodynamics, other mechanisms were suggested as improving metabolic parameters,7,8) and increasing oxygenation of tubular cells.9,10) Among them, the impact on an increase of natriuresis is a hemodynamically important factor,11) and it might play a similar role to that of diuretics in HF management. Pharmacologic inhibition of SGLT2 in the kidney reduces the capacity for renal glucose reabsorption.12) As SGLT2 reabsorbs sodium and glucose in a cotransport manner, SGLT2 inhibitors also cause natriuresis and are associated with an antihypertensive effect.13) Based on these mechanisms, in addition to CV outcome, SGLT2i is approved for lowering renal outcomes among patients with chronic kidney disease (CKD), regardless of the presence or absence of diabetes in the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial.14)
In the outpatient clinic setting with HF patients, intensification of HF therapy can also be an indicator of HF worsening in a broad sense. In particular, requiring intensification of diuretic therapy can be a representative indicator of worsening HF and has been demonstrated through a recent prespecified analysis of DAPA-HF study.15) There is a lack of studies that analyze the effect of SGLT2i on the dose change of diuretics in HF patients in an outpatient clinic setting. Therefore, the researchers analyzed the effect of empagliflozin on diuretic dose in outpatient HF patients.
METHODS
Study design and study population
This was a retrospective, observational single-center study. The researchers reviewed the medical records of 612 patients who were treated with empagliflozin (10 mg/day) for the first time and concomitant using diuretics between January 2019 and December 2021 at Kosin University Gospel Hospital. Patients who were not treated with empagliflozin for at least 6 months were excluded. Also excluded were patients who did not meet the criteria for HF, patients who had never undergone echocardiography (n=9), those who did not perform an N-terminal pro brain natriuretic peptide (NT-proBNP) test, or those with less than 125 pg/dL (n=347). Two cardiologists analyzed the echocardiography results and judged the presence or absence of structural/functional abnormalities in the heart based on the guideline.4) Five patients without definite structural/functional abnormalities were excluded. Changes in the type and dose of diuretics were investigated in those who used empagliflozin for more than 6 months. Two cardiologists retrospectively reviewed the data to evaluate whether the diuretic dose was clearly decreased or increased from initiation of empagliflozin. If the diuretic dose was decreased or stopped during the follow-up period, it was classified as a ‘reduced dose’ group, and if the diuretic dose was increased or another type of diuretic was added, it was classified as an ‘increased dose’ group. If there was no change in the type and dose of diuretics during the observation period, they were classified into the ‘same dose’ group and if the diuretic type was changed to different class of diuretics without definitive dose change, it was defined as ‘switched diuretics’ group. Demographic and comorbidity data were obtained from the medical records. Baseline characteristics of age, sex, presence of diabetes, hypertension, ischemic heart disease, CKD, stroke, and atrial fibrillation (AF) were assessed. The researchers also analyzed laboratory tests such as NT-proBNP, serum creatinine, estimated glomerular filtration rate (GFR), hemoglobin A1c, and echocardiography parameters. We also reviewed events of hospital admission or urgent emergency room visit due to acute decompensated HF. Ischemic heart disease was defined as myocardial infarction and angina that had significant coronary artery stenosis (luminal narrowing ≥70%) with or without coronary artery bypass graft or percutaneous coronary intervention.
This study was approved by the ethics committee of Kosin University Gospel Hospital, Busan, South Korea (No. 2021-10-013). The need for written informed consent was waived because of the retrospective nature of the study.
