Abstract
Garlic saccharides have prebiotic activity, but the association between their function and structure is still poorly known. In present study, four different garlic saccharides were obtained from garlic polysaccharides (GPs) after acidolysis by ultrafiltration. Obtained GPs were constituted by different monosaccharides, among which fructose and glucose were the main components, while galactose was a major component of GPs-U6. All four saccharides were partly degraded by the simulated digestive system, and most could reach the large intestine to be utilized by the gut microbiota. Except for GPs-U6, the other three garlic saccharide fractions had good prebiotic activity in vitro and in vivo. Furthermore, GPs-U0.3 with lower molecular weight (Mw) showed better prebiotic activity, including promoting the production of short-chain fatty acids (SCFAs), increasing the abundance of beneficial bacteria such as Bifidobacterium, Lachnospiraceae NK4A136 group and Phoscolarctobacterium, and inhibiting the growth of potentially harmful bacteria. In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway enrichment analysis showed that GPs-U0.3 could reduce the risk of cancer and cardiovascular diseases. Overall, this findings of the present study revealed the digestive properties of GPs, as well as the potential association between their chemical structures and fermentation characteristics by gut microbiota. Thus, it can be stated that GPs-U0.3 can be used as potential prebiotics in functional foods, which provides a theoretical basis for the targeted preparation of functionalized garlic saccharides.
Keywords: Garlic polysaccharides, Acidolysis, Digestive properties, Prebiotic activity, Gut microbiota, Short chain fatty acids
Graphical abstract
Highlights
-
•
Four garlic saccharides of different Mw could pass through the digestive system and reach the large intestine safely.
-
•
GPs-U2, GPs-U1 and GPs-U0.3 significantly modulate the composition and abundance of gut microbiota.
-
•
GPs-U2, GPs-U1 and GPs-U0.3 significantly enhance the production of SCFAs.
-
•
GPs-U0.3 exhibit better probiotic activity in vitro and in vivo.
1. Introduction
Garlic (Allium sativum L.) is an edible bulb plant of the family of Liliaceae, and has been widely used as spice, food, and folk medicine (Bo et al., 2021). Over 200 substances have been identified in garlic, including polysaccharides, proteins, lipids, vitamins, trace elements (such as selenium), flavonoids and organosulfur compounds (Fei et al., 2015). Among these, garlic polysaccharides (GPs) are among the main active components in garlic (approximately 26–30% of fresh weight), and exhibit a variety of biological activities, such as immunomodulatory (Li et al., 2017a), antitumor (Li and Huang, 2017), hepatoprotective (Wang et al., 2018), and anti-inflammatory (Shao et al., 2020). Previous studies reported that GPs were constituted of fructans whose main chain consist of a (2 → 1)-β-D-fructopyranose connected to a terminal (2 → 1)-β-D-glucopyranose at the non-reducing end and of (2 → 6)-β-D-fructopyranose branched chains with a degree of polymerization of 28 (Chen et al., 2013). It has been suggested that nondigestible polysaccharides and oligosaccharides can function as prebiotics, improving host health by selectively stimulating the growth and activity of a single or a limited number of bacteria in the colon (mainly Lactobacillus and Bifidobacterium) (Gibson and Roberfroid, 1995).
The gut microbiota living in the intestine establishes a close and beneficial relationship with its host (Veiga et al., 2014). As the largest and most complex “organ” of the body, the gut microbiota has been extensively studied for its powerful functions. The equilibrium between the host and the gut microbiota not only could prevent obesity and improve immune function (Ding et al., 2021; Thaiss et al., 2016), but also influences the emergence of certain diseases, including diabetes Lin et al. (2021), obesity (Zhang et al., 2021), colitis (Gong et al., 2022), heart cerebrovascular disease (Yoshida et al., 2018) and cancer (Peng et al., 2020; Wang et al., 2020a), among others.
It is widely known that the prebiotic activity of saccharides is closely associated with their molecular weight (Mw). Dou et al. (2019) found that moderately degraded blackberry polysaccharides showed better prebiotic activity, and low Mw blackberry polysaccharides could be better utilized by the gut microbiota. Moreover, inulin-type fructan with a lower degree of polymerization could be easily utilized by Bifidobacterium strains, and the metabolites produced were also significantly different due to differences in saccharides Mw. Acetic acid and lactic acid are the fermentation products of fructooligosaccharides (FOS), while butyrate is mainly a result of inulin-type fructan (Fu et al., 2019). Preliminary studies conducted by our research group suggested that garlic oligosaccharides obtained from acidolysis of GPs could better promote the growth of Lactobacillus when compared to unhydrolyzed GPs (Lu et al., 2021). Thus, it can be suggested that the prebiotic activity of garlic saccharides is closely related to their Mw.
Studies have shown that Mw, chemical composition and spatial structure of polysaccharides change under the influence of pH, bile salts and enzymes in the presence of salivary and gastrointestinal digestion (Yuan et al., 2019). Therefore, the study of the activity of saccharides should accompany that of their digestive properties.
Therefore, the aim of this study was to explore the effect of Mw of garlic saccharides on their prebiotic activity. Membrane technology has been widely used to separate polysaccharides based on Mw owing to its environmental and efficiency advantages (Cai et al., 2021). For this, four ultrafiltration membrane with cut-off Mw of 6000 Da, 2000 Da, 1000 Da and 300 Da selected could divide the acid hydrolyzed garlic saccharides into four fractions, high-Mw polysaccharides (GPs-U6), low-Mw polysaccharides (GPs-U2), high-Mw oligosaccharides (GPs-U1) and low-Mw oligosaccharides (GPs-U0.3). The physicochemical properties and digestive characteristics of these saccharides were investigated. Furthermore, the relationship between the Mw of these four saccharide fractions and their prebiotic activity was evaluated based on the growth of Lactobacillus and Bifidobacterium in vitro, as well as on gut microbiota community composition in healthy male C57BL/6 mice. These results described herein shed a light on the relationship between structure and prebiotic activity of garlic saccharides, and provide theoretical guidance for the production of garlic saccharides with higher prebiotic activity.
2. Materials and methods
2.1. Materials
Fresh garlic was purchased from Laiwu (Shandong, China). Simulated saliva fluid (SSF), simulated gastric fluid (SGF) and simulated small intestinal fluid (SIF) were provided by Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China). FOS was produced by Meiji Seika Kaisha, Ltd. (Japan), which was composed of 6.5% of fructosylfructosyl nystose (GF5), 43.4% of 1F-β-fructofuranosyl nystose (GF4), 40.9% of nystose (GF3), 7.1% of 1-kestose (GF2), and 2.1% glucose and fructose. Glucose-free De Man-Rogosa-Sharpe (MRS) broth and anaerobic culture bags were purchased from Haibo Biotechnology Co., Ltd. (Qingdao, China). Chemical standards (i.e., acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid and isovaleric acid) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). All other chemicals used in the experiments were of analytical grade.
2.2. Preparation of garlic saccharides of different Mw
2.2.1. Preparation of GPs
GPs was extracted from fresh garlic using the method described by Jiang et al. (2006) with some modifications. Briefly, fresh garlic were pulped and treated with deionized water (1:6, w/v) at 80 °C for 2.5 h. Then, 4-fold volume of absolute ethanol was added to the supernatant and incubated overnight at 4 °C to precipitate. The obtained precipitate was then collected and redissolved in deionized water. Then, pectin and protein were removed from crude saccharides extract by the addition of 10% CaCl2 solution and by the Sevag method, respectively. Finally, GPs were lyophilized and stored until further use.
2.2.2. Acidolysis and ultrafiltration of GPs
GPs aqueous solution (5%, w/v) at pH 3 adjusted with 1 mol/L HCl was submitted to hydrolysis at 70 °C for 3 h. The reaction was immediately terminated by adjusting pH to 7 with 1 mol/L NaOH. Then, the hydrolysate was fractionated using an ultrafiltration system (Shandong Bona Biotechnology Group Co., Ltd., Jinan, China) at 0.3 MPa or less of pressure. Ultrafiltration membranes of four different Mw cut-offs (i.e., 6000 Da, 2000 Da, 1000 Da and 300 Da) were used, hence yielding four saccharide fractions, namely GPs-U6 (Mw > 6 kDa); GPs-U2 (Mw 2–6 kDa); GPs-U1 (Mw 1–2 kDa); and GPs-U0.3 (Mw 0.3–1 kDa), which were lyophilized for subsequent experiments.
2.3. Chemical composition analysis
Total sugar content in GPs fractions was determined using the phenol-sulfuric acid method with glucose as the standard. The content of reducing sugar (CR) was determined based on the 3, 5-dinitrosalicylic acid (DNS) method (Miller, 1959). Protein content was determined by the Bradford method with bovine serum albumin as the standard (Bradford, 1976).
