Abstract
Autoimmune diseases are caused by a dysfunction of the acquired immune system. In a subset of autoimmune diseases, B cells escaping immune tolerance present autoantigen and produce cytokines and/or autoantibodies, resulting in systemic or organ-specific autoimmunity. Therefore, B cell depletion with monoclonal Abs targeting B cell lineage markers is standard care therapy for several B cell-mediated autoimmune disorders. In the last 5 years, genetically-engineered cellular immunotherapies targeting B cells have shown superior efficacy and long-term remission of B cell malignancies compared to historical clinical outcomes using B cell depletion with monoclonal Ab therapies. This has raised interest in understanding whether similar durable remission could be achieved with use of genetically-engineered cell therapies for autoimmunity. This review will focus on current human clinical trials using engineered cell therapies for B cell-associated autoimmune diseases.
Keywords: Autoimmunity, Cell therapy, Chimeric antigen receptor, Clinical trials, Pemphigus, Myasthenia gravis, Lupus, Neuromyelitis optica, Systemic sclerosis, Sjogren’s syndrome
INTRODUCTION
Autoimmune diseases are caused by a dysfunction of the acquired immune system, leading T and B lymphocytes to attack normal cells or proteins in the body. Although autoimmune diseases are classified as being predominantly T or B cell-mediated, both T and B cells contribute to pathogenesis in many autoimmune diseases. B cells can contribute to autoimmunity through Ag presentation, cytokine production, and the production of autoantibodies, which normally protect against infection by several mechanisms, such as: 1) direct neutralization to prevent infection; 2) opsonization and phagocytosis to clear pathogens; and 3) Ab-dependent cellular cytotoxicity, and 4) complement-dependent cytotoxicity to lyse bacteria or virus-infected cells. T cells differentiate into several subtypes including: 1) cytotoxic T cells that recognize infected cells and lead to cell death; 2) helper T cells that directly engage and activate B cells or indirectly activate B cells or cytotoxic T cells through cytokine production; and 3) regulatory T cells that suppress a broad range of effector immune functions to maintain homeostasis.
Current therapies for autoimmune disease aim to suppress the immune system using small molecules that inhibit immune cell activation or proliferation and biological drugs such as monoclonal Abs that target immune cells or their downstream effectors, such as complement (1,2,3). In particular, B cell depletion with drugs such as the anti-CD20 monoclonal Ab rituximab, which was initially approved for certain types of B cell malignancies, has now been established as a treatment for B cell-mediated autoimmune diseases including rheumatoid arthritis, pemphigus vulgaris, and vasculitides such as granulomatosis with polyangiitis and microscopic polyangiitis (4). However, repetitive rituximab infusions are required for maintenance of disease control, resulting in chronic B cell depletion and risk of infections due to chronic immunosuppression, with autoimmune disease registries indicating 5.3 serious infections per 100 person-years and a 1.9% lifetime risk of fatal infection (5).
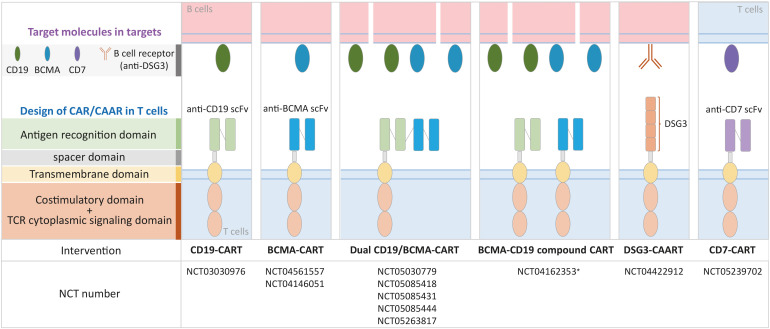
Chimeric Ag receptor T (CART) cells are genetically-engineered T cell immunotherapies that express a synthetic receptor consisting of 4 major domains: 1) an Ag recognition domain, such as an anti-CD19 single-chain variable fragment Ab; 2) an extracellular hinge or spacer domain; 3) a transmembrane domain; and 4) an endodomain consisting of a costimulatory domain and T cell receptor cytoplasmic signaling domain (6). Upon binding of Ag-expressing target cells, such as malignant or healthy CD19+ B cells, chimeric Ag receptors (CARs) direct target cell lysis as well as CART cell differentiation into long-lived memory CART cells. In this review, we will discuss the clinical trials using CART cell therapy for autoimmune diseases, as well as a novel technology for Ag-specific B cell depletion known as chimeric autoantibody receptor T (CAART) cell therapy (Fig. 1). We searched the clinicaltrials.gov database using the keywords CAR, CAAR, and autoimmune disease and identified 10 clinical trials registered as of April 8, 2022 (Table 1).
Figure 1. CAR and CAAR T cell therapies in clinical development for autoimmune diseases (ClinicalTrials.gov).
*Clinical development for multiple myeloma and plasmacytoid lymphoma.
Table 1. Clinical trials using CAR or CAAR T cells in autoimmune diseases.