Echocardiography measurement
Standard 2-dimensional echocardiography was performed on all subjects while they lay in the left lateral decubitus position using a 3.5-MHz transducer (Vivid E9, GE Healthcare, Boston, MA, USA; and Philips iE33, Philips Medical Systems, Bothell, WA, USA). LVEF was measured using M-mode and Simpson’s method. Pulsed-wave doppler imaging of trans-mitral LV inflow was performed in the apical 4-chamber view, with the sample volume placed at the level of the mitral valve tips; doppler variables were analyzed during 3 consecutive beats. The following measurements of global LV diastolic function were also recorded: peak early (E) and late (A) diastolic mitral flow velocity. Tissue doppler imaging was used to assess mitral annulus velocities at septal and lateral mitral annuli, and their ratio of E/A and early (E’) diastolic mitral annular velocity were measured. Structural or functional abnormalities were evaluated as LVEF, LV mass index ≥95 g/m2 in females and ≥115 g/m2 in males, left atrial (LA) volume index >34 mL/m2 or LA dimension >40 mm, E/e’ ratio ≥13, and a mean e’ septal and lateral wall <9 cm/s.16,17)
Statistical analysis
Baseline clinical and laboratory characteristics and echocardiographic parameters of the study patients were collected and analyzed. Values are expressed as the mean (± standard deviation [SD]) for numerical variables or as the number of participants and the percentage for categorical variables. Continuous variables were expressed as the mean value with SD, as indicated. In comparison of parameters between HFrEF and HFpEF, continuous variables were compared using the Student’s t-test. The analysis of categorical data was performed using the χ2 test. Two-tailed p values less than 0.05 were considered to be statistically significant. Statistical analyses were performed using the IBM SPSS Statistics software Version 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
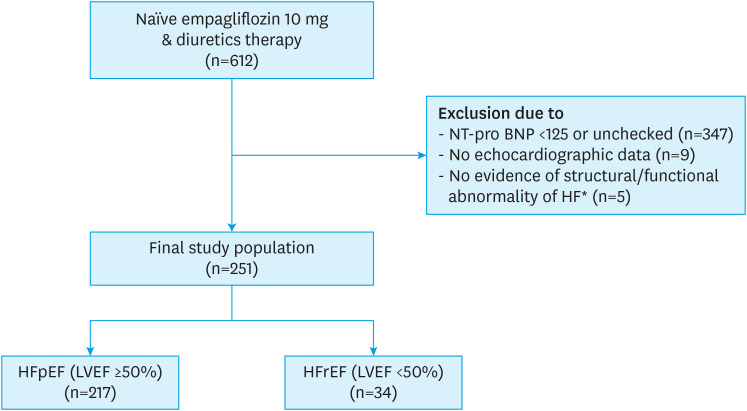
Of 612 patients, 361 were excluded, and 251 patients were analyzed (Figure 1). Mean follow-up duration was 430.0±175.4 days. Table 1 shows baseline characteristics. The mean age was 69.3 and 51.8% were female. Of the patients, 93.2% had diabetes, 80.5% had hypertension, 29.1% had ischemic heart disease, and 74.1% had AF. In laboratory tests, the mean NT-proBNP level was 1,451.3±2,795.1 pg/mL. Regarding concomitant medication, 63.7% were taking angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, 88.0% were taking a beta-blocker, and 27.5% were taking an angiotensin receptor neprilysin inhibitor (ARNI). Among the patients, 217 were categorized as HFpEF (LVEF ≥50%) and 34 as HFrEF (LVEF <50%). Patients with LVEF <50% showed higher NT-proBNP level (3,684.0±5,908.4 vs. 1,101.4±1,673.7 pg/mL, p=0.016) compared to HFpEF patients.
Figure 1. Flow diagram of the study population.
NT-proBNP = N-terminal pro brain natriuretic peptide; HF = heart failure; HFpEF = heart failure preserved ejection fraction; HFrEF = heart failure reduced ejection fraction; LVEF = left ventricular ejection fraction; LV = left ventricular; LA = left atrial.
*Structural/functional abnormality of HF: abnormal/normal LVEF with other abnormalities: LV mass index ≥95 g/m2 in females and ≥115 g/m2 in males, LA volume index >34 mL/m2 or LA dimension >40 mm, E/e’ ratio ≥13 and a mean e’ septal and lateral wall <9 cm/s.
