Abstract
Objective
Cisplatin resistance is a huge problem encountered in ovarian cancer treatment. Our study probed the roles and the underlying mechanisms of lncRNA MCF2L-AS1 in ovarian cancer cisplatin-resistance.
Methods
SKOV3 and IGROV-1 cells were subjected to gradually increasing concentrations of cisplatin to construct ovarian cancer cisplatin-resistance cells. Cell proliferation was evaluated by cell counting kit-8 and colony formation assays. Cell apoptosis was assessed using Annexin V and PI staining. The relationships between SP1, MCF2L-AS1 and insulin-like growth factor-2 mRNA binding protein 1 (IGF2BP1) were verified by RNA pull-down, RIP, ChIP and dual-luciferase reporter gene assay, respectively. Tumor xenograft experiment was employed to evaluate the effects of MCF2L-AS1 silencing on ovarian cancer cisplatin-resistance in vivo. TUNEL staining and immunohistochemistry were performed in tumor tissue.
Results
MCF2L-AS1 and IGF2BP1 were upregulated in cisplatin-resistant cells. MCF2L-AS1 silencing suppressed cell proliferation of cisplatin-resistant cells, while promoted the apoptosis, suggesting that MCF2L-AS1 knockdown suppressed ovarian cancer cells cisplatin-resistance. Meanwhile, MCF2L-AS1 silencing enhanced cisplatin sensitivity in ovarian cancer parental cells and IGF2BP1 overexpression impaired cisplatin sensitivity of parental cells. MCF2L-AS1 activated IGF2/MEK/ERK pathway through interacting with IGF2BP1. Transcription factor SP1 activated MCF2L-AS1 expression. MCF2L-AS1 knockdown inhibited ovarian cancer cisplatin-resistance in vivo.
Conclusion
SP1-induced MCF2L-AS1 promoted ovarian cancer cisplatin-resistance through activation of IGF2/MEK/ERK pathway via interacting with IGF2BP1.
Keywords: Ovarian Cancer, Cisplatin, LncRNA MCF2L-AS1, SP1, IGF2BP1, IGF2/MEK/ERK Signaling Pathway
Synopsis
LncRNA MCF2L-AS1 and insulin-like growth factor-2 mRNA binding protein 1 (IGF2BP1) were upregulated in cisplatin-resistant cells. LncRNA MCF2L-AS1 silencing suppressed ovarian cancer cell cisplatin resistance. SP1-induced lncRNA MCF2L-AS1 activated the IGF2/MEK/ERK signaling pathway by targeting IGF2BP1.
INTRODUCTION
Ovarian cancer is one of the lethal gynecological malignancies. Cisplatin is a well-known antitumor agent [1]. However, most patients with ovarian cancer will eventually die of recurrent and progressive disease because of resistance to cisplatin [2]. As reported, cisplatin resistance in ovarian cancer is a multifactorial process, which may result from a series of dysregulation of gene expression; however, the specific mechanism of cisplatin resistance in ovarian cancer is still poorly understood.
Transcription factor SP is overexpressed in many cancers and is associated with poor prognosis, which both activates and suppresses the expression of a number of essential oncogenes and tumor suppressors [3]. As previously reported, SP1 was markedly upregulated in ovarian cancer, and SP1 knockdown could suppress cell migration, cell invasion and chemoresistance [4,5]. It is suggested that SP1 facilitates the malignant behavior of ovarian cancer cells, while its role in regulating ovarian cancer cell cisplatin resistance is unknown, which deserves further study.
LncRNA refers to a single stranded RNA (ssRNA) with the transcripts more than 200 nts and function in many biological processes [6]. As widely reported, the development of ovarian cancer is accompanied with changes in expression pattern of large set of lncRNAs [7,8]. For instance, Xu et al. [9] demonstrated that lncRNA EBIC promoted ovarian cancer cisplatin-resistance. In addition, lncRNA CHRF was markedly upregulated in cisplatin resistant ovarian cancer cells and patients with resistant disease [10]. LncRNA MCF.2 cell line derived transforming sequence like-antisense RNA 1 (MCF2L-AS1) was confirmed as an oncogene in colorectal cancer and lung cancer [11,12]. However, the expression and the role of lncRNA MCF2L-AS1 in ovarian cancer were never reported before. Herein, through the investigation of GEPIA database, it was found that lncRNA MCF2L-AS1 expression markedly increased in ovarian cancer, which caught our attention. Recent studies have revealed that SP1 could bind to the promoter of non-coding RNA to promote ovarian cancer progression [4,13]. In the current study, SP1 was predicted to have a binding site to lncRNA MCF2L-AS1 promoter by using JASPAR. However, whether SP1 could positively regulate lncRNA MCF2L-AS1 expression to promote ovarian cancer cisplatin-resistance needs further study.
Insulin-like growth factor-2 mRNA binding protein 1 (IGF2BP1) is a member of the conserved RNA binding protein family [14]. Much evidence has confirmed that IGF2BP1 is involved in tumor cell proliferation, invasion, and chemo-resistance in a series of malignant tumors, including ovarian cancer [15,16]. IGF2BP1 binds to targets such as insulin-like growth factor 2 (IGF2) in the cytoplasm [17]. Elevated IGF2 expression is associated with increased risk of developing various cancers, and IGF2 promotes the malignant behaviors of tumors by activating downstream pathways, such as MEK/ERK signaling pathway [18,19]. As previously reported, IGF2BP1 promoted the proliferation of tongue squamous cell carcinoma cells by activating the IGF2/MEK/ERK signaling pathway [20]. RNA-protein interaction prediction (RPISeq) showed high affinity of MCF2L-AS1 to IGF2BP1, suggesting that lncRNA MCF2L-AS1 could interacted with IGF2BP1 to regulate IGF2/MEK/ERK signaling pathway. However, the regulatory relationship between lncRNA MCF2L-AS1, IGF2BP1 and IGF2/MEK/ERK in ovarian cancer and their roles in ovarian cancer remains unclear.
Herein, we found that SP1-induced MCF2L-AS1 promoted ovarian cancer cisplatin-resistance through activation of the IGF2/MEK/ERK signaling pathway via interacting with IGF2BP1. Our findings provided new insights into the regulatory mechanisms of lncRNA MCF2L-AS1 in ovarian cancer cisplatin-resistance.
MATERIALS AND METHODS
1. Cell culture and induction of cisplatin resistance
Human ovarian cancer cells (SKOV3 and IGROV-1 cells) were purchased from ATCC (VA, Manassas, USA). All cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Gibco) with 5% CO2 at 37°C. SKOV3/DDP and IGROV-1/DDP cells were constructed as previously described [21]. Cells were treated with cisplatin for 2 days as a cycle. After completing 3 cycles of stimulation with the same concentration of cisplatin, the dose was increased. The initial concentration of cisplatin was 5 μM, and the final concentration of cisplatin was 70 μM. Only when cells remained the resistance to cisplatin after cultured in medium without cisplatin for at least 6 months, they were used for subsequent experiment. In subsequent experiments, for cisplatin treatment, SKOV3/DDP cells were subjected to 30 μM cisplatin, and IGROV-1/DDP cells were subjected to 36 μM cisplatin.