| NCT number | Title | Status | Conditions | Interventions | Study start | Study completion | Study report | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT03030976 | A Study of CD19 Redirected Autologous T Cells for CD19 Positive Systemic Lupus Erythematosus (SLE) | Unknown | SLE | CD19 CAR T-cells | Mar-17 | Mar-18 | No results posted | I |
| NCT05030779 | A Study of CD19/BCMA Chimeric Antigen Receptor T Cells Therapy for Patients With Refractory Systemic Lupus Erythematosus | Recruiting | SLE | CD19/BCMA CAR T-cells | Sep-21 | Sep-22 | Active | I |
| NCT05085418 | A Study of CD19/BCMA Chimeric Antigen Receptor T Cells Therapy for Patients With Refractory Immune Nephritis | Recruiting | Immune Nephritis | CD19/BCMA CAR T-cells | Nov-21 | Nov-24 | Active | I |
| NCT04422912 | Open-label Study to Determine the Maximum Tolerated Dose of DSG3-CAART in Mucosal-dominant PV Patients (mPV) | Recruiting | mPV | DSG3-CAART | Sep-20 | Sep-26 | Active | I |
| NCT05085431 | A Study of CD19/BCMA Chimeric Antigen Receptor T Cells Therapy for Patients With Refractory Sjogren's Syndrome | Recruiting | Syndrome | CD19/BCMA CAR T-cells | Nov-21 | Nov-24 | Active | I |
| NCT05085444 | A Study of CD19/BCMA Chimeric Antigen Receptor T Cells Therapy for Patients With Refractory Scleroderma | Recruiting | Scleroderma | CD19/BCMA CAR T-cells | Oct-21 | Oct-24 | Active | I |
| NCT04561557 | Safety and Efficacy of CT103A Cells for Relapsed/Refractory Antibody-associated Idiopathic Inflammatory Diseases of the Nervous System | Recruiting | Neuromyelitis Optica Spectrum Disorder | BCMA CAR T-cells (CT103A) | Sep-20 | Dec-23 | Active | I |
| NCT04146051 | Descartes-08 CAR-T Cells in Generalized Myasthenia Gravis (MG) | Recruiting | Generalized MG | BCMA CAR T-cells (Descartes-08) | Dec-19 | Dec-23 | Active | I/II |
| NCT05263817 | A Clinical Study of CD19/BCMA CAR-T Cells in the Treatment of Refractory POEMS Syndrome, Amyloidosis, Autoimmune Hemolytic Anemia, and Vasculitis | Recruiting | Refractory POEMS Syndrome, Amyloidosis, Autoimmune Hemolytic Anemia, and Vasculitis | CD19/BCMA CAR T cells | Oct-21 | Oct-24 | Active | I |
| NCT05239702 | Clinical Study of Targeting CD7 CAR-T Cells in the Treatment of Autoimmune Diseases | Recruiting | Crohn’s Disease, Ulcerative Colitis, Dermatomyositis, Still's Disease, Autoimmune Diseases | CD7 CAR T cells | Feb-22 | Dec-24 | Active | I |
| NCT05451212 | Open-label Study to Determine the Maximum Tolerated Dose of MuSK-CAART for MuSK Myasthenia Gravis | Not yet recruiting | MuSK MG | MuSK-CAART | Oct-22 | Oct-28 | Active | I |
B CELL TARGETING STRATEGIES IN AUTOIMMUNE DISEASES
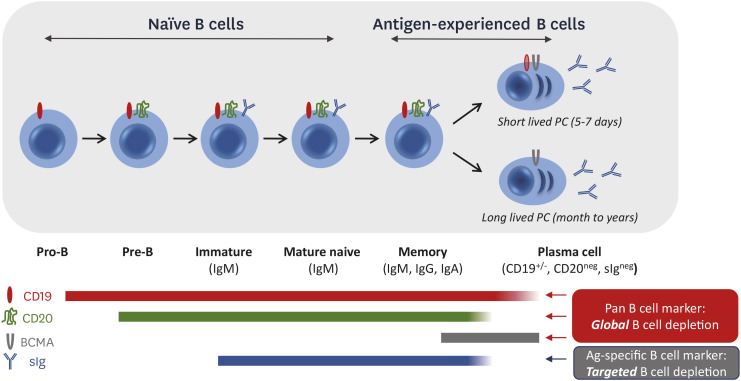
B cells expressing self-reactive B cell receptors (BCRs) are normally eliminated by central or peripheral tolerance mechanisms (7,8). Autoreactive B cells that escape immune tolerance checkpoints can activate and differentiate into plasma cells (PCs), which can be either long-lived (LLPCs) or short-lived (SLPCs). LLPCs persist for years, whereas SLPCs typically survive for only a few days after differentiation and are replenished by memory B cells. CD19 is expressed from the pro-B cell stage and is downregulated on SLPCs and LLPCs, but a subset of PCs retains CD19 expression (Fig. 2) (9). CD20 is first expressed at the pre-B cell stage and is downregulated on PCs. The BCR, a surface Ig (sIg) that dictates a B cell’s Ag specificity, is first expressed at the immature B cell stage as surface IgM and can undergo class switch to IgG or IgA (or remain as IgM) in memory B cells. B cell maturation Ag (BCMA) is expressed in SLPCs and LLPCs and critical for their survival.
Figure 2. B cell marker expression during development and differentiation.
As a result of these Ag expression patterns, the anti-CD20 monoclonal Ab rituximab indirectly targets SLPCs by depleting precursor memory B cells, but does not target LLPCs. CD19-targeting agents target pro-B cells and can reduce SLPCs and LLPCs. BCMA-targeting agents deplete PCs. There are currently 4 CART therapies targeting CD19 (CD19-CART) and 2 CART therapies targeting BCMA (BCMA-CART) that are approved for clinical use in oncologic indications. Ag loss or downregulation in target B cells has been observed with both CD19 and BCMA after CART therapy (10). Dual CD19/BCMA-CART products are also in development to enhance B cell depletion.
CLINICAL TRIALS EVALUATING ENGINEERED CELLULAR THERAPIES FOR AUTOIMMUNE DISEASES
Clinical trials evaluating CART therapy for systemic lupus erythematosus (SLE)
SLE is a systemic autoimmune disease characterized by polyclonal B cell activation and autoantibody production, with over 100 autoantibody specificities having been described (11,12,13). Current treatments include corticosteroids, adjunctive immunosuppressants, antimalarials such as hydroxychloroquine, and monoclonal Ab therapies targeting soluble B lymphocyte stimulator (belimumab) and type I IFN receptor (anifrolumab) (14,15). The importance of B cells in the pathogenicity of SLE has been established by the failure of disease development in the absence of B cells in various mouse models (16,17,18). Although B cell depletion with rituximab failed to show clinical efficacy in two independent SLE randomized clinical trials (19), the recent preclinical and clinical success of B cell depletion with anti-CD19 CART cells (CD19-CART) has sparked interest in further exploring the role of B cell depletion with CART cells in SLE.
Engineered cellular therapy using CD19-CART has been tested in SLE mouse models, including murine CD8+ CD19-CART in both NZBxNZWF1 and MRL-lpr mouse models and murine CD19-CART in the MRL-lpr mouse model (20,21). In these 2 studies, CD19-CART caused CD19+ B cell depletion and decreased titers of autoantibodies such as anti-nuclear Ab (ANA) and anti-double stranded DNA (dsDNA). However, incomplete depletion of autoantibodies was observed after syngeneic CD19-CART treatment (20). In further analysis, the authors identified the presence of IgMlowCD19− B cells in CD19-CART treated mice. Residual CD138+ PCs were observed in the latter study after CD19-CART treatment, although almost all circulating CD19+ B cells in the blood were depleted (21), suggesting that LLPCs may mediate autoantibody production or that other factors in the mouse models evaluated may have prevented complete remission of serologic disease.
Recently, a report of the successful treatment of a severe refractory SLE patient with CD19-CART using a CD137 costimulatory domain was published (22). A 20-year-old woman with lupus nephritis, nephrotic syndrome, pericarditis, pleurisy, rash, arthritis, and a history of Libman-Sacks endocarditis (22), who had failed hydroxychloroquine, high-dose glucocorticoids, cyclophosphamide, mycophenolate mofetil, tacrolimus, rituximab, and belimumab, was treated with 1.1×106 CD19-CART cells/kg, after a preconditioning regimen of three daily doses of 25 mg/m2 fludarabine and one 1000 mg infusion of cyclophosphamide. The Systemic Lupus Erythematosus Disease Activity Index score with the Safety of Estrogens in Lupus National Assessment modification (SLEDAI-SELENA) decreased from 16 at baseline to 0 by day 44 after infusion, alongside a decrease of anti-dsDNA autoantibodies from 5,000 to 4 U/mL within 5 weeks after treatment. CD19-CART related adverse events such as cytokine release syndrome, neurotoxicity, or prolonged cytopenias were not observed. Although it remains unclear to what extent the fludarabine and cyclophosphamide preconditioning before CD19-CART infusion contributed to the patient’s disease remission, an updated report demonstrating long-term remission of the patient’s disease up to 10 months after CD19-CART infusion, plus additional patients who achieved complete or near complete disease remission after CD19-CART therapy, support favorable outcomes of CD19-CART treatment regimens for refractory SLE (23).