Table 1. Baseline characteristics of the study population.
| Variables | Total (n=251) | HFpEF (n=217) | HFrEF (n=34) | p value | |
|---|---|---|---|---|---|
| Age (years) | 69.3 ± 11.0 | 70.0 ± 10.4 | 65.1 ± 13.7 | 0.056 | |
| Female sex | 130 (51.8) | 116 (53.5) | 14 (41.2) | 0.200 | |
| Height (cm) | 161.8 ± 9.1 | 161.6 ± 9.1 | 162.9 ± 9.4 | 0.431 | |
| Weight (kg) | 66.8 ± 12.5 | 66.8 ± 12.2 | 66.7 ± 14.4 | 0.962 | |
| Blood pressure (mmHg) | |||||
| Systolic | 116.7 ± 18.8 | 116.9 ± 18.5 | 115.4 ± 21.2 | 0.695 | |
| Diastolic | 66.3 ± 11.3 | 66.4 ± 10.8 | 66.0 ± 14.3 | 0.876 | |
| Heart rate (beats/minute) | 72.4 ± 13.4 | 71.5 ± 13.0 | 77.9 ± 14.3 | 0.017 | |
| Hypertension | 202 (80.5) | 178 (82.0) | 24 (70.6) | 0.160 | |
| Diabetes mellitus | 234 (93.2) | 200 (92.2) | 34 (100) | 0.139 | |
| Coronary artery disease | 73 (29.1) | 60 (27.6) | 13 (38.2) | 0.226 | |
| Previous CVA | 9 (3.6) | 8 (3.7) | 1 (2.9) | 0.828 | |
| CKD | 36 (14.3) | 27 (12.4) | 9 (26.5) | 0.061 | |
| Atrial fibrillation | 186 (74.1) | 168 (77.4) | 18 (52.9) | 0.005 | |
| Laboratory measurements | |||||
| HbA1c (%A1c) | 6.1 ± 0.9 | 6.1 ± 0.9 | 6.0 ± 0.5 | 0.463 | |
| Serum Cr (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.9 | 0.9 ± 0.3 | 0.660 | |
| eGFR (mL/min/1.73 m2) | 77.1 ± 25.0 | 76.4 ± 24.9 | 81.4 ± 25.8 | 0.345 | |
| Serum K+ (mg/dL) | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.4 ± 0.4 | 0.097 | |
| NT-proBNP (pg/mL) | 1,451.3 ± 2,795.1 | 1,101.4 ± 1,673.7 | 3,684.0 ± 5,908.4 | 0.016 | |
| Medications | |||||
| Beta-blocker | 221 (88.0) | 191 (88.0) | 30 (88.2) | 0.971 | |
| ACEi/ARB | 160 (63.7) | 151 (69.8) | 9 (26.5) | <0.001 | |
| Sacubitril/Valsartan | 69 (27.5) | 45 (20.7) | 24 (70.6) | <0.001 | |
| CCB | 143 (57.0) | 133 (61.3) | 10 (29.4) | 0.001 | |
| Statin | 243 (96.8) | 211 (97.2) | 32 (94.1) | 0.296 | |
| Diuretics | |||||
| Furosemide alone | 62 (24.7) | 49 (22.6) | 13 (38.2) | ||
| Spironolactone alone | 52 (20.7) | 48 (22.1) | 4 (11.8) | ||
| Hydrochlorothiazide alone | 90 (35.9) | 80 (36.9) | 10 (29.4) | ||
| Combination of furosemide and spironolactone | 24 (9.6) | 22 (10.1) | 2 (5.9) | ||
| Combination of spironolactone and hydrochlorothiazide | 11 (4.4) | 10 (4.6) | 1 (2.9) | ||
| Others* | 13 (5.2) | 9 (4.2) | 4 (11.8) | ||
Continuous variables were expressed as the mean ± standard deviation, and categorical variables were expressed as number (%).
HFpEF = heart failure preserved ejection fraction; HFrEF = heart failure reduced ejection fraction; CVA = cerebrovascular attack; CKD = chronic kidney disease; HbA1c = hemoglobin A1c; Cr = creatinine; eGFR = estimated glomerular filtration rate; K+ = potassium; NT-proBNP = N-terminal pro brain natriuretic peptide; ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; CCB = calcium channel blocker.
*Acetazolamide alone, torsemide alone, or combination containing one of 2 drugs.
