Abstract
Background
Sentinel lymph node (SLN) mapping has been suggested as an alternative surgical technique to full lymphadenectomy for early-stage endometrial cancer. However, the survival outcomes of SLN mapping compared with lymphadenectomy have not been established via a prospective study.
Methods
A multi-center, single-blind, randomized controlled trial has been designed to determine the prognostic value of SLN mapping alone compared with conventional lymphadenectomy for patients with clinical stage I-II endometrial cancer. Eligible participants will be randomly assigned in a 1:1 ratio between the group to undergo SLN mapping using indocyanine green and the conventional lymph node dissection group. A high-risk group will undergo a 2-step SLN mapping procedure.
The primary endpoint is the 3-year disease-free survival (DFS). The secondary endpoints are 3-year overall survival (OS), 5-year DFS, 5-year OS after surgery, pattern of recurrence, immediate surgical outcomes, success rate of SLN mapping, postoperative lymph-related complications, postoperative quality of life, and postoperative cost effectiveness. The role of pathologic ultrastaging of SLNs will also be assessed.
Trial Registration
ClinicalTrials.gov Identifier (NCT number): NCT04845828
Keywords: Endometrial Cancer, Sentinel Lymph Node, Indocyanine Green, Lymphadenectomy, Prognosis
INTRODUCTION
According to the 2015 Cancer Statistical Report of the American Cancer Society, endometrial cancer ranks fourth in terms of cancer incidence among women and first among female genital cancers [1]. Standardized treatment for early endometrial cancer consists of total hysterectomy, bilateral salpingo-oophorectomy, and lymph node dissection (LND), depending on risk factors [2]. Although pelvic LND is helpful for staging and preparation for adjuvant treatment, its therapeutic effect has not been proven [3,4]. In 2 recent randomized controlled trials, routine pelvic LND did not improve survival in early endometrial cancer [3,4]. Moreover, LND can cause complications in many patients and is associated with reduced quality of life (QOL) [5]. Therefore, it is important to develop a method that can reveal the status of lymph nodes in a less invasive way.
Efforts to develop a sentinel lymph node (SLN) mapping strategy for endometrial cancer and cervical cancer are continuing [6,7,8]. In a recent large-scale prospective endometrial cancer study, SLN mapping using indocyanine green (ICG) and fluorescent imaging was successful in 86% of cases, and its sensitivity (patient-by-patient analysis) for diagnosing lymph node metastasis was reported to be 100%, with a 3% false negative rate [6]. Given the encouraging finding that the accuracy of SLN mapping for endometrial cancer has been similar to that associated with breast cancer or vulvar cancer, it has been proposed that SLN mapping should be included in routine treatment of endometrial cancer [6]. According to a recent National Comprehensive Cancer Network guideline, SLN mapping is recommended in the treatment of endometrial cancer, including for high-risk patients [2].
Laparoscopic surgery and robotic surgery have become part of the management of most endometrial cancer patients, and this has introduced a conducive environment for performing SLN mapping using ICG. Consequently, the procedure’s sensitivity and detection rate have improved compared with previous methods [9]. To date, there have been no randomized trials investigating the effects of SLN mapping alone on prognostic outcomes among endometrial cancer patients; therefore, a prospective study is necessary. We aim to compare the survival outcomes between patients subjected to SLN mapping vs. those treated with LND for clinical stage I-II endometrial cancer.
MATERIALS AND METHODS
1. Objectives
This study aims to compare outcomes between patients who undergo SLN mapping vs. those who undergo conventional LND for preoperative clinical stage I-II endometrial cancer.
2. Endpoints
The primary endpoint is the 3-year disease-free survival (DFS). The secondary endpoints are 3-year overall survival (OS), 5-year DFS, 5-year OS after surgery, pattern of recurrence, immediate surgical outcomes, SLN mapping success rate, postoperative lymph-related complications, postoperative QOL, and the cost-effectiveness of SLN mapping vs. LND.