Another case study was recently published using a BCMA-CD19 compound CART to treat a 41-year-old woman with a 20-year history of SLE who was diagnosed with stage IV diffuse large B cell lymphoma (24). The clinical trial (NCT04162353) is evaluating the safety and efficacy of a compound CART product expressing tandem anti-BCMA and anti-CD19 CARs with a CD137 costimulatory domain, separated by a P2A ribosome skip site for equimolar expression in the same T cell. The patient was preconditioned with fludarabine and cyclophosphamide before CART infusion. Although B cells began to repopulate approximately 28 weeks after treatment, ANA titers remained undetectable at 37 weeks, and the patient’s SLE remained stable until 20 months after treatment despite receiving no additional systemic therapies.
Currently, 3 clinical trials are registered to evaluate CART therapies for SLE. A single-arm open-label phase 1 study to assess the safety and efficacy of CD19-CART cells engineered from autologous T cells with an anti-CD19 CAR containing CD137 as a costimulatory domain in subjects with SLE (NCT03030976) is intending to enroll 5 subjects. A cyclophosphamide preconditioning regimen (0.5 g/m2) is dosed two days prior to an initial infusion of 1−10×106 CART cells/kg. Adverse events, overall response rates, and CART persistence in the peripheral blood, detected by quantitative PCR, will be assessed during the 6-week follow-up period.
Two additional clinical trials sponsored by the same institutions are evaluating dual CD19/BCMA-CART cells for subjects with refractory SLE (NCT05030779) and refractory immune nephritis (NCT05085418), as well as numerous other immune conditions including Sjogren’s syndrome, scleroderma, autoimmune hemolytic anemia (AIHA), and vasculitis, described further below. The primary endpoints are dose-limiting toxicity (1×106 to 4×106 cells/kg) up to 28 days and the incidence of treatment-emergent adverse events up to 90 days after CD19/BCMA-CART infusion.
Clinical trials using chimeric autoantibody receptor T cell therapy for mucosal pemphigus vulgaris
Pemphigus vulgaris is a B cell-mediated autoimmune disease in which autoantibodies against the desmosomal protein desmoglein 3 (DSG3) interfere with keratinocyte adhesion, leading to epithelial blistering (25,26,27,28). Standard care therapy for pemphigus vulgaris includes corticosteroids and rituximab, with repeated infusions every 6 months to maintain disease control (29,30). Anti-DSG3 Abs drop to the undetectable range after rituximab treatment, indicating that SLPCs are the main source of autoantibodies in pemphigus vulgaris patients (31,32). However, chronic B cell depletion from the rituximab and corticosteroid regimen was associated with a 4%–9% annual rate of severe infections (29,30), indicating that a therapeutic strategy to deplete only anti-DSG3 B cells may be desirable to achieve a targeted remission of disease without global immune suppression.
DSG3 chimeric autoantibody receptor T cell (DSG3-CAART) therapy for Ag-specific B cell depletion was developed by replacing the anti-CD19 extracellular domain of an anti-CD19 CAR with CD137-CD3z costimulatory and activation domains with the extracellular domains of the DSG3 autoantigen, which directs T cell cytotoxicity against autoreactive B cells expressing an anti-DSG3 BCR (33,34). DSG3-CAART showed specific cytotoxicity against anti-DSG3 BCR-positive target cells and no off-target cytotoxic interactions in various in vitro assays and in vivo mouse models (33,34). A difference between the clinical applications of CART and CAART therapy for autoantibody-mediated disease is the presence of soluble Abs that could theoretically have numerous effects including: 1) inhibition of CAAR and BCR interaction, either by direct blocking or CAAR internalization; 2) Ab-mediated CAART lysis, which is not expected in pemphigus vulgaris as anti-DSG3 Abs are IgG4 predominant and hence do not bind complement or activating Fc gamma receptors; and/or 3) CAART activation leading to IFN-γ production and CAART proliferation, which could risk cytokine release syndrome but could also provide a survival signal in vivo to enhance persistence of DSG3-CAART. Given that DSG3-CAART demonstrated activity in in vitro assays even in the presence of soluble anti-DSG3 Abs and in an experimental autoimmune pemphigus vulgaris model with circulating anti-DSG3 Ab titers comparable to higher than those observed in pemphigus vulgaris patients, a phase 1 clinical trial of DSG3-CAART without preconditioning or removal of soluble anti-DSG3 Abs was initiated to evaluate its safety and preliminary efficacy in subjects with anti-DSG3 Ab-positive mucosal pemphigus vulgaris (NCT04422912).
Early clinical data from the first four cohorts of the DSG3-CAART trial have shown no dose-limiting toxicities up to a dose of 2.5×109 CAAR+ T cells and a dose-related increase in DSG3-CAART persistence in the 28 days after DSG3-CAART infusion (35), approaching the lower range of persistence levels observed among responders receiving anti-CD19 CART plus lymphodepletion for B cell leukemias (36), indicating that soluble Abs do not induce cytokine release syndrome or mediate CAART destruction. Transient improvements in clinical disease activity scores up to 2 months after DSG3-CAART infusion and variable effects on anti-DSG3 Ab titers were reported. Dose escalation in this study is currently ongoing.
Clinical trials using CART therapy for Sjogren’s syndrome
Sjogren’s syndrome is a chronic autoimmune disease characterized by the impaired secretory function of exocrine glands leading to ocular and mouth dryness and can be associated with multiple organ system dysfunction (37). B cell hyperactivity is a hallmark of the disease as evidenced by altered circulating levels of B cells, increase in serum B cell-activating factor (BAFF) levels, hypergammaglobulinemia, and various autoantibodies, most notably those targeting ribonucleoproteins SS-A/Ro and SS-B/La (38). Currently, no disease-modifying drug has been approved for the treatment of Sjogren’s syndrome. Limited benefits of rituximab were reported in 2 large randomized controlled trials, including Tolerance and Efficacy of Rituximab in primary Sjogren’s syndrome (TEARS) and Trial of Anti-B-Cell Therapy In subjects with primary Sjogren’s Syndrome (TRACTISS) (39,40). The TEARS randomized clinical trial (NCT00740948) was designed to evaluate the efficacy of a 1-g dose of rituximab given 2 weeks apart compared to placebo. Although rituximab-treated subjects did not achieve the primary end point (≥30-mm decrease in ≥2 of 4 visual analogue scale (VAS) scores for global disease, pain, fatigue, or dryness) at week 24, rituximab treatment resulted in transient clinical improvements in fatigue at week 6 (39). The TRACTISS study evaluated 1 g rituximab treatment at week 0 and 2, repeated at week 24 and week 26 to extend the period of B cell depletion (40,41). The TRACTISS study also did not achieve its primary outcome (30% reduction at 48 weeks in VAS scores), however, stable glandular secretory function was observed in rituximab treated groups, while it worsened in the placebo-treated group (41,42). It is unknown whether LLPCs residing in the salivary glands of Sjogren’s syndrome patients may explain the partial therapeutic results of CD20+ B cell depletion with rituximab for Sjogren’s syndrome (43). Although CART therapy has not been evaluated in animal models of Sjogren’s syndrome, a clinical trial (NCT05085431) evaluating CD19/BCMA-CART for refractory Sjogren’s syndrome has been initiated as a single-arm, open-label study to assess safety and efficacy. The primary outcomes of this clinical trial are dose-limiting toxicities (1×106 to 4×106 cells/kg) up to 28 days and incidence of treatment-emergent adverse events up to 90 days after CD19/BCMA-CART infusion.