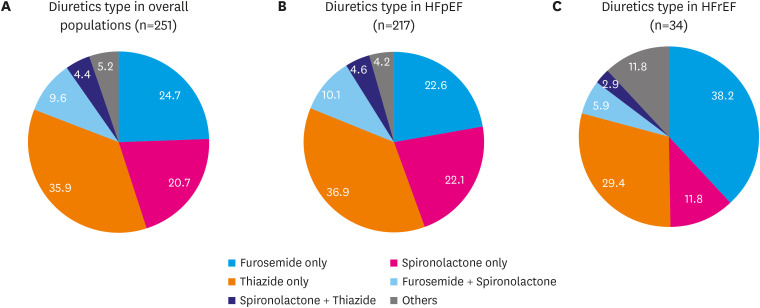
Figure 2 shows the distribution of initial using diuretic type in use when starting empagliflozin. Furosemide alone comprised 24.7%, spironolactone alone 20.7%, and thiazide alone 35.9%. Twenty-four patients (9.6%) were taking a combination of furosemide and spironolactone and 11 patients were taking of spironolactone and thiazide combination. If none of these combinations, it classified as others. When classified according to LVEF, patients with HFrEF showed a higher distribution of furosemide alone use (38.2%) than patients with HFpEF (22.6%).
Figure 2. Type of diuretics when empagliflozin was started: (A) in overall population; (B) in HFpEF; (C) in HFrEF.
HFpEF = heart failure preserved ejection fraction; HFrEF = heart failure reduced ejection fraction.
In echocardiographic parameters (Table 2), the mean LVEF was 58.9% and the mean LV end diastolic dimension was 49.5 mm; the LA dimension by M-mode was 43.9 mm and E/e’ was 16.9. Patients with LVEF <50% showed significant lower mean LVEF (34.4±8.6 vs. 62.7±5.3%, p<0.001) compared to HFpEF patients. When patients were classified according to LV geometry, only 20.7% had normal LV geometry, and the others had concentric remodeling (26.3%) or LV hypertrophy (17.9% were concentric hypertrophy and 35.1% were eccentric hypertrophy).
Table 2. Echocardiographic parameters.
| Echocardiographic measurements | Total (n=251) | Preserved LVEF (n=217) | Reduced LVEF (n=34) | p value | |
|---|---|---|---|---|---|
| LVEF (%) | 58.9 ± 11.3 | 62.7 ± 5.3 | 34.4 ± 8.6 | <0.001 | |
| LVEDD (mm) | 49.5 ± 7.8 | 47.9 ± 5.9 | 59.9 ± 10.1 | <0.001 | |
| IVSd (mm) | 11.0 ± 2.3 | 11.1 ± 2.4 | 10.1 ± 1.2 | <0.001 | |
| PWd (mm) | 9.9 ± 1.5 | 9.9 ± 1.5 | 9.7 ± 1.5 | 0.546 | |
| LAd (mm) | 43.9 ± 7.9 | 43.6 ± 8.1 | 45.8 ± 6.6 | 0.126 | |
| LVMI (g/m2) | 111.7 ± 32.7 | 106.8 ± 28.1 | 142.9 ± 42.2 | <0.001 | |
| Mild abnormality | 51 (20.3) | 46 (21.2) | 5 (14.7) | ||
| Moderate abnormality | 32 (12.7) | 28 (12.9) | 4 (11.8) | ||
| Severe abnormality | 50 (19.9) | 32 (14.7) | 18 (52.9) | ||
| E (m/s) | 0.9 ± 0.8 | 0.9 ± 0.9 | 0.7 ± 0.3 | 0.161 | |
| E/e’ | 16.9 ± 13.3 | 16.9 ± 13.8 | 16.8 ± 9.0 | 0.982 | |
| MR ≥ grade 2 | 53 (21.1) | 39 (18.0) | 14 (41.2) | 0.002 | |
| TR ≥ grade 2 | 50 (19.9) | 44 (20.3) | 6 (17.6) | 0.721 | |
| Global longitudinal strain (%) | −15.5 ± 4.3 | 16.5 ± 3.6 | 10.0 ± 3.6 | <0.001 | |
| LV geometry | |||||
| Normal LV geometry | 52 (20.7) | 46 (21.2) | 6 (17.6) | ||
| Concentric remodeling | 66 (26.3) | 65 (30.0) | 1 (2.9) | ||
| Concentric hypertrophy | 45 (17.9) | 44 (20.3) | 1 (2.9) | ||
| Eccentric hypertrophy | 88 (35.1) | 62 (28.6) | 26 (76.5) | ||
Continuous variables were expressed as the mean ± standard deviation, and categorical variables were expressed as number (%).
