Abstract
Pulmonary hamartoma is a benign lung tumor. However, it is difficult to distinguish this lesion from other diseases via imaging. Three patients with pathologically confirmed pulmonary hamartoma in our department were analyzed. We believe it is necessary to combine imaging, pathology, clinical testing, and individual patient assessments to enable an earlier and more definitive diagnosis of pulmonary hamartoma. Therefore, it is necessary to analyze and summarize the clinical manifestations and imaging features of patients with pulmonary hamartoma to improve the early recognition of the disease by clinicians.
Keywords: Pulmonary hamartoma, pulmonary nodule, imaging performance, pathology, case report, lung cancer, differential diagnosis
Introduction
Approximately 8% of lung tumors are benign, and pulmonary hamartoma accounts for 75% to 77% of benign lung tumors.1 The poor specificity of imaging and the lack of appropriate etiologic markers for pulmonary hamartoma have led to a high rate of clinical misdiagnosis and omission of this disease.2 The imaging presentation of pulmonary hamartoma has many similarities with that of non-small cell lung cancer and tuberculosis spheroids (tumor), which makes it difficult to differentiate pulmonary hamartoma from other diseases. Therefore, this study summarized and analyzed the clinical data, imaging manifestations, and pathological findings of three patients with pulmonary hamartoma diagnosed by our pathology department in an effort to provide a reference for the diagnosis of this disease and reduce the rates of misdiagnosis.
Case presentation
This case report was approved by the Hebei General Hospital Ethics Committee Application for Approval of Research (protocol number: No. 2022091). Written approval was obtained from the Institutional Review Board for publication of this study. Patients provided oral consent for the publication of their data. The reporting of this study conforms to CARE guidelines.3
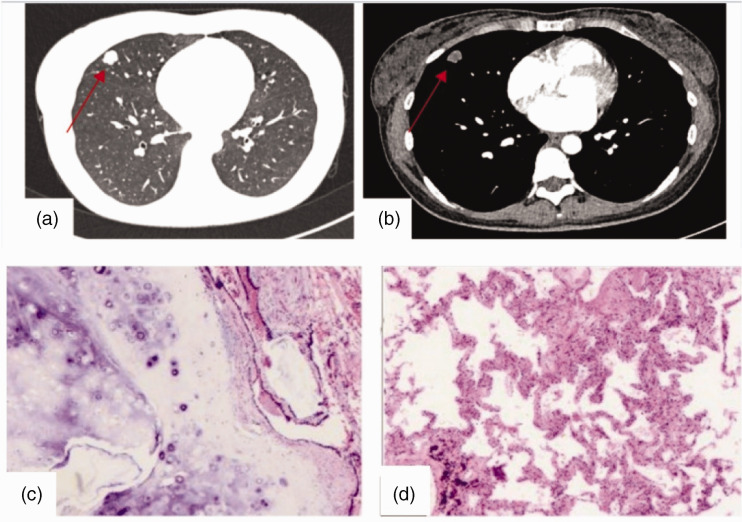
Case 1 was a 37-year-old woman admitted to our hospital on 8 February 2022 because of a “right lung nodule found on physical examination for more than 20 days.” At that time, the patient did not have chills, fever, chest tightness, shortness of breath, chest pain, cough, or sputum. There was no previous history of hypertension or diabetes mellitus. Physical examination did not reveal any significant abnormalities. After admission, the patient’s blood count, biochemistry, hematocrit, pulmonary tumor 4, gastrin-releasing peptide precursor, immunofluorescence staining diagnosis (cytokine 7), lymphocyte immunoassay 5, and lymphocyte immunoassay (regulatory T cells) results were approximately normal. Head magnetic resonance imaging (MRI), abdominal B-ultrasound, cardiac ultrasound, and pulmonary function testing revealed no significant abnormalities. Chest computed tomography (CT) suggested the presence of an elliptical nodular shadow (approximately 11 × 15 × 15 mm3 in size) with lobulated margins and clear borders in the lateral segment of the middle lobe of the right lung, which was considered a possible neoplasm (Figure 1a–b). Because of the patient’s age and CT findings, the possibility of pulmonary malignancy could not be excluded. To clarify the diagnosis, wedge resection of the middle lobe of the right lung was performed under general intravenous resuscitation anesthesia. Regarding the postoperative pathology, a mass of approximately 2 ×1.5 × 1.2 cm3 was visible at the subpleural dissection tether line by the naked eye, and a diagnosis of pulmonary hamartoma was rendered (Figure 1c–d). After surgery, the patient recovered well, and she was discharged from the hospital. Repeat CT of both lungs revealed no significant abnormalities.