2.4. Structural characterization
2.4.1. Molecular weight determination
Mw of saccharides was determined by HPLC in an LC-20A system (Shimadzu, Kyoto, Japan) equipped with the tandem chromatography columns of Shodex Ohpak SB-803HQ, Shodex Ohpak SB-802.5HQ and Shodex Ohpak SB-802HQ (300 × 8 mm, 6 μm). After filtration of saccharides samples (5 mg/mL) with a 0.45 μm filter, elution was performed with 0.3 mol/L NaNO3 at a flow rate of 0.3 mL/min and column temperature at 30 °C. Mw of saccharides was calculated based on the calibration curves of a set of dextran standards (180, 342, 1000, 2000, 3000, 6000, 9000 and 20000 Da).
2.4.2. Fourier-transform infrared spectroscopy (FTIR) analysis
FTIR spectra of saccharides samples were obtained in a FTIR spectrophotometer (Thermo Nicolet IS10, USA) equipped with a universal ATR. Briefly, the dried sample (3 mg) was pressed onto the diamond crystal and scanned within the range of 400–4000 cm−1. Scans were performed in triplicate for each sample. The results were analyzed using OMNIC 8.2.0.387 software.
2.4.3. Monosaccharide composition determination
Monosaccharide composition of saccharides was determined by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) (Thermo Fisher ICS-5000+, USA) according to the method proposed by Li et al. (2021). Briefly, saccharides sample (10 mg) was submitted to hydrolysis with 2 mL of 2.0 mol/L trifluoroacetic acid (TFA) at 80 °C for 4 h. After removing the excess of TFA by rotary evaporation, sample volume was adjusted to 10 mL with deionized water. Subsequently, the samples was purified using SupelClean™ LC-18 tubes (500 mg/6 mL) (Supelco, USA) and 0.22 μm filters, and then analyzed in an AminoPac™ PA10 (Dionex, 3 × 250 mm) column with 17 mmol/L NaOH at 30 °C at a flow rate of 0.25 mL/min.
2.5. In vitro simulated saliva-gastrointestinal digestion
The digestion of saccharides was carried out with a method previously described (Wu et al., 2021a). Briefly, saccharides (80 mg/mL) and SSF (with salivary amylase 200 U/mL) were added to a centrifuge tube at a ratio of 1:1 (v/v) and incubated at 37 °C for 1 h to stimulate the digestive process in the oral cavity. The process was conducted for a longer period than the average time required for oral digestion to evaluate the digestibility of garlic saccharides in the oral cavity. Samples were collected at different digestion times (0 , 0.5 and 1 h) and heated at 100 °C for 5 min to inactivate salivary amylase.
Subsequently, the saliva digestion solution was mixed with SGF at a ratio of 1:1 (v/v) and the pH of the mixture was immediately adjusted to 2.0. The digestion process was performed at 37 °C for 6 h. Then, the pH of the saliva-gastric digestive fluid was adjusted to 7.0 (by the addition of 1 M NaHCO3 dropwise), and the digested mixture was mixed with SIF at a ratio of 10:3 (v/v) and incubated at 37 °C for 6 h. An aliquot samples (2 mL) was obtained from the mixture at different times (1, 2, 4 and 6 h) of gastric and intestinal digestion, and boiled for 5 min to inactivate enzymes for further analysis. All experiments were carried out in triplicate. The hydrolysis degree of garlic saccharides was calculated as follows:
| Hydrolysis degree (%) = (final reducing sugar content - initial reducing sugar content)/(total sugar content - initial reducing sugar content) |
2.6. Prebiotic activity of garlic saccharides in vitro
2.6.1. Effect of garlic saccharides of different Mw on the growth of probiotic bacteria
Lactobacillus acidophilus L3-003, Lactobacillus plantarum L1-006, and Lactobacillus rhamnosus L2-005, as well as Bifidobacterium longum B1-0013, Bifidobacterium animal B3-001 and Bifidobacterium youth B2-001 were provided by Shandong Baolai-leelai Biotechnology Co., Ltd. (Taian, China). All strains were preserved in MRS broth and activated at 37 °C for 16 h prior to use.
The effect of garlic saccharides on the growth of probiotic bacteria was determined following a method reported by Nobre et al. (2019) with a minor modification. In brief, the different GPs fractions were sterilized with 0.22 μm filters and added to sterile glucose-free MRS broth at a ratio of 1:9 (v/v) to a final concentration of 2% (w/v). Then, previously activated probiotic bacteria inoculated twice at 2% (v/v) were inoculated to media with different carbon sources (glucose and FOS as control) and cultured under anaerobic conditions at 37 °C for 48 h. Samples were obtained at 0, 2, 4, 8, 12, 16, 24, 36 and 48 h of incubation time, and turbidity of culture media indicative of growth of probiotic bacteria was measured in a microplate reader at 600 nm. pH of samples was determined using a pH meter.
2.6.2. Determination of SCFAs and lactic acid contents
In brief, the fermentation medium was centrifuged (10,000 rpm for 15 min at 4 °C) and extracted with 1 mL of ethyl acetate. Then, SCFAs were analyzed by GC-MS (Shimadzu, Japan) equipped with an Agilent HP-INNOWax column (30 m × 0.25 mm × 0.25 μm) using high purity helium as carrier gas. The initial column temperature was 100 °C, which was increased to 140 °C at a rate of 3.5 °C/min and 200 °C at the rate of 30 °C/min, and then maintained for 9 min. The temperature of the injector and electron bombardment ion source was 250 °C and 220 °C, respectively. In addition, a HPLC system (Shimadzu, Kyoto, Japan) equipped with Extend C-18 column (250 × 4.6 mm, Agilent, USA) was used to determine the content of lactic acid at 210 nm. After the fermentation supernatant was ten-fold diluted with deionized water, the analysis was directly performed using 95% NaH2PO4 (10 mM, pH = 2.7) and 5% methanol as the mobile phase at a flow rate of 0.8 mL/min and 30 °C as column temperature.
2.7. Prebiotic activity of garlic saccharides of different Mw in vivo
2.7.1. Animals experiment design
Six-week-old healthy male C57BL/6 mice (20 ± 0.5 g) were purchased from Jinan Pengyue Laboratory Animal Breeding Co. Ltd. (Shandong, China). The mice were housed in cages under controlled temperature and humidity conditions with a 12 h light/dark cycle, and had free access to food and water. After 7 days of acclimation, mice were randomly divided into 4 groups (10 mice per group), as follows: the control group and three experiment groups (GPs-U2, GPs-U1 and GPs-U0.3). Mice in the three experimental groups received different saccharides (4 g/kg of body weight) once daily by oral gavage; animals in the control group were provided with the same volume of 0.9% NaCl solution instead of saccharide solution. Body weight, food intake and health status of mice were recorded during 21 days of intervention. Finally, fecal samples of each mice were collected in individual sterile EP tubes and stored at −80 °C until further analysis. All mice were sacrificed by cervical dislocation after the experiment. All methods and procedures were approved by Institutional Animal Care and Use Committee of Shangdong Agricultural University (Approval No.2020-04), Shandong, China.
2.7.2. Determination of SCFAs in feces
Fecal samples were accurately weighed (100 mg) and transferred to tube containing 200 μL of 10% H2SO4 and 800 μL of ethyl acetate. After the mixture was shaken for 10 min, SCFAs contents were determined as described in 2.6.2.
2.7.3. DNA extraction and 16S rDNA sequencing
According to the manufacturer's instructions, microbial genome in fecal samples was carried out using the E.Z.N.A. soil DNA kit (Omega Bio⁃tek, Norcross, GA, USA). The V3–V4 hypervariable region of the bacterial 16S rDNA was amplified by PCR using total extracted DNA and primers. Based on the Illumina MiSeq sequencing platform, a small fragment library was constructed and paired-end sequencing was performed. QIIME (version 1.17) software and UCLUST were used for sequence analysis and chimera removal on clean reads, respectively. Then, duplicate sequences were clustered into OTUs with 97% identification, and representative sequences of OTUs were annotated by Greengenes or SILVA databases. Rarefaction curve and Shannon curve were used to evaluate the reliability of sequencing data. Principal coordinate analysis (PCoA) was performed using weighted UniFrac. In addition, functional characteristics of microbial communities were conducted using PICRUSt based on the KEGG database.
2.8. Statistical analysis
One-way analysis of variance (ANOVA) and Duncan's test were used to determine differences in the means between groups using SPSS software (Version 21, SPSS Inc. Chicago, USA). Differences in taxa relative abundance in gut microbial community profiles among sample groups were determined based on the Kruskal–Wallis test with a false-discovery rate (FDR) correction by STAMP software. Statistical significance was considered at P values < 0.05.
