Abstract
The hypermucoviscosity/hypervirulent K. pneumoniae (hvKP) is a dominant cause of pyogenic liver abscess (PLA) and has contributed to the endemicity of disease in Asian country. The siderophore aerobactin (iucA) is highly expressed in hvKP and acting virulence role during hvKP infection. However, its role in the PLA is poorly understood. We constructed iucA deletion mutant (ΔiucA-hvKP852) and used animal study to characterize the role of siderophore iucA in K. pneumoniae liver abscess. The animal experiments showed that ΔiucA-hvKP852 strain had lower virulence in mice compared to hvKP852 wild type strain. At 24 h after infection, only two of ten mice developed liver abscess during infection with ΔiucA-hvKP852 strain, while nine of ten mice infected with wild type hvKP852 strain showed multiple lesions of liver abscess. The liver tissue infected with ΔiucA-hvKP852 exhibited low reactive oxygen stress levels compared to those infected by wild type hvKP852 strain (P < 0.05). The results suggest that siderophore iucA play an important role in the liver abscess by inducing oxidative stress.
Keywords: Hypermucoviscosity K. pneumoniae, Siderophore, Aerobactin, Oxidative stress, A liver abscess
Highlights
-
•
iucA positive strains produces more siderophore than iucA negative hvK. pneumoniae.
-
•
Siderophore production is positively related with Oxidative stress in hvK. pneumoniae.
-
•
iucA enhances oxidative stress in liver and forms liver abscess during hvK. pneumoniae infection.
1. Introduction
In the past three decades, the new variant of hypervirulent (hypermucoviscosity) K. pneumoniae (hvKP) widely emerged. It poses a great threat to human health and has been thought of as an emerging-reemerging pathogen [1,2]. Of interest, is that compared with the classical K. pneumoniae (cKP), hvKP is more likely to cause a bacterial liver abscess [3]. It is also noteworthy that it causes distinctive metastatic complications of hvKP, especially, endophthalmitis and meningitis, which has significant morbidity and mortality [[4], [5], [6]]. Studies have shown that hvKP is more virulent than cKP characterized by expressing capsular polysaccharide and carrying the virulence factors including mgaA, transcriptional regulators rmpA/rmpA2 [[7], [8], [9]]. Nevertheless, the enhanced virulence of hvKP is largely depended on the iron acquisition molecule [10].
Siderophores are low molecular weight and high-affinity iron chelators for bacterial replication and full virulence. Siderophore secreted by K. pneumoniae during infection can impact tissue localization, systemic dissemination, and host survival [11]. However, the impact of siderophore secreted by K. pneumoniae on the host during infection is still unknown. Genome sequence analysis showed that hvKP has four putative iron acquisition-related genes, enterobactin, salmochelin, yersiniabactin (ybt), and aerobactin [12]. Aerobactin has the iucABCD/iutA system and highly expressed in hvKP, is acting virulence determinant during hvKP infection [8,13]. The gene encoding aerobactin is iucA, and it is the main element of siderophore production in K. pneumoniae [14]. We have previously reported that the siderophore of K. pneumoniae induces bacterial resistance to ciprofloxacin by reducing the level of oxidative stress [15]. Studies have also indicated that the siderophore enterobactin of E. coli can act as a protective agent against oxidative stress to promote the formation of colonies of E. coli [16], suggesting that there is a regulatory relationship between siderophore and oxidative stress.
Oxidative stress is one of the important factors leading to infection [17]. The susceptibility of tissues to oxidative stress depends on the dynamic balance between oxidant and antioxidant agents. Long term oxidative stress can lead to an overabundance of free radicals in the liver, contributing to liver injury [18]. However, the connection between siderophore iucA and oxidative stress on liver damage with hvKP infections is still unknown. Whereby we constructed the iucA deletion mutant ΔiucA-hvKP and used mice infection model to investigate whether iucA induces oxidative stress and further damage the liver in hvKP infection.
2. Materials and methods
2.1. Bacterial isolates and growth condition
Fifty-six of K. pneumonia clinical isolates were collected from patients at two hospitals in Harbin, Heilongjiang Province, the People's Republic of China between June 2016 and October 2017. The strain were cultured in LB broth for overnight at 37 °C. The wildtype strain of hypermucoviscosity-hvKP852 (K2, ST65, carry rmpA1/rmpA2 and iucA) was isolated from liver abscess aspirate from inpatient. Our previous study has indicated that hvKP852 shows high virulence in mice (OD50:1 × 103 CFU/mL in mice) [8].