Figure 1.
Imaging and pathological images of Case 1. (a–b) Images of the pulmonary window and mediastinal window, respectively. (c–d) Pathological smears (magnification, ×100).
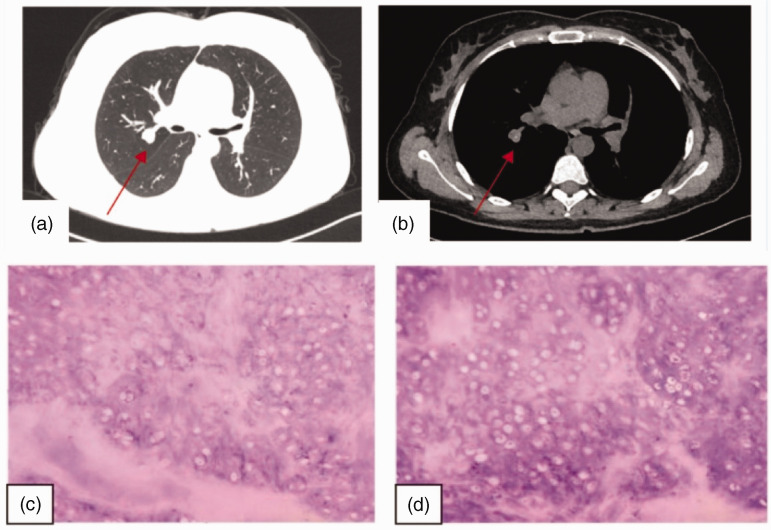
Case 2 was a 28-year-old woman who was admitted to our hospital on 31 July 2021 with “pulmonary nodules found on physical examination for 2 weeks.” The nodule was found in the posterior segment of the upper lobe of the right lung, and the adjacent branch of the pulmonary artery near the nodule was slightly compressed. At that time, there was no cough, sputum, chest tightness, shortness of breath, fever, fatigue, chest pain, dyspnea, dizziness, or headache. There was no previous history of hypertension or diabetes mellitus. Physical examination did not uncover any significant abnormalities. The patient’s NK cell (CD3−CD16+CD56+) count was slightly reduced after admission, whereas her B cell (CD3−CD19+) count was elevated. Routine blood count, complete biochemistry, blood sedimentation, lung tumor 4, and gastrin-releasing peptide precursor data were approximately normal. Cranial MRI, abdominal ultrasound, cardiac ultrasound, and pulmonary function testing did not reveal any significant abnormalities. Chest CT identified a solid nodular shadow in the posterior segment of the upper lobe of the right lung next to the hilum, with a shallow lobulated margin and dotted linear calcification foci in the center, and the nodule size was approximately 16 × 14 mm2 (Figure 2a–b). Based on the patient’s age and imaging features, the possibility of pulmonary malignancy could not be excluded. To clarify the nature of the pulmonary nodule, wedge resection of the upper lobe of the right lung was performed under combined sedation and anesthesia. Postoperatively, a free nodule of approximately 1.5 cm in size was visible by the naked eye. A diagnosis of pulmonary hamartoma with calcification was considered (Figure 2c–d). After surgery, the patient recovered well, and she was discharged from the hospital. Repeat CT of both lungs revealed no significant abnormalities.
Figure 2.
Imaging and pathological images of Case 2. (a–b) Images of the pulmonary window and mediastinal window, respectively. (c–d) Pathological smears (magnification, ×100).