3. Trial design
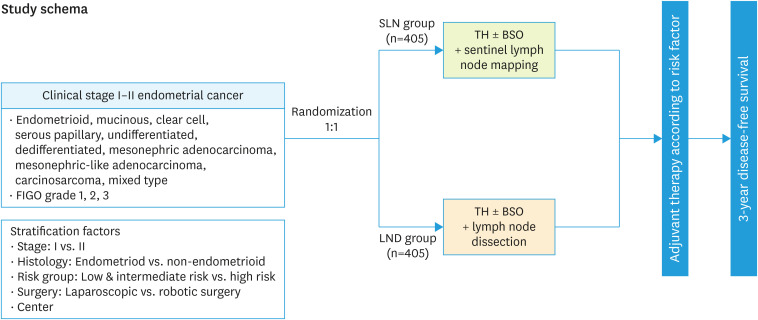
This multi-center, single-blind, randomized controlled trial is a Korean Gynecologic Oncology Group (KGOG) 2029 trial. The leading research institute is Asan Medical center, and 24 institutions in Korea are participating. All institutions are members of the KGOG (Fig. 1).
Fig. 1. Trial schema.
BSO, bilateral salpingo-oophorectomy; FIGO, International Federation of Obstetrics and Gynecology; LND, lymph node dissection; SLN, sentinel lymph node; TH, total hysterectomy.
4. Participants
Eligibility criteria
Study patients must satisfy all of the following selection criteria: 1) woman aged 20–80 years; 2) previously untreated histologically diagnosed endometrial cancer; 3) histological type is endometrioid, mucinous, serous, clear cell, undifferentiated, dedifferentiated, mesonephric adenocarcinoma, mesonephric-like adenocarcinoma, carcinosarcoma, or mixed type; 4) histological differentiation graded as 1, 2, or 3; 5) preoperative clinical stage I-II according to the International Federation of Obstetrics and Gynecology (FIGO) staging system; 6) planning to undergo laparoscopic or robotic hysterectomy and pelvic LND; 7) pelvic and para-aortic lymph nodes less than 15 mm in preoperative magnetic resonance imaging (MRI) or computed tomography (CT); 8) Eastern Cooperative Oncology Group (ECOG) performance status 0–2; 9) and American Society of Anesthesiologists (ASA) physical status classification 0–2. 10) For patients with adequate organ function, the following inclusion criteria will apply: white blood cell count ≥3,000/mm3; platelet count ≥100,000/mm3; creatinine ≤2.0 mg/dL; bilirubin ≤1.5× the institutional upper normal limit; and serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, and alkaline phosphatase ≤3× the institutional upper limit normal. 11) Finally, patients must voluntarily provide written informed consent to participate in the study.
Exclusion criteria
Patients meeting any of the following exclusion criteria will be ineligible to participate: 1) predicted FIGO stages III-IV; 2) neuroendocrine tumor histology; 3) disease involving the lymphatic system; 4) lymphedema of the lower extremities or perineum; 5) previous pelvic or para-aortic LND; 6) history of radiation therapy to the abdominal cavity or pelvic area; 7) history of chemotherapy for tumors in the abdominal cavity or pelvis; 8) history of having received cancer treatment within 5 years, except for non-melanoma skin cancer, cervical intraepithelial tumor, and superficial cancer of the stomach or bladder; 9) serious underlying disease or complications; 10) hypersensitivity to ICG; 11) pregnant or breastfeeding.
Surgeon proficiency criteria
All participating surgeons must submit unedited surgical video of SLN mapping to the research center, and they should be evaluated according to the evaluation criteria.
5. Sample size
The primary endpoint of this study is the 3-year DFS after surgery, and the study aims to confirm that the 3-year DFS rate associated with SLN mapping is non-inferior to that associated with conventional LND for early endometrial cancer patients. Referring to previous studies, we predicted the 3-year DFS rate associated with traditional lymphadenectomy to be 87% [4,10], and assuming an exponential distribution of the survival function, we calculated the hazard rate as 0.0039. For this study, the non-inferiority hazard ratio was be set to 1.6 (non-inferiority margin of 3-year DFS=7%) to confirm the non-inferiority of the 3-year DFS rate associated with SLN mapping compared with conventional LND. Accrual time will be 36 months, follow-up time will be 36 months, and the level of significance is α=0.025 (1-sided). Type II error, β=0.2, that is, power=80%. The sample size calculation specifies that 375 patients per group will be required, for a total of 750 patients. Considering a dropout rate of 10%, we intend to enroll 405 patients per group, for a total of 810 patients.
6. Randomization and blinding
If the selection criteria are satisfied, randomization will be performed in a 1:1 ratio between the SLN mapping group and the conventional LND group. A randomization table will be prepared using SAS (SAS Institute, Cary, NC, USA) software by an independent statistician, and study patients will be assigned using the stratified block randomization method with the following stratification factors: 1) stage (stage I vs. II), 2) histological type (endometrioid vs. non-endometrioid), 3) risk of lymph node metastasis (low/intermediate-risk group vs. high-risk group), 4) surgical method (laparoscopic surgery vs. robotic surgery), and 5) participating research institutes. This will be a single-blind study, meaning that the study patients will not know which group they belong to (SLN or LND).