2. Cell transfection
The overexpression plasmid of SP1 (Oe-SP1), the overexpression plasmid of IGF2BP1 (Oe-IGF2BP1), the short hairpin RNA of lncRNA MCF2L-AS1 (sh-MCF2L-AS1) and their negative controls were obtained from GenePharma (Shanghai, China). SKOV3, IGROV-1, SKOV3/DDP and IGROV-1/DDP cells were transfected with above plasmids using Lipofectamine™ 3000 (Invitrogen, Carlsbad, CA, USA).
3. Cell counting kit-8 (CCK-8) assay
Cells were seeded in 96-well plates with 5×103 cells per well and then incubated with CCK-8 reagent (10 μL, Sangon) for 2 hours. Absorbance was examined at 450 nm with the microplate spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
4. Colony formation assay
For colony formation analysis, 1×105/well cells were seeded on the 6-well plates plate and incubated for 1 week at 37°C. Then cells were stained with 0.1% crystal violet for 10 minutes (Solarbio, Beijing, China), and the colonies formed were counted manually.
5. Cell apoptosis assay
Cells were collected and re-suspended in 500 μL of 1X Annexin-binding buffer (Beyotime, Shanghai, China). Then cells were incubated with 10 μL Annexin V-FITC and 5 μL PI stain (Beyotime) for 10 minutes. Samples were immediately analyzed using flow cytometry (BD Biosciences, New York, NY, USA).
6. RNA pull-down assay
LncRNA MCF2L-AS1 or NC was labeled with biotin (Roche, Basel, Switzerland) and transcribed with the Biotin RNA Labeling Mix (Roche) and T7 RNA polymerase (Roche). About 2×107 cells were dissolved in the soft lysis buffer plus 80 U/mL RNasin (Promega, Madison, WI, USA). Cell extract (2 μg) was incubated with biotinylated RNA (100 pmol) for 1 hour at 4°C. Washed streptavidin-coupled agarose beads (Invitrogen) were added to each binding reaction and further incubated for 1 hour to isolate the RNA-protein complex. The retrieved protein was assessed using western blot.
7. RNA immunoprecipitation (RIP) assay
Cells were lysed with a complete RIP lysis buffer (Millipore, Burlington, MA, USA). Cell extract was incubated with immunoglobulin G (IgG) (1:50, ab172730; Abcam, Waltham, MA, USA) and IGF2BP1 (1:50, ab184305; Abcam) antibody at 4°C overnight. RNA samples were purified by phenol chloroform extraction, followed by quantitative real-time polymerase chain reaction (qRT-PCR) to determine MCF2L-AS1 transcripts enrichment.
8. Chromatin immunoprecipitation (ChIP) assay
ChIP assay was conducted by using ChIP kit (Beyotime). Briefly, cells were treated with 1% formaldehyde solution for 10 minutes and quenched with 125 mM glycine for 5 minutes. DNA was fragmented by sonication. Cell lysate was subsequently incubated with anti-SP1 (1:100, ab231778; Abcam) or anti-IgG (1:100, ab172730; Abcam) at 4°C overnight. Then, dynabeads protein G (Invitrogen) was added for 2 hours for DNA enrichment. Finally, immunoprecipitated DNAs were analyzed by qRT-PCR.
9. Dual-luciferase reporter gene assay
The SP1-binding site in the promoter of lncRNA MCF2L-AS1 was predicted using JASPAR database (http://jaspar.genereg.net/). Site-directed mutagenesis of the SP1 binding site in lncRNA MCF2L-AS1 promoter was performed using a site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Wild-type (wt) and mutant-type (mut) reporter plasmids of lncRNA MCF2L-AS1 sequences were cloned into PGL3 vector (GenePharma). Then, cells were co-transfected with lncRNA MCF2L-AS1-wt or lncRNA MCF2L-AS1-mut plasmids and oe-SP1 or oe-NC by Lipofectamine™ 3000 (Invitrogen). The luciferase activity was examined using a dual-luciferase reporter assay system (Promega).
10. Tumor xenograft in vivo
A total of 24 BALB/c nude mice were assigned to 3 groups: SKOV3/DDP group (injected with SKOV3/DDP cells), sh-NC group (injected with SKOV3/DDP cells with sh-NC transfection) and sh-MCF2L-AS1 group (injected with sh-MCF2L-AS1 cells with sh-MCF2L-AS1 transfection) (8 mice/group). 0.2 mL of above cell suspension containing 2×104 cells was injected into the back of each mouse. At 7 days after inoculation, all mice received 4 mg/kg cisplatin treatment every 3 day. The tumor volumes were determined by measuring their length (l) and width (w) and calculating the volume (V) as follows: V=lw2/2. At 21 days, the mice were euthanized and tumor tissues were weighted. The animal studies were approved by Animal Ethics Committee of The Fourth Affiliated Hospital of Nantong University.
11. Immunohistochemistry (IHC)
The tumor tissues were fixed in 10% paraformaldehyde and tumor sections (4 μm in thickness) were prepared. After deparaffinization and antigen retrieval (Dako, Santa Clara, CA, USA), sections were then blocked with goat serum, avidin solution and biotin solution. Then sections were incubated with antibody against Ki67 (1:200, ab15580; Abcam) overnight followed by incubation with an appropriate secondary antibody (1:500, ab150077; Abcam) for 1 h. The sections were stained with diaminobenzidine and then counterstained with hematoxylin, dehydrated and mounted. Then, the sections were observed under an inverted microscope (Nikon, Tokyo, Japan). The number of positive cells was counted using ImageJ software version 1.8 (National Institute of Health, Bethesda, MD, USA).
12. TdT-mediated dUTP nick-end labeling (TUNEL) staining
The experimental operation was performed in accordance to the instructions of the TUNEL staining kit (Sigma-Aldrich, St. Louis, MO, USA). Sections were photographed using a microscope (Olympus, Tokyo, Japan).
13. qRT-PCR
Total RNA was isolated with TRIzol reagent (Thermo Fisher Scientific). cDNA was synthesized with HiFiScript cDNA synthesis kit (Life Technologies, Carlsbad, CA, USA). Then, the cDNA was used for qRT-PCR assay using SYBR (Thermo Fisher Scientific). The relative expressions of mRNAs were normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated by 2−ΔΔCT method. The primers used in the study were listed as follows (5′-3′):
SP1 (F): 5′-GACAGGACCCCCTTGAGCTT-3′,
SP1 (R): 5′-GGCACCACCACCATTACCAT-3′;
LncRNA MCF2L-AS1 (F): 5′-GATCAACGTTCAATCCACCG-3′,
LncRNA MCF2L-AS1 (R): 5′-CGTCAAGATAGCGCAGCTTCC-3′;
IGF2BP1 (F): 5′-CCAAGGAGGAAGTGAAGCTG-3′,
IGF2BP1 (R): 5′-ATGTCTCGGATCTTCCGTTG-3′;
GAPDH (F): 5′-CCAGGTGGTCTCCTCTGA-3′,
GAPDH (R): 5′-GCTGTAGCCAAATCGTTGT-3′.