Additionally, a CAAR strategy targeting La/SSB-reactive B cells has been reported (44). Approximately 25%–40% of Sjogren’s syndrome patients demonstrate anti-La/SSB autoantibodies (45). A NK cell line (NK92MI) was engineered to express a CAAR comprising an immunodominant domain of the La/SSB protein (44). Although no in vivo data were reported, La-CAAR NK92MI cells showed selective cytotoxicity against anti-La BCR+ target cell lines, as well as a partial decrease in B cell frequency by flow cytometry after co-culture of whole blood samples obtained from anti-La seropositive patients with La-CAAR NK92MI cells, while no change in B cell frequency was observed in the same experimental system using whole blood samples from healthy donors.
Clinical trials of CART therapy for scleroderma
Scleroderma is an autoimmune disease characterized by the hardening and tightening of the skin. The disease overlaps with systemic sclerosis (SSc) when the fibrosis extends to lung or other internal organs (46). Upregulated CD19 expression in B cells may contribute to activation and hyperreactivity of memory B cells, which is associated with autoantibody production and fibrosis (47).
A clinical trial evaluating rituximab effects on skin and lung fibrosis in subjects with SSc was reported from the European Scleroderma Trial And Research (EUSTAR) group (48). This post hoc nested case control observational study of 63 rituximab treated patients demonstrated improvement of skin fibrosis (mean 24.0 point decrease in modified Rodnan skin score compared to 7.7 point decrease in controls) and prevention of lung fibrosis progression (forced vital capacity increase of 0.4% in rituximab-treated subjects compared to 7.7% decrease in controls). An open-label comparative study of rituximab (n=33) versus oral immunosuppressive therapies such as azathioprine (n=2), methotrexate (n=6), and mycophenolate mofetil (n=10) for SSc-associated interstitial lung disease also demonstrated favorable clinical improvements in lung function and skin fibrosis with rituximab (49). Additionally, a double-blind, placebo-controlled study of rituximab evaluated 56 subjects with SSc randomly assigned to receive rituximab (n=28) or placebo (n=28) (50). Subjects in the rituximab treated group demonstrated a 6.3 point decrease in modified Rodnan Skin Score compared to a 2.1 point increase in the placebo treated group.
A single-arm open-label clinical trial (NCT05085444) to evaluate the safety and efficacy of CD19/BCMA-CART in the treatment of refractory scleroderma is currently enrolling. The primary outcome is the determination of dose-limiting toxicity (1×106 to 4×106 cells/kg) up to 28 days and observing the incidence of treatment-emergent adverse events up to 90 days after CD19/BCMA-CART infusion.
Clinical trials of CART therapy for AIHA and vasculitis
AIHA is an autoimmune disease characterized by severe anemia due to autoantibodies against red blood cell Ags, most often of the IgG isotype in warm AIHA and the IgM isotype in cold AIHA, classified based on the temperature at which autoantibodies bind maximally to red blood cells. Although randomized controlled trials of rituximab have not been performed in AIHA, several case series and retrospective studies report favorable results of rituximab treatment for both warm and cold AIHA (51,52).
Vasculitis is an autoimmune inflammatory disease of the blood vessel wall associated with autoantibodies against anti-neutrophil cytoplasmic Abs (ANCA). A randomized controlled trial of rituximab versus cyclophosphamide for ANCA-associated vasculitis demonstrated superiority of rituximab in inducing remission of relapsing disease at 6 months (67% in the rituximab treated group [n=51] compared to 42% in the control group [n=50]) (53). Another randomized controlled trial of rituximab with a 2-year follow-up indicated similar rates of relapse between rituximab-treated and cyclophosphamide-treated groups (54), suggesting that repeated rituximab infusions may be necessary for maintenance of disease control or that CART therapy may provide a more durable therapeutic option.
Based on this rationale, a basket clinical trial (NCT05263817) has been registered to evaluate CD19/BCMA-CART for subjects with AIHA and vasculitis. The primary outcome of this clinical trial is the determination of dose-limiting toxicity (1×106 to 4×106 cells/kg) up to 28 days and observing the incidence of treatment-emergent adverse events up to 90 days after CD19/BCMA-CART infusion.
Clinical trials of CART therapy for neuromyelitis optica spectrum disorders (NMOSD)
NMOSD is a debilitating autoimmune inflammatory disease of the central nervous system in which lesions in the spinal cord and optic nerves can lead to loss of sensation, paralysis, and blindness. Over 70% of NMOSD patients have autoantibodies against the water channel aquaporin-4 (AQP4) (55). The therapeutic effect of plasmapheresis for NMOSD patients support the importance of autoantibodies in disease pathogenesis (56). A double-blind randomized controlled trial of rituximab for NMOSD was conducted in which subjects received rituximab (n=19) or placebo (n=19) followed by concomitant oral corticosteroid taper; the primary outcome was time to first relapse (57). None of the rituximab treated subjects relapsed during the 72 week follow up period compared to 7 (37%) of 19 subjects who relapsed in the placebo-treated group.
In 2020, the anti-CD19 monoclonal Ab inebilizumab was approved by the FDA for the treatment of NMOSD. In the N-Momentum double-blind randomized clinical trial (NCT02200770), 213 (93%) of the 230 NMOSD subjects were seropositive for AQP4 autoantibodies and were treated with either inebilizumab (n=174) or placebo (n=56) (58). The benefit of inebilizumab for preventing NMOSD attacks was 12% in the inebilizumab-treated group versus 39% in placebo: 39%, with comparable incidence of serious adverse events between the two groups (5% with inebilizumab versus 9% with placebo).
An open-label clinical trial (NCT04561557) has been registered to evaluate the safety and efficacy of BCMA-CART in the treatment of NMOSD. The study design includes treatment of 3 subjects with an initial dose of 0.5×106 CAR+ T cells per kg. If no dose-limiting toxicity occurs and at least one subject benefits from the first cohort, then the subsequent 3 subjects will be infused with either the same dose (0.5×106 CART cells per kg) or an increased dose (1×106 CART cells per kg) based on the available clinical data.