7. Surgical treatment
Surgical treatment will be performed within 4 weeks of randomization. Both laparoscopic and robotic laparoscopic procedures are possible. In the case of laparoscopic surgery, both single-port surgery and multi-port surgery are possible. The type of robotic surgical equipment for robotic laparoscopic surgery is irrelevant, and both single-site surgery and multi-port surgery are possible.
Classification of patients into risk groups according to their risk of lymph node metastasis
Study patients will be classified into low/intermediate-risk and high-risk groups according to the risk of lymph node metastasis. High-risk patients are those with at least 1 of the following 3 criteria on preoperative biopsy and MRI: 1) High-grade histology (endometrioid grade 3, serous papillary, clear cell, undifferentiated, dedifferentiated, mesonephric adenocarcinoma, mesonephric-like adenocarcinoma, carcinosarcoma, and mixed type with high-grade histology); 2) myometrial invasion >50% on MRI; or 3) cervical invasion on MRI).
SLN mapping group
The SLN mapping group will use ICG and fluorescence imaging. Before starting SLN mapping, intraperitoneal washing cytology will be performed, and bilateral tubal ligation will be performed on the ampulla. At this time, if the isthmic portion is ligated, the lymphatic vessels coming out of the uterus can be ligated together, so ligation should be performed as close to the tip of the fallopian tube as possible. Patients in the low/intermediate-risk group will only undergo pelvic SLN mapping, and cervical injection methods will be used as described below. Patients in the high-risk group will undergo 2-step SLN mapping consisting of para-aortic SLN mapping (first step) and pelvic SLN mapping (second step) [11]. For the first step, 1.25 mg/mL ICG will be injected into the cornual area (0.5 to 1 cm in depth) on both sides of the uterine body (3 mL each for a total of 6 mL), and the para-aortic SLNs will be harvested first. SLNs will be sent for histopathological examination. In the para-aortic stations wherein mapping is not achieved, even after 20 minutes have passed after injecting ICG and massaging the uterus and lymphatic vessels to move the lymphatic fluid, re-injecting of ICG into the cornual area of the ipsilateral uterine corpus for mapping will be implemented. If mapping is not achieved after 2 injections, this will be considered a mapping failure, and para-aortic LND will be performed.
Then, for the second step, ICG with a concentration of 1.25 mg/mL will be administered at the 3 o'clock and 9 o'clock positions of the cervix: submucosal (1-3 mm in depth) 1 mL and intrastromal (1–2 cm in depth) 1 mL, respectively, for a total of 4 mL. After ICG injection into the cervix, a uterine manipulator can be installed if necessary. First, without opening the peritoneum, guided by a fluorescence image, the lymph fluid will be observed to be drained. If the fluorescent lymph fluid does not drain after about 20 minutes, ICG will be injected into the cervix again. When the lymphatic vessels on both sides of the pelvis are sufficiently dyed with fluorescent fluid as the fluorescent-colored lymph flows, the pelvic peritoneum covering the pelvic lymph nodes will be incised. Careful dissection of the pelvic space will be performed so as not to damage the fine lymphatic vessels and to facilitate the observation of lymphatic flow from the uterus. In each hemipelvis, the lymph nodes directly connected to the lymphatic vessels emanating from the cervix will be defined as SLNs. Then, pelvic SLNs will be identified and harvested. For any hemipelvis that is not mapped, even 20 minutes after ICG injection, mapping via re-injection of ICG into the cervix on the ipsilateral side will be performed. If mapping is not achieved after 2 injections, this will be considered mapping failure, and LND will be performed for the hemipelvis.
In addition to SLNs, if there are lymph nodes larger than 1 cm in short diameter or lymph nodes suspected to be metastatic, they must be removed together and sent as pathological “non-sentinel enlarged lymph nodes.”
All SLNs retrieved will be evaluated using an ultrastaging protocol.
Conventional LND group
In the conventional LND group, only pelvic LND will be performed for the low/intermediate-risk group, and pelvic and para-aortic LND will be performed for the high-risk group.
After ligation of the fallopian tubes on both sides before starting lymphadenectomy, in the case of laparoscopic or robotic laparoscopic surgery, if necessary, a uterine manipulator will be applied, and peritoneal cytology will be performed. LND will be performed as specified in the KGOG Surgical Manual [12].
