Abstract
Introduction and objectives:
Leishmania donovani is the causative organism of leishmaniasis in Sri Lanka. Studies on the immunopathology of leishmaniasis due to L. donovani are limited. The objective of this study was to describe the immunopathological characteristics of cutaneous leishmaniasis in a cohort of Sri Lankan patients.
Methodology:
Fifty skin biopsies of cutaneous leishmaniasis confirmed by detection of organisms by histology, culture, slit-skin smear, and/or polymerase chain reaction were reviewed. The inflammatory infiltrate was characterized by immunohistochemical staining for CD4, CD8, CD20, and CD68. Associations and correlations between immunohistochemical staining pattern and the parasitic load, and patterns of inflammation were determined.
Results:
The majority of biopsies showed a CD8+/CD4− T lymphocyte predominant infiltrate (84%, n = 42). A CD68 predominant infiltrate was seen in 16%(n = 8). The mean percentage of CD8+, CD4+, CD20+, and CD68+ inflammatory cells in the biopsies were 56.1% (SD = 16.5%), 2.6% (SD = 4.5%), 12.3% (SD = 10.9%), and 25.7% (SD = 15.8%) respectively. There was no association between the predominant inflammatory cell and the degree of inflammation (P = .173), presence of high RPI (P = .922), MRI(P = .367) or presence of granuloma (P = .247).The percentage of CD4+ cells showed a positive correlation with granuloma formation (Correlation coefficient = .411, P = .03). The percentage of CD20+ cells in the infiltrate showed a positive correlation with the degree of inflammation (Correlation coefficient = .491, P = .02) and the RPI (Correlation coefficient = .334, P = .018).
Discussion and Conclusion:
Skin biopsies from cutaneous leishmaniasis due to L. donovani infection showed a CD8+/CD4− predominant infiltrate. This is similar to the findings of studies on cutaneous leishmaniasis due to some other species and suggests that the cytotoxic T cell response plays a role in infections due to L. donovani.
Keywords: Cutaneous leishmaniasis, pathology, immune response, Sri Lanka, L. donovani infection
Introduction
Leishmaniasis is a neglected tropical disease caused by the intra cellular protozoan of the genus Leishmania. It has 3 main forms of clinical presentation: cutaneous leishmaniasis (CL) mucocutaneous leishmaniasis and visceral leishmaniasis1 CL is the predominant form seen in Sri Lanka.
Evidence has shown that CD4+ and CD8+ T lymphocytes play different roles in the control of Leishmania infection. CD4+ cells are believed to be important in controlling parasite growth, but the strong inflammatory response elicited by these cells also contributes to the immunopathogenesis of lesions. Studies on CD8+ T lymphocytes show that they play both a protective and pathogenetic role in leishmaniasis. CD8+ T cells have been shown to be important in the healing process and in developing resistance to infection in leishmaniasis and in bringing about tissue damage through cytotoxic mechanisms.2
Leishmania donovani MON-37 is the causative organism of both cutaneous and visceral leishmaniasis in Sri Lanka.3,4 Localized infections, such as CL are regarded as having a well-balanced cellular immune response with a very high level of resistance to infection. However, there is still no agreement as to which type of T cell (CD4+ or CD8+) is predominant in the inflammatory infiltrate of the cutaneous lesions.5 -10
Immunophenotypic evaluation of the lymphoid infiltrate in the tissue section is helpful in demonstrating the host immune response occurring at tissue level. This has not been studied in CL due to L. donovani in Sri Lanka. This study aimed to characterize the inflammatory cell infiltrate in a cohort of Sri Lankan patients and to correlate the immunopathological characteristics with the organism load.
Methodology
Fifty biopsies from skin lesions with CL were included in the present study. These biopsies were collected during a 1 year period from November 2018 to 2019. The methods of sample collection and the clinical and histopathological features of this cohort have been described previously.11 All biopsies were handled by the Department of Pathology, Faculty of Medicine, University of Colombo. Cases of confirmed CL based on the presence of the organism on at least 2 of the following: histology, direct microscopy, culture or polymerase chain reaction (PCR) were included. Cases with inadequate residual tissue left for immunohistochemical evaluation and cases in which tissue was unsuitable for histological analysis due to excessive crushing and autolysis were excluded. Ethical approval for the current study was obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo (EC/20/101).