14. Western blot
The proteins were isolated from cells by using RIPA buffer, and concentrations of protein were determined by a BCA Kit (Beyotime), which further transferred to a PVDF membrane (Millipore). Then, membranes incubated overnight with antibodies against Bax (1:1,000, ab32503; Abcam), B-cell lymphoma-2 (Bcl-2) (1:1,000, ab182858; Abcam), Cleaved Caspase-3 (1:1,000, ab2302; Abcam), IGF2BP1 (1:1,000, ab100999; Abcam), IGF2 (1:1,000, ab177467; Abcam), p-MEK (1:1,000, 2338; Cell Signaling Technology, Danvers, MA, USA), mitogen-activated protein kinase (MEK) (1:1,000, 4694; Cell Signaling Technology), p-ERK (1:1,000, 4370; Cell Signaling Technology), extracellular regulated protein kinase (ERK) (1:1,000, ab32537; Abcam) and GAPDH antibody (1:10,000, SAB2701826; Sigma-Aldrich). After washed with PBS-T, membranes were then incubated with the corresponding secondary antibody (1:10,000, ab7090; Abcam) for 60 minutes. The membranes were visualized and imaged by GEL imaging system (Bio-Rad, Hercules, CA, USA). The quantification of proteins was analyzed by the software Image J.
15. Data analysis
All data were obtained from at least 3 replicate experiments. Results were expressed as mean±standard deviation (SD). Statistical analysis was performed using SPSS 19.0 (IBM, Armonk, NY, USA). The comparisons of 2 different group parameters and multi-group were determined using Student’s t-test and one-way analysis of variance (ANOVA), respectively. The p-values less than 0.05 were considered significant.
RESULTS
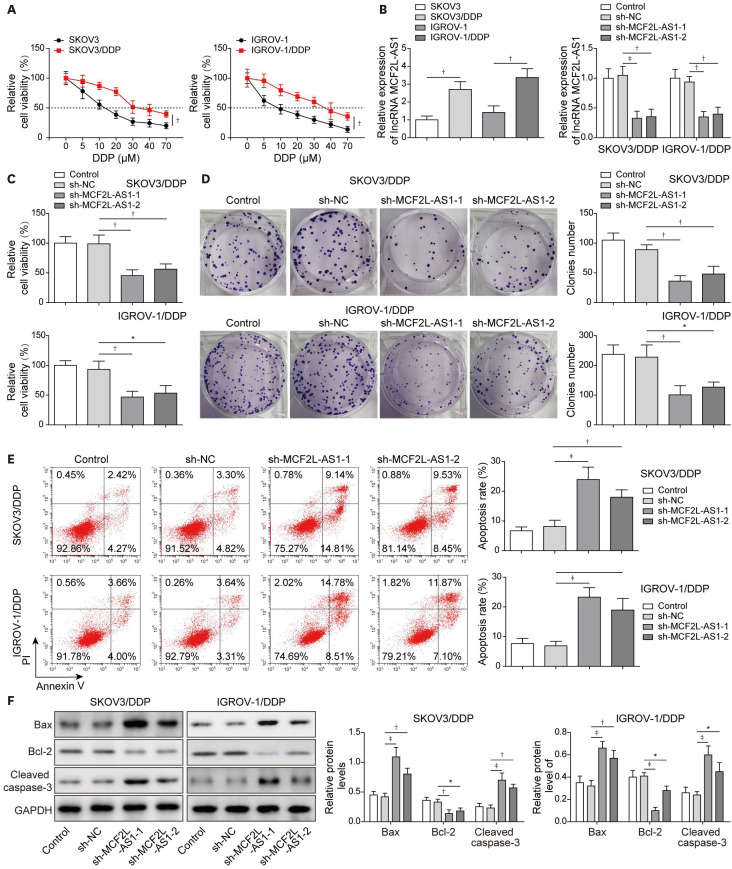
1. LncRNA MCF2L-AS1 knockdown suppressed cisplatin resistance in ovarian cancer cells
Through GEPIA database, we found that MCF2L-AS1 was markedly upregulated in ovarian cancer tumor tissues compared to non-tumor tissues, indicating that MCF2L-AS1 was involved in ovarian cancer development. However, whether MCF2L-AS1 participates in ovarian cancer cisplatin-resistance remains unknown. To probe the roles of MCF2L-AS1 in regulating cisplatin resistance in ovarian cancer cells, cells were exposed to increasing concentrations of cisplatin as previously described [21] to construct SKOV3/DDP and IGROV-1/DDP cells. The results displayed that the half maximal inhibitory concentration (IC50) of cisplatin-resistant cells was greater than that of the parental cells (Fig. 1A). As revealed in Fig. 1B, MCF2L-AS1 expression was significantly elevated in resistant ones compared to parental cells. To further probe the relationship between MCF2L-AS1 expression and cisplatin resistance in ovarian cancer, we knocked down MCF2L-AS1 expression in SKOV3/DDP and IGROV-1/DDP cells (Fig. 1B). The results from CCK-8 assay and colony formation assay subsequently demonstrated that MCF2L-AS1 knockdown remarkably inhibited proliferation and colony formation of cisplatin-resistant cells (Fig. 1C-D). In addition, MCF2L-AS1 silencing promoted cell apoptosis of cisplatin-resistant cells (Fig. 1E). Similarly, MCF2L-AS1 knockdown significantly upregulated Bax and Cleaved Caspase-3 levels, while reduced Bcl-2 level (Fig. 1F). Moreover, we detected the effect of MCF2L-AS1 knockdown on cisplatin sensitivity in parental cells (SKOV3 and IGROV-1 cells). The expression of MCF2L-AS1 in SKOV3 and IGROV-1 cells was decreased after transfected with sh-MCF2L-AS1 (Fig. S1A). As shown in Fig. S1B, the IC50 of SKOV3 and IGROV-1 cells was significantly reduced after MCF2L-AS1 knockdown, suggesting that cisplatin sensitivity in parental cells was enhanced by MCF2L-AS1 silencing. Moreover, MCF2L-AS1 knockdown remarkably inhibited parental cell proliferation (Fig. S1C) and promoted cell apoptosis (Fig. S1D). Taken together, MCF2L-AS1 silencing remarkably inhibited cisplatin resistance in ovarian cancer cells.
Fig. 1. LncRNA MCF2L-AS1 knockdown suppressed cisplatin resistance in ovarian cancer cells.
SKOV3 and IGROV-1 cells were exposed to increasing concentrations of cisplatin as previously described to construct cisplatin-resistant cells. (A) Cell proliferation was evaluated using CCK-8 assay. Then, cisplatin-resistant cells were transfected with sh-NC, sh-MCF2L-AS1-1 or sh-MCF2L-AS1-2. (B) MCF2L-AS1 expression was determined using qRT-PCR. (C) CCK-8 assay was employed to evaluate cell proliferation. (D) Colony formation assay was employed to evaluate colony formation. (E) Cell apoptosis was evaluated by flow cytometry assay. (F) Bax, Bcl-2 and Cleaved Caspase-3 levels were assessed using western blot. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
CCK-8, cell counting kit-8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, normal control; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation; sh-, short hairpin RNA of.
*p<0.05, †p<0.01, ‡p<0.001.
