Abstract
Introduction
Frailty is an important predictor of outcomes after noncardiac surgery. The 5-factor Modified Frailty Index (mFI-5) is a recently developed frailty metric that has not been adequately evaluated in relation to surgical therapy for lung cancer. We evaluated whether the mFI-5 is predictive of clinical and administrative outcomes after anatomical lung resection for cancer.
Methods
Data in the Society of Thoracic Surgeons Database were used to evaluate the relationship of mFI-5 to outcomes of patients undergoing elective anatomical lung resection for cancer from 2015 to 2018 using logistic regression analyses. Results were compared with validated risk predictors, including the American Society of Anesthesiologists Physical Status Classification and the Charlson Comorbidity Index.
Results
The mFI-5 score could be calculated for 36,587 patients. On univariate analyses, mFI-5 was significantly associated with all clinical and administrative outcomes in an incremental pattern (p < 0.0001 for each). On multivariate analyses, mFI-5 was significantly associated in an incremental pattern with 13 of 15 postoperative complication and administrative outcome categories; the exceptions were cardiovascular complications and 30-day mortality. The overall performance of the frailty metric mFI-5 was similar to that of the American Society of Anesthesiologists and the Charlson Comorbidity Index.
Conclusions
The mFI-5 is independently predictive of almost all outcomes after lung resection for cancer. It can be calculated from data typically collected for thoracic surgical patients. Assessment of surgical candidates using mFI-5 may be useful in risk prediction and may identify patients who would benefit from mitigation of increased surgical risk related to frailty.
Keywords: Frailty, Lung resection, Lung cancer surgery, Surgical complications, Surgical risk
Introduction
Surgeons are increasingly presented with the task of evaluating operative candidacy for older vulnerable patients. With an aging population,1 preoperative assessment is important in improving risk stratification for informed decision making, helping to reduce postoperative morbidity and mortality. Surgical risk assessment tools such as the Charlson Comorbidity Index (CCI) and the American Society of Anesthesiologists (ASA) Physical Status Classification are most often used to aid in surgical decision making for patients who are candidates for anatomical resection for NSCLC.2, 3, 4, 5 In addition, a variety of screening methods recently have been used to evaluate for frailty, the presence of which correlates strongly with both mortality and morbidity for a broad spectrum of operations.6, 7, 8, 9, 10 Instruments used to screen for frailty include the FRAIL score,11 Fried’s Frailty Phenotype scale,12 the Clinical Frail Scale,13 and the Short Physical Performance Battery.14,15
Among the important frailty screening tools used for potential surgical patients is the Modified Frailty Index (mFI), which was developed as a scale of 11 variables from the American College of Surgeons National Surgical Quality Improvement Project that were mapped against variables in the Canadian Study of Health and Aging Frailty Index.16,17 Tsiouris et al.18 validated the predictive value of mFI in patients who had undergone lung cancer resection with open lobectomy. The mFI was later condensed to five factors (5-factor mFI [mFI-5]), which has predictive abilities for outcomes similar to those of the mFI across multiple surgical specialties.19 Nevertheless, no study has focused on the predictive value of mFI-5 for postoperative outcomes in patients with lung cancer resection.
In this study, we mapped the mFI-5 variables to variables captured in the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database to enable assignment of frailty scores in patients undergoing anatomical lung resection for cancer. We evaluated the relationship of mFI-5 to acute postoperative outcomes and compared its performance with that of CCI and ASA.
Materials and Methods
Data
The use of a limited data set from the STS General Thoracic Surgery Database was approved by The University of Chicago Institutional Review Board (protocol #IRB20-1709; November 11, 2020), and the need for the consent process and consent documentation was waived. We queried the STS database (version 2.3) for all patients with a diagnosis of lung cancer undergoing elective anatomical lung resection from January 1, 2015, to December 31, 2018. Data were collected for demographic, physiological, operative, and outcome variables. Comorbidities and ASA status were also collected. Patients were excluded if they underwent sleeve (carinal) pneumonectomy, extrapleural pneumonectomy, resection of an apical lung tumor including chest wall resection, completion pneumonectomy, chest wall reconstruction with muscle flap, or lung volume reduction surgery, or if they had a history of previous cardiothoracic surgery. Patients were excluded if there were any missing data necessary for calculation of mFI-5.
Complications
Complications were categorized as pulmonary, cardiovascular, infectious, neurologic, gastrointestinal, urinary, surgical, and in-hospital mortality (Supplementary Table 1). Composite outcomes included major postoperative complications and any postoperative event (Supplementary Table 2). Perioperative administrative outcomes that were abstracted included length of postoperative hospital stay, duration of operation, total intensive care unit days, 30-day mortality, unexpected admission to the intensive care unit, readmission within 30 days of discharge, and discharge location other than home.