3. Results and discussion
3.1. Structural characterization of garlic saccharides of different Mw
3.1.1. Chemical composition
Four saccharide fractions of different Mw were obtained by acidolysis and ultrafiltration (Fig. S1). Their total sugar content was greater than 85%, thus indicating high purity (Table 1). The protein contents in GPs-U6, GPs-U2, GPs-U1, and GPs-U0.3 were 9.99 ± 0.26%, 0.46 ± 0.08%, 0.26 ± 0.06%, and 0.24 ± 0.08%, respectively, indicating that the fractions GPs-U2, GPs-U1, and GPs-U0.3 had low amounts of proteins. The higher protein content in GPs-U6 might be due to the combination of polysaccharides and proteins.
Table 1.
Chemical composition, Mw and monosaccharide compositions of GPs and garlic saccharides with different Mw.
| GPs | GPs-U6 | GPs-U2 | GPs-U1 | GPs-U0.3 | |
|---|---|---|---|---|---|
| Chemical composition | |||||
| Total sugar content (%) | 93.10 ± 0.69 | 86.57 ± 0.69 | 92.49 ± 2.72 | 96.15 ± 0.87 | 93.59 ± 0.59 |
| Protein content (%) | 2.60 ± 0.14 | 9.99 ± 0.26 | 0.46 ± 0.08 | 0.26 ± 0.06 | 0.24 ± 0.08 |
| Molecular weight | |||||
| Mn (g/mol) | 1836 | 8384 | 2221 | 1026 | 551 |
| Mw (g/mol) | 3656 | 45796 | 2723 | 1247 | 731 |
| Monosaccharide composition (%) | |||||
| Fructose | 79.23 | 57.47 | 88.09 | 75.70 | 79.89 |
| Glucose | 18.92 | 9.75 | 11.71 | 23.85 | 19.56 |
| Galactose | 1.01 | 24.38 | 0.19 | 0.21 | 0.24 |
| Arabinose | 0.84 | 0.79 | – | 0.24 | 0.31 |
| Xylose | – | 0.52 | – | – | – |
Data was expressed as mean ± standard deviation (SD). The percentage of monosaccharides was calculated based on all detected monosaccharides.
3.1.2. Monosaccharide composition
As shown in Table 1, fructose and glucose were the main monosaccharides in GPs, a finding which was similar to previous studies showing that GPs were fructan polymers (Yan et al., 2021). GPs-U2, GPs-U1 and GPs-U0.3 showed similar monosaccharide profiles, and were mainly composed of fructose and glucose, similarly to GPs, but their specific proportions were slightly different. In contrast, GPs-U6 contained a higher proportion of galactose in addition to fructose and glucose. It has been reported that galactan can also be found in garlic (Li et al., 2017b). Liang et al. (2022) revealed that the monosaccharide composition of polysaccharides of different Mw obtained by acidolysis of black garlic polysaccharides also differed, which was similar to the results of the presented study. This may be due to differences in the ability of glycans to resist acidolysis, and galactan could exhibit higher stability under acidic conditions.
3.1.3. FTIR analysis
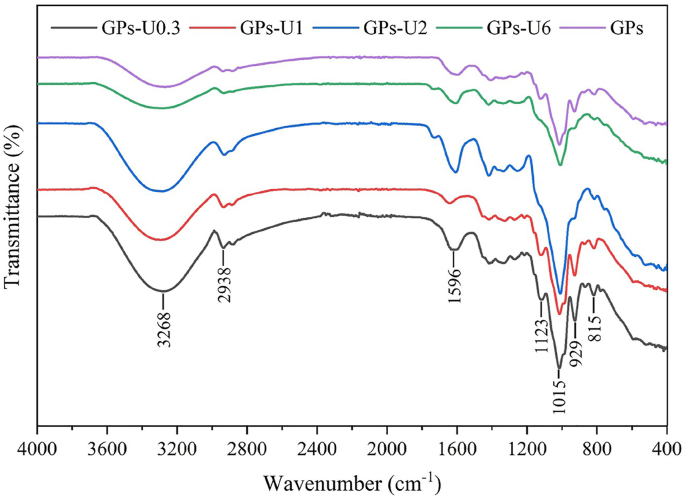
FTIR spectra of GPs and the four different fractions had similar IR bands within the range of 4000–400 cm−1 (Fig. 1). The absorption bands at around 3268−1, 2938−1, 1596−1 and 1000–800 cm−1 represented O–H stretching vibrations of hydroxyl groups, C–H stretching vibrations, C O stretching vibrations of ionic carboxyl groups, and O–C–O and C–O–C stretching vibrations of glycosidic linkages and rings, respectively (Hospodarova et al., 2018). The absorption bands around 929−1 and 815 cm−1 represented fructose with β-type glycosidic bond (Lu et al., 2021). After acid hydrolysis and ultrafiltration, certain differences were also found. The absorption intensity near the band 3268 cm−1 increased with the decrease of Mw, which might be due to the release of an increasing number of O–H groups via the disruption of glycosidic bonds, thus resulting in increased O–H absorption intensity of lower Mw saccharides (Xu et al., 2018). Furthermore, the absorption bands at 1123 cm−1 and 1929 cm−1 gradually became less pronounced with the increase in Mw. Collectively, these results suggested that the fractioning with acidolysis and ultrafiltration did not significantly change the primary structure of GPs, and differences were only observed in the absorption intensity of several bands.
Fig. 1.
FTIR spectra of GPs and different saccharide fractions.
3.2. Effect of in vitro digestion on Mw and the degree of hydrolysis of garlic saccharides
Fig. S2 shows the dynamic changes in Mw of garlic saccharides of different Mw during in vitro digestion. The peaks attributed to GPs, GPs-U2, GPs-U1 and GPs-U0.3 did not change significantly during simulated oral and small intestine digestion, which indicates that Mw of polysaccharides in these fractions might not decrease significantly during these conditions, which was consistent with the results of the degree of hydrolysis (Table 2). However, a decrease in saccharides Mw and the generation of a new peak I was observed during gastric simulated digestion, suggesting that these saccharides could be partially degraded by the gastric juice. Moreover, their degree of hydrolysis increased significantly (P < 0.05), which indicated that the decrease in Mw might be due to the breakage of glycosidic bonds, and that the lower the Mw of the saccharide, the higher the degree of hydrolysis it undergoes. In addition, glycosidic bonds are broken under acidic conditions. Therefore, the reduced Mw of GPs, GPs-U2, GPs-U1 and GPs-U0.3 might be related to the acidic environment in the simulated environment of the stomach, which was similar to the findings revealed in previous study that showed that mulberry fruit polysaccharides could be degraded during gastric digestion (Chen et al., 2016). However, a significant increase in the degree of hydrolysis was observed for GPs-U6 in simulated oral digestion (P < 0.05) due to amylase hydrolysis. No significant changes were observed in the degree of hydrolysis and Mw for GPs-U6 in simulated gastrointestinal digestion, which was similar to the results of in vitro digestion of polysaccharides obtained from Fuzhuan brick tea that only slightly degraded during salivary and gastrointestinal digestion (Chen et al., 2018). Thus, it can be stated that differences in the digestive properties of the saccharides were related to the type of the glycosidic bond, Mw, and monosaccharide composition. Taken together, GPs, GPs-U6, GPs-U2, GPs-U1 and GPs-U0.3 showed great stability during in vitro digestion, with a degrees of hydrolysis of only 9.82 ± 1.36, 1.60 ± 0.42, 9.47 ± 0.27, 11.72 ± 1.32 and 13.67 ± 0.74, respectively, thus indicating that they could potentially reach large intestines to be utilized by gut microbiota.
Table 2.