Clinical trials of CART and CAART therapy for myasthenia gravis (MG)
MG is a chronic autoimmune neuromuscular disorder caused by autoantibodies to the acetylcholine receptor (AChR), muscle-specific tyrosine kinase (MuSK), or low-density lipoprotein receptor-related protein (LRP4) expressed in postsynaptic muscle cells (59). Approximately 80% of MG patients demonstrate AChR autoantibodies, and approximately 40% of anti-AChR Ab-seronegative MG patients demonstrate autoantibodies against MuSK. Seronegative MG patients may demonstrate reactivity to LRP4 or other proteins, or may have low-level reactivity against AChR or MuSK (60). Depending on the affected muscle groups, MG is classified as generalized or ocular. Passive transfer of Abs is sufficient to induce the MG phenotype, indicating the primary role of autoantibodies in disease pathogenesis (61).
In a single-center retrospective study of rituximab treatment for MG (62), MuSK MG patients experienced earlier time to remission, fewer disease exacerbations and hospitalizations post-rituximab treatment than AChR MG patients, although both groups experienced clinical improvement after rituximab infusion. The randomized phase 2 B cell targeted treatment in MG (BeatMG) study to treat AChR autoantibody-positive generalized MG showed that 4 weekly 375 mg/m2 infusions of rituximab were safe and well tolerated but did not achieve the primary outcome of ≥75% reduction in mean daily prednisone dose from weeks 48–52 (63).
An open-label clinical trial (NCT04146051) is currently enrolling to assess the maximum tolerated dose (MTD) until day 28 and efficacy until day 168 after BCMA-CART (named Descartes-08) infusion. Descartes-08 is engineered by electroporating autologous CD8+ T cells with RNA encoding a BCMA-CAR, which allows temporal expression of the BCMA-CAR in T cells. Early clinical results have shown that treatment is well-tolerated, with no serious adverse events (64). The mean improvement in the Myasthenia Gravis Composite (MGC) scale was over 50% three months after Descartes-08 treatment. Two subjects enrolled into the weekly dosing cohort received 6 infusions of Descartes-08. At the week 10 visit, one subject demonstrated improvement in the MGC score from 27 to 2 and the other subject improved their MGC score from 23 to 3.
An independent open-label clinical trial (NCT04561557) is concurrently enrolling to evaluate the safety and efficacy of BCMA-CART to treat generalized MG, starting with an initial dose of 0.5×106 CAR+ T cells per kg in the first cohort and a dose of 0.5−1×106 CAR-T cells per kg for subsequent cohorts pending clinical data.
The same sponsor that developed DSG3-CAART for mucosal pemphigus vulgaris announced plans to initiate a clinical trial of MuSK-CAART for MuSK autoantibody-positive MG patients in 2022 (NCT05451212) (65), based on preclinical studies leading to FDA clearance of the MuSK-CAART Investigational New Drug application (66). B cell depletion with rituximab preferentially reduces anti-MuSK IgG relative to total IgG (67), indicating that like pemphigus vulgaris, autoantibodies in MuSK MG patients are produced by SLPCs (68,69). Therefore, targeted depletion of anti-MuSK BCR-positive B cells by MuSK-CAART may prevent the replenishment of PCs producing anti-MuSK autoantibodies, while retaining healthy B cells.
Clinical trials of CART therapy for chronic immune demyelinating polyradiculoneuropathy (CIDP) and immune-mediated necrotizing myopathy (IMNM)
A basket clinical trial (NCT04561557) has been initiated to evaluate BCMA-CART treatment of autoimmune inflammatory diseases of the nervous system (CIDP and IMNM). CIDP is an autoimmune disease of the peripheral nerves and nerve root characterized by symmetric loss of motor and sensory function (70). Autoantibodies targeting NF155, CNTN1, CASPR1, and Ranvier’s nodal proteins NF140 and NF186 have been described. Current off-label treatments for CIDP include glucocorticoids, intravenous immunoglobulin, plasmapheresis, and rituximab (70,71). Plasma exchange is effective for both chronic progressive and relapsing CIDP, although 66% of plasma exchange responders relapsed within 2 weeks after stopping plasma exchange (72). Rituximab treatment also has been reported to be effective for CIDP (73,74). IMNM is a subtype of idiopathic inflammatory myopathies (75) characterized by high serum creatine kinase and necrotic muscle fibers (76), associated with autoantibodies against signal recognition particle (SRP) or 3-hydroxy-3-methylglutaryl-coA-reductase (HMGCR). Although there are no randomized clinical trials evaluating rituximab in IMDM, a systematic review reported 61.8% of patients reported across 12 individual studies improved after rituximab therapy for IMDM (76). Patients presenting with anti-SRP Abs were more responsive (77.8%) to rituximab treatment than those who have anti-HMGCR Abs (43.8%).
An open-label clinical trial (NCT04561557) is currently enrolling to evaluate the safety and efficacy of BCMA-CART to treat CIDP or IMNM, starting with an initial dose of 0.5×106 CAR+ T cells per kg in the first cohort and a dose of 0.5−1×106 CAR-T cells per kg for subsequent cohorts pending clinical data.
FUTURE DIRECTIONS
To date, CART therapy has demonstrated its greatest clinical impact against malignancies of the B cell lineage, which has increased both academic and industry interest in pursuing CART or CAART approaches to B cell-mediated autoimmune diseases. Current clinical trials using CART technology are mainly focused on the depletion of B cells targeting B cell lineage markers such as CD19 or BCMA, which can result in partial or complete B cell aplasia after treatment. Next-generation technologies that allow for temporal control of CART-mediated B cell depletion, such as suicide cassettes or transient CAR surface expression, may be required to avoid chronic immunosuppression associated with long-term CART engraftment. CAART technology is designed for Ag-specific memory B cell depletion, which aims to avoid B cell aplasia by targeting only autoreactive B cells and sparing healthy B cells, although diseases mediated by long-lived plasma cells are not targetable by the CAART approach. Future data from ongoing clinical trials will inform the field on the persistence of CAART cells in the absence of lymphodepletion and how factors unique to autoimmunity versus cancer, such as soluble autoantibody and rare target cells, may influence clinical outcomes.
Another major frontier for the field is whether T cell mediated autoimmune diseases can be addressed through CART therapy. CART therapy for malignancies of the T cell lineage have not yet received clinical approval, in part due to the challenge of using a T cell therapeutic to target a T cell-mediated disease, although CART therapies targeting the T cell markers CD5 and/or CD7 have been developed for the treatment of T cell malignancies in preclinical and clinical studies (77,78,79). To avoid fratricide, anti-CD5/CD7 CARTs employ strategies to suppress endogenous CD5 and/or CD7 expression, such as CRISPR/Cas9-based gene editing or intracellular retention of CD7 by a CD7-binding protein having an endoplasmic reticulum retention signal sequence (77,79). CD7-CART has been evaluated in a clinical study of subjects with relapsed or refractory T cell acute lymphoblastic leukemia (T-ALL) (NCT04689659), which demonstrated that 18 (90%) of 20 T-ALL subjects who received CD7-CART achieved complete remission at 30-day follow-up and 15 subjects remained in remission at subsequent follow-up (average, 6.3 months; range, 4.0–9.2 months) (79). CD7− T cell expansion was observed in these subjects, suggesting selective cytotoxicity and persistence of CD7-CART cells. CD7− T cells are found in several autoimmune diseases such as RA (80), psoriasis (81), and autoimmune enteropathy (82). A case report suggested that the reduction of CD8+CD7− T cell population is related with the improvement of disease phenotype in autoimmune enteropathy (82). A clinical trial (NCT05239702) has been registered to evaluate the safety and efficacy of CD7-CART for the treatment of autoimmune diseases such as Crohn disease, ulcerative colitis, dermatomyositis, and Still disease (Table 1), which will be valuable to determine whether infusion of activated CD7− T cells can have beneficial therapeutic effect by depleting CD7+ T cells without flaring the underlying autoimmune disease activity.