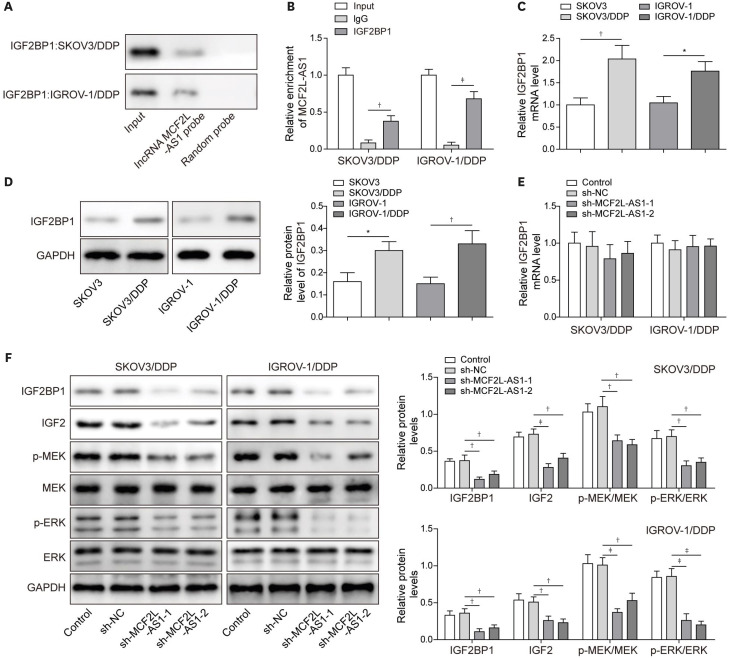
2. LncRNA MCF2L-AS1 regulated the IGF2/MEK/ERK signaling pathway through interacting with IGF2BP1
We sought to identify the target of MCF2L-AS1 to explore the molecular mechanisms by which MCF2L-AS1 exerted its effects on ovarian cancer cisplatin-resistance. RPISeq prediction was first employed to identify MCF2L-AS1 interaction proteins and revealed that IGF2BP1 was a potential binding protein of MCF2L-AS1 (prediction using RF classifier: 0.7 score; prediction using SVM classifier: 0.77 score). RNA pull-down and RIP assay subsequently displayed that MCF2L-AS1 directly interacted with IGF2BP1 (Fig. 2A and B). As demonstrated in Fig. 2C and D, IGF2BP1 expression was significantly elevated in cisplatin-resistant cells. We observed that sh-MCF2L-AS1 transfection did not have any impact on the mRNA level of IGF2BP1 in cisplatin-resistant cells (Fig. 2E). However, the protein level of IGF2BP1 was markedly reduced by MCF2L-AS1 silencing, as well as the protein levels of IGF2/MEK/ERK signaling pathway-related proteins (IGF2, p-MEK and pERK) (Fig. 2F). All these results suggested that lncRNA MCF2L-AS1 activated the IGF2/MEK/ERK signaling pathway through interacting with IGF2BP1 protein.
Fig. 2. LncRNA MCF2L-AS1 regulated the IGF2/MEK/ERK signaling pathway through interacting with IGF2BP1.
(A, B) RNA pull-down and RIP assay were employed to verify the binding relationship between lncRNA MCF2L-AS1 and IGF2BP1. (C, D) IGF2BP1 expression was assessed by qRT-PCR and western blot. Cisplatin-resistant cells were transfected with sh-NC or sh-MCF2L-AS1. (E) The mRNA level of IGF2BP1 was determined using qRT-PCR. (F) IGF2BP1, IGF2, p-MEK, MEK, p-ERK and ERK levels were evaluated using western blot. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
ERK, extracellular regulated protein kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IGF2, insulin-like growth factor 2; IGF2BP1, insulin-like growth factor-2 mRNA binding protein 1; IgG, immunoglobulin G; MEK, mitogen-activated protein kinase kinase; NC, normal control; qRT-PCR, quantitative real-time polymerase chain reaction; RIP, RNA immunoprecipitation; SD, standard deviation; sh-, short hairpin RNA of.
*p<0.05, †p<0.01, ‡p<0.001.
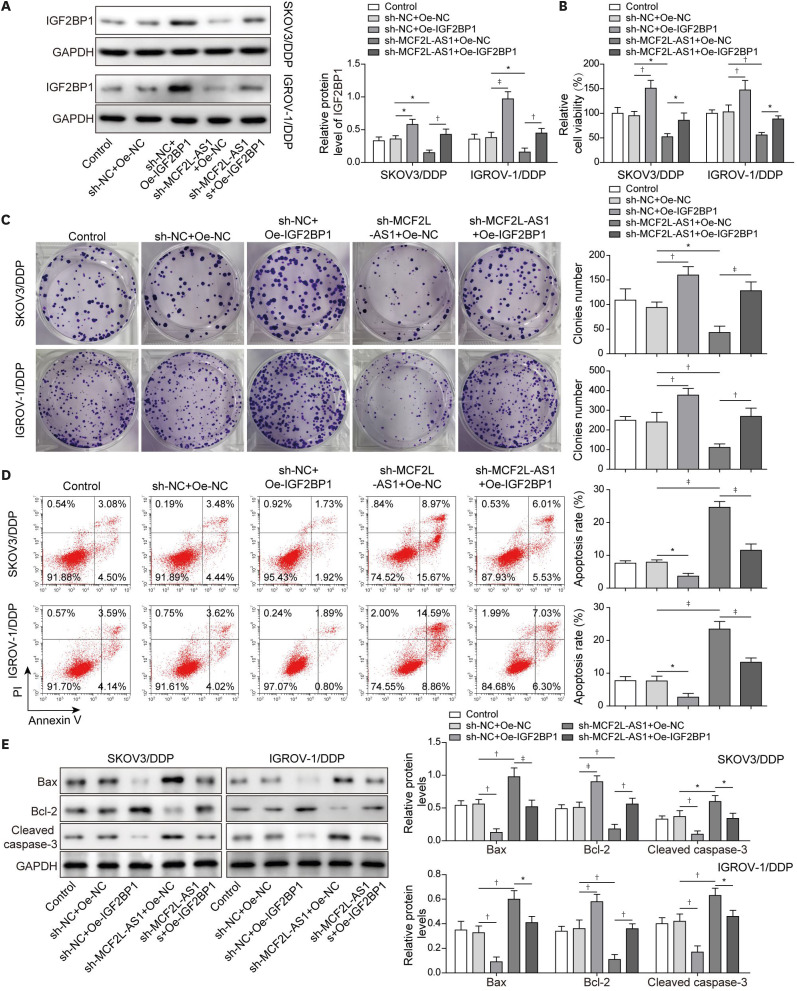
3. IGF2BP1 overexpression reversed the effects of MCF2L-AS1 silencing on cisplatin-resistance ovarian cancer
Next, we aimed to explore the effects of IGF2BP1 on MCF2L-AS1-mediated biological functions in vitro. Thus, we overexpressed IGF2BP1 alone and co-transfected with sh-MCF2L-AS1 and oe-IGF2BP1 in SKOV3/DDP and IGROV-1/DDP cells. First, IGF2BP1 expression was markedly reduced following sh-MCF2L-AS1 transfection, while IGF2BP1 expression was significantly elevated in sh-MCF2L-AS1 + Oe-IGF2BP1 group compared to sh-MCF2L-AS1 + Oe-NC group (Fig. 3A). Overexpression IGF2BP1 remarkably promoted proliferation and colony formation of cisplatin-resistant cells, which could abolish the inhibitory effects of MCF2L-AS1 silencing on proliferation and colony formation of cisplatin-resistant cells (Fig. 3B and C). Besides, cell apoptosis of cisplatin-resistant cells was inhibited after IGF2BP1 overexpression, which reversed the promotion effect of MCF2L-AS1 silencing on cell apoptosis of cisplatin-resistant cells (Fig. 3D). Finally, IGF2BP1 overexpression significantly inhibited Bax and Cleaved Caspase-3 expressions, while promoted Bcl-2 expression, and the effects of MCF2L-AS1 silencing on the levels of these apoptosis-related protein were eliminated (Fig. 3E). Moreover, we detected the effect of IGF2BP1 overexpression on cisplatin sensitivity in parental cells (SKOV3 and IGROV-1 cells). It was observed that the IC50 of SKOV3 and IGROV-1 cells was significantly increased after IGF2BP1 overexpression (Fig. S2), suggesting that cisplatin sensitivity in parental cells was weakened by IGF2BP1 overexpression. In summary, IGF2BP1 overexpression could promoted cisplatin resistance, and reverse the effects of MCF2L-AS1 silencing on cisplatin resistance in ovarian cancer.