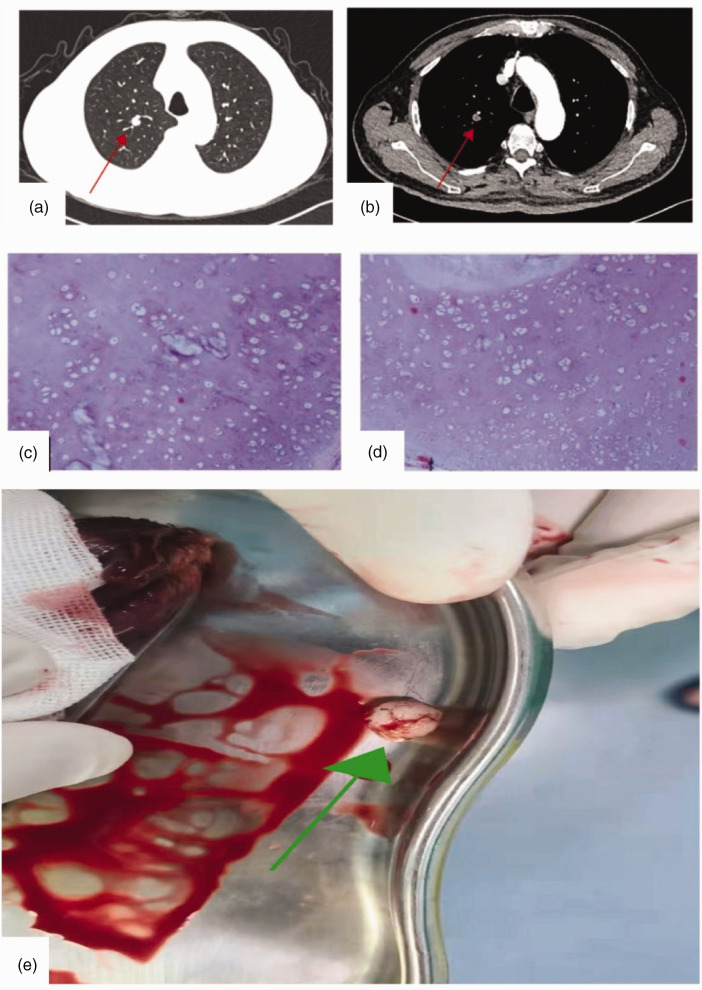
Case 3 was a 65-year-old man who was admitted to our hospital on 8 February 2022 with a “right lung nodule found on physical examination for more than 5 years.” The nodule was slightly dense, and it was located in the apical segment of the upper lobe of the right lung. At that time, he had occasional cough and sputum, chest tightness, and shortness of breath but not chills, fever, or other discomfort. He had a history of hypertension for more than 20 years, diabetes mellitus for more than 10 years, and coronary artery disease for more than 2 years. There was no significant abnormality on physical examination. The patient was admitted to our hospital with a mild elevation of cytokeratin 19 fragments in the lung tumor quadruple test and mild decreases of total T lymphocyte (CD3+) and B cell (CD3-CD19+) counts in the lymphocyte immunoassay quintuple test (regulatory T cells). Gastrin-releasing peptide precursor levels and immunofluorescence staining for diagnosis (cytokines VII) were approximately normal. Abdominal B-ultrasound, cardiac ultrasound, and pulmonary function data were not significantly abnormal. Cranial MRI suggested small chronic ischemic foci in the left parietal lobe. A cyst in the left maxillary sinus was possible. Chest CT uncovered a mildly heterogeneous enhancing nodule (approximately 10 × 8 mm2 in size) with clear borders in the apical segment of the right upper lobe (Figure 3a–b). Based on the patient’s advanced age combined with the results of relevant tests, preoperative CT, and imaging, pulmonary malignancy was highly suspected. To clarify the diagnosis, wedge resection of the upper lobe of the right lung was performed under general intravenous resuscitation anesthesia. Postoperatively, a free mass measuring 1 × 0.8 × 0.8 cm3 with a solid grayish-white brittle cut surface was visible to the naked eye, leading to a diagnosis of pulmonary hamartoma (Figure 3c–d). The patient’s lung malignancy is presented in Figure 3e. The patient had a good postoperative recovery, and she was discharged from the hospital. Repeat CT of both lungs revealed no significant abnormalities.
Figure 3.
Radiographic, pathological, and hamartoma images of Case 3. (a–b) Images of the pulmonary window and mediastinal window, respectively. (c–d) Pathological smears (magnification, ×100) and (e) Image of the pulmonary hamartoma lesion.
Discussion
Pulmonary hamartoma originates from bronchial-associated primitive mesenchymal cells, and it is currently classified as a true benign mesenchymal tumor with the ability to differentiate into a variety of mature mesenchymal components (e.g., adipocytes, smooth muscle cells, chondrocytes). The lesion often presents with reactive hyperplasia.4 Pulmonary hamartoma is pathologically divided into cartilaginous and non-chondrogenic types (smooth muscle type). In total, 91% of pulmonary hamartomas contain cartilaginous components, whereas the proportion of other components is highly variable. For example, 10% to 30% of pulmonary hamartomas are accompanied by calcification, and the proportion of fat in endobronchial type pulmonary hamartoma is higher than in intrapulmonary type intrapulmonary hamartoma. Clinically, this malignancy can be divided into intrapulmonary and endobronchial types, and the endobronchial type is uncommon, accounting for 1.40% to 10.0% of cases.5,6 In a retrospective study of patients with pulmonary hamartoma, the male-to-female ratio was approximately 3:4, the most common age group at diagnosis was 41 to 60 years (69.3%), and the vast majority (80.7%) of patients were asymptomatic, with lesions frequently discovered during hospitalization or physical examination for other diseases.7 The three cases in this report were all found accidentally during physical examination. Pulmonary hamartoma can arise in all lobes and segments of the lungs, and most lesions are <25 mm in diameter.8 In this study, the three patients who were surgically treated and finally diagnosed with pulmonary hamartoma by pathological biopsy were atypical cases. It is noteworthy that all three patients in this study had pulmonary nodules smaller than 20 mm, and they presented with atypical imaging and symptoms, making it difficult to reach a definitive diagnosis before surgery.