Metrics
The mFI-5 is a cumulative deficit scale in which the presence of any of five factors is summed to yield a score from 0 to 5. It was evaluated by mapping seven variables in the STS database to the following five factors: hypertension, congestive heart failure, respiratory problems, changes in everyday activity, and diabetes (Table 1). We used a complete case analysis approach to evaluate preoperative characteristics and postoperative outcomes and then performed a sensitivity analysis by including “missing” as a value for mFI-5 where appropriate. The modified CCI was calculated as previously described, expanding the inclusion criteria for cardiac disease to include all forms of coronary artery disease.20 ASA status was abstracted from the STS data, which used a standard definition.21
Table 1.
STS Database Variables Mapped to the mFI-5
| mFI-5 Component | Matched Variable(s) in STS Database Version 2.3 |
|---|---|
| Arterial hypertension | Hypertension |
| Congestive heart failure | Congestive heart failure |
| Respiratory problems | Chronic obstructive pulmonary disease or FEV1 predicted < 70% or Category of disease - secondary (pneumonia) |
| Changes in everyday activity | Zubrod score (scores 2–5: with symptoms, not fully ambulatory; bedridden; moribund) |
| History of diabetes mellitus | Diabetes |
FEV1, forced expiratory volume during the first second; mFI-5, 5-factor Modified Frailty Index; STS, Society of Thoracic Surgery.
Statistical Techniques
Preoperative patient characteristics were compared among different mFI-5, CCI, and ASA categories using analysis of variance. Univariate analyses of outcomes compared patients in different mFI-5, CCI, and ASA categories using analysis of variance for continuous variables and the chi-square test for categorical variables. We used logistic regression models to evaluate all postoperative outcomes performing both multivariate analyses. Covariates used in the multivariable model for mFI-5 included extent of resection (pneumonectomy or bilobectomy), renal dysfunction (on dialysis or creatinine >2 mL/dL before operation), induction therapy (preoperative chemotherapy for the current thoracic malignancy and previous radiation therapy to the chest for any reason), steroid use, sex, body mass index (BMI) category (underweight [<18.5 kg/m2], normal [18.5–24.9 kg/m2], overweight [25–29.9 kg/m2], obese I [30–34.9 kg/m2], obese II [35–39.9 kg/m2], or obese III [≥40 kg/m2]), coronary artery disease, cerebrovascular disease, smoking status, age (10-y increments), forced expiratory volume in the first second expressed as a percent of predicted (FEV1%; 10-point increments), and diffusing capacity of the lung for carbon monoxide expressed as a percentage of predicted and diffusing capacity of the lung for carbon monoxide (DLCO) expressed as a percentage of predicted (DLCO%; 10-point increments).22 Similar modeling was performed for CCI and ASA.
Performance of mFI-5, CCI, and ASA as risk metrics was also analyzed by evaluating the marginal probability of events within individual outcome categories related to each level of each risk metric. A progressive increase in the marginal probability as risk scores within a metric increased was interpreted as a desirable characteristic. Monotonicity was classified as strictly monotonic (progression between all ORs), partially monotonic (lack of progression between any two consecutive ORs), or nonmonotonic. Receiver operating characteristic (ROC) analysis was performed for each metric with results for each outcome reported as the area under the curve (AUC). All statistical analyses were performed using R software version 3.3.0.
Results
Patients
A total of 55,261 patients were identified in the STS General Thoracic Surgery Database according to the inclusion criteria. After applying the exclusion criteria, 36,587 patients were identified who had all the factors necessary for calculation of mFI-5; all these patients had information available for CCI, and all but 10 patients had ASA status recorded (Supplementary Fig. 1). Patients who lacked the necessary data to calculate mFI-5 were most similar demographically and clinically to patients with an mFI-5 score of 1 or 2, particularly with regard to age, sex, ASA status, smoking status, coronary artery disease, renal failure, incidence of important weight loss, and BMI. Notable exceptions were pulmonary conditions, including interstitial fibrosis and shortness of breath, which were more similar in frequency to patients with an mFI-5 score of 3 (Supplementary Table 3). We compared the excluded cohort with the included cohort and found no evidence of important clinical differences other than those who had mFI-5 calculated had a different distribution of T stages compared with those who did not (Supplementary Table 4).