Fig. 3. IGF2BP1 overexpression reversed the effects of MCF2L-AS1 silencing on ovarian cancer cisplatin-resistance.
Cisplatin-resistant cells were transfected with sh-NC + Oe-NC, sh-NC + Oe-IGF2BP1, sh-MCF2L-AS1 + Oe-NC and sh-MCF2L-AS1 + Oe-IGF2BP1. (A) Western blot was performed to evaluate IGF2BP1 level. (B) Cell proliferation was evaluated using CCK-8 assay. (C) Colony formation assay was performed to detect colony formation. (D) Cell apoptosis was evaluated by flow cytometry assay. (E) Bax, Bcl-2 and Cleaved Caspase-3 levels were assessed using western blot. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
CCK-8, cell counting kit-8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IGF2BP1, insulin-like growth factor-2 mRNA binding protein 1; NC, normal control; Oe-, overexpression plasmid of; SD, standard deviation; sh-, short hairpin RNA of.
*p<0.05, †p<0.01, ‡p<0.001.
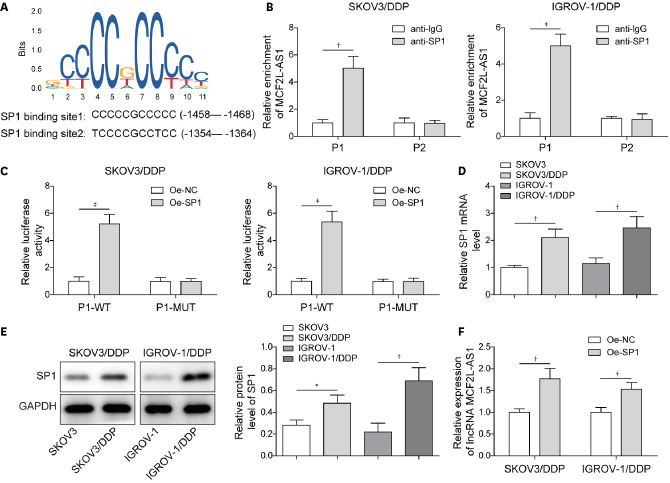
4. SP1 promoted lncRNA MCF2L-AS1 expression at the transcriptional level
Much evidence has suggested that several transcription factors contribute to lncRNAs dysregulation in human malignancies [22]. The factors involved in MCF2L-AS1 dysregulation in ovarian cancer remained elusive. Through JASPAR database, we found that a classic transcription factor SP1 had potential binding sites to MCF2L-AS1 promoter region (Fig. 4A). ChIP assay subsequently revealed that SP1 could bind to P1 site of MCF2L-AS1 promoter (Fig. 4B). Subsequently, SP1 overexpression upregulated the luciferase activity of P1-WT, while the luciferase activity of P1-MUT was not affected (Fig. 4C), further suggesting that SP1 could bind to P1 site of MCF2L-AS1 promoter. Moreover, SP1 expression was significantly elevated in cisplatin-resistant cells (Fig. 4D and E). Subsequently, MCF2L-AS1 expression in cisplatin-resistant cells was markedly elevated after SP1 overexpression (Fig. 4F). In conclusion, SP1 activated MCF2L-AS1 expression in cisplatin-resistant ovarian cancer cells.
Fig. 4. SP1 promoted lncRNA MCF2L-AS1 expression at the transcriptional level.
(A) The predicted positions of puative SP1 binding motif in human MCF2L-AS1 promoter. (B, C) ChIP assay and dual luciferase reporter gene assay were performed to verify the binding relationship between MCF2L-AS1 promoter and SP1. (D, E) SP1 expression was detected using qRT-PCR and western blot. (F) MCF2L-AS1 expression was determined using qRT-PCR. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
ChIP, chromatin immunoprecipitation; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, normal control; Oe-, overexpression plasmid of; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation.
*p<0.05, †p<0.01, ‡p<0001.
5. LncRNA MCF2L-AS1 knockdown mitigated the promotion effects of SP1 overexpression on cisplatin resistance of ovarian cancer
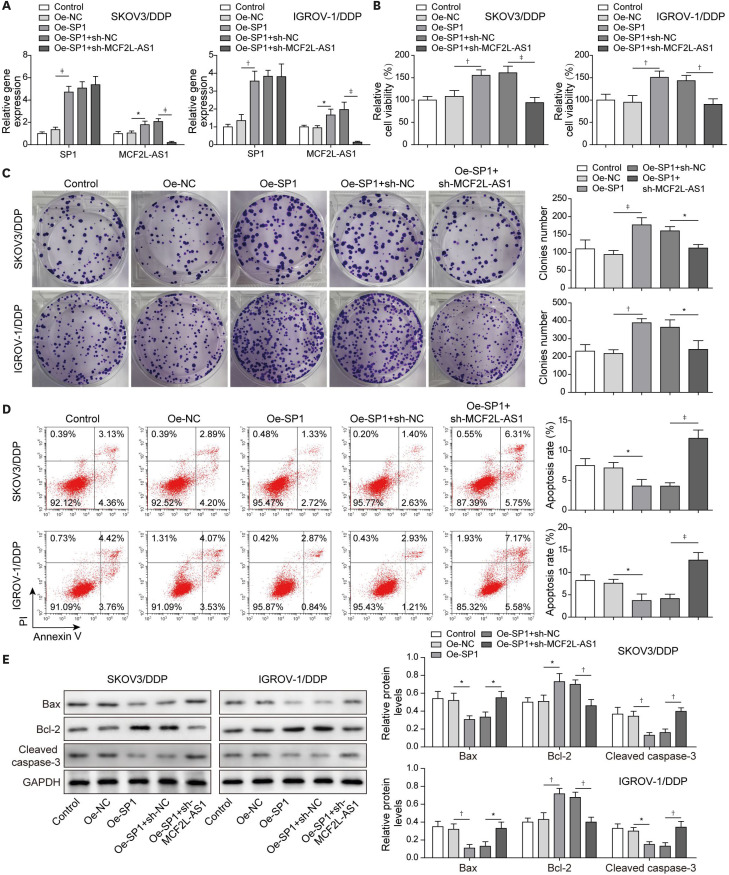
Next, we aimed to probe the effects of the SP1/lncRNA MCF2L-AS1 axis on cisplatin resistance in ovarian cancer. Thus, we co-transfected with oe-SP1 and sh-MCF2L-AS1 in cisplatin-resistant cells. First, SP1 and MCF2L-AS1 expressions significantly increased after SP1 overexpression; MCF2L-AS1 expression in oe-SP1 + sh-MCF2L-AS1 group was markedly reduced compared to oe-SP1 + sh-NC group, while SP1 expression was not significantly different (Fig. 5A). SP1 overexpression remarkably promoted proliferation and colony formation of SKOV3/DDP and IGROV-1/DDP cells, while this phenomenon was abolished by MCF2L-AS1 knockdown (Fig. 5B and C). Cell apoptosis were markedly suppressed by oe-SP1 transfection, while it was eliminated by sh-MCF2L-AS1 transfection in SKOV3/DDP and IGROV-1/DDP cells (Fig. 5D). After SP1 overexpression, Bax and Cleaved Caspase-3 levels in cisplatin-resistant cells were obviously reduced and Bcl-2 level was significantly elevated, while MCF2L-AS1 silencing attenuated this effect (Fig. 5E). In total, MCF2L-AS1 knockdown mitigated the promotion effects of SP1 overexpression on ovarian cancer cisplatin-resistance.