Changes of degree of hydrolysis during garlic saccharides with different Mw simulated digestion in vitro.
| Time/h | Hydrolysis degree/% |
|||||
|---|---|---|---|---|---|---|
| GPs | GPs-U6 | GPs-U2 | GPs-U1 | GPs-U0.3 | ||
| Saliva | 0 | 0.16 ± 0.24e | 0.10 ± 0.04c | 0.17 ± 0.07e | 0.11 ± 0.14e | 0.32 ± 0.10e |
| 0.5 | 0.21 ± 0.20e | 0.42 ± 0.08b | 0.14 ± 0.07e | 0.39 ± 0.22e | 0.31 ± 0.29e | |
| 1 | 0.20 ± 0.05e | 0.92 ± 0.13a | 0.26 ± 0.11e | 0.37 ± 0.09e | 0.22 ± 0.18e | |
| Gastric | 1 | 2.27 ± 0.94d | 1.30 ± 0.49a | 3.27 ± 0.44d | 5.19 ± 0.15d | 6.77 ± 0.57d |
| 2 | 3.40 ± 1.14c | 1.33 ± 0.18a | 5.81 ± 0.94c | 6.74 ± 0.76c | 9.96 ± 0.95c | |
| 4 | 4.84 ± 0.80b | 1.30 ± 0.60a | 7.02 ± 0.51b | 8.41 ± 0.47b | 11.51 ± 0.71b | |
| 6 | 8.25 ± 1.54a | 1.60 ± 0.20a | 8.90 ± 0.58a | 12.91 ± 0.92a | 13.43 ± 0.98a | |
| Intestinal | 1 | 9.78 ± 1.00a | 2.04 ± 0.70a | 9.58 ± 0.20a | 13.93 ± 0.80a | 14.31 ± 0.30a |
| 2 | 9.77 ± 0.98a | 1.46 ± 0.78a | 10.23 ± 1.13a | 14.57 ± 0.28a | 13.47 ± 1.03a | |
| 4 | 8.92 ± 1.37a | 1.48 ± 0.78a | 9.91 ± 0.92a | 12.63 ± 0.76a | 12.40 ± 0.37a | |
| 6 | 9.82 ± 1.36a | 1.60 ± 0.42a | 9.47 ± 0.27a | 11.72 ± 1.32a | 13.67 ± 0.74a | |
Data were expressed as mean ± standard deviation. The values with different letters in the same column were significantly different at P < 0.05.
3.3. In vitro prebiotic activity
3.3.1. Effect of garlic saccharides of different Mw on the growth of probiotic bacteria
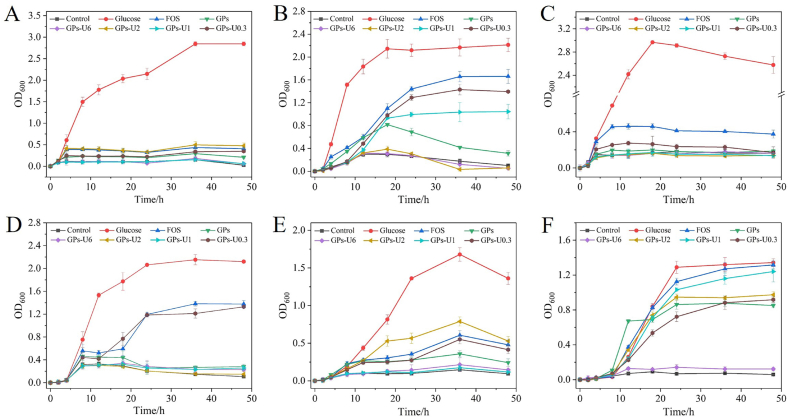
The prebiotic activity of saccharides is attributed to their capacity of being selective metabolize by probiotics microorganisms hence stimulating their growth (de Figueiredo et al., 2020). As shown in Fig. 2, all six probiotic strains had the greatest growth rate in culture media with glucose as carbon source. For L. acidophilus L3-003, OD600 values of media supplemented with FOS, GPs, GPs-U6, GPs-U2, GPs-U1 and GPs-U0.3 were 1.66, 0.31, 0.05, 0.06, 1.05 and 1.39 after 48 h of incubation, respectively. Among GPs and the four saccharide fractions, GPs-U0.3 showed the best growth-promoting effect, followed by GPs-U1, which also reflected the preference of L. acidophilus L3-003 for low-Mw saccharides. For L. plantarum L1-006 and L. rhamnosus L2-005 strains, only glucose showed a good ability to promote bacterial growth. Compared with Lactobacillus strains, the lag phase of growth of the three Bifidobacteria strains was longer. For B. longum B1-0013, the growth-promoting effect of GPs-U0.3 was comparable to FOS, and was more remarkable than the other saccharide components. Apart from GPs-U6, all saccharide components were able to promote the growth of B. adolescentis B2-001 based on the following order of preference: glucose > FOS > GPs-U1 > GPs-U2 = GPs-U0.3 > GPs > GPs-U6. In contrast, GPs-U2 showed a greater prebiotic potential than the other saccharide fractions from GPs for B. animalis B3-001. Therefore, the prebiotic activity of saccharides was strain-dependent due to the presence of different carbohydrate hydrolases, and that saccharides of low Mw tended to show a stronger growth-promoting effect (Hu et al., 2013).
Fig. 2.
Growth curves of L. plantarum L1-006 (A), L. acidophilus L3-003 (B), L. rhamnosus L2-005 (C), B. longum B1-0013 (D), B. animalis B3-001 (E) and B. adolescentis B2-001 (F) with glucose, FOS, GPs or different sugar fractions as the sole carbon source.
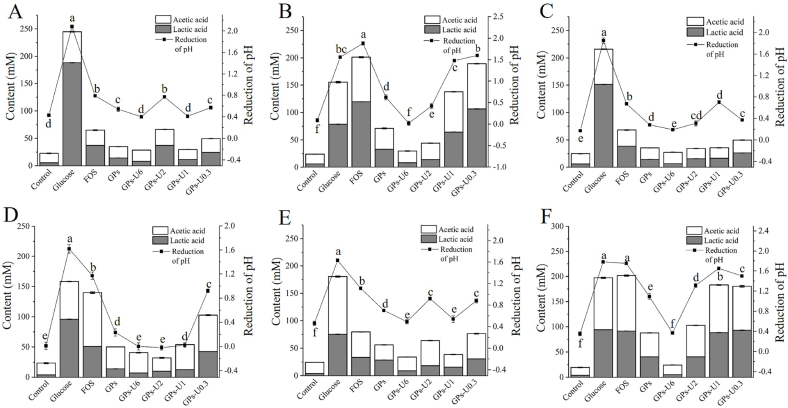
3.3.2. Determination of SCFAs, lactic acid and pH
Prebiotics alter the microbial composition in the colon and induce the production of fermentation products such as lactic acid and SCFAs, which lower the pH of the surroundings and inhibit the growth of pathogenic bacteria (Teferra, 2021). As shown in Fig. 3, acetic acid was the main SCFAs produced in the culture medium, and other SCFAs were not detected. The effects of different carbon sources on the pH of the culture medium and the levels of lactic acid and acetic acid were closely related to the degree of utilization of carbon compounds by bacterial strains. No significant increase was observed in the biomass of the six probiotics strains evaluated in sugar-free and GPs-U6-supplemented culture media, thus leading to a slight decrease in pH and production of lactic and acetic acid. For L. acidophilus L3-003 and B. longum B1-0013 strains, the most remarkable decrease in pH and increase in the production of lactic acid and acetic acid were observed in the medium containing GPs-U0.3 compared with the other garlic saccharides, but lower than that of GPs-U1 for B. adolescentis B2-001.
Fig. 3.
The content (mM) of lactic acid and acetic acid at the end of fermentation (48 h) and changes in pH in media with different sugars as the sole carbon source. (A) L. plantarum L1-006, (B) L. acidophilus L3-003, (C) L. rhamnosus L2-005, (D) B. longum B1-0013, (E) B. animalis B3-001 and (F) B. adolescentis B2-001. The values with different letters (a–f) were significantly different at P < 0.05.
Thus, the differences in the prebiotic activity of the same bacterial strain could be related to the structure (especially Mw) of saccharides (Wang et al., 2020b). Moreover, the saccharides factions with a lower Mw (i.e., GPs-U0.3) tended to exhibit higher probiotic potential than the other saccharides in vitro, based on its the growth-promoting effect, capacity for the production of SCFAs and decrease in pH. In contrast, GPs-U6 showed little prebiotic activity among all six selected strains. Therefore, GPs-U2, GPs-U1 and GPs-U0.3 were selected for use in further in vivo studies.
3.4. Effect of different saccharides on the healthy mice
3.4.1. Effect of different saccharides on body weight, food intake, and water intake
Body weight, food intake and water intake of mice in each group were monitored during 21-days. As shown in Table S1, the intake of GPs-U2, GPs-U1 and GPs-U0.3 did not show a significant effect on the body weight, food intake, and water intake of the mice compared to the control group.
3.4.2. Microbial diversity and OTU distribution in feces
A total of 1,562,029 clean sequences were obtained from 24 fecal samples with an average length of 421 bp. Non-repeated sequences were aggregated into 1535 OTUs based on 97% sequence similarity. The rarefaction and Shannon index curve of each group showed that the curve gradually reached an equilibrium as the number of reads in samples increased (Fig. S3). Thus, sequencing depth was sufficient to characterize the gut microbiota of mice in experimental and control groups.