The histological features were evaluated on the hematoxylin and eosin and Giemsa-stained sections. The degree of the inflammatory infiltrate was categorized as mild, moderate, and severe. The presence of granulomas was documented as absent, ill-defined, and well-defined. The organisms were quantified based on the Ridley parasitic index,12 and the overall histological pattern was categorized according to modified criteria originally published by Ridley et al.13
All biopsies were evaluated by immunohistochemical staining for CD4, CD8, CD68, and CD20 using the following prediluted primary monoclonal antibodies: mouse anti-human CD20 (Clone L26) and CD4 (Clone 4B12), CD8 (Clone C8/144B) and CD 68 (Clone KP 1) (Dako, Denmark A/S). The primary antibody was detected using the Mouse and Rabbit Specific HRP/DAM (ABC) Detection IHC kit (abcam, ab64264, United Kingdom) according to manufacturer instructions. Appropriate controls were run with each test.
In brief, 4 μm thick sections were cut from paraffin-embedded blocks and transferred onto positively charged slides (Hydrophilic Plus, Bio SB). The sections were then deparaffinized in xylene and rehydrated with absolute and 95% alcohol and placed in 3% hydrogen peroxide and absolute methanol mixed at a 1:9 ratio for 15 minutes to achieve an endogenous peroxidase block; following which they were washed well in running tap water for 10 minutes and transferred to distilled water. Antigen retrieval was achieved by heat induced epitope retrieval using citrate buffer and pressure cooker method and sections were transferred to the working phosphate buffered saline (PBS) solution. The hydrogen peroxide block available in the IHC detection kit (abcam, ab64264, United Kingdom) was applied for 10 minutes and the slides were then washed 3 times in PBS. This was followed by application of the protein block available in the IHC kit (abcam, ab64264, United Kingdom) for 10 minutes followed by washing in PBS 3 times. The primary bodies CD4, CD8, CD68, and CD20 were then added to the sections, and the slides were incubated in a humidity chamber for 60 minutes. The sections were washed in PBS and then allowed to react for 10 minutes with biotinylated goat anti-polyvalent (abcam, ab64264, United Kingdom), following which they were rinsed in PBS and subsequently incubated with streptavidin peroxidase (abcam, ab64264, United Kingdom) for the next 25 minutes. After a 10 minutes rinse in PBS, slides were developed using the DAB system and counterstained with Mayer’s hematoxylin.
Slides were analyzed under a light microscope at a magnification of 400. For each of the 4 antibodies, the positively stained cells were expressed as a percentage of mononuclear cells in the infiltrate in each sample. Associations between the predominant cell in the inflammatory infiltrate and the presence of granuloma, modified Ridley index(MRI), and Ridley’s parasitic index(RPI) were determined by chi square analysis. Correlations between percentage of CD8+, CD68+, and CD20+ cells and the RPI, presence of granuloma and degree of inflammation were assessed using the Spearman’s rank correlation test. Statistical analysis was done using SPSS 28.0
Results
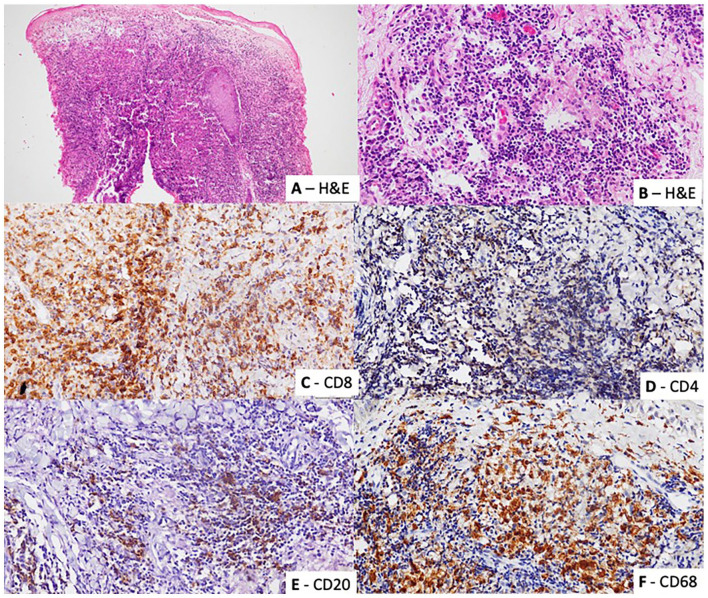
Fifty skin biopsies that fulfilled the inclusion and exclusion criteria were identified. The majority of biopsies showed a CD8+/CD4− T lymphocyte predominant infiltrate (84%, n = 42). A CD68 predominant infiltrate was seen in 16% (n = 8) (Figure 1).