Fig. 5. LncRNA MCF2L-AS1 knockdown mitigated the promotion effects of SP1 overexpression on ovarian cancer cisplatin-resistance.
Cisplatin-resistant cells were transfected with Oe-NC, Oe-SP1, Oe-SP1 + sh-NC and Oe-SP1 + sh-MCF2L-AS1. (A) SP1 and MCF2L-AS1 expressions were assessed using qRT-pCR. (B) CCK-8 assay was employed to evaluate cell proliferation. (C) Colony formation assay was performed to detect colony formation. (D) Cell apoptosis was evaluated by flow cytometry assay. (E) Western blot was employed to determine Bax, Bcl-2 and Cleaved Caspase-3 levels. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
CCK-8, cell counting kit-8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC, normal control; Oe-, overexpression plasmid of; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation; sh-, short hairpin RNA of.
*p<0.05, †p<0.01, ‡p<0.001.
6. LncRNA MCF2L-AS1 knockdown inhibited ovarian cancer cisplatin-resistance in vivo
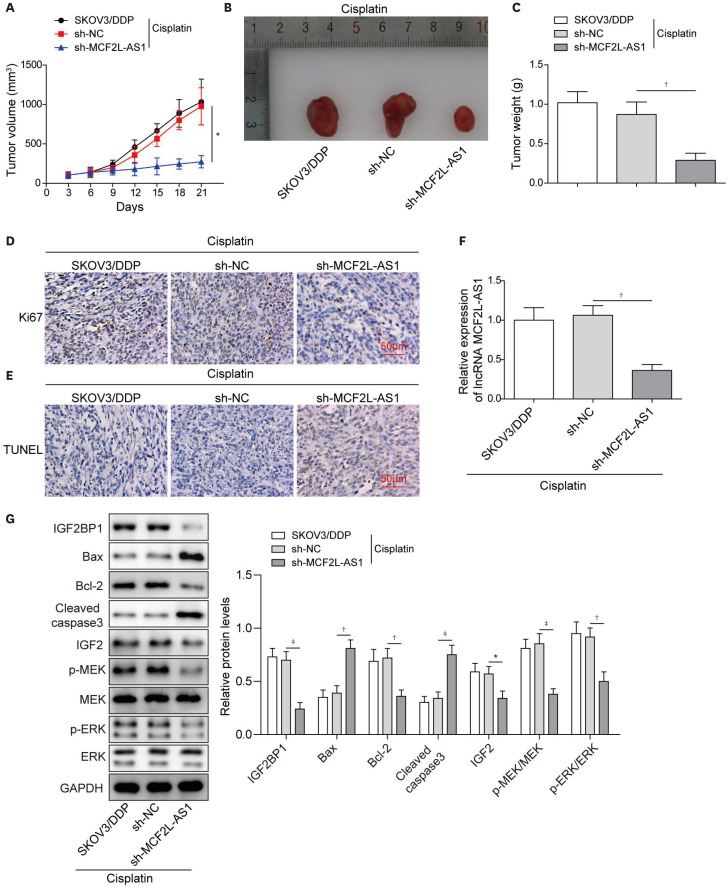
To probe the effect of MCF2L-AS1 on cisplatin resistance in vivo, SKOV3/DDP stably transfected with sh-NC or sh-MCF2L-AS1 were injected into the back of nude mice, and then all mice were received cisplatin treatment. As displayed in Fig. 6A-C, after cisplatin treatment, tumor injected with sh-MCF2L-AS1 grew slower than controls, suggesting that MCF2L-AS1 knockdown promoted the cell cytotoxicity induced by cisplatin treatment in vivo. In addition, IHC displayed that the protein level of Ki67 (proliferation marker) in tumor tissues was obviously decreased following MCF2L-AS1 knockdown (Fig. 6D). TUNEL positive cell in tumor tissues were increased by MCF2L-AS1 knockdown (Fig. 6E). As shown in Fig. 6F, MCF2L-AS1 expression in tumor tissues was significantly reduced after MCF2L-AS1 knockdown. Finally, we observed that Bax and Cleaved Caspase-3 levels increased, while the protein levels of IGF2/MEK/ERK signaling pathway-related proteins, IGF2BP1 and Bcl-2 in tumor tissues were significantly reduced after MCF2L-AS1 knockdown (Fig. 6G). In summary, MCF2L-AS1 silencing suppressed ovarian cancer cisplatin-resistance in vivo.
Fig. 6. LncRNA MCF2L-AS1 knockdown inhibited ovarian cancer cisplatin-resistance in vivo.
SKOV3/DDP cells transfected with sh-MCF2L-AS1 and sh-NC were inoculated into nude mice, and then all mice were received cisplatin treatment. (A-C) The tumors were collected, and the size and weight of tumors were measured. (D) Ki67 level in tumor tissues was evaluated using IHC. (E) Tumor tissues apoptosis was evaluated using TUNEL staining. (F) qRT-PCR was performed to detect MCF2L-AS1 expression in tumor tissues. (G) Western blot was employed to determine Bax, Bcl-2, Cleaved Caspase-3, IGF2BP1, IGF2, p-MEK, MEK, p-ERK and ERK levels in tumor tissues. The data were expressed as mean ± SD (n=8).
ERK, extracellular regulated protein kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IGF2BP1, insulin-like growth factor-2 mRNA binding protein 1; IHC, immunohistochemistry; MEK, mitogen-activated protein kinase kinase; NC, normal control; qRT-PCR, quantitative real-time polymerase chain reaction; SD, standard deviation; sh-, short hairpin RNA of; TUNEL, TdT-mediated dUTP nick-end labeling.
*p<0.05, †p<0.01, ‡p<0.001.
DISCUSSION
For researchers, the mechanism of ovarian cancer cisplatin-resistance is still elusive. Although many molecular medical studies have been performed to explain the mechanism of drug resistance in ovarian cancer, it is difficult to distinguish effective targets for regulating drug resistance. The epigenetic regulation of ovarian cancer may unravel this mystery. Herein, we found that SP1-induced MCF2L-AS1 promoted cisplatin resistance in ovarian cancer through targeting the IGF2BP1/IGF2/MEK/ERK signaling pathway.