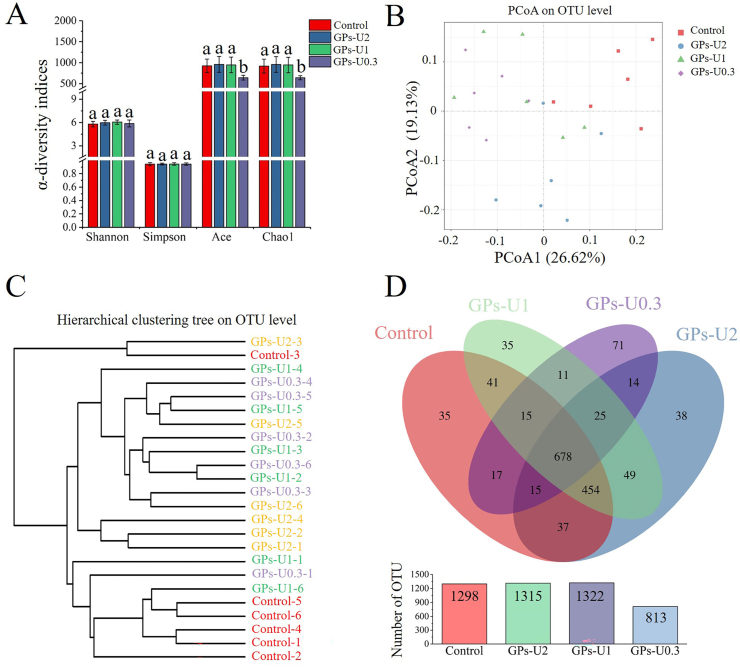
Shannon, Simpson, Ace and Chao 1 indices of α-diversity were calculated to reflect richness and diversity of the microbial community in samples (Han et al., 2021). Ace and Chao 1 indices showed that the supplementation of GPs-U0.3 significantly reduced the abundance of microorganisms compared with the control group (P < 0.05), which was similar to the findings of a previous study in which the richness and diversity of the small gut microbiota community of mice fed with FOS-rich goat milk were significantly reduced (Ma et al., 2021). However, no significant changes were observed in Ace and Chao 1 indices in GPs-U2 and GPs-U1-treated mice, and no significant changes were observed in Shannon and Simpson indices in all treatment groups (Fig. 4A).
Fig. 4.
Effect of GPs-U2, GPs-U1 and GPs-U0.3 on α- and β-diversity of gut microbiota of the mice. (A)α-diversity indices (Shannon, Simpson, Ace and Chao 1); (B) PCoA analysis of β-diversity; (C) the cluster tree analysis of β-diversity; (D) Venn diagram based on OTUs. The values with different letters were significantly different at P < 0.05.
Furthermore, PCoA and phylogenetic tree analysis based on β-diversity were used to assess differences in gut microbiota composition between the different treatment groups. PCoA results showed that the control group was mainly distributed in the upper right quadrant and could be distinctly separated from treatment groups (Fig. 4B). Moreover, the short distance between GPs-U1 and GPs-U0.3-treated mice indicated that the gut microbiota in these mice might be similar. In contrast, significant differences were observed between GPs-U2-treated mice and the other groups. The cluster tree results (Fig. 4C) showed similar results to the PCoA.
In addition, the number of OTUs indicates species richness in the samples, and a Venn diagram was generated based on the number of unique and shared OTUs. As shown in Figs. 4D and 678 of all OTUs were commonly found in all groups, whereas 35, 38, 35 and 71 unique OTUs were found in the control group, GPs-U2, GPs-U1, and GPs-U0.3-treated groups, respectively. Compared with the control group, the reduction of OTUs in GPs-U0.3-treated mice indicated a decrease in the abundance of species in the gut microbiota, which was consistent with the results of Ace and Chao 1 indices.
3.4.3. Composition of gut microbiota
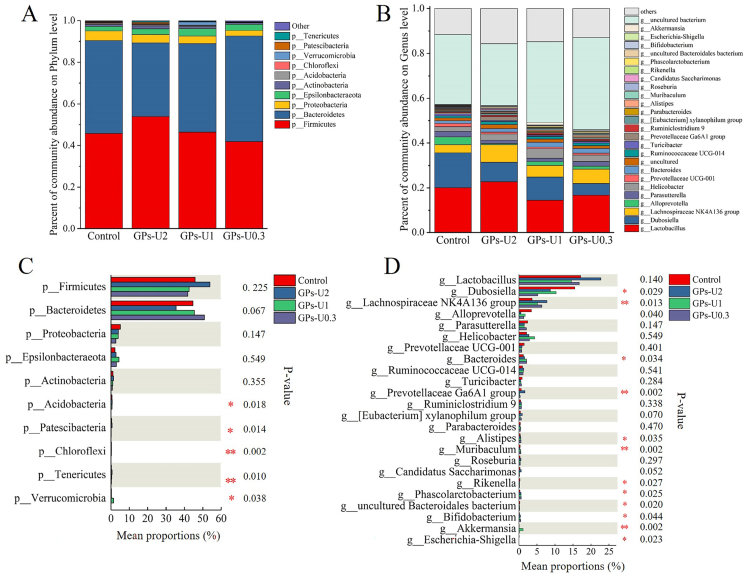
At the phylum level, Firmicutes, Bacteroidetes and Proteobacteria were the main phyla in mice fecal samples, and 5 phyla in the four experimental groups were observed to be significantly different (Fig. 5A and C). The supplementation of GPs-U0.3 increased the abundance of Bacteroidetes (P > 0.05), which includes representatives capable of producing high levels of acetic acid and propionic acid (Macfarlane and Macfarlane, 2003). After the intake of GPs-U2, the abundance of Firmicutes, Patescibacteria and Tenericutes significantly increased (P < 0.05), while that of Bacteroidetes decreased (P < 0.05). Interestingly, higher ratio of Firmicutes and Bacteroides (F/B) was observed only in GPs-U2-treated mice (P < 0.05), which could promote the increase in the levels of SCFAs in the gut and reduce the risk of infection (Molist et al., 2012). Moreover, F/B has been reported to be positively correlated with body mass index (Koliada et al., 2017). In mice treated with GPs-U0.3, the abundance of Proteobacteria, Acidobacteria and Chloroflexi was significantly reduced (P < 0.05). It is known that nearly all species within the phylum Proteobacteria are pathogenic bacteria (such as Escherichia coli and Salmonella), which are also related to diabetes, inflammation and cancer. Therefore, the decrease in the abundance of Proteobacteria indicated that GPs-U0.3 components could potentially inhibit the proliferation of harmful bacteria and reduce the risk of such diseases (Lange et al., 2016). Moreover, the phylum Verrucomicrobia was significantly increased in the GPs-U1-treated mice (P < 0.05), which could be mainly due to a significant increase in the content of Akkermansia.
Fig. 5.
Effect of GPs-U2, GPs-U1 and GPs-U0.3 on the composition and differences in intestinal microbial taxa at the level of phylum (A, C) and genus (B, D). The differences in the abundance of microbial communities were determined by the Kruskal-Wallis test; *P < 0.05, **P < 0.01.
At the genus level, 12 genera were found differently enriched in the four experimental groups (Fig. 5B and D). All treatments resulted in a significant increase in the abundance of Bifidobacterium and Phascolarctobacterium (P < 0.05), but only in the GPs-U0.3-treated mice the level of Lachnospiraceae NK4A136 group was increased (P < 0.05). In addition, a significant reduction in the abundance of Rikenella was observed in mice in all treatments, which could prevent the emergence of disorders of the immune system (Wu et al., 2020b). The significant increase in the abundance of Bifidobacterium in the intestine is known to promote human health, including maintaining intestinal homeostasis and enhancing barrier and immune functions of the intestine (Ding et al., 2019). Lachnospiraceae NK4A136 group has been reported to play a key anti-inflammatory role (Hu et al., 2019). Furthermore, the supplementation of GPs-U2 led to a significantly increased in the abundance of Eubacterium Xylanophilum group and Prevotellaceae Ga6A1 group (P < 0.05), which knowingly utilize polysaccharides and improve glucose metabolism, thus preventing cardiovascular disease (Zhang et al., 2012; Precup and Vodnar 2019). The genus Alitipes has been suggested to play a very important role in the microbial treatment of inflammatory bowel disease (Dziarski et al., 2016). Moreover, representatives of the genus Bacteroides exhibited an extraordinary ability to utilize polysaccharides and were positively correlated with the production of propionic acid (Salonen et al., 2014). Additionally, in a sodium dextran sulfate-induced mice model, the relative abundance of Muribaculum has been shown to be negatively correlated with pro-inflammatory cytokines and positively correlated with the expression levels of genes coding for tight junction protein and mucin2 (Yan et al., 2019). In mice treated with GPs-U0.3 and GPs-U1, the abundance of three bacteria genera was significantly increased (P < 0.05). Interestingly, a significant decrease in the abundance of Parasutterella was observed in mice treated with GPs-U2 and GPs-U1 (P < 0.05). In addition, the abundance of Dubosiella was significantly decreased in GPs-U0.3-treated mice (P < 0.05), whose association with intestinal inflammation has been previously suggested (Sheng et al., 2020). Akkermansia has been considered a probiotic genus with beneficial effects on immune and metabolic functions (Yu et al., 2021; Zou and Chen, 2020), whose abundance was significantly increased due to the supplementation of GPs-U1 (P < 0.05). Finally, it is widely known that Escherichia-Shigella might cause inflammation via the production of lipopolysaccharides; a decrease in its abundance was observed in the GPs-U0.3-treated mice (P < 0.05) (Yang et al., 2020).