Most comorbidities increased in frequency as mFI-5 scores increased, partly as a function of their role in contributing to those scores (Supplementary Table 5). BMI had an inconsistent relationship with mFI-5: underweight status was fairly evenly distributed across scores, with the lowest frequency among the higher mFI-5 scores, whereas overweight and obese categories had a progressive increase in frequency as mFI-5 increased. There was a downward trend in the likelihood of having received preoperative chemotherapy or radiation therapy as mFI-5 increased, which seemed unrelated to differences in T and N status. The distribution of mFI-5 among representative demographic, clinical, and cancer variables is depicted in Supplementary Figure 2.
The distribution of scores for the three metrics is displayed in Table 2. The median score for ASA and CCI was 3, whereas it was 1 for mFI-5. The mFI-5 and CCI scores were reasonably well distributed among four score categories, with sparse population of the two remaining categories for mFI-5 and among the six remaining categories for CCI that were affected in this study. Of note, CCI scores were always 2 or greater because all patients had a diagnosis of lung cancer. ASA was well distributed among three categories, with the remaining three categories being sparsely populated.
Table 2.
Distribution of Scores for the Different Risk Assessment Metrics Among 36,587 Patients
| Category | Metric |
||
|---|---|---|---|
| ASA | CCI | mFI-5 | |
| Median | 3 | 3 | 1 |
| Score | Numbers of patients | ||
|---|---|---|---|
| 0 | n/a | 0 | 7835 |
| 1 | 85 | 0 | 14,069 |
| 2 | 5946 | 14,985 | 11,045 |
| 3 | 28,037 | 13,155 | 3316 |
| 4 | 2488 | 5642 | 306 |
| 5 | 14 | 2075 | 16 |
| 6 | 7 | 560 | n/a |
| 7 | n/a | 145 | n/a |
| 8–12 | n/a | 25 | n/a |
ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; mFI-5, 5-factor Modified Frailty Index; n/a, not applicable.
Univariate Analyses
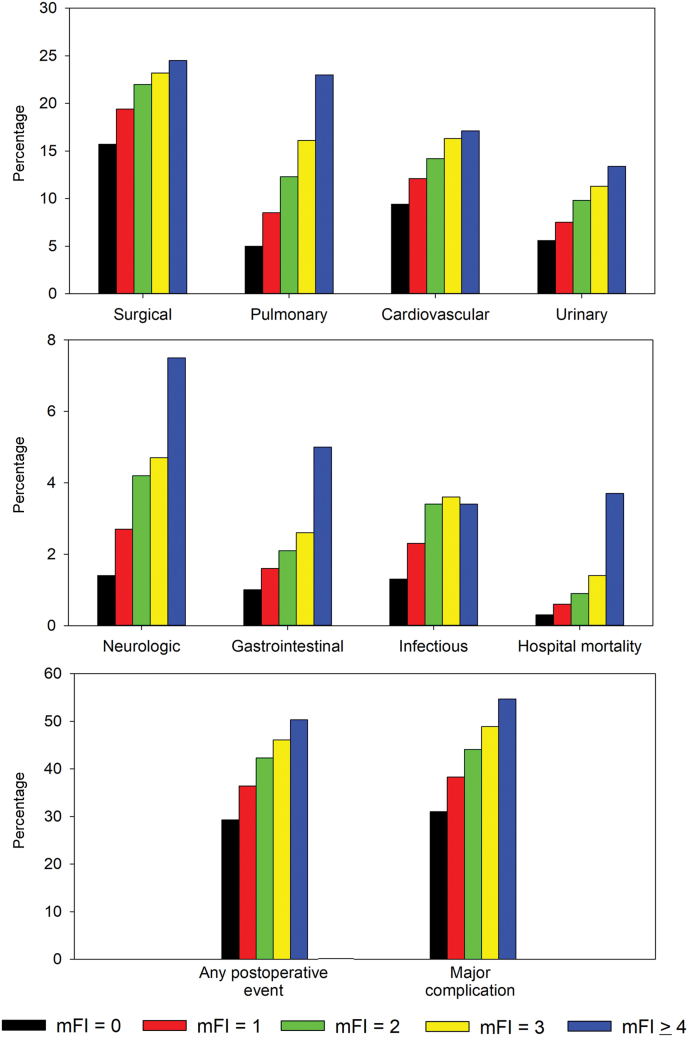
On univariate analysis, mFI-5 (categorized as 0, 1, 2, 3, ≥4) was significantly associated with all postoperative complication categories and composite measures in an incremental pattern (p < 0.0001 for each). Similarly, mFI-5 was significantly associated with all perioperative administrative outcomes in an incremental pattern (p < 0.0001 for each; Fig. 1).