LVEF = left ventricular ejection fraction; LVEDD = left ventricular end-diastolic diameter; IVSd = diastolic interventricular septal wall dimension; PWTd = diastolic posterior wall dimension; LAd = left atrial dimension; LVMI = left ventricular mass index; E = peak early diastolic mitral filling velocity; e’ = early diastolic mitral annular velocity; MR = mitral regurgitation; T =, tricuspid regurgitation; LV = left ventricular.
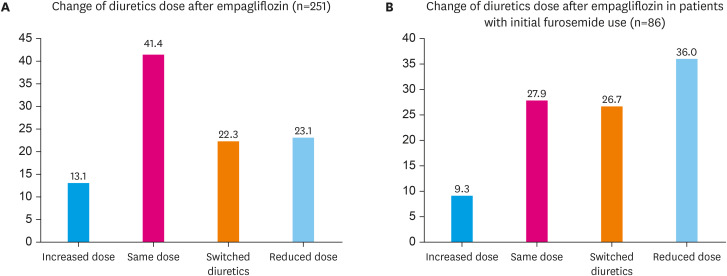
Figure 3 shows the changes in diuretic dose after empagliflozin prescription. During the study observation period, 23.1% of patients reduced diuretic dose, 13.1% increased diuretic dose, 41.4% continued at the same diuretic dose, and 22.3% switched to different diuretics (Figure 3A). Details of the switched diuretics group are presented in the Supplementary Table 1. Among them, when analyzing the patients who were using furosemide (N=86), 36.0% of patients reduced diuretics dose, and 9.3% increased diuretics dose (Figure 3B). Among the reduced group, mean duration of diuretics reduction was 10.9 months.
Figure 3. Changes of diuretics dose after empagliflozin treatment in overall patients. (A) Changes of diuretics dose in overall population. (B) Changes of diuretics dose in patients using furosemide.
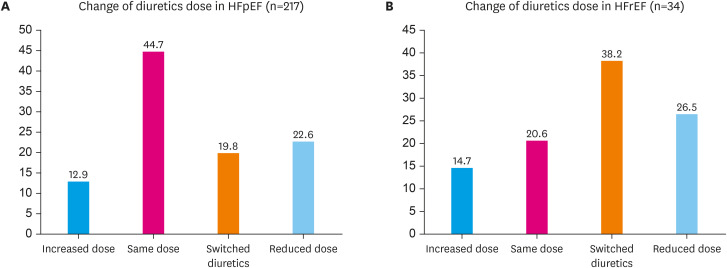
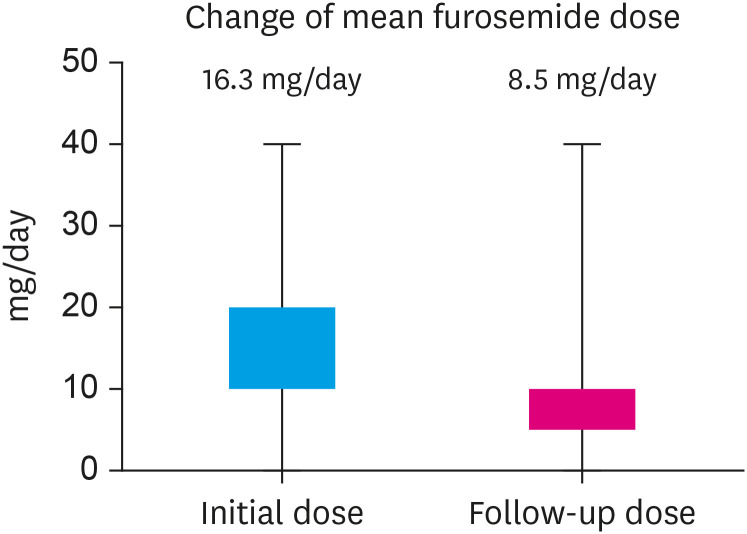
When we analyzed according the LVEF group, there was a diuretic reduction in 22.6% of patients with HFpEF and 26.5% of patients with HFrEF (Figure 4). Comparing the average values of furosemide doses of all patients who were using furosemide at the start of empagliflozin and at the time of follow-up showed a decrease from 16.3 mg/day to 8.5 mg/day (Figure 5).