3.4.4. Functional prediction analysis of the gut microbial metagenomes
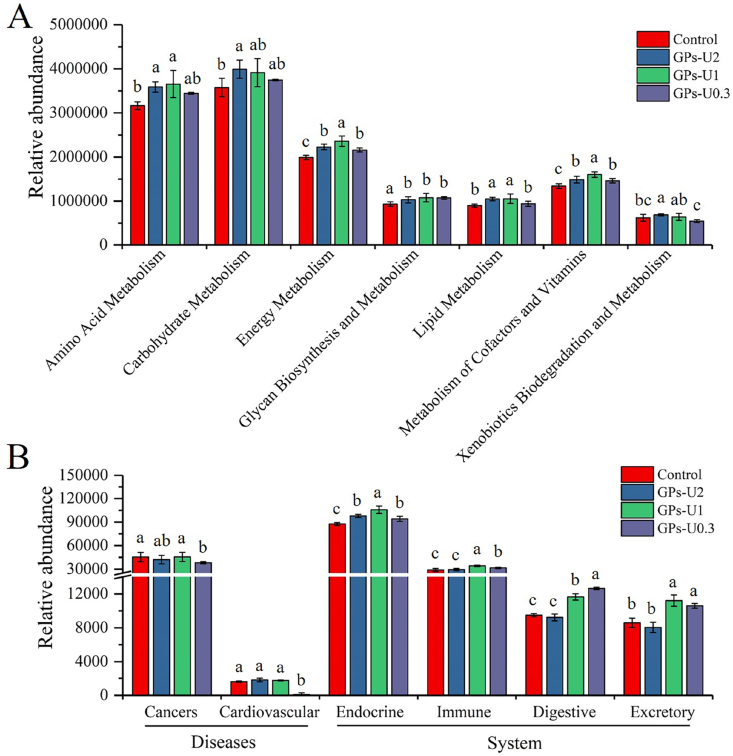
As shown in Fig. 6A, all treatments up-regulated the abundance of genes related to energy metabolism, glycan biosynthesis and metabolism, and metabolism of cofactors and vitamins (P < 0.05). Glycan metabolism has been shown to shape human gut microbiota and confer immunomodulatory properties to the host (Koropatkin et al., 2012). Therefore, it can be stated that garlic saccharides play important roles in providing immunomodulatory properties to the host. For GPs-U2-treated mice, enriched genes were found involved in amino acid metabolism, carbohydrate metabolism, lipid metabolism, and xenobiotics biodegradation and metabolism (P < 0.05). In contrast, the abundance of genes involved in amino acid metabolism was significantly reduced in the GPs-U1-treated mice (P < 0.05). Thus, it can be suggested that GPs-U2, GPs-U1 and GPs-U0.3 can improve metabolic activity of microorganisms.
Fig. 6.
Functional predictions of metabolism (A), disease and systemic (B) gene abundance in the control GPs-U2, GPs-U1 and GPs-U0.3 groups. Histograms are shown as mean ± standard deviation (n = 6). The values with different letters were significantly different at P < 0.05.
Moreover, the results of disease prediction showed that the intake of GPs-U0.3 significantly reduced the risk of cancer and cardiovascular diseases (P < 0.05) (Fig. 6B). In terms of system function prediction, the abundance of genes related to endocrine system, immune system, digestive system and excretion system was significantly increased in the GPs-U1-treated and GPs-U0.3-treated mice (P < 0.05). In addition, the intake of GPs-U2 significantly reduced the abundance of genes related to the endocrine system (P < 0.05).
3.4.5. Levels of SCFAs
Acetic acid, butyric acid and propionic acid were the main SCFAs found in mice fecal samples (Table 3). SCFAs can maintain the stability of the intestinal environment and protect the colon. In particular, acetic acid and propionic acid have crucial anti-inflammatory and anticancer effects (Maslowski et al., 2009); propionic acid can also affect liver and cholesterol metabolism (Venter et al., 1990), and butyric acid can exert anti-inflammatory effects by modulating inflammatory pathways and increasing the integrity of the intestinal barrier (Canani et al., 2011). Compared with the control group, the intake of GPs-U2, GPs-U1 and GPs-U0.3 led to significant increase in the content of acetic acid, propionic acid and total SCFAs (P < 0.05). In particular, the content of acetic acid was 1.19, 1.23 and 1.35-fold higher in the GPs-U2, GPs-U1 and GPs-U0.3-treated mice than in the control group, respectively. In addition, the content of total SCFAs in GPs-U2, GPs-U1 and GPs-U0.3 groups were 1.12, 1.18 and 1.31-fold higher than that in the control group, respectively. Notably, the highest content of acetic acid, propionic acid and total SCFAs was found in GPs-U0.3-treated mice, which suggested that all components could increase the levels of SCFAs in the intestines, especially GPs-U0.3.
Table 3.
The content of SCFAs in feces.
| Control | GPs-U2 | GPs-U1 | GPs-U0.3 | |
|---|---|---|---|---|
| Acetic acid | 6.00 ± 0.54c | 7.11 ± 0.42b | 7.38 ± 0.13b | 8.08 ± 0.19a |
| Propionic acid | 1.27 ± 0.10c | 1.54 ± 0.18b | 1.49 ± 0.11b | 1.75 ± 0.20a |
| Butyric acid | 1.04 ± 0.08ab | 0.71 ± 0.23b | 1.02 ± 0.34ab | 1.11 ± 0.23a |
| Isobutyric acid | 0.06 ± 0.01a | 0.05 ± 0.01a | 0.05 ± 0.02a | 0.06 ± 0.02a |
| Valeric acid | 0.10 ± 0.04ab | 0.10 ± 0.01ab | 0.09 ± 0.04b | 0.13 ± 0.02a |
| Isovaleric acid | 0.10 ± 0.03a | 0.07 ± 0.01a | 0.06 ± 0.03a | 0.08 ± 0.03a |
| Total SCFAs | 8.56 ± 0.54d | 9.58 ± 0.35c | 10.10 ± 0.12b | 11.21 ± 0.30a |
Data were expressed as mean ± standard deviation (n = 6). The values with different letters in the same column were significantly different at P < 0.05.
3.4.6. Relationship between Mw, SCFAs and bacterial genera
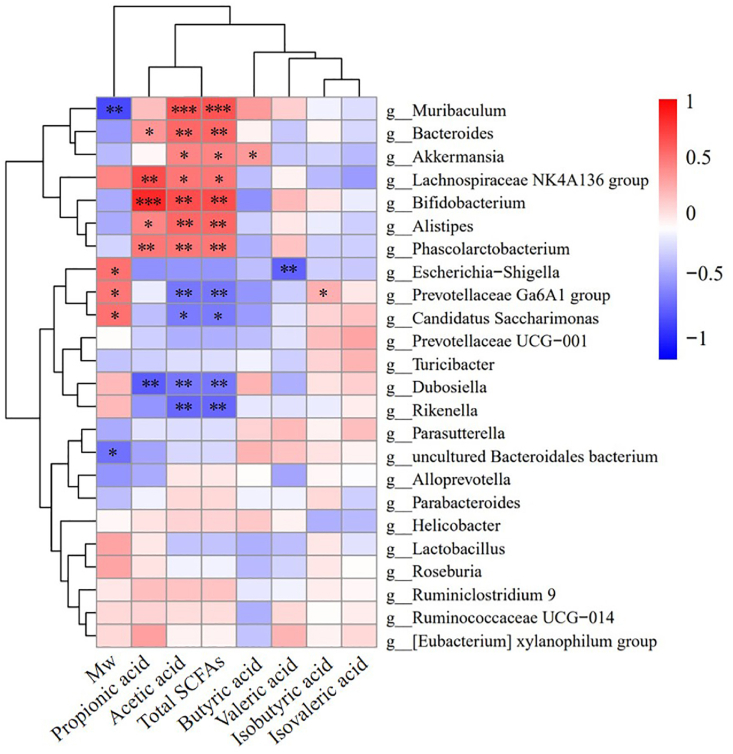
Fig. 7 shows the relationship between Mw, SCFAs and gut microbiota composition. Mw of saccharides was positively correlated with the abundance of Prevotellaceae Ga6A1 group (P < 0.05), Candidatus Saccharimonas (P < 0.05) and Escherichia-Shigella (P < 0.05), but was negatively correlated with Muribaculum (P < 0.01) and uncultured Bacteroidales (P < 0.05), which was consistent with the results of dominant microorganisms in the composition of the gut microbiota of each group (Fig. 5B and D). Moreover, it also indicated that Mw of saccharides is an important factor affecting the composition of the gut microbiota of mice due to the preference of different microorganisms for specific substrates.