2.2. Construction of unmarked iucA deletion hv K.pneumoniae
The iucA hvKP deletion procedures was modified from Hsieh et al. [19]. In brief, the PCR product of the mixed iucA upstream gene (780 bp, primers K10 and K11) and the iucA downstream gene (828 bp, primers K12 and K13) was used as a template, and the overlap PCR was performed using primers K10 and K13 to obtain an iucA-deficient PCR product of 1500 bp (Table 1). The iucA deletion PCR products were sequenced and cloned into the pHSG412 vector (Fig. 1A). The iucA deletion of recombinant plasmid (pHSG412-ΔiucA) was transformed into hvKP852 (K2, ST65) by electroporation, and screening of target colonies using kanamycin selective medium (Fig. 1B–C). Integration and excision of the gene resulted in the generation of iucA deletion mutant (ΔiucA-hvKP852) which were further confirmed by PCR(Fig. 1D).
Table 1.
Primers used in this study.
| Primers | Sequences |
|---|---|
| iucA for | GTACATCCGTGGCAGTGGCAG |
| iucA rev | CAAGCGCGGCATAGCCTTCAT |
| ybtS for | CAAAAATGGGCGGTGGATTC |
| ybtS rev | CCTGACGGAACATAAACGAGCG |
| iroN for | GCATTGGTATTCCAGTTCAGACG |
| iroN rev | GAAAGGCAACGGTTGTCCAAA |
| EntB for | TGAAGACGATACCGTGCTGGTGGA |
| EntB rev | GTCGGCGACAAAGAACGGTTTGAT |
| K10 | GGGCTGCAGTTCCGCCGCCCAGTAGATTTC |
| K11 | GCGCAGTGCTTCCTTAATTCCTGGAACGTAAAAGGTAACTTT |
| K12 | AAAGTTACCTTTTACGTTCCAGGAATTAAGGAAGCACTGCGC |
| K13 | GGGGGATCCCCGCGGTGACGTGCCAGG |
Key: Restriction enzymes are highlighted by boldface. For, forward. rev, reverse.
Fig. 1.
Construction of iucA deletion strain. A. The construction scheme of the iucA gene deleted strain. B. Confirmation of the iucA deletion by PCR (1. BAF fraction; 2. BF fraction; 3. pHSG412-ΔiucA recombinant plasmid; 4. ΔiucA-hvKP852 strain). C&D. Growth of ΔiucA-hvKP852 strain in LB medium with or without kanamycin. BAF, 3800bp fragment; BF,1500bp fragment; Kna, kanamycin.
2.3. Animal experiments
Strains of ΔiucA-hvKP852 and hvKP852 were suspended in 0.5 mL of PBS, containing 3 × 104–1 × 106 CFU of bacteria and subsequently injected intra-peritoneally at different dilutions into 6–7 week old female BALB/C mice (10 mice/group, Vitalriver, Beijing, China) [8,20,21]. The survival rate of the mice which was described by Kaplan-Meier curves was recorded seven days after infection [22]. The load of the bacteria in the organs of the infected 6–8 week old BALB/C female mice was determined 24 h after infection. The mice were sacrificed, the organs; liver, lung and spleen were collected and 0.9 mL/g PBS added, homogenized and spread in LB agar plate. The exact numbers of bacteria in organs were determined using colony-forming units (CFU/mL) on LB plates in duplicate at adequate dilutions. All animal experiments comply with the guidelines (Laboratory Animals of the National Researh council) and crried out in accordance with the guidelines of the Ethics Review Committee of Animal experiments of Harbin Medical University (protocol number of Animal experimentation Ethical Inspection, HMUIRB2013002).
2.4. Histology
Liver and lung tissues were fixed in 4% paraformaldehyde for 24 h and dehydrated by gradient alcohol from 75% to 100%. The small pieces of paraffin-embedded specimens were processed for conventional histological assessment by Hematoxylin and Eosin (H&E) staining, and the bright-field microscopy images were acquired using a DP74 microscope, equipped with an Olympus BX53 camera [23].
2.5. DNA isolation and siderophore genes amplification
Chromosomal DNA was extracted from the K. pneumonia clinical isolates as described previously [24]. Genomic DNA from strains was used as a template for PCR. The four siderophore genes (iucA, iroN, ybtS, and enterobactin) were amplified using specific primers (Table 1).
2.6. Detection of siderophore production
The qualitative detection of siderophore was confirmed by CAS (chrome azurols) overlay plate method [15]. Briefly, an overnight culture of K. pneumoniae was spot inoculated onto a CAS agar plate and incubated for 24 h at 37 °C, the production of orange zones surrounding the colonies were measured.
2.7. Biofilm formation
Crystal violet staining and spectrophotometry was used to detect biofilms. a fresh single colony was selected and inoculated into LB medium for shaking culture at 37 °C for 16 h Then add diluted bacteria (OD595 value 0.09–0.12) to each 96 well plate, respectively, and incubated at 37 °C for 36 h. Add 150 μL 0.1% crystal viole staining solution, let stand for 30 min after carefully discard supernatant ashed by DDW for three times. After adding 1% SDS (w/v) and shaking for 10 min, its value was detected by spectophotomery (OD595) [25].