Figure 1.
Incidence of surgical complications and administrative outcomes related to mFI-5 category. mFI, Modified Frailty Index; mFI-5, 5-factor Modified Frailty Index.
Multivariate Analyses
On multivariate analysis, mFI-5 (categorized as 0, 1, 2, ≥3) was significantly associated with almost all postoperative complication categories and administrative outcomes (Table 3) except for cardiovascular complications and 30-day mortality. Sensitivity analyses of multivariate outcomes, including “missing” as a value for mFI-5 where appropriate, revealed no important changes in the findings reported using the complete case analysis approach, although ORs and p values did change somewhat (Supplementary Table 6). Multivariate analyses for ASA revealed a significant association with all postoperative and administrative outcomes (Supplementary Table 7). CCI was significantly associated with all postoperative and administrative outcomes except for unanticipated surgical approach conversion (Supplementary Table 8).
Table 3.
Multivariate Analysis for Postoperative Outcomes
| Outcomes | OR Relative to mFI = 0 |
|||||
|---|---|---|---|---|---|---|
| mFI = 1 | p Value | mFI = 2 | p Value | mFI ≥ 3 | p Value | |
| Postoperative complications | ||||||
| Pulmonary (n = 32,109) | 1.17 (1.03–1.33) | 0.0194 | 1.41 (1.23–1.61) | <0.0001 | 1.69 (1.44–1.98) | <0.0001 |
| Cardiovascular (n = 32,109) | 1.00 (0.90–1.10) | 0.9293 | 1.05 (0.94–1.17) | 0.3668 | 1.12 (0.98–1.29) | 0.1049 |
| Infectious (n = 32,109) | 1.30 (1.03–1.66) | 0.0311 | 1.78 (1.39–2.29) | <0.0001 | 1.66 (1.22–2.25) | 0.0012 |
| Neurologic (n = 32,109) | 1.38 (1.10–1.75) | 0.0068 | 1.79 (1.41–2.29) | <0.0001 | 1.93 (1.46–2.57) | <0.0001 |
| Gastrointestinal (n = 32,109) | 1.24 (0.94–1.66) | 0.1313 | 1.51 (1.13–2.05) | 0.0065 | 1.87 (1.31–2.67) | 0.0006 |
| Urinary (n = 32,109) | 1.05 (0.93–1.20) | 0.4247 | 1.30 (1.14–1.49) | 0.0001 | 1.47 (1.24–1.74) | <0.0001 |
| Surgical (n = 32,109) | 1.07 (0.99–1.17) | 0.1062 | 1.15 (1.05–1.26) | 0.0023 | 1.23 (1.09–1.39) | 0.0007 |
| Inhospital mortality (n = 32,109) | 1.13 (0.69–1.91) | 0.6401 | 1.62 (0.99–2.76) | 0.0615 | 2.27 (1.31–4.06) | 0.0042 |
| Perioperative administrative outcomes | ||||||
| 30-d mortality (n = 32,016) | 0.94 (0.65–1.38) | 0.7308 | 1.23 (0.84–1.82) | 0.2985 | 1.53 (0.99–2.40) | 0.0567 |
| Unexpected ICU admission (n = 12,102) | 1.27 (1.00–1.63) | 0.0525 | 1.61 (1.26–2.08) | 0.0002 | 1.99 (1.50–2.67) | <0.0001 |
| Readmission within 30 d (n = 30,172) | 1.05 (0.92–1.19) | 0.4765 | 1.16 (1.01–1.33) | 0.0325 | 1.43 (1.21–1.70) | <0.0001 |
| Unanticipated surgical approach conversiona (n = 31,488) | 1.12 (0.99–1.27) | 0.0730 | 1.11 (0.97–1.27) | 0.1235 | 1.23 (1.04–1.46) | 0.0179 |
| Discharge to home (n = 32,109) | 0.77 (0.65–0.92) | 0.0042 | 0.58 (0.49–0.70) | <0.0001 | 0.42 (0.34–0.52) | <0.0001 |
| Composite events | ||||||
| Any postoperative event (n = 32,102) | 1.06 (0.99–1.13) | 0.1134 | 1.18 (1.09–1.27) | <0.0001 | 1.29 (1.17–1.42) | <0.0001 |
| Any major complication (n = 32,109) | 1.06 (0.99–1.14) | 0.0735 | 1.18 (1.09–1.27) | <0.0001 | 1.34 (1.21–1.48) | <0.0001 |
ICU, intensive care unit; mFI, 5-F=factor Modified Frailty Index; VATS, video-assisted thoracic surgery.