Figure 4. Changes of diuretics dose after empagliflozin treatment according to left ventricular ejection fraction. (A) Changes of diuretics dose in HFpEF. (B) Changes of diuretics dose in HFrEF.
HFpEF = heart failure preserved ejection fraction; HFrEF = heart failure reduced ejection fraction.
Figure 5. Change of mean furosemide dose after empagliflozin treatment.
DISCUSSION
The baseline characteristics of patients enrolled in this study showed that most had diabetes, and there was a high prevalence of hypertension and AF. Although the mean LVEF was relatively high and the majority (86.5%) was classified as HFpEF, most have diastolic dysfunction or LV hypertrophy/LA enlargement. Specifically, about 80% of patients have LV hypertrophy, and this seems to be associated with a higher prevalence of diabetes and hypertension. The mean LA diameter was 44.0 mm, and this is thought to be due to the very high prevalence of AF patients. In addition, considering that most patients have taken an RAS inhibitor/ARNI and beta-blockers, it seems to try to the optimal medical treatment of HF. In this population, we showed that about 23.1% of the patients taking empagliflozin reduced their diuretics and the mean dose of furosemide was reduced by about half.
As various classes of medications must be used in management of HF patients, reduction of diuretics has important clinical implications, especially in combination with other medications. Treatment with RAS blockers or ARNI is frequently accompanied by hypotension as well as renal dysfunction.18) Mineralocorticoid receptor antagonists (MRAs) also have the risk of reducing GFR. Therefore, use of these drugs is limited, or there is reluctance to up-titration when combined with other diuretics. In this respect, reducing the diuretic dose is significant in that it not only reduces the side effects, but also provides an opportunity to more actively use other medications. When SGLT2i and MRA are used together, they have a diuretic effect, so there is a concern about safety such as renal dysfunction. However, recent analysis of “DAPA-HF” showed that dapagliflozin was similarly efficacious and safe in patients with HFrEF taking or not taking an MRA, supporting the use of the drugs together.19) Although there is concern that SGLT2i initially reduces GFR, it has been demonstrated that the decrease in GFR is less than that of the placebo group in the long-term period.14)
According to a recent review about the efficacy and safety of diuretics in HFpEF, SGLT2i was also included as a kind of diuretic and showed a relatively low rate of hypovolemic events in terms of safety outcomes.20) It has been demonstrated in a well-designed previous prospective study that empagliflozin can reduce the dose of furosemide in small number of HF patients.21) Compared to that study, our study, although of a retrospective design, is meaningful in that it analyzed a longer period of time targeting outpatient HF patients with significant structural or functional abnormality suitable for HF through echocardiography. Furosemide as well as other diuretics were analyzed, and the analysis was performed according to EF.
Recent updated 2021 ESC guidelines4) suggest initial simultaneous combination of evidence-based medications; however, there is no definitive conclusion as to which drug should be used first. Interestingly, beta-blockers have an absolute advantage in HF, but there is a concern about fluid retention in the condition’s early stages. The volume reduction advantage of SGLT2i can compensate for this concern of beta-blockers. A recent expert’s review proposed an accelerated 3-step approach, which consists of simultaneous initiation of a beta-blocker and an SGLT2i, followed one to 2 weeks later by initiation of ARNI and one to 2 weeks later by an MRA in HFrEF patients.22) Our study result that empagliflozin can reduce diuretic dose supports the recent suggestion for initial combination of beta-blockers and SGLT2i in management of HF.