In summary, a recent plethora of clinical trials using gene-engineered cellular immunotherapy has entered clinical stage development. Emerging clinical results from these studies will offer critical in vivo human data on the potential to program the immune system to durably reverse autoimmunity after a single treatment, a therapeutic ideal that has not been previously achievable.
ACKNOWLEDGEMENTS
Dr. Oh was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (2019R1A6A3A03033057).
Abbreviations
- AChR
acetylcholine receptor
- AIHA
autoimmune hemolytic anemia
- ANA
anti-nuclear Ab
- ANCA
anti-neutrophil cytoplasmic Abs
- AQP4
aquaporin-4
- BAFF
B cell-activating factor
- BCMA
B cell maturation Ag
- BCMA-CART
CART therapies targeting BCMA
- BCR
B cell receptor
- BeatMG
B cell targeted treatment in MG
- CAART
chimeric autoantibody receptor T
- CAR
chimeric Ag receptor
- CART
chimeric Ag receptor T
- CD19-CART
CART therapies targeting CD19
- CIDP
chronic immune demyelinating polyradiculoneuropathy
- dsDNA
double stranded DNA
- DSG3
desmoglein 3
- DSG3-CAART
DSG3 chimeric autoantibody receptor T cell
- EUSTAR
European Scleroderma Trial And Research
- HMGCR
3-hydroxy-3-methylglutaryl-coA-reductase
- IMNM
immune-mediated necrotizing myopathy
- LLPC
long-lived plasma cell
- LRP4
low-density lipoprotein receptor-related protein
- MG
myasthenia gravis
- MGC
Myasthenia Gravis Composite
- MTD
maximum tolerated dose
- MuSK
muscle-specific tyrosine kinase
- NMOSD
neuromyelitis optica spectrum disorders
- PC
plasma cell
- sIg
surface Ig
- SLE
systemic lupus erythematosus
- SLEDAI-SELENA
Systemic Lupus Erythematosus Disease Activity Index score with the Safety of Estrogens in Lupus National Assessment modification
- SLPC
short-lived plasma cell
- SRP
signal recognition particle
- SSc
systemic sclerosis
- T-ALL
T cell acute lymphoblastic leukemia
- TEARS
Tolerance and Efficacy of Rituximab in primary Sjogren’s syndrome
- TRACTISS
Trial of Anti-B-Cell Therapy In subjects with primary Sjogren’s Syndrome
- VAS
visual analogue scale
Footnotes
Conflict of Interest: Dr. Payne has equity, payments, grant funding, patent licensing from Cabaletta Bio; has patent licensing from Novartis; and work as a consultant for Janssen.
Dr. Oh has patent licensing from Cabaletta Bio.
- Conceptualization: Oh S, Payne AS.
- Data curation: Oh S, Payne AS.
- Supervision: Payne AS.
- Writing - original draft: Oh S.
- Writing - review & editing: Oh S, Payne AS.
References
- 1.Lallana EC, Fadul CE. Toxicities of immunosuppressive treatment of autoimmune neurologic diseases. Curr Neuropharmacol. 2011;9:468–477. doi: 10.2174/157015911796557939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li P, Zheng Y, Chen X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front Pharmacol. 2017;8:460. doi: 10.3389/fphar.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DS, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20:179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kötter I, Haas J, Unger L, Lovric S, Haubitz M, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis Res Ther. 2011;13:R75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayaraman J, Mellody MP, Hou AJ, Desai RP, Fung AW, Pham AH, Chen YY, Zhao W. CAR-T design: elements and their synergistic function. EBioMedicine. 2020;58:102931. doi: 10.1016/j.ebiom.2020.102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodnow CC, Adelstein S, Basten A. The need for central and peripheral tolerance in the B cell repertoire. Science. 1990;248:1373–1379. doi: 10.1126/science.2356469. [DOI] [PubMed] [Google Scholar]
- 8.Nemazee D. Mechanisms of central tolerance for B cells. Nat Rev Immunol. 2017;17:281–294. doi: 10.1038/nri.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, Obstfeld AE, Lacey SF, Melenhorst JJ, Nazimuddin F, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128:360–370. doi: 10.1182/blood-2016-01-694356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 11.Poole BD, Schneider RI, Guthridge JM, Velte CA, Reichlin M, Harley JB, James JA. Early targets of nuclear RNP humoral autoimmunity in human systemic lupus erythematosus. Arthritis Rheum. 2009;60:848–859. doi: 10.1002/art.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tebbe B, Orfanos CE. Epidemiology and socioeconomic impact of skin disease in lupus erythematosus. Lupus. 1997;6:96–104. doi: 10.1177/096120339700600204. [DOI] [PubMed] [Google Scholar]
- 13.Yaniv G, Twig G, Shor DB, Furer A, Sherer Y, Mozes O, Komisar O, Slonimsky E, Klang E, Lotan E, et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev. 2015;14:75–79. doi: 10.1016/j.autrev.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Bruce IN, Golam S, Steenkamp J, Wang P, Worthington E, Desta B, Psachoulia K, Erhardt W, Tummala R. Indirect treatment comparison of anifrolumab efficacy versus belimumab in adults with systemic lupus erythematosus. J Comp Eff Res. 2022;11:765–777. doi: 10.2217/cer-2022-0040. [DOI] [PubMed] [Google Scholar]
- 15.Murphy G, Isenberg D. Effect of gender on clinical presentation in systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:2108–2115. doi: 10.1093/rheumatology/ket160. [DOI] [PubMed] [Google Scholar]
- 16.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob N, Guo S, Mathian A, Koss MN, Gindea S, Putterman C, Jacob CO, Stohl W. B cell and BAFF dependence of IFN-α-exaggerated disease in systemic lupus erythematosus-prone NZM 2328 mice. J Immunol. 2011;186:4984–4993. doi: 10.4049/jimmunol.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy V, Jayne D, Close D, Isenberg D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res Ther. 2013;15(Suppl 1):S2. doi: 10.1186/ar3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, Ghani QU, Balazs L, Beranova-Giorgianni S, Giorgianni F, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11:eaav1648. doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, Xiao L, Han Y, Lu L. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2021;18:1896–1903. doi: 10.1038/s41423-020-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, Böltz S, Manger B, Mackensen A, Schett G. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–569. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]
- 23.Schett G, Boeltz S, Müller F, Kleyer A, Völkl S, Aigner M, Gary R, Kretschmann S, Simon D, Kharboutli S, et al. OP0279 CAR-T cell treatment of refractory systemic lupus erythematosus- safety and preliminary efficacy data from the first four patients. Ann Rheum Dis. 2022;81:185. [Google Scholar]