Fig. 7.
Heat maps displaying the relationship between Mw, SCFAs and microbiota. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, a correlation was observed between the levels of SCFAs and the abundance of microorganisms in the gut of treated mice. Muribaculum (P < 0.001), Bacteroides (P < 0.01), Akkermansia (P < 0.05), Lachnospiraceae NK4A136 group (P < 0.05), Bifidobacterium (P < 0.01), Alitipes (P < 0.01) and Phascolarctobacterium (P < 0.01) had a significant positive correlation with acetic acid content and total SCFAs. In addition, Bacteroides (P < 0.01), Bifidobacterium (P < 0.01), Lachnospiraceae NK4A136 group (P < 0.05), Alistipes (P < 0.05), and Phascolarctobacterium (P < 0.05) were significantly correlated with propionic acid. In contrast, Prevotellaceae Ga6A1 group (P < 0.05) and Akkermansia (P < 0.05) were significantly associated with increased levels of isobutyric acid and butyric acid, respectively. These findings were also consistent with those obtained in our previous studies, which showed that Muribaculum, Bacteroides, Phascolarctobacterium, Bifidobacterium, among other genera could promote the production of SCFAs.
4. Conclusions
Four saccharide fractions with different Mw and monosaccharide compositions were obtained by acid hydrolysis and ultrafiltration of GPs. In addition, the main primary structure in these polysaccharides and fractions were highly similar as indicated by FTIR analysis. Simultaneously, garlic saccharides of different Mw were only slightly degraded in a simulated digestion, and most could effectively reach the large intestine to be utilized by the gut microbiota. All saccharides exhibited a certain prebiotic activity, except for GPs-U6 in vitro, especially, GPs-U0.3 showed better prebiotic activity, since it promoted the growth of L. acidophilus L3-003, B. longum B1-0013 and B. adolescentis B2-001, as well as was found associated with increased production of lactic acid and SCFAs. In vivo, GPs-U0.3 was shown to be associated with high production of SCFAs and could selectively stimulate the growth of certain gut microbiota, including Bifidobacterium, Lachnospiraceae NK4A136 group, Phoscolarctobacterium, among others, whereas it led to a decrease in the abundance of Dubosiella, Escherichia-Shigella and Rikenella. Collectively, this study elucidated the relationship between Mw and prebiotic activity of garlic saccharides, and showed that GPs-U0.3 could be potentially used as a prebiotic by the functional food industry.
CRediT authorship contribution statement
Renjie Zhao: Writing – original draft, Formal analysis, Investigation. Zhichang Qiu: Writing – review & editing, Supervision. Xinyan Bai: Writing – review & editing, Supervision, Conceptualization. Lu Xiang: Writing – review & editing, Project administration, Supervision, Conceptualization. Yiteng Qiao: Writing – review & editing, Methodology. Xiaoming Lu: Project administration, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31801559), the Key R & D project of Shandong Province (2021TZXD007), the Major Scientific and Technological Innovation Projects of Key R&D Program of Shandong Province (2019JZZY020607) and the Special Fund for Leading Talent in Mount Tai of Shandong Province (tscy20200121).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.10.022.
Contributor Information
Yiteng Qiao, Email: marsttt@163.com.
Xiaoming Lu, Email: xxalxm@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bo R., Ji X., Yang H., Liu M., Li J. The characterization of optimal selenized garlic polysaccharides and its immune and antioxidant activity in chickens. Int. J. Biol. Macromol. 2021;182:136–143. doi: 10.1016/j.ijbiomac.2021.03.197. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai M., Xing H., Tian B., Xu J., Li Z., Zhu H., Yang K., Sun P. Characteristics and antifatigue activity of graded polysaccharides from Ganoderma lucidum separated by cascade membrane technology. Carbohydr. Polym. 2021;118329 doi: 10.1016/j.carbpol.2021.118329. [DOI] [PubMed] [Google Scholar]
- Canani R.B., Di Costanzo M., Leone L., Pedata M., Meli R., Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17(12):1519. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang B., Fu X., You L.J., Abbasi A.M., Liu R.H. The digestibility of mulberry fruit polysaccharides and its impact on lipolysis under simulated saliva, gastric and intestinal conditions. Food Hydrocolloids. 2016;58:171–178. doi: 10.1016/j.foodhyd.2016.02.033. [DOI] [Google Scholar]
- Chen G., Xie M., Wan P., Chen D., Ye H., Chen L., Zeng X., Liu Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018;244:331–339. doi: 10.1016/j.foodchem.2017.10.074. [DOI] [PubMed] [Google Scholar]
- Chen J., leong Cheong K., Song Z., Shi Y., Huang X. Structure and protective effect on UVB-induced keratinocyte damage of fructan from white garlic. Carbohydr. Polym. 2013;92(1):200–205. doi: 10.1016/j.carbpol.2012.09.068. [DOI] [PubMed] [Google Scholar]
- de Figueiredo F.C., de Barros Ranke F.F., de Oliva-Neto P. Evaluation of xylooligosaccharides and fructooligosaccharides on digestive enzymes hydrolysis and as a nutrient for different probiotics and Salmonella typhimurium. LWT--Food Sci. Technol. 2020;118 doi: 10.1016/j.lwt.2019.108761. [DOI] [Google Scholar]
- Ding G., Gong Q., Ma J., Liu X., Wang Y., Cheng X. Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci. 2021;112(10) doi: 10.1111/cas.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Yan Y., Peng Y., Chen D., Mi J., Lu L., Luo Q., Li X., Zeng X., Cao Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019;125:751–760. doi: 10.1016/j.ijbiomac.2018.12.081. [DOI] [PubMed] [Google Scholar]
- Dou Z., Chen C., Fu X. Digestive property and bioactivity of blackberry polysaccharides with different molecular weights. J. Agric. Food Chem. 2019;67(45):12428–12440. doi: 10.1021/acs.jafc.9b03505. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Park S.Y., Kashyap D.R., Dowd S.E., Gupta D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei M.L., Tong L.I., Wei L.I., De Yang L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crop. Prod. 2015;69:137–142. doi: 10.1016/j.indcrop.2015.02.021. [DOI] [Google Scholar]
- Fu X., Liu Z., Zhu C., Mou H., Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019;59(Suppl. 1):S130–S152. doi: 10.1080/10408398.2018.1542587. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gong L., Hu L., Wang H., Chen R., Wang J. Protective effect of feruloylated oligosaccharides on dextran sulfate sodium-induced ulcerative colitis in rats. Food Front. 2022;2022 doi: 10.1002/fft2.140. [DOI] [Google Scholar]
- Han Y., Ma H., Liu Y., Zhao Y., Li L. Effects of goat milk enriched with oligosaccharides on microbiota structures, and correlation between microbiota and short-chain fatty acids in the large intestine of the mouse. J. Dairy Sci. 2021;(3):2773–2786. doi: 10.3168/jds.2020-19510. 04. [DOI] [PubMed] [Google Scholar]
- Hospodarova V., Singovszka E., Stevulova N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Anal. Chem. 2018;9(6):303–310. doi: 10.4236/ajac.2018.96023. [DOI] [Google Scholar]
- Hu J.L., Nie S.P., Li C., Xie M.Y. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota. Food Hydrocolloids. 2013;33(2):384–392. doi: 10.1016/j.foodhyd.2013.04.006. [DOI] [Google Scholar]
- Hu S., Wang J., Xu Y., Yang H., Wang J., Xue C., Yan X., Su L. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019;10(3):1736–1746. doi: 10.1039/c8fo02364f. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Zhang Z., Qiao X. Separation and purification of garlic neutral polysaccharide (Ⅱ) J. Chin. Inst. Food Sci. Technol. 2006;6:54–58. doi: 10.3969/j.issn.1009-7848.2006.02.010. [DOI] [Google Scholar]
- Koliada A., Syzenko G., Moseiko V., Budovska L., Puchkov K., Perederiy V., Gavalko Y., Dorofeyev A., Romanenko M., Tkach S., Sineok L., Lushchak O., Vaiserman A. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K., Buerger M., Stallmach A., Bruns T. Effects of antibiotics on gut microbiota. Dig. Dis. 2016;34(3):260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- Li L., Huang T. Growth inhibitory effects of garlic polysaccharide on human HepG2 cells. Agric. Sci. Technol. 2017;18(6):988–992. [Google Scholar]