Pulmonary hamartoma consists of a combination of components from different germ layers, and therefore, the tumor has a variety of imaging presentations.1 On chest CT, pulmonary hamartoma often appears as round or oval nodules with smooth margins and an intact envelope or mildly lobulated nodules with uniform or heterogeneous density. They are often <4 cm in diameter, and no infiltrates or satellite foci are present in the surrounding lung tissue. The postoperative pulmonary hamartoma image of Case 3 was consistent with its CT imaging presentation.2 Most tumors arise closer to the periphery, near the pleura, or even directly next to the pleura, but they rarely exhibit pleural traction or pleural depression. The pulmonary hamartoma is easier if two typical signs, namely fat and typical calcification, are present. By contrast, peripheral pulmonary hamartoma without typical signs is more difficult to differentiate from peripheral lung cancer and tuberculosis spheres.4 When there are more fibrous and cartilaginous components in the lesion, it presents as a homogeneous nodular shadow, whereas when there are more fatty and calcified components, it presents as a heterogeneous nodular shadow. There are four forms of calcification in isolated pulmonary nodules, namely laminar calcification, central calcification, scattered granular calcification, and popcorn-like calcification.9 The distinctive cartilage-like calcification in malignant nodules is popcorn-like calcification, which is a main indicator for the diagnosis of pulmonary hamartoma.10 All three patients in this report had lobulated lung nodules with smooth margins. However, Case 3 displayed special signs such as fatty calcification in addition to signs such as lobar signs and smooth margins. This also provided reliable evidence for the differential diagnosis.
Differentiation from primary lung cancer should be noted when diagnosing pulmonary hamartoma. Primary lung cancer mostly appears as isolated nodules in the lungs with clear borders, mostly with lobes and short burrs on CT, as well as with vacuolar signs and pleural traction. Some lesions present with vascular collection signs, whereas no fatty density is present. Calcification is rare, and it might be accompanied by enlargement of hilar and mediastinal lymph nodes. Enhancement of parenchymal components on CT is obvious, and the CT values are typically 20 HU or higher. Regarding the differentiation from tuberculosis spheres, it is important to note that tuberculosis spheres are usually located in the posterior segment of the upper lobe or the dorsal segment of the lower lobe, and they are rounded with smooth edges or long burrs and surrounded by satellite foci. These lesions are mainly cheese-like material, and the CT value is usually lower than that of the soft tissue. Concerning the differentiation from sclerosing pneumocytoma, pulmonary sclerosing pneumocytoma (PSP), formerly known as pulmonary sclerosing hemangioma, is a rare lung tumor. This tumor is presumed to be of vascular origin. Immunohistochemical results confirmed that the epithelial origin was the respiratory epithelium. Radiological studies can support the diagnosis. This tumor is actually a marginal, solitary, well-defined mass that is located in the lower lobe with overlying blood vessels and surrounding ground-glass opacities.
Pulmonary hamartoma is benign tumor that rarely becomes malignant.11 However, because pulmonary hamartoma is often a hard lesion, CT-guided aspiration biopsy is more likely to cause pneumothorax. Therefore, surgical resection is necessary for isolated nodules with an unclear imaging diagnosis. Surgery is the primary treatment for benign lung tumors, but the need for surgery in patients with biopsy-proven asymptomatic pulmonary hamartoma is controversial.12 Some scholars reviewed a large body of literature and found no evidence of malignant transformation in any of the follow-up studies, suggesting that the diagnosis of hamartoma with an unclear nature needs to be combined with imaging and puncture biopsy pathology and that patients with biopsy-proven asymptomatic pulmonary hamartoma should be followed up and monitored rather than targeted for surgical resection.13 Surgery can be considered in the following cases: 1) isolated lesions >2.5 cm in diameter; 2) no typical imaging features to exclude the possibility of malignancy; 3) the possibility of recurrence or enlargement on follow-up; 4) no change in the lung lesion after drug treatment; and 5) an excessive psychological burden. In recent years, minimally invasive surgery with a small incision has been increasing selected because of the advantages, including less bleeding, a shorter operative time, less impact on lung function, fewer complications, faster recovery, and shorter postoperative hospital stay. Under the concept of rapid recovery surgery, minimally invasive surgery has now become the mainstream of thoracic surgery. A study compared the surgical results of different incision types (conventional open chest, video-assisted thoracoscopic surgery [VATS], or lateral incision surgery) for performing tumor debulking, wedge resection, and lobectomy, and the results illustrated that the intraoperative bleeding and postoperative drainage within 24 hours was significantly lower for the lateral incision or VATS than for conventional open chest surgery.14 All three patients in this study underwent resection of pulmonary hamartoma through minimally invasive surgery, and they were discharged successfully. Lung CT was repeated after discharge, and good postoperative lung recovery was confirmed.