- 24.Zhang W, Feng J, Cinquina A, Wang Q, Xu H, Zhang Q, Sun L, Chen Q, Xu L, Pinz K, et al. Treatment of systemic lupus erythematosus using BCMA-CD19 compound CAR. Stem Cell Rev Rep. 2021;17:2120–2123. doi: 10.1007/s12015-021-10251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu A, Ishiko A, Ota T, Tsunoda K, Koyasu S, Amagai M, Nishikawa T. Ultrastructural changes in mice actively producing antibodies to desmoglein 3 parallel those in patients with pemphigus vulgaris. Arch Dermatol Res. 2002;294:318–323. doi: 10.1007/s00403-002-0341-z. [DOI] [PubMed] [Google Scholar]
- 27.Kasperkiewicz M, Ellebrecht CT, Takahashi H, Yamagami J, Zillikens D, Payne AS, Amagai M. Pemphigus. Nat Rev Dis Primers. 2017;3:17026. doi: 10.1038/nrdp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders SL, Nelson CT. Pemphigus and pemphigoid. Med Clin North Am. 1965;49:681–694. doi: 10.1016/s0025-7125(16)33314-4. [DOI] [PubMed] [Google Scholar]
- 29.Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, Caillot F, Golinski ML, Labeille B, Picard-Dahan C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 30.Werth VP, Joly P, Mimouni D, Maverakis E, Caux F, Lehane P, Gearhart L, Kapre A, Pordeli P, Chen DM, et al. Rituximab versus mycophenolate mofetil in patients with pemphigus vulgaris. N Engl J Med. 2021;384:2295–2305. doi: 10.1056/NEJMoa2028564. [DOI] [PubMed] [Google Scholar]
- 31.Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850–2858. doi: 10.1038/jid.2008.172. [DOI] [PubMed] [Google Scholar]
- 32.Lunardon L, Tsai KJ, Propert KJ, Fett N, Stanley JR, Werth VP, Tsai DE, Payne AS. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031–1036. doi: 10.1001/archdermatol.2012.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Lundgren DK, Mao X, Manfredo-Vieira S, Nunez-Cruz S, Williams EF, Assenmacher CA, Radaelli E, Oh S, Wang B, et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J Clin Invest. 2020;130:6317–6324. doi: 10.1172/JCI138416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang DJ, Micheletti R, Maverakis E, Marinkovich MP, Porter DL, Abedi M, Weng WK, Hoffman K, Volkov J, Nunez D, et al. A phase 1 trial of DSG3-CAART cells in mucosal-dominant pemphigus vulgaris (mPV) patients: early cohort clinical and translational data. J Invest Dermatol. 2022;142:S1. [Google Scholar]
- 36.Mueller KT, Maude SL, Porter DL, Frey N, Wood P, Han X, Waldron E, Chakraborty A, Awasthi R, Levine BL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornec D, Devauchelle-Pensec V, Tobón GJ, Pers JO, Jousse-Joulin S, Saraux A. B cells in Sjögren’s syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun. 2012;39:161–167. doi: 10.1016/j.jaut.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Mielle J, Tison A, Cornec D, Le Pottier L, Daien C, Pers JO. B cells in Sjögren’s syndrome: from pathophysiology to therapeutic target. Rheumatology (Oxford) 2019 doi: 10.1093/rheumatology/key332. [DOI] [PubMed] [Google Scholar]
- 39.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puéchal X, Le Guern V, Sibilia J, Gottenberg JE, Chiche L, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014;160:233–242. doi: 10.7326/M13-1085. [DOI] [PubMed] [Google Scholar]
- 40.Brown S, Navarro Coy N, Pitzalis C, Emery P, Pavitt S, Gray J, Hulme C, Hall F, Busch R, Smith P, et al. The TRACTISS protocol: a randomised double blind placebo controlled clinical trial of anti-B-cell therapy in patients with primary Sjögren’s Syndrome. BMC Musculoskelet Disord. 2014;15:21. doi: 10.1186/1471-2474-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhury F, Tappuni A, Bombardieri M. Biological therapy in primary Sjögren’s syndrome: effect on salivary gland function and inflammation. Front Med (Lausanne) 2021;8:707104. doi: 10.3389/fmed.2021.707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowman SJ, Everett CC, O’Dwyer JL, Emery P, Pitzalis C, Ng WF, Pease CT, Price EJ, Sutcliffe N, Gendi NS, et al. Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren’s syndrome. Arthritis Rheumatol. 2017;69:1440–1450. doi: 10.1002/art.40093. [DOI] [PubMed] [Google Scholar]
- 43.Szyszko EA, Brokstad KA, Oijordsbakken G, Jonsson MV, Jonsson R, Skarstein K. Salivary glands of primary Sjögren’s syndrome patients express factors vital for plasma cell survival. Arthritis Res Ther. 2011;13:R2. doi: 10.1186/ar3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng H, Sun X, Song Y, Zou J, An G, Jin Z, Yang L. La/SSB chimeric autoantibody receptor modified NK92MI cells for targeted therapy of autoimmune disease. Clin Immunol. 2018;192:40–49. doi: 10.1016/j.clim.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjögren’s syndrome. Rheum Dis Clin North Am. 2016;42:419–434. doi: 10.1016/j.rdc.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lescoat A, Roofeh D, Kuwana M, Lafyatis R, Allanore Y, Khanna D. Therapeutic approaches to systemic sclerosis: recent approvals and future candidate therapies. Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshizaki A. Pathogenic roles of B lymphocytes in systemic sclerosis. Immunol Lett. 2018;195:76–82. doi: 10.1016/j.imlet.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, Distler O EUSTAR Rituximab study group. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74:1188–1194. doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 49.Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T, Georgiou P, Andonopoulos AP, Drosos AA, Sakkas L, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2017;46:625–631. doi: 10.1016/j.semarthrit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Ebata S, Yoshizaki A, Oba K, Kashiwabara K, Ueda K, Uemura Y, Watadani T, Fukasawa T, Miura S, Yoshizaki-Ogawa A, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3:e489–e497. doi: 10.1016/S2665-9913(21)00107-7. [DOI] [PubMed] [Google Scholar]
- 51.Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol. 2008;141:149–169. doi: 10.1111/j.1365-2141.2008.07054.x. [DOI] [PubMed] [Google Scholar]
- 52.Rodrigo C, Rajapakse S, Gooneratne L. Rituximab in the treatment of autoimmune haemolytic anaemia. Br J Clin Pharmacol. 2015;79:709–719. doi: 10.1111/bcp.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. 2015;74:1178–1182. doi: 10.1136/annrheumdis-2014-206404. [DOI] [PubMed] [Google Scholar]