Surgical staging
In the SLN mapping group, total hysterectomy and bilateral salpingo-oophorectomy will be performed after the SLN mapping is completed. In the LND group, after traditional lymphadenectomy, total hysterectomy and bilateral salpingo-oophorectomy will be performed.
In the case of laparoscopic surgery or robotic laparoscopic surgery, total hysterectomy is possible with both total laparoscopic hysterectomy and laparoscopically assisted vaginal hysterectomy. For women with stage I disease and women under the age of 40 years, ovarian preservation can be performed. In this case, both salpinges will be removed. For women with stage II disease, radical hysterectomy will be performed. For serous carcinoma, clear cell carcinoma, and carcinosarcoma, omental biopsy will be performed.
8. Adjuvant treatment
Adjuvant therapy will be offered according to the Korean Society of Gynecological Oncology (KSGO) treatment guideline, considering the pathologic risk factors after surgery [13].
Chemotherapy will be started 3 weeks after surgery, and radiation therapy will be started within 4 weeks after surgery. In stage I patients, aged 60 years or older, lymph-vascular space invasion, tumor size, and lower uterine involvement are established as risk factors, and adjuvant treatment can be subdivided according to the presence of these risk factors. For stage IA with no or less than half of the myometrial invasion, patients with no risk factors can choose between follow-up (grade 1) or follow-up or vaginal brachytherapy (grade 2–3). If there are risk factors, follow-up or vaginal brachytherapy will be performed for patients with grade 1 differentiation, and for patients with grade 2–3 differentiation, the choice will be between follow-up or vaginal brachytherapy and/or pelvic irradiation. If there are no risk factors in stage IB patients with myometrial wall invasion greater than half, follow-up or vaginal brachytherapy will be performed for grade 1–2 patients, and vaginal brachytherapy and/or pelvic irradiation is recommended for grade 3, and follow-up is also considered. If there are risk factors in stage IB patients, follow-up or vaginal brachytherapy and/or pelvic irradiation is recommended for grades 1–2, and pelvic irradiation and/or vaginal brachytherapy ± chemotherapy is preferred for grade 3.
For stage II patients with surgical staging, postoperative adjuvant treatment is combined with pelvic radiation therapy ± vaginal brachytherapy for grades 1–2, and pelvic radiation therapy ± vaginal brachytherapy for grade 3 with chemotherapy can be optionally administered. For patients with only benign ascites cancer cells, follow-up is recommended for grades 1–2. For endometrial cancer with benign ascites, cancer cells, and grade 3 endometrial cancer, the choice is between follow-up or vaginal brachytherapy or pelvic radiotherapy ± vaginal brachytherapy ± chemotherapy. For stage III patients with surgical staging, chemotherapy or chemoradiation is recommended over radiotherapy alone. Adjuvant treatment is not considered for patients with only isolated tumor cells of the lymph node. In the case of lymph node micrometastasis, it is up to the physician to decide whether to administer adjuvant treatment. Chemotherapy is recommended for adjuvant treatment after surgery for stage IV patients who have undergone surgical staging. For serous papillary carcinoma, clear cell carcinoma, and carcinosarcoma, postoperative adjuvant treatment may include follow-up or chemotherapy, or it may include pelvic radiation if the lesion is confined to the endometrium. Chemotherapy and/or pelvic radiation therapy are performed for patients with myometrial invasion or for patients with advanced disease.
9. Postoperative follow-up
Follow-up will be performed every 3 months for 3 years after surgery. History taking, examination, and CA 125 testing will be performed every 3 months, and Pap smears will be performed as needed. Abdominopelvic CT + chest CT or chest X-ray (CXR) will be performed every 6 months. Ultrasound, MRI, and positron emission tomography (PET)-CT can be performed if necessary. Imaging studies, including APCT + chest CT or CXR, will start from the first 3-4 weeks after surgery and will also be conducted at the end of follow-up treatment. If there are symptoms or signs of recurrence, the tests will be performed regardless of the period.
10. Statistical analysis
We plan to form a Data Safety Monitoring Board (DSMB) for professional review of safety data for this study. The DSMB will evaluate the safety-related matters of registered study patients during the entire study period and provide advice on research progress, and it will operate through the KGOG. During this study, a total of 3 interim analyses will be conducted to review safety outcomes (adverse reactions), and the results of the interim analysis will be reviewed by DSMB. The first interim analysis will be performed when 200 study patients are enrolled.