- Li L., Qiu Z., Dong H., Ma C., Qiao Y., Zheng Z. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: a comparison. Int. J. Biol. Macromol. 2021;182:187–196. doi: 10.1016/j.ijbiomac.2021.03.177. [DOI] [PubMed] [Google Scholar]
- Li M., Yan Y.X., Yu Q.T., Deng Y., Wu D.T., Wang Y., Ge Y.Z., Li S.P., Zhao J. Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 macrophages. J. Food Sci. 2017;82(3):765–771. doi: 10.1111/17503841.13589. [DOI] [PubMed] [Google Scholar]
- Liang J., Zhao Y., Yang F., Zheng L., Ma Y., Liu Q., Cai L., Gong W., Wang B. Preparation and structure-activity relationship of highly active black garlic polysaccharides. Int. J. Biol. Macromol. 2022;220:601–612. doi: 10.1016/j.ijbiomac.2022.08.115. [DOI] [PubMed] [Google Scholar]
- Lin H., Zhang J., Li S., Zheng B., Hu J. Polysaccharides isolated from Laminaria japonica attenuates gestational diabetes mellitus by regulating the gut microbiota in mice. Food Front. 2021;2(2):208–217. doi: 10.1002/fft2.79. [DOI] [Google Scholar]
- Lu X., Li N., Zhao R., Zhao M., Cui X., Xu Y., Qiao X. In vitro prebiotic properties of garlic polysaccharides and its oligosaccharide mixtures obtained by acid hydrolysis. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.798450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Zhao Y., Li L., Liu Y. Effects of combinations of goat milk and oligosaccharides on altering the microbiota, immune responses, and short chain fatty acid levels in the small intestines of mice. J. Agric. Food Chem. 2021;69(31):8828–8837. doi: 10.1021/acs.jafc.1c03408. [DOI] [PubMed] [Google Scholar]
- Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62(1):67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D., Xavier R.J., Teixeira M.M., Mackay C.R. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Molist F., Manzanilla E.G., Pérez J.F., Nyachoti C.M. Coarse, but not finely ground, dietary fibre increases intestinal Firmicutes:Bacteroidetes ratio and reduces diarrhoea induced by experimental infection in piglets. Br. J. Nutr. 2012;108(1):9–15. doi: 10.1017/S0007114511005216. [DOI] [PubMed] [Google Scholar]
- Nobre C., do Nascimento A.K.C., Silva S.P., Coelho E., Coimbra M.A., Cavalcanti M.T.H., Teixeira J.A., Porto A.L.F. Process development for the production of prebiotic fructo-oligosaccharides by Penicillium citreonigrum. Bioresour. Technol. 2019;282:464–474. doi: 10.1016/j.biortech.2019.03.053. [DOI] [PubMed] [Google Scholar]
- Peng M., Lee S.H., Rahaman S.O., Biswas D. Dietary probiotic and metabolites improve intestinal homeostasis and prevent colorectal cancer. Food Funct. 2020;11(12):10724–10735. doi: 10.1039/D0FO02652B. [DOI] [PubMed] [Google Scholar]
- Precup G., Vodnar D.C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br. J. Nutr. 2019;122(2):131–140. doi: 10.1017/S0007114519000680. [DOI] [PubMed] [Google Scholar]
- Salonen A., Lahti L., Salojärvi J., Holtrop G., Korpela K., Duncan S.H., Date P., Farquharson F., Johnstone A.M., Lobley G.E., Louis P., Flint H.J., De Vos W.M. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8(11):2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X., Sun C., Tang X., Zhang X., Han D., Liang S., Qu R., Hui X., Shan Y., Hu L., Fang H., Zhang H., Wu X., Chen C. Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativum L.) polysaccharides toward dextran sodium sulfate-induced colitis. J. Agric. Food Chem. 2020;68(44):12295–12309. doi: 10.1021/acs.jafc.0c04773. [DOI] [PubMed] [Google Scholar]
- Sheng K., Zhang G., Sun M., He S., Kong X., Wang J., Zhu F., Zha X., Wang Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020;11(9):7817–7829. doi: 10.1039/D0FO01418D. [DOI] [PubMed] [Google Scholar]
- Teferra T.F. Possible actions of inulin as prebiotic polysaccharide: a review. Food Front. 2021;2(4):407–416. doi: 10.1002/fft2.92. [DOI] [Google Scholar]
- Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Veiga P., Pons N., Agrawal A., Oozeer R., Guyonnet D., Brazeilles R., Faurie J., van Hylckama Vlieg J.E.T., Houghton L.A., Whorwell P.J., Ehrlich S.D., Kennedy S.P. Changes of the human gut microbiome induced by a fermented milk product. Sci. Rep. 2014;4(1):1–9. doi: 10.1038/srep06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter C.S., Vorster H.H., Cummings J.H. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol. 1990;85(5) [PubMed] [Google Scholar]
- Wang L.S., Mo Y.Y., Huang Y.W., Echeveste C.E., Wang H.T., Chen J., Oshima K., Yearsley M., Simal-Gandaraf J., Battino M., Xiao J., Chen J., Sun C., Yu J., Bai W. Effects of dietary interventions on gut microbiota in humans and the possible impacts of foods on patients' responses to cancer immunotherapy. efood. 2020;1(4):279–287. doi: 10.2991/efood.k.200824.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Pan J., Zhang Z., Yan X. Investigation of dietary fructooligosaccharides from different production methods: interpreting the impact of compositions on probiotic metabolism and growth. J. Funct.Foods. 2020;69 doi: 10.1016/j.foodchem.2020.127934. [DOI] [Google Scholar]
- Wang Y., Guan M., Zhao X., Li X. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018;56(1):325–332. doi: 10.1080/13880209.2018.1479868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.T., Fu Y., Guo H., Yuan Q., Nie X.R., Wang S.P., Gan R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021;168:733–742. doi: 10.1016/j.ijbiomac.2020.11.130. [DOI] [PubMed] [Google Scholar]
- Wu M., Wang F., Yang J., Li P., Yan D., Yang Y., Zhang W., Ren J., Zhang Z., Wang M. The responses of the gut microbiota to MBL deficiency. Mol. Immunol. 2020;122:99–108. doi: 10.1016/j.molimm.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Xu Y., Niu X., Liu N., Gao Y., Wang L., Xu G., Li X., Yang Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018;243:26–35. doi: 10.1016/j.foodchem.2017.09.107. [DOI] [PubMed] [Google Scholar]
- Yan J.K., Wang C., Yu Y.B., Wu L.X., Chen T.T., Wang Z.W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.128081. [DOI] [PubMed] [Google Scholar]
- Yan S., Yang B., Zhao J., Zhao J., Stanton C., Ross R.P., Zhang H., Chen W. A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019;10(3):1595–1608. doi: 10.1039/C9FO00014C. [DOI] [PubMed] [Google Scholar]
- Yang Y., Xing S., Li S., Niu Y., Li C., Huang T., Liao X. Potential regulation of small RNAs on bacterial function activities in pig farm wastewater treatment plants. J. Environ. Sci. 2020;91:292–300. doi: 10.1016/j.jes.2020.02.014. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Yamashita T., Hirata K.I. Gut microbiome and cardiovascular diseases. Diseases. 2018;6(3):56. doi: 10.3390/diseases6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Lu J., Sun L., Lyu X., Chang X.Y., Mi X., Hu M.G., Wu C., Chen X. Akkermansia muciniphila: a potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111014. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Li C., Zheng Q., Wu J., Zhu K., Shen X., Cao J. Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity, molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota) Food Hydrocolloids. 2019;89:735–741. doi: 10.1016/j.foodhyd.2018.11.040. [DOI] [Google Scholar]
- Zhang X., Zhao Y., Zhang M., Pang X., Xu J., Kang C., Li M., Zhang C., Zhang Z., Zhang Y., Li X., Ning G., Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xie Q., You L., Cheung P.C.K., Zhao Z. Behavior of non‐digestible polysaccharides in gastrointestinal tract: a mechanistic review of its anti‐obesity effect. eFood. 2021;2(2):59–72. doi: 10.2991/efood.k.210310.001. [DOI] [Google Scholar]
- Zou Y., Chen T. Engineered Akkermansia muciniphila: a promising agent against diseases. Exp. Ther. Med. 2020;20(6) doi: 10.3892/etm.2020.9415. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.