Conclusion
Pulmonary hamartoma is a benign tumor with a good prognosis, and it occurs in the middle-aged and elderly populations. Most patients have no clinical or specific symptoms. Pulmonary hamartoma without characteristic imaging manifestations needs to be differentiated from peripheral lung cancer, tuberculosis spheres, and PSP,15 and the diagnosis can be further clarified by chest CT combined with other ancillary examinations. Patients with characteristic imaging manifestations can be diagnosed by CT. Patients diagnosed with pulmonary hamartoma by imaging or percutaneous lung aspiration biopsy who have no symptoms can choose conservative treatment and regular follow-up observation. Patients with concomitant symptoms who failed to respond to conservative treatment should undergo surgery. Because of the high misdiagnosis rate of pulmonary hamartoma, patients with an unclear diagnosis and high suspicion of pulmonary hamartoma should opt for surgical resection of the lesion for pathological diagnosis, and the goal of surgery is complete tumor removal and preservation of the lung tissue as much as possible. For pulmonary hamartoma, minimally invasive surgery is preferred to reduce patient pain.
Author contributions: Xiao-Peng Zhang conceived and designed the study. Zhi-Fei Xin and Wen-Fei Xue performed the clinical study. Bo-Wen Li wrote the paper and reviewed and edited the manuscript. All authors read and approved the manuscript.
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
ORCID iD: Xiaopeng Zhang https://orcid.org/0000-0002-9646-5245
Ethics statement
This case report was approved by the Hebei General Hospital Ethics Committee Application for Approval of Research (approval number: No. 2022091). Written approval was obtained from the Institutional Review Board for the publication of this paper. Participants provided oral consent to the publication of their data.
References
- 1.Ettinger DS. Ten years of progress in non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10: 292–295. [DOI] [PubMed] [Google Scholar]
- 2.Novello S, Asamura H, Bazan J, et al. Early stage lung cancer: progress in the last 40 years [corrected]. J Thorac Oncol 2014; 9: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 3.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 4.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin GD, Roos JE, Tall M, et al. Characterizing search, recognition, and decision in the detection of lung nodules on CT scans: elucidation with eye tracking. Radiology 2015; 274: 276–286. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Feng X, Chi W, et al. Deep learning aided decision support for pulmonary nodules diagnosing: a review. J Thorac Dis 2018; 10: S867–S875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017; 284: 228–243. [DOI] [PubMed] [Google Scholar]
- 8.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014; 371: 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lien YC, Hsu HS, Li WY, et al. Pulmonary hamartoma. J Chin Med Assoc 2004; 67: 21–26. [PubMed] [Google Scholar]
- 10.Ekinci GH, Hacıömeroğlu O, Ersev A, et al. The frequency of lung cancer in patients with pulmonary hamartomas: An evaluation of clinical, radiological, and pathological features and follow-up data of 96 patients with pulmonary hamartomas. Rev Port Pneumol (2006) 2017; 23: 280–286. [DOI] [PubMed] [Google Scholar]
- 11.Manser R, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev 2013; 2013: CD001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salminen US. Pulmonary hamartoma. A clinical study of 77 cases in a 21-year period and review of literature. Eur J Cardiothorac Surg 1990; 4: 15–18. [DOI] [PubMed] [Google Scholar]
- 13.Al-Ameri A, Malhotra P, Thygesen H, et al. Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 2015; 89: 27–30. [DOI] [PubMed] [Google Scholar]
- 14.Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax 2008; 63: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trabucco SMR, Brascia D, Cazzato G, et al. Pulmonary Sclerosing Pneumocytoma: A Pre and Intraoperative Diagnostic Challenge. Report of Two Cases and Review of the Literature. Medicina (Kaunas) 2021; 57: 524. doi: 10.3390/medicina57060524. PMID: 34071040; PMCID: PMC8224668. [DOI] [PMC free article] [PubMed] [Google Scholar]