The important function of lncRNAs in regulating ovarian cancer cisplatin-resistance is obvious to all. For example, Miao et al. revealed that lncRNA ANRIL reduced the sensitivity of ovarian cancer to cisplatin by targeting miR-let-7a [23]. LncRNA MCF2L-AS1 was previously confirmed as an oncogene in several human malignancies, such as colorectal cancer [24] and lung cancer [12]. However, the role of MCF2L-AS1 in regulating cisplatin resistance in ovarian cancer remains unknown. Herein, our results revealed that MCF2L-AS1 was markedly upregulated in cisplatin-resistant cells. Besides, we found that MCF2L-AS1 silencing remarkably inhibited cell growth and promoted cell apoptosis of ovarian cancer parental cells and cisplatin-resistant cells at the first time. Additionally, our results firstly confirmed that MCF2L-AS1 silencing suppressed cisplatin resistance in ovarian cancer in vivo. It has been widely described that lncRNAs also function in regulating cisplatin sensitivity of ovarian cancer. For instance, Miao et al. revealed that lncRNA ANRIL silencing improved cisplatin-sensitivity of ovarian cancer cells [23]. LncRNA LINC01125 enhanced cisplatin sensitivity of ovarian cancer via acting on miR-1972 [25]. In the present study, our results revealed that the IC50 of SKOV3 and IGROV-1 cells was significantly reduced after MCF2L-AS1 knockdown, suggesting that cisplatin sensitivity in parental cells was enhanced by MCF2L-AS1 silencing. Moreover, MCF2L-AS1 knockdown remarkably inhibited parental cell proliferation and promoted cell apoptosis. Collectively, our results suggested that MCF2L-AS1 downregulation contributed to cisplatin sensitivity of parental cells and impaired cisplatin resistance of cisplatin-resistant cells. Platine-based chemotherapy agents are major drugs in oncology and are currently used in most solid malignancies. Of these, cisplatin and carboplatin have been the most widely used over past years. Many trials performed comparing the efficacy of carboplatin and cisplatin in ovarian cancer, either as single agent first-line therapy or in combination chemotherapy suggest equivalent results [26]. However, superior results in terms of either progression-free survival or overall survival have been obtained with cisplatin in 2 larger randomized studies of combination chemotherapy [26]. Our study aimed to explore the drug resistance mechanism of ovarian cancer, which layed a foundation for clinical research. Considering that the mechanism of action of these 2 drugs for ovarian cancer is similar, and the efficacy of cisplatin is better than that of carboplatin, it is more beneficial to explore the regulatory mechanism of cisplatin resistance in ovarian cancer. In the future, we will also conduct research on carboplatin resistance in ovarian cancer.
Recent studies have suggested that SP1 is a key transcription factor regulating the abnormal expressions of lncRNAs in cancers [27,28]. Meanwhile, it was widely reported that SP1 is a risk factor promoting cisplatin resistance in ovarian cancer [5,29]. Herein, SP1 was confirmed to bind to MCF2L-AS1 promoter by using ChIP and luciferase reporter assays, which was never reported before. In addition, as expected, MCF2L-AS1 knockdown could mitigate the promotion effects of SP1 overexpression on ovarian cancer cisplatin-resistance. For the first time, our study revealed that SP1 contributed to ovarian cancer cisplatin-resistance by activating the transcriptional activity of MCF2L-AS1.
IGF2BP1 is a type of oncofetal RNA binding protein, which is upregulated in a variety of solid tumors, including ovarian cancer [16]. Meanwhile, the high expression of IGF2BP1 in ovarian cancer was also related to cisplatin resistance, specifically IGF2BP1 overexpression could enhance cisplatin resistance in ovarian cancer [30]. In the present study, IGF2BP1 expression was markedly elevated in SKOV3/DDP and IGROV-1/DDP cells. Moreover, this current report was the first one to indicate that MCF2L-AS1 could interact with IGF2BP1 protein. Our results revealed that MCF2L-AS1 knockdown significantly reduced IGF2BP1 protein level but not the mRNA level in cisplatin-resistant cells. It was suggested that MCF2L-AS1 might be involved in regulating the post-transcriptional level of IGF2BP1 through other regulatory mechanisms, such as acetylation, ubiquitination and other modifications. As reported, lncRNA NEAT1 could regulate the DDX5 protein to enhance its stability in colorectal cancer cells [31]. In addition, lncRNA HOTAIR regulated the ATXN1 protein but not the mRNA by ubiquitination [32]. However, the specific regulatory mechanism of MCF2L-AS1 on the post-transcriptional level of IGF2BP1 remains unknown and needs further study. By analyzing the functions in depth, it was evident that IGF2BP1 overexpression promoted cisplatin resistance in cisplatin-resistant cells and reversed the effects of MCF2L-AS1 silencing on cisplatin resistance. Moreover, our results revealed that IGF2BP1 overexpression resulted in increased IC50 of SKOV3 and IGROV-1 cells, suggesting that cisplatin sensitivity in parental cells was weakened by IGF2BP1 overexpression. According to reports, IGF2BP1 usually achieves its biological function in cancers by regulating the expression of target mRNA. IGF2, one of the targets of IGF2BP1, is associated with an acquired drug resistance in cancer [33]. Yang et al. [20] also revealed that IGF2BP1 could promote cell proliferation ongue squamous cell carcinoma cells through the activation of IGF2/MEK/ERK signaling pathway. Herein, we found that MCF2L-AS1 silencing markedly inhibited IGF2 level, as well as MEK/ERK signaling pathway-related proteins (p-MEK and pERK). Therefore, we speculated that lncRNA MCF2L-AS1 promoted cisplatin resistance in ovarian cancer through regulating IGF2BP1/IGF2/MEK/ERK axis.
In summary, our study displayed that SP1-induced MCF2L-AS1 promoted ovarian cancer cisplatin-resistance through activation of IGF2/MEK/ERK pathway via interacting with IGF2BP1, which illustrated that MCF2L-AS1 had a potential therapeutic value for the treatment of cisplatin resistance in ovarian cancer patients.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Z.Y., C.Y.
- Data curation: Z.Y., Y.L.
- Formal analysis: Z.Y.
- Investigation: Z.Y., W.J.
- Supervision: C.Y.
- Writing - original draft: Z.Y., L.Y.
- Writing - review & editing: L.Y., C.Y.