Another aspect of this study was that HFpEF patients in the outpatient clinic were the main subjects. According to the subgroup analysis of the ‘Emperor Preserved trial,’ empagliflozin’s effect did not show statistical significance in patients with LVEF of 60% or more.5) This finding indicates that patients with HF mild reduced EF (LVEF 40–50%) would have contributed to improving the entire primary CV outcome. Thus, it is limiting to interpret empagliflozin’s effect as proven in patients with more than 60% of EF as completely normal. However, in our study, among HFpEF patients (mean LVEF 62.7%), the diuretic was reduced in about 22.6%, which seems to have clinical significance. In addition, among patients who were classified as switched to different diuretics, some were considered to have undergone equivalent diuretic reduction as well as a change in diuretic type. It is possible that the diuretic reduction effect of empagliflozin can be predicted to be higher than the suggested result value.
This study had several limitations. The most important is that there is no control group for empagliflozin. In addition, we did not include patients’ symptoms and physical signs in the enrollment process. The study also involved a single center, and it had a retrospective design. In addition, due to the small number of HFrEF patients, individual preference for diuretics may have influenced the results. Finally, the duration of medication was not constant among all patients. However, to the best of the researchers’ knowledge, this is the first study to describe the effect of empagliflozin on diuretic dose in outpatient HF patients in Korea.
In conclusion, among outpatient clinic HF patients treated with both diuretics and empagliflozin, 23.1% had their diuretics reduced, and the mean dose of furosemide was reduced by about half. This suggests that empagliflozin has clinical advantages in managing outpatient HF patients. A large-scale prospective study is needed in the future.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim BJ.
- Data collection: Kim SJ, Kim BJ.
- Data analysis: Kim SJ.
- Supervision: Im SI, Kim HS, Heo JH.
- Writing - original draft: Kim SJ.
- Writing - review & editing: Kim BJ.
SUPPLEMENTARY MATERIAL
Details of switched diuretics group
References
- 1.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 3.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 4.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 6.Wanner C. EMPA-REG OUTCOME: the nephrologist’s point of view. Am J Cardiol. 2017;120:S59–S67. doi: 10.1016/j.amjcard.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res. 2018;122:1439–1459. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZ. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler J, Hamo CE, Filippatos G, et al. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19:1390–1400. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 10.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–847. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heerspink HJ, Kosiborod M, Inzucchi SE, Cherney DZ. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39. doi: 10.1016/j.kint.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62:3324–3328. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–225. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 15.Docherty KF, Jhund PS, Anand I, et al. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA-HF. Circulation. 2020;142:1623–1632. doi: 10.1161/CIRCULATIONAHA.120.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galderisi M, Cosyns B, Edvardsen T, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Packer M, Kessler PD, Gottlieb SS. Adverse effects of converting-enzyme inhibition in patients with severe congestive heart failure: pathophysiology and management. Postgrad Med J. 1986;62(Suppl 1):179–182. [PubMed] [Google Scholar]
- 19.Shen L, Kristensen SL, Bengtsson O, et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. JACC Heart Fail. 2021;9:254–264. doi: 10.1016/j.jchf.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Agarwal A, Wafford QE, Shah SJ, Huffman M, Khan S. Efficacy and safety of diuretics in heart failure with preserved ejection fraction: a scoping review. Heart. 2022;108:593–605. doi: 10.1136/heartjnl-2021-319643. [DOI] [PubMed] [Google Scholar]
- 21.Shirakabe A, Matsushita M, Kiuchi K, Okazaki H, Inami T, Takayasu T, et al. Empagliflozin administration can decrease the dose of loop diuretics and prevent the exacerbation of renal tubular injury in patients with compensated heart failure complicated by diabetes. Circ Rep. 2020;2:565–575. doi: 10.1253/circrep.CR-20-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Packer M, McMurray JJ. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23:882–894. doi: 10.1002/ejhf.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of switched diuretics group