- 55.Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, Geraldes R, Vale T, Jacob A, Palace J, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913–919. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, Fujihara K, Itoyama Y. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128–132. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 57.Tahara M, Oeda T, Okada K, Kiriyama T, Ochi K, Maruyama H, Fukaura H, Nomura K, Shimizu Y, Mori M, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:298–306. doi: 10.1016/S1474-4422(20)30066-1. [DOI] [PubMed] [Google Scholar]
- 58.Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, Fujihara K, Paul F, Cutter GR, Marignier R, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 59.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJ. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 60.Lazaridis K, Tzartos SJ. Myasthenia gravis: autoantibody specificities and their role in MG management. Front Neurol. 2020;11:596981. doi: 10.3389/fneur.2020.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behin A, Le Panse R. New pathways and therapeutic targets in autoimmune myasthenia gravis. J Neuromuscul Dis. 2018;5:265–277. doi: 10.3233/JND-170294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litchman T, Roy B, Kumar A, Sharma A, Njike V, Nowak RJ. Differential response to rituximab in anti-AChR and anti-MuSK positive myasthenia gravis patients: a single-center retrospective study. J Neurol Sci. 2020;411:116690. doi: 10.1016/j.jns.2020.116690. [DOI] [PubMed] [Google Scholar]
- 63.Nowak RJ, Coffey CS, Goldstein JM, Dimachkie MM, Benatar M, Kissel JT, Wolfe GI, Burns TM, Freimer ML, Nations S, et al. Phase 2 trial of rituximab in acetylcholine receptor antibody-positive generalized myasthenia gravis: the BeatMG study. Neurology. 2021;98:e376–e389. doi: 10.1212/WNL.0000000000013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stachowiak S, Galatioto J. Cartesian therapeutics to present late-breaking data from phase 1/2a trial of RNA CAR T-cell therapy in patients with generalized myasthenia gravis. Cartesian Therapeutics [Internet] 2022. May 10, [accessed on 24 June 2022]. Available at https://www.cartesiantherapeutics.com/cartesian-therapeutics-to-present-late-breaking-data-from-phase-1-2a-trial-of-rna-car-t-cell-therapy-in-patients-with-generalized-myasthenia-gravis/
- 65.Marda A, McCabe S. U.S. Food and Drug Administration Grants Cabaletta Bio Fast Track Designation for MuSK-CAART. Cabaletta Bio [Internet] 2022. Mar 01, [accessed on 24 June 2022]. Available at https://www.cabalettabio.com/news-media/press-releases/detail/61/u-s-food-and-drug-administration-grants-cabaletta-bio-fast.
- 66.Oh SM, Manfredo-Vieira S, Choi EJ, Herzberg U, Patel D, Cottman-Thomas E, Lee J, Basu S, O’Connor KC, Milone MC, et al. Muscle-specific tyrosine kinase chimeric autoantibody receptor T cells (MuSK-CAART): a precision cellular immunotherapy for antigen-specific B cell depletion in MuSK myasthenia gravis. Mol Ther. 2022;30:15. [Google Scholar]
- 67.Marino M, Basile U, Spagni G, Napodano C, Iorio R, Gulli F, Todi L, Provenzano C, Bartoccioni E, Evoli A. Long-lasting rituximab-induced reduction of specific-but not total-IgG4 in MuSK-positive myasthenia gravis. Front Immunol. 2020;11:613. doi: 10.3389/fimmu.2020.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zografou C, Vakrakou AG, Stathopoulos P. Short- and long-lived autoantibody-secreting cells in autoimmune neurological disorders. Front Immunol. 2021;12:686466. doi: 10.3389/fimmu.2021.686466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight. 2017;2:e94263. doi: 10.1172/jci.insight.94263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolbert J, Cheng MI, Meyer zu Horste G, Su MA. Deciphering immune mechanisms in chronic inflammatory demyelinating polyneuropathies. JCI Insight. 2020;5:e132411. doi: 10.1172/jci.insight.132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bright RJ, Wilkinson J, Coventry BJ. Therapeutic options for chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review. BMC Neurol. 2014;14:26. doi: 10.1186/1471-2377-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn AF, Bolton CF, Pillay N, Chalk C, Benstead T, Bril V, Shumak K, Vandervoort MK, Feasby TE. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham-controlled, cross-over study. Brain. 1996;119:1055–1066. doi: 10.1093/brain/119.4.1055. [DOI] [PubMed] [Google Scholar]
- 73.Roux T, Debs R, Maisonobe T, Lenglet T, Delorme C, Louapre C, Leblond V, Viala K. Rituximab in chronic inflammatory demyelinating polyradiculoneuropathy with associated diseases. J Peripher Nerv Syst. 2018;23:235–240. doi: 10.1111/jns.12287. [DOI] [PubMed] [Google Scholar]
- 74.Querol L, Rojas-García R, Diaz-Manera J, Barcena J, Pardo J, Ortega-Moreno A, Sedano MJ, Seró-Ballesteros L, Carvajal A, Ortiz N, et al. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm. 2015;2:e149. doi: 10.1212/NXI.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stuhlmüller B, Schneider U, González-González JB, Feist E. Disease specific autoantibodies in idiopathic inflammatory myopathies. Front Neurol. 2019;10:438. doi: 10.3389/fneur.2019.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong A, Yang G, Song Z, Xiong C, Liu D, Shuai Y, He L, Zhang L, Guo Z, Shuai S. Rituximab in the treatment of immune-mediated necrotizing myopathy: a review of case reports and case series. Ther Adv Neurol Disorder. 2021;14:1756286421998918. doi: 10.1177/1756286421998918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai Z, Mu W, Zhao Y, Cheng J, Lin H, Ouyang K, Jia X, Liu J, Wei Q, Wang M, et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduct Target Ther. 2022;7:85. doi: 10.1038/s41392-022-00898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, Rouce RH, Bao G, Brenner MK, Mamonkin M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–296. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan J, Tan Y, Wang G, Deng B, Ling Z, Song W, Seery S, Zhang Y, Peng S, Xu J, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J Clin Oncol. 2021;39:3340–3351. doi: 10.1200/JCO.21.00389. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moll M, Reinhold U, Kukel S, Abken H, Müller R, Oltermann I, Kreysel HW. CD7-negative helper T cells accumulate in inflammatory skin lesions. J Invest Dermatol. 1994;102:328–332. doi: 10.1111/1523-1747.ep12371791. [DOI] [PubMed] [Google Scholar]
- 82.Bishu S, Arsenescu V, Lee EY, Vargas HD, de Villiers WJ, Arsenescu R. Autoimmune enteropathy with a CD8+ CD7− T-cell small bowel intraepithelial lymphocytosis: case report and literature review. BMC Gastroenterol. 2011;11:131. doi: 10.1186/1471-230X-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]