SUPPLEMENTARY MATERIALS
SKOV3 and IGROV-1 cells were transfected with sh-NC or sh-MCF2L-AS1. (A) MCF2L-AS1 expression was determined using qRT-PCR. (B) IC50 was evaluated using CCK-8 assay. (C) CCK-8 assay was employed to evaluate cell proliferation. (D) Cell apoptosis was evaluated by flow cytometry assay. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
SKOV3 and IGROV-1 cells were transfected with Oe-NC or Oe-IGF2BP1, and IC50 was evaluated using CCK-8 assay. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
References
- 1.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vargas-Hernández VM, Moreno-Eutimio MA, Acosta-Altamirano G, Vargas-Aguilar VM. Management of recurrent epithelial ovarian cancer. Gland Surg. 2014;3:198–202. doi: 10.3978/j.issn.2227-684X.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safe S, Abbruzzese J, Abdelrahim M, Hedrick E. Specificity protein transcription factors and cancer: opportunities for drug development. Cancer Prev Res (Phila) 2018;11:371–382. doi: 10.1158/1940-6207.CAPR-17-0407. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Li Y, Sun S, Cai J, Cao J. Sp1 promotes ovarian cancer cell migration through repressing miR-335 expression. Biochem Biophys Res Commun. 2020;524:211–216. doi: 10.1016/j.bbrc.2020.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Yan G, Lei J, Chen Y, Wang T, Gong J, et al. The SP1-12LOX axis promotes chemoresistance and metastasis of ovarian cancer. Mol Med. 2020;26:39. doi: 10.1186/s10020-020-00174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang H, Yu T, Han Y, Jiang H, Wang C, You T, et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer. 2018;17:119. doi: 10.1186/s12943-018-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JY, Lu AQ, Chen LJ. LncRNAs in ovarian cancer. Clin Chim Acta. 2019;490:17–27. doi: 10.1016/j.cca.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Xu QF, Tang YX, Wang X. LncRNA EBIC promoted proliferation, metastasis and cisplatin resistance of ovarian cancer cells and predicted poor survival in ovarian cancer patients. Eur Rev Med Pharmacol Sci. 2018;22:4440–4447. doi: 10.26355/eurrev_201807_15495. [DOI] [PubMed] [Google Scholar]
- 10.Tan WX, Sun G, Shangguan MY, Gui Z, Bao Y, Li YF, et al. Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling. Sci Rep. 2020;10:14768. doi: 10.1038/s41598-020-71153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang FK, Zheng CY, Huang LK, Lin CQ, Zhou JF, Wang JX. Long non-coding RNA MCF2L-AS1 promotes the aggressiveness of colorectal cancer by sponging miR-874-3p and thereby up-regulating CCNE1. J Gene Med. 2021;23:e3285. doi: 10.1002/jgm.3285. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Lin L. Long noncoding RNA MCF2L-AS1 promotes the cancer stem cell-like traits in non-small cell lung cancer cells through regulating miR-873-5p level. Environ Toxicol. 2021;36:1457–1465. doi: 10.1002/tox.23142. [DOI] [PubMed] [Google Scholar]
- 13.Cui PH, Li ZY, Li DH, Han SY, Zhang YJ. SP1-induced lncRNA DANCR contributes to proliferation and invasion of ovarian cancer. Kaohsiung J Med Sci. 2021;37:371–378. doi: 10.1002/kjm2.12316. [DOI] [PubMed] [Google Scholar]
- 14.Bell JL, Wächter K, Mühleck B, Pazaitis N, Köhn M, Lederer M, et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70:2657–2675. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Zhang H, Guo X, Zhu Z, Cai H, Kong X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J Hematol Oncol. 2018;11:88. doi: 10.1186/s13045-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bley N, Schott A, Müller S, Misiak D, Lederer M, Fuchs T, et al. IGF2BP1 is a targetable SRC/MAPK-dependent driver of invasive growth in ovarian cancer. RNA Biol. 2021;18:391–403. doi: 10.1080/15476286.2020.1812894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hämmerle M, Gutschner T, Uckelmann H, Ozgur S, Fiskin E, Gross M, et al. Posttranscriptional destabilization of the liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding protein 1 (IGF2BP1) Hepatology. 2013;58:1703–1712. doi: 10.1002/hep.26537. [DOI] [PubMed] [Google Scholar]
- 18.Ji Y, Wang Z, Chen H, Zhang L, Zhuo F, Yang Q. Serum from chronic hepatitis B patients promotes growth and proliferation via the IGF-II/IGF-IR/MEK/ERK signaling pathway in hepatocellular carcinoma cells. Cell Physiol Biochem. 2018;47:39–53. doi: 10.1159/000489744. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20:R321–R339. doi: 10.1530/ERC-13-0231. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Fu G, Liu F, Hu C, Lin J, Tan Z, et al. LncRNA THOR promotes tongue squamous cell carcinomas by stabilizing IGF2BP1 downstream targets. Biochimie. 2019;165:9–18. doi: 10.1016/j.biochi.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Wei X, Shi J, Lin Q, Ma X, Pang Y, Mao H, et al. Targeting ACLY attenuates tumor growth and acquired cisplatin resistance in ovarian cancer by inhibiting the PI3K-AKT pathway and activating the AMPK-ROS pathway. Front Oncol. 2021;11:642229. doi: 10.3389/fonc.2021.642229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Huo X, Yang XR, He J, Cheng L, Wang N, et al. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer. 2017;16:136. doi: 10.1186/s12943-017-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao JT, Gao JH, Chen YQ, Chen H, Meng HY, Lou G. LncRNA ANRIL affects the sensitivity of ovarian cancer to cisplatin via regulation of let-7a/HMGA2 axis. Biosci Rep. 2019;39:BSR20182101. doi: 10.1042/BSR20182101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Zhang Z, Yang W, Li N, Chen X, Ma F, Yang J, et al. LncRNA MCF2L-AS1 aggravates proliferation, invasion and glycolysis of colorectal cancer cells via the crosstalk with miR-874-3p/FOXM1 signaling axis. Carcinogenesis. 2021;42:263–271. doi: 10.1093/carcin/bgaa093. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Pan H. Long noncoding RNA LINC01125 enhances cisplatin sensitivity of ovarian cancer via miR-1972. Med Sci Monit. 2019;25:9844–9854. doi: 10.12659/MSM.916820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermorken JB, ten Bokkel Huinink WW, Eisenhauer EA, Favalli G, Belpomme D, Conte PF, et al. Advanced ovarian cancer. Carboplatin versus cisplatin. Ann Oncol. 1993;4(Suppl 4):41–48. [PubMed] [Google Scholar]
- 27.Wang ZQ, Cai Q, Hu L, He CY, Li JF, Quan ZW, et al. Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis. 2017;8:e2839. doi: 10.1038/cddis.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang S, Sun L, Feng G. SP1-mediated long noncoding RNA POU3F3 accelerates the cervical cancer through miR-127-5p/FOXD1. Biomed Pharmacother. 2019;117:109133. doi: 10.1016/j.biopha.2019.109133. [DOI] [PubMed] [Google Scholar]
- 29.Zou W, Ma X, Yang H, Hua W, Chen B, Cai G. Hepatitis B X-interacting protein promotes cisplatin resistance and regulates CD147 via Sp1 in ovarian cancer. Exp Biol Med (Maywood) 2017;242:497–504. doi: 10.1177/1535370216685007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin X, Sun L, Wang J. Restoration of microRNA-708 sensitizes ovarian cancer cells to cisplatin via IGF2BP1/Akt pathway. Cell Biol Int. 2017;41:1110–1118. doi: 10.1002/cbin.10819. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan C, et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11:113. doi: 10.1186/s13045-018-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon JH, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga-Yamanaka K, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa T, Ogawa K, Shiga K, Furukawa T, Nagase H, Hashimoto S, et al. Upregulation of IGF2 is associated with an acquired resistance for cis-diamminedichloroplatinum in human head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2010;267:1599–1606. doi: 10.1007/s00405-010-1257-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SKOV3 and IGROV-1 cells were transfected with sh-NC or sh-MCF2L-AS1. (A) MCF2L-AS1 expression was determined using qRT-PCR. (B) IC50 was evaluated using CCK-8 assay. (C) CCK-8 assay was employed to evaluate cell proliferation. (D) Cell apoptosis was evaluated by flow cytometry assay. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.
SKOV3 and IGROV-1 cells were transfected with Oe-NC or Oe-IGF2BP1, and IC50 was evaluated using CCK-8 assay. The data were expressed as mean ± SD. All data were obtained from at least 3 replicate experiments.