Figure 1.
The biopsies showed a chronic inflammatory cell infiltrate composed of lymphocytes, histiocytes and plasma cells ((A) Hematoxylin and eosin ×100 and (B) Hematoxylin and eosin ×400). The infiltrate in each biopsy was characterized with immunohistochemical staining for (C) CD8 (CD8 ×400), (D) CD4 (CD4 ×400), (E) CD20 (CD20 ×400), and (F) CD68 (CD68 ×400).
The mean percentage of CD8+, CD4+, CD20+, and CD68+ inflammatory cells in the biopsies were 56.1% (SD = 16.5%), 2.6% (SD = 4.5%), 12.3% (SD = 10.9%) and 25.7% (SD = 15.8%) respectively. There was no association between the predominant inflammatory cell and the degree of inflammation (P = .173), presence of high RPI (P = .922), MRI (P = .367) or presence of granuloma (P = .247) on Chi-square analysis.
Spearman’s rank correlation showed a positive correlation between the degree of inflammation and the RPI (Correlation coefficient = .594, P < .001). The presence of granuloma showed a negative correlation with the degree of inflammation (Correlation coefficient = −0.399, P = .04) and the RPI (Correlation coefficient = − 0.381, P = .006).
The percentage of CD4+ cells showed positive correlation with granuloma formation (Correlation coefficient = .411, P = .03). The percentage of CD20+ cells in the infiltrate showed a positive correlation with the degree of inflammation (Correlation coefficient = .491, P = .02) and the RPI (Correlation coefficient = .334, P = .018). The percentage of CD8+ and C68+ cells showed no correlation with the presence of granuloma, degree of inflammation or the RPI.
Discussion
CD8+ T cells were more frequent than CD4+ in the lesions of localized CL that were studied. The role of CD8+ T cells during Leishmania infections is controversial. There is a discrepancy among findings depending on the Leishmania species and the type of disease caused.14 Studies have shown varying patterns of distribution of T cell subtypes in the infiltrate of lesions of CL with the predominant type of T cell (CD4+ or CD8+) varying according to the Leishmania species (Table 1). A predominance of CD8 over CD4 was seen in CL due to L. braziliensis, L. amazonensis, and L. panamensis.6,8,9
Table 1.
Studies on the characterization of T cell infiltrate present in skin lesions of cutaneous leishmaniasis.
| Study | Number of specimens | Leishmania species | Result |
|---|---|---|---|
| Esterre et al5 | 50 | Leishmania braziliensis guyanesis | CD4/CD8 ratio = 1.05 ± 0.7 T helper cell (CD4+ CD45RO+) > suppressor/inducer subtype (CD4+ CD45 RA+) |
| Isaza et al6 | - | Leishmania panamensis | CD4/CD8 –0.80 ± 0.06 |
| Gaafar et al7 | 47 | Leishmania major | CD4 > CD8. Higher number of CD8+ cells at diagnosis than at healing. |
| Vierra et al8 | 15 | Leishmania braziliensis | CD8 > CD4 Higher frequency of B cells in localized form. |
| Silveira et al9 | - |
L.(V.)braziliensis and
L. (L.) amazonensis |
CD8V> CD4 |
| Jabbour et al10 | 33 |
Leishmania tropica
Leishmania major |
Majority CD3+, CD4−, CD8− lymphocytes |
Studies in patients with lesions of CL have shown that interferon γ is associated with a T helper-1 response (CD4+, Th1) and plays a crucial role in developing resistance to leishmania infection whilst interleukin-4 is associated with a T helper-2 response (CD4+, Th2) and increased susceptibility to leishmanial infections.15 Patients with localized CL are believed to have adequate cell mediated immunity with a predominance of CD4+ Th1 immune response.16,17
Th1 cells producing interferon γ are essential in controlling L. major infection. Failure to develop an adequate Th1 response, results in susceptibility to the disease.14 Studies in Sri Lanka, where cutaneous leishmaniasis is due to L donovani, have also demonstrated a Th 1 cell response with increased interferon γ levels in healing lesions.18 In a study by Vierra et al8 higher levels of interferon γ and increased numbers of B cells were identified in localized CL as compared to diffuse CL, leading the authors to speculate that the higher interferon γ expression stimulates B cells to secrete antibodies, bring about opsonization and more efficient phagocytosis by macrophages, leading to better parasite control.