A second interim analysis will be performed when 400 patients are enrolled. The third interim analysis will be performed when participant enrollment is complete.
For each interim analysis, safety analysis including adverse events will be performed, and in the second interim analysis, the proportions of participants enrolled (stratified by lymph node metastasis risk level) will be reviewed, and a decision will be made whether to adjust the high-risk group registration rate in the future. In the third interim analysis, data on complications related to surgery during and after surgery will also be reviewed. Since all 3 interim analyses will be for safety monitoring, type I error correction due to multiple tests will not be required. All target patients will be registered, and final analyses will be performed on data for patients who have completed or dropped out of follow-up for 3 years. In the interim analysis and final analysis, evaluations of patient characteristics, complication analyses, and safety analyses will be included, and efficacy analysis will be performed only at the final analysis.
The demographic data and baseline characteristics of all patients enrolled in this study will be summarized by group, continuous variables will be summarized using descriptive statistics, and categorical variables will be summarized using frequencies and percentages.
To compare the differences between groups, t-tests or the Wilcoxon rank sum test will be performed for continuous variables. For categorical variables, the chi-square test or Fisher’s exact test will be performed.
The ratio of complications that occurred during and after surgery will be presented according to study group, and the chi-square test or Fisher’s exact test will be performed to determine if there will be any intergroup differences in the incidence of complications.
For the 3-year DFS rate after surgery, which is the primary efficacy endpoint, the survival curves and medians for each group (generated and calculated using the Kaplan-Meier method), along with 95% confidence intervals, will be presented. Additionally, the 95% confidence intervals of the hazard ratios will be presented, and if the upper limit of the confidence interval is less than the non-inferiority hazard ratio, 1.6, SLN mapping will be judged to be non-inferior to conventional LND.
Additionally, subgroup analysis will be performed for each stratification factor to analyze whether there are intergroup differences in terms of the 3-year DFS rate after surgery.
For secondary efficacy endpoints, using the Kaplan-Meier method, we will present survival curves and median values for each group, as well as 95% confidence intervals. For adverse events, the number of affected participants, expression rate (%), and number of occurrences for each group (for adverse reactions, adverse drug reactions, and serious adverse reactions) will be presented, and 95% confidence intervals for the expression rates will be presented. The chi-square test or Fisher’s exact test will be performed to test whether there is a difference in the incidence of adverse events between groups. If necessary, t-tests or the Wilcoxon rank sum test can be performed for continuous data to determine whether there are differences in other safety endpoints between groups, and the χ2 test or Fisher’s exact test can be performed for categorical variables.
11. Ethics
This trial will be conducted in accordance with the pharmaceutical clinical trial management standards (KFDA Notice No. 2008-39) and the Declaration of Helsinki, 59th World Medical Association General Assembly, Seoul, October 2008, and International Council for Harmonisation Good Clinical Practice standards.
DISCUSSION
This trial aims to determine if the prognostic value of SLN mapping alone is not inferior to that of conventional lymphadenectomy for clinical stage I-II endometrial cancer. Moreover, immediate surgical complications, postoperative lymph-related complications, postoperative QOL, and the cost-effectiveness of SLN mapping will be investigated. The role of pathologic ultrastaging of SLNs will also be assessed.
Similar to our study, there are currently ongoing studies, such as the SNEC trial and the ENDO-3 trial, evaluating the oncologic outcomes of SLN mapping alone in the treatment of endometrial cancer [14,15]. The SNEC trial is a multi-center, open-label, randomized controlled trial to evaluate whether SLN mapping alone, based on National Comprehensive Cancer Network (NCCN) guidelines, is non-inferior (in terms of progression) to lymphadenectomy for intermediate/high-risk endometrial cancer clinically confined to the uterus [14]. Another phase III, open-label, randomized clinical trial aims to investigate if SLN biopsy alone using ICG can achieve non-inferiority compared with no retroperitoneal lymphadenectomy for early-stage endometrial cancer [15].
In this study, a 2-step SLN mapping procedure with ICG will be performed specifically for high-risk endometrial cancer patients with clinical stage I-II. The 2-step SLN mapping technique has been proposed to improve the intraoperative localization of para-aortic SLNs and has shown feasibility in a recent study [11]. Based on the results of the study outlined herein, we can also identify the efficacy of 2-step SLN mapping.
All the participants in this study should receive adjuvant treatment according to KSGO guidelines, which are consistent with the recent NCCN guidelines. In the control group, the high-risk patients will undergo pelvic and para-aortic LND. Although para-aortic LND is not mandatory in the guidelines, the expert board of this trial strongly recommended para-aortic LND for high-risk endometrial cancer.