VATS to open or robotic to open.
Assessment of Performance as Risk Metrics
Monotonicity of marginal probability was strict or partial among all risk metrics for all outcome categories. Strict monotonicity favored ASA, CCI, and mFI-5 in that order, although ASA was at less risk for partial monotonicity on the basis of having only three categories compared with four categories for mFI-5 and CCI (Supplementary Table 9). ROC analyses revealed “good” AUC values (0.70–0.80) for all three metrics related to mortality and discharge to other than home, whereas AUC values for other outcomes were in the fair category (0.60–0.70; Table 4). There were no clinically important differences among the AUC values for the three metrics for any of the outcomes.
Table 4.
Results of ROC Analyses
| Complication/Outcome Category | AUC |
p Values |
||||
|---|---|---|---|---|---|---|
| mFI | ASA | CCI | mFI vs. ASA | mFI vs. CCI | ASA vs. CCI | |
| Postoperative complications | ||||||
| Pulmonary | 0.69 | 0.69 | 0.69 | 0.191 | 0.656 | 0.110 |
| Cardiovascular | 0.65 | 0.65 | 0.65 | 0.342 | 0.260 | 0.793 |
| Infectious | 0.65 | 0.65 | 0.65 | 0.602 | 0.438 | 0.973 |
| Neurologic | 0.69 | 0.69 | 0.69 | 0.900 | 0.257 | 0.515 |
| Gastrointestinal | 0.65 | 0.64 | 0.64 | 0.659 | 0.320 | 0.779 |
| Urinary | 0.65 | 0.65 | 0.65 | 0.026 | 0.297 | 0.096 |
| Surgical | 0.64 | 0.64 | 0.64 | 0.499 | 0.002 | 0.012 |
| Inhospital mortality | 0.80 | 0.79 | 0.79 | 0.491 | 0.547 | 0.762 |
| Perioperative administrative outcomes | ||||||
| 30-d mortality | 0.77 | 0.77 | 0.77 | 0.492 | 0.946 | 0.478 |
| Unexpected ICU admission | 0.62 | 0.62 | 0.62 | 0.285 | 0.288 | 0.695 |
| Readmission with 30 d | 0.61 | 0.61 | 0.61 | 0.664 | 0.132 | 0.605 |
| Unanticipated surgical approach conversion | 0.60 | 0.61 | 0.60 | 0.081 | 0.594 | 0.037 |
| Discharge to home | 0.75 | 0.75 | 0.74 | 0.429 | 0.010 | 0.364 |
| Composite events | ||||||
| Any postoperative event | 0.63 | 0.63 | 0.63 | 0.384 | 0.074 | 0.051 |
| Major complication | 0.64 | 0.64 | 0.64 | 0.440 | 0.039 | 0.047 |
ASA, American Society of Anesthesiologists; AUC, area under the curve; ROC, receiver operating characteristics; CCI, Charlson Comorbidity Index; ICU, intensive care unit; mFI, Modified Frailty Index.
Discussion
In many surgical fields, frailty has a close association with postoperative complications, discharge to institutional care, and mortality among geriatric patients.23,24 The mFI was developed to improve the practicality of use of frailty assessment in both clinical and research settings. To date, no study has investigated the predictive value of mFI-5 focusing on patients undergoing lung resection. The current study evaluated whether frailty as assessed by mFI-5 is independently associated with postoperative outcomes in patients after an anatomical lung resection for lung cancer.
Previous studies have reported an association between frailty and clinical outcomes after lung resection using the 11-factor mFI index on the basis of the National Surgical Quality Improvement Project variables and using frailty-defining diagnoses as outlined by the Johns Hopkins Adjusted Clinical Groups.19,25 Our study aimed to improve the applicability of frailty assessment in surgical management of lung cancer by using a simplified scoring system while also expanding the surgical case pool to include minimally invasive and more extensive lung operations in the analyses compared with previous studies of frailty in lung resection patients.
We found mFI-5 to be an independent predictor of almost all postoperative complications. The differences in outcomes were closely related to incremental changes in mFI-5 and were clinically meaningful. We also found that mFI-5 was associated with a wide variety of administrative outcomes. Increasing mFI-5 scores were associated with incremental changes in the odds of these outcomes on multivariable analyses and were clinically meaningful.