Studies, including those conducted in Sri Lanka have shown that the presence of granuloma is associated with reduction in parasite counts and that the mean percentage of T cells increased with the formation of granuloma and a reduction in parasite counts.19,20 In the present study too, there was a positive correlation between the degree of inflammation and the RPI, but granuloma formation showed a negative correlation with the RPI, indicating that the presence of granuloma led to reduction in the parasitic load. Although the CD4+ infiltrate was not prominent in this cohort, the percentage of CD4+ cells showed a positive correlation with the formation of granuloma. This supports the theory that interferon γ, produced by CD4+ cells, plays an important role in granuloma formation and control of the parasite. A higher percentage of CD20 was seen with higher parasitic counts and more severe inflammation. It is possible that the higher organism load stimulates a greater inflammatory response and an increased CD20+ infiltrate.
The percentage of CD8+ cells did not show a correlation with the degree of inflammation, presence of granuloma or the RPI. However, since the dominant T cell present in the infiltrate is a CD8+ T cell, it is possible that the CD8+ T cells play a role in stimulating the release of interferon γ in L. donovani infection. This has been demonstrated in studies of CL due to L. major, which have shown that the immune response is mediated not only through the expansion of antigen-specific interferon γ producing CD4+ Th1 cells, but also through that interferon γ producing CD8+ T cells.21 Large numbers of CD8+ T cells have been observed in the lesions of L. major patients during both the acute phase and the healing process.7,22 Higher number of CD8+ T cells were seen in localized CL compared to patients with diffuse cutaneous leishmaniasis due to L. mexicana. Additionally, the CD8+ T cells, in diffuse CL produced little interferon γ upon stimulation in comparison to localized CL.23 Studies conducted in patients with visceral leishmaniasis due to L. donovani have also shown evidence to support the role of CD8+ T cells in resistance to Leishmania24 with lesions of healed leishmaniasis demonstrating high levels of Th1 cytokines and granzyme B and activated CD+ T cells. Studies in visceral leishmaniasis due to L. donovani have also shown CD8+NKT cells may play more of a protective role in contact with target cells than CD4+NKT cells.25 This evidence provides further support for a protective role of CD 8+ T cells in CL due L. donovani.
Conclusion
This study demonstrated that the dominant T cell present in the infiltrate in skin biopsies of patients with CL due to L. donovani infection was the CD8+ T cell. Although the CD4+ T cell response was not prominent, CD4+ cells were correlated with granuloma formation, suggesting that the CD4+ cells play an important role in control of the organism and immune pathogenesis. The role of the CD8+ T cells in controlling the organism is unclear. However, the presence of these cells in high numbers suggests that they play a role in the control of the organism and further studies on their role in CL due to L. donovani this are recommended.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Colombo [Research Grant no. AP/3/2/2020/SG/10].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Harshima Wijesinghe: Conceptualization, Methodology, Investigation, Analysis, Writing original draft, editing and funding acquisition
Kokila Wijesinghe: Investigation, Writing review and editing
Deepika Fernando: Resources, Writing review and editing, Supervision
Chandu de Silva: Conceptualization, Methodology, Investigation, Writing review and editing, Supervision
Research Data: The research data is available with the investigators and can be shared following an individual request.