Our multi-center, single-blind, randomized controlled trial will rely on collaboration between 24 institutions to achieve a large sample size. All centers are members of the KGOG and are accredited as facilities that provide high-quality surgery. This trial will provide a high level of evidence about the prognostic value of SLN mapping alone using ICG compared with conventional lymphadenectomy in the treatment of early-stage endometrial cancer.
Footnotes
Funding: This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC21C0012).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: P.J.Y., B.M.H., P.E., K.S.W.
- Funding acquisition: P.J.Y.
- Methodology: P.J.Y., K.J.H., B.M.H., P.E., K.S.W.
- Supervision: P.J.Y.
- Writing - original draft: P.J.Y., K.J.H., B.M.H., P.E., K.S.W.
- Writing - review & editing: P.J.Y., K.J.H., B.M.H., P.E., K.S.W.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Rustum NR, Yashar CM, Bradley K, Campos SM, Chon HS, Chu C, et al. Uterine neoplasm, version 2.2020, NCCN clinical practice guidelines in oncology. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. [Google Scholar]
- 3.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK ASTEC Study Group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 5.Bae HS, Lim MC, Lee JS, Lee Y, Nam BH, Seo SS, et al. Postoperative lower extremity edema in patients with primary endometrial cancer. Ann Surg Oncol. 2016;23:186–195. doi: 10.1245/s10434-015-4613-1. [DOI] [PubMed] [Google Scholar]
- 6.Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 7.Balaya V, Guani B, Morice P, Querleu D, Fourchotte V, Leblanc E, et al. Long-term oncological safety of sentinel lymph node biopsy in early-stage cervical cancer: a post-hoc analysis of SENTICOL I and SENTICOL II cohorts. Gynecol Oncol. 2021;164:53–61. doi: 10.1016/j.ygyno.2021.10.074. [DOI] [PubMed] [Google Scholar]
- 8.Frumovitz M, Plante M, Lee PS, Sandadi S, Lilja JF, Escobar PF, et al. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018;19:1394–1403. doi: 10.1016/S1470-2045(18)30448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagen B, Valla M, Aune G, Ravlo M, Abusland AB, Araya E, et al. Indocyanine green fluorescence imaging of lymph nodes during robotic-assisted laparoscopic operation for endometrial cancer. A prospective validation study using a sentinel lymph node surgical algorithm. Gynecol Oncol. 2016;143:479–483. doi: 10.1016/j.ygyno.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810–1818. doi: 10.1200/JCO.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eoh KJ, Lee YJ, Kim HS, Lee JY, Nam EJ, Kim S, et al. Two-step sentinel lymph node mapping strategy in endometrial cancer staging using fluorescent imaging: a novel sentinel lymph node tracer injection procedure. Surg Oncol. 2018;27:514–519. doi: 10.1016/j.suronc.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Korean Gynecologic Oncology Group. Surgical manual for gynecologic oncology [Internet] Seoul: Korean Gynecologic Oncology Group; 2016. [cited 2022 May 16]. Available from: http://www.kgog.org/popup/file/Surgical_manual_of_KGOG.pdf. [Google Scholar]
- 13.Korean Society of Gynecologic Oncology. Practice guideline for uterine corpus cancer [Internet] Seoul: Korean Society of Gynecologic Oncology; 2020. [cited 2022 May 16]. Available from: http://cdn.medsoft.co.kr/201/date/guid_04_03.pdf. [Google Scholar]
- 14.Guan J, Xue Y, Zang RY, Liu JH, Zhu JQ, Zheng Y, et al. Sentinel lymph Node mapping versus systematic pelvic lymphadenectomy on the prognosis for patients with intermediate-high-risk Endometrial Cancer confined to the uterus before surgery: trial protocol for a non-inferiority randomized controlled trial (SNEC trial) J Gynecol Oncol. 2021;32:e60. doi: 10.3802/jgo.2021.32.e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obermair A, Nicklin J, Gebski V, Hayes SC, Graves N, Mileshkin L, et al. A phase III randomized clinical trial comparing sentinel node biopsy with no retroperitoneal node dissection in apparent early-stage endometrial cancer - ENDO-3: ANZGOG trial 1911/2020. Int J Gynecol Cancer. 2021;31:1595–1601. doi: 10.1136/ijgc-2021-003029. [DOI] [PubMed] [Google Scholar]