Selecting covariates for multivariate analyses is sometimes challenging in studies such as this. There are a number of known factors that are associated with adverse clinical outcomes after resection for lung cancer which have been derived through use of the STS General Thoracic Surgery Database. These include both induction therapy for more advanced cancers and cancer stage. It is generally accepted that more advanced cancer stage is related to poorer long-term outcomes after lung cancer resection, and this has been supported by analyses of the STS General Thoracic Surgery Database data.26 Nevertheless, surgical studies using STS data tend to focus on induction therapy as a perioperative risk factor in analyses of postoperative complications, as induction therapy is a surrogate for more advanced stage but also carries its own added risk of increased postoperative complications.22 It is for this reason that we selected induction therapy rather than tumor stage as a covariate in the multivariate analyses.
It is likely that the frequency of each mFI-5 factor in determining the degree of frailty will depend on the population being studied. The mFI-5 weights each individual component equally, whereas differential weighting may produce a more accurate metric. Whether different weighting should be considered for different populations is also an interesting consideration. The current study did not consider weighting the factors, as the intent of the study was to evaluate the utility of mFI-5 as originally developed in assessing frailty and its association with risk.
It is not always clear why functional elements represented in frailty metrics are closely associated with postoperative complications. Although many frailty indices consisted of functional elements, the underlying pathophysiology of frailty is related to poor reserve associated with a condition of chronic systemic inflammation.27 When a complex system is tested by a major physical insult such as lung resection, the reduced ability of a frail individual to respond to even small perturbations is related to this lack of physiological reserve and underlying chronic inflammation. We observe these failed or imperfect responses manifested as a wide variety of postoperative complications.
Cardiovascular complication was the only surgical complication category not independently predicted by mFI-5. The calculation of mFI-5 includes variables for hypertension and heart failure, and these therefore could not be included in our modeling of outcomes. The already low risk of major adverse cardiac events after lung resection is further reduced when minimally invasive approaches are used,28 which was the case in 71% of our patients. Adverse cardiovascular events in this study were primarily related to atrial fibrillation (82.6% of 4599 events), and variables in the STS database that predict this outcome do not include any of the elements that comprise mFI-5.29 Fortunately, patients at high risk for important cardiovascular outcomes can be identified using a different metric, the thoracic-specific revised cardiovascular risk index.30,31 The mFI-5 metric was also not significantly associated with 30-day mortality, which is an important outcome metric for reporting and analyses. The reasons for this lack of association are not clear.
The frailty assessment tool mFI-5 performed similarly overall as a surgical risk metric compared with the validated instruments ASA and CCI. There were no important differences among the metrics on ROC analyses. There may be some benefit in using mFI-5 as a risk assessment tool: it categorizes patient status more evenly across a convenient number of categories, is easier to calculate than CCI, is not reliant on combing through a long list of possible comorbidities to ensure accuracy and is more objective than ASA. There is an added benefit of its assessment of frailty, which helps identify patients who are candidates for prehabilitation, provides specific targets for frailty mitigation, and indicates for which patients perioperative order sets and subsequent discharge instructions focused on frail status are useful. For example, prehabilitation often includes a variety of interventions such as nutritional repletion and exercise focused on strength, endurance, and balance. Patients identified as having respiratory problems contributing to their mFI score may benefit from additional focused attention on strengthening muscles of respiration, whereas those found to have changes in activity contributing to their frailty score may benefit from interventions focused on increasing energy and elevating mood. Nevertheless, the value of mFI does not exceed that of ASA or CCI when used exclusively to evaluate the risk of lung resection.
The impact of using a frailty score to predict outcomes after lung resection for cancer may be important in a variety of domains, including costs of care, arranging appropriate resources for perioperative care, shared decision making, and mitigating risk through prehabilitation. The potential of mFI-5 to identify patients at risk of postoperative complication may help predict appropriate resources for perioperative care. Preoperative rehabilitation is effective in mitigating frailty.32,33 Thus, mFI-5 may help identify patients who would best benefit from prehabilitation. Finally, our observation of mFI-5 as an independent predictor of postoperative complications may help guide future shared decision making with the patients and their families before committing to surgery.
There are potential limitations to this study. Within the STS database, there were a large number of missing values related to some outcome and covariate variables. The STS database includes a relatively small number of participating centers in North America, thus our findings may not be based on truly representative data from North America. Data on postdischarge status are limited, largely restricting our analyses to inhospital outcomes. It is likely that complication rates are lower when using a minimally invasive approach rather than an open approach, as is being studied in the ongoing VIOLET trial.34 Nevertheless, we did not evaluate surgical approach (open, video-assisted thoracic surgery, robotic) and its possible effect on the utility of mFI-5 in predicting complications, as this is the topic of a separate ongoing study using STS database data. Similarly, we did not evaluate the extent of lung resection (segment versus lobe versus bilobe) on outcomes. Recent randomized trials have revealed no important difference in acute outcomes related to the extent of parenchymal resection.35,36 Finally, we limited our study to version 2.3 of the STS database owing to changes in collected variables that permitted calculation of the mFI-5 score using only this version.