References
- 1. WHO Leishmaniasis. Key facts. 2022. Accessed July 4, 2022. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- 2. da Silva Santos C, Brodskyn CI. The role of CD4 and CD8 T cells in human cutaneous leishmaniasis. Front Public Health. 2014;2:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karunaweera ND, Pratlong F, Siriwardane HV, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003;97:380-381. [DOI] [PubMed] [Google Scholar]
- 4. Ranasinghe S, Zhang WW, Wickremasinghe R, et al. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog Glob Health. 2012;106:421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esterre P, Dedet JP, Frenay C, Chevallier M, Grimaud JA. Cell populations in the lesion of human cutaneous leishmaniasis: a light microscopical, immunohistochemical and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1992;421:239-247. [DOI] [PubMed] [Google Scholar]
- 6. Isaza DM, Restrepo M, Restrepo R, Caceres-Dittmar G, Tapia FJ. Immunocytochemical and histopathologic characterization of lesions from patients with localized cutaneous leishmaniasis caused by Leishmania panamensis. Am J Trop Med Hyg. 1996;55:365-369. [DOI] [PubMed] [Google Scholar]
- 7. Gaafar A, Veress B, Permin H, Kharazmi A, Theander TG, el Hassan AM. Characterization of the local and systemic immune responses in patients with cutaneous leishmaniasis due to Leishmania major. Clin Immunol. 1999;91:314-320. [DOI] [PubMed] [Google Scholar]
- 8. Vieira MG, Oliveira F, Arruda S, et al. B-cell infiltration and frequency of cytokine producing cells differ between localized and disseminated human cutaneous leishmaniases. Mem Inst Oswaldo Cruz. 2002;97:979-983. [DOI] [PubMed] [Google Scholar]
- 9. Silveira FT, Lainson R, Corbett CE. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz. 2004;99:239-251. [DOI] [PubMed] [Google Scholar]
- 10. Jabbour MN, Issa G, Charafeddine K, et al. The immune microenvironment in cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2015;29:1170-1179. [DOI] [PubMed] [Google Scholar]
- 11. Wijesinghe H, Gunathilaka N, Semege S, et al. Histopathology of cutaneous leishmaniasis caused by Leishmania donovani in Sri Lanka. Biomed Res Int. 2020;2020:4926819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ridley DS, Ridley MJ. The evolution of the lesion in cutaneous leishmaniasis. J Pathol. 1983;141:83-96. [DOI] [PubMed] [Google Scholar]
- 13. Ridley DS, Peters W, Killick-Kendrick R, eds. The Leishmaniases in Biology and Medicine. Academic Press; 1997. [Google Scholar]
- 14. Stäger S, Rafati S. CD8(+) T cells in leishmania infections: friends or foes? Front Immunol. 2012;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143-148. [DOI] [PubMed] [Google Scholar]
- 16. Cáceres-Dittmar G, Tapia FJ, Sánchez MA, et al. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993;91:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carvalho EM, Correia Filho D, Bacellar O, Almeida RP, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273-277. [DOI] [PubMed] [Google Scholar]
- 18. Manamperi NH, Oghumu S, Pathirana N, et al. In situ immunopathological changes in cutaneous leishmaniasis due to Leishmania donovani. Parasite Immunol. 2017;39:m.12413. doi: 10.1111/pim.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azadeh B, Samad A, Ardehali S. Histological spectrum of cutaneous leishmaniasis due to Leishmania tropica. Trans R Soc Trop Med Hyg. 1985;79:631-636. [DOI] [PubMed] [Google Scholar]
- 20. Herath CH, Ratnatunga NV, Waduge R, Ratnayake P, Ratnatunga CN, Ramadasa S. A histopathological study of cutaneous leishmaniasis in Sri Lanka. Ceylon Med J. 2010;55:106-111. [DOI] [PubMed] [Google Scholar]
- 21. Nateghi Rostami M, Keshavarz H, Edalat R, et al. CD8+ T cells as a source of IFN-γ production in human cutaneous leishmaniasis. PLoS Negl Trop Dis. 2010;4:e845. Published 2010. Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Da-Cruz AM, Bittar R, Mattos M, et al. T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol. 2002;9:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernández-Ruiz J, Salaiza-Suazo N, Carrada G, et al. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaushal H, Bras-Gonçalves R, Negi NS, Lemesre JL, Papierok G, Salotra P. Role of CD8(+) T cells in protection against Leishmania donovani infection in healed visceral leishmaniasis individuals. BMC Infect Dis. 2014;14:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumari S, Jamal F, Shivam P, et al. Leishmania donovani skews the CD56(+) Natural Killer T cell response during human visceral leishmaniasis. Cytokine. 2015;73:53-60. [DOI] [PubMed] [Google Scholar]