Whether mFI-5 is a good frailty metric or is more accurately a surgical risk predictor in disguise is a valid question. Distinguishing between the two is important. A standalone metric that is useful primarily for calculating risk for adverse postoperative outcomes does not provide important information regarding a potential underlying frail condition. In contrast, an instrument that accurately estimates surgical risk and simultaneously screens for degree of frailty provides the added benefit of indicating the need for and possibly the methods of mitigating underlying frailty through personalized interventions. Frailty is frequently related to underweight status and sarcopenia, and in the current study mFI-5 did not have a strong association with underweight status. The relationship between mFI-5 and other frailty metrics and the association between sarcopenia and mFI-5 need to be further investigated. If such investigations reveal that mFI-5 is an effective surgical risk index but only fair at evaluating frailty, a change in the name of this metric may be warranted.
In conclusion, we found that mFI-5 is independently predictive of most clinical and administrative outcomes after anatomical lung resection for cancer. Assessment of potential lung resection candidates at risk for frailty using mFI-5 may be useful in identifying patients who would benefit from preoperative optimization, may help with shared decision making, and could assist with identification of appropriate perioperative and postdischarge resources.
CRediT Authorship Contribution Statement
Andy Chao Hsuan Lee: Data curation, Investigation, Methodology, Resources, Visualization, Roles/writing—original draft, Writing—review and editing.
Sang Mee Lee: Data curation, Formal analysis, Methodology, Software, Writing—review and editing.
Mark K. Ferguson: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing—review and editing.
Acknowledgments
Funding was provided by the Donald J. Ferguson, MD, Surgical Research Fund of The University of Chicago. The data for this research were provided by The Society of Thoracic Surgeons’ National Database Participant User File Research Program.
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Lee ACH, Lee SM, Ferguson MK. Frailty is associated with adverse postoperative outcomes after lung cancer resection. JTO Clin Res Rep. 2022;3:100414.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100414.
Supplementary Data
References
- 1.US Census Bureau National population projections tables: main series. census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html
- 2.Pei G., Zhou S., Han Y., Liu Z., Xu S. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis. 2014;6:1230–1238. doi: 10.3978/j.issn.2072-1439.2014.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green A., Hauge J., Iachina M., Jakobsen E. The mortality after surgery in primary lung cancer: results from the Danish Lung Cancer Registry. Eur J Cardiothorac Surg. 2016;49:589–594. doi: 10.1093/ejcts/ezv107. [DOI] [PubMed] [Google Scholar]
- 4.Vaz Souza R., Bassi M., Mantovani S., et al. Comparison of preoperative scores predicting outcome in elderly undergoing lung malignancies resection. J Thorac Dis. 2020;12:7083–7088. doi: 10.21037/jtd-20-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosi D., Nosotti M., Bonitta G., et al. Anatomical segmentectomy versus pulmonary lobectomy for stage I non-small-cell lung cancer: patients selection and outcomes from the European Society of Thoracic Surgeons database analysis. Interact Cardiovasc Thorac Surg. 2021;32:546–551. doi: 10.1093/icvts/ivaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee D.H., Buth K.J., Martin B.J., Yip A.M., Hirsch G.M. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 7.Farhat J.S., Velanovich V., Falvo A.J., et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72:1526–1531. doi: 10.1097/TA.0b013e3182542fab. [DOI] [PubMed] [Google Scholar]
- 8.Visser L., Banning L.B.D., El Moumni M., Zeebregts C.J., Pol R.A. The effect of frailty on outcome after vascular surgery. Eur J Vasc Endovasc Surg. 2019;58:762–769. doi: 10.1016/j.ejvs.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Seib C.D., Rochefort H., Chomsky-Higgins K., et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153:160–168. doi: 10.1001/jamasurg.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko F.C. Preoperative frailty evaluation: a promising risk-stratification tool in older adults undergoing general surgery. Clin Ther. 2019;41:387–399. doi: 10.1016/j.clinthera.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abellan van Kan G., Rolland Y.M., Morley J.E., Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49 doi: 10.1093/geronj/49.2.m85. M85–M94. [DOI] [PubMed] [Google Scholar]
- 15.Hanada M., Yamauchi K., Miyazaki S., et al. Short-Physical Performance Battery (SPPB) score is associated with postoperative pulmonary complications in elderly patients undergoing lung resection surgery: a prospective multicenter cohort study. Chron Respir Dis. 2020;17 doi: 10.1177/1479973120961846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obeid N.M., Azuh O., Reddy S., et al. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. 2012;72:878–883. doi: 10.1097/TA.0b013e31824d0f70. [DOI] [PubMed] [Google Scholar]
- 17.Mitnitski A.B., Mogilner A.J., Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsiouris A., Hammoud Z.T., Velanovich V., Hodari A., Borgi J., Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183:40–46. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam S., Aalberg J.J., Soriano R.P., Divino C.M. New 5-Factor Modified Frailty Index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226:173–181.e8. doi: 10.1016/j.jamcollsurg.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Birim O., Maat A.P., Kappetein A.P., van Meerbeeck J.P., Damhuis R.A., Bogers A.J. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:30–34. doi: 10.1016/s1010-7940(02)00721-2. [DOI] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists ASA physical status classification system. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- 22.Kozower B.D., Sheng S., O’Brien S.M., et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875–883. doi: 10.1016/j.athoracsur.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 23.Hadaya J., Mandelbaum A., Sanaiha Y., Benharash P. Impact of frailty on clinical outcomes and hospitalization costs following elective colectomy. Am Surg. 2021;87:1589–1593. doi: 10.1177/00031348211024233. [DOI] [PubMed] [Google Scholar]
- 24.Joseph B., Pandit V., Zangbar B., et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149:766–772. doi: 10.1001/jamasurg.2014.296. [DOI] [PubMed] [Google Scholar]
- 25.Karunungan K.L., Hadaya J., Tran Z., et al. Frailty is independently associated with worse outcomes after elective anatomic lung resection. Ann Thorac Surg. 2021;112:1639–1646. doi: 10.1016/j.athoracsur.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson M.K., Mitzman B., Derstine B., et al. A morphomic index is an independent predictor of survival after lung cancer resection. Ann Thorac Surg. 2020;109:873–878. doi: 10.1016/j.athoracsur.2019.10.064. [DOI] [PubMed] [Google Scholar]
- 27.Vatic M., von Haehling S., Ebner N. Inflammatory biomarkers of frailty. Exp Gerontol. 2020;133 doi: 10.1016/j.exger.2020.110858. [DOI] [PubMed] [Google Scholar]
- 28.Sandri A., Petersen R.H., Decaluwé H., et al. Coronary artery disease is associated with an increased mortality rate following video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg. 2017;154:352–357. doi: 10.1016/j.jtcvs.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Onaitis M., D’Amico T., Zhao Y., O’Brien S., Harpole D. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90:368–374. doi: 10.1016/j.athoracsur.2010.03.100. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson M.K., Saha-Chaudhuri P., Mitchell J.D., Varela G., Brunelli A. Prediction of major cardiovascular events after lung resection using a modified scoring system. Ann Thorac Surg. 2014;97:1135–1140. doi: 10.1016/j.athoracsur.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Thomas D.C., Blasberg J.D., Arnold B.N., et al. Validating the Thoracic revised Cardiac Risk Index following lung resection. Ann Thorac Surg. 2017;104:389–394. doi: 10.1016/j.athoracsur.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Xu X., Cheung D.S.T., Smith R., Lai A.Y.K., Lin C.C. The effectiveness of pre- and post-operative rehabilitation for lung cancer: a systematic review and meta-analysis on postoperative pulmonary complications and length of hospital stay. Clin Rehabil. 2022;36:172–189. doi: 10.1177/02692155211043267. [DOI] [PubMed] [Google Scholar]
- 33.Steffens D., Beckenkamp P.R., Hancock M., Solomon M., Young J. Preoperative exercise halves the postoperative complication rate in patients with lung cancer: a systematic review of the effect of exercise on complications, length of stay and quality of life in patients with cancer. Br J Sports Med. 2018;52:344. doi: 10.1136/bjsports-2017-098032. [DOI] [PubMed] [Google Scholar]
- 34.Lim E., Batchelor T., Shackcloth M., et al. Study protocol for VIdeo assisted thoracoscopic lobectomy versus conventional open lobectomy for lung cancer, a UK multicentre randomised controlled trial with an internal pilot (the VIOLET study) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altorki N.K., Wang X., Wigle D., et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503) Lancet Respir Med. 2018;6:915–924. doi: 10.1016/S2213-2600(18)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki K., Saji H., Aokage K., et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158:895–907. doi: 10.1016/j.jtcvs.2019.03.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.