2.8. Test for reactive oxidative stress level and antioxidant factors
Quantitative measurement of ROS was performed by DCFH-DA method [26]. Freshly collected liver, lung and spleen of the mice infected with K. pneumoniae were homogenated and filtered using 300 meshes of nylon mesh. The collected cell suspension was treated with 2% NP40 for 10 min. They were rinsed twice with PBS and 5 × 104 cells were inoculated into 96-well cell culture plate. DCFH-DA (Chengjian Nanjing, China) was added at final concentration of 10 μM and incubated for 60 min at 37 °C. The fluorescence intensity of the cells in each group was measured by fluorescence microplate at 485 nm absorption wavelength. The bacterial strains ROS level was also detected using DCFH-DA method. DCFH-DA fluorescent dye was added to the iucA positive and negative K.pneumoniae strains cultured in iron starvation for 30 min to clean the unbounded fluorescence. NanoDrop 3300 was used to detect the ROS level of the bacteria. RFU (relative fluorescence units) was used to detect ROS level of strains [15]. The T-SOD (total superoxide dismutase), GSH (glutathione), and MDA (malondialdehyde) were assessed using the method following manufacturer's instructions. (Chengjian Nanjing, China). Freshly extracted supernatants of tissue homogenates determined the level of protein and used for detection of antioxidants. The results measured by chemiluminescence and showed fluorescence values per milligram of tissue proteins. The organs three replicate wells were set in each group, and the experiment was repeated twice.
2.9. Statistical analysis
All experimental data were statistically analyzed using SPSS 26.0 software. Differences in various of indicators between the two groups of KP852 and ΔiucA-KP852 strains were calculated by independent samples t-test. Kendall's tau rank coefficient is a nonparametric statistic used to measure the relationship between ROS level and siderophore production by strains. Survival of animal groups was estimated using the Kaplan-Meier method and log-rank (Mantel-Cox) test. Results are shown as mean ± SEM (standard error of mean). The difference was statistically significant at P < 0.05.
3. Results
3.1. iucA knockout of K. Pnumoniae reduces siderphores production
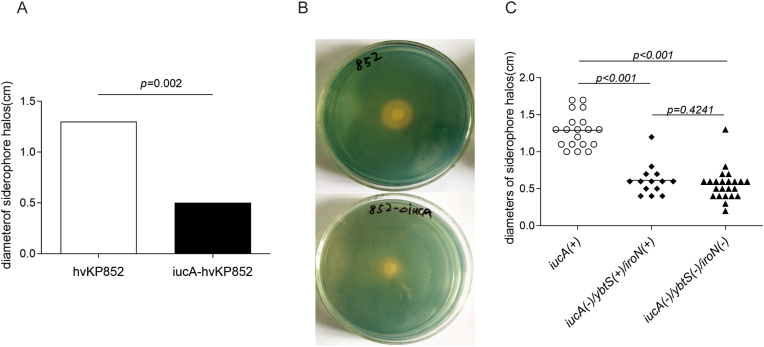
The siderophore production was detected using CAS assay which was measured using the diameter of orange zones to indicate the production of siderophore. The results showed that the orange zones of ΔiucA-hvKP852 (0.5 ± 0.07 cm) were significantly smaller than the wild-type hvKP85 (1.3 ± 0.07 cm) (Fig. 2A–B) (P < 0.001). In order to determine whether iucA was the main factor of siderophore production, we collected 56 K. pneumoniae clinical isolates to detect the siderophore related genes and siderophores production. Among the 56 K. pneumoniae isolates, the distribution of iucA, iroN, ybtS and enterobactin were 33.93% (19/56), 32.12% (18/56), 48.21% (27/56) and 85.71% (48/56), respectively, and all 13 strains of hypermucoviscous K. pneumoniae carried iucA gene (100%) (Table 2). The siderophore production of iucA-negative strains (0.614 ± 0.207 cm, 0.557 ± 0.213 cm, respectively) was significantly lower than that of iucA-positive clinical isolates (1.389 ± 0.231 cm) (P < 0.01, P < 0.001, respectively), (Fig. 2C). However, there is no significant difference of siderophore production in iucA negative strains, with or without iroN/ybtS genes (P = 0.413) (Fig. 2C).
Fig. 2.
iucA reduces siderophore production in hvK. pneumoniae. A-B. Comparison of siderophore production of ΔiucA-hvKP852 and wild-type hvKP852; C. Comparison of siderophore production of iucA positive and negative K. pneumoniae clinical isolates.
Table 2.
Distribution of siderophore related genes in clinical isolates of K. pneumoniae.
| Genes | No. of isolates % |
||
|---|---|---|---|
| hv KP (N = 13) | Non-hvKP (N = 43) | Total (N = 56) | |
| iucA | 100 (13/13) | 13.95 (6/43) | 33.93 (19/56) |
| iroN | 53.85 (7/13) | 25.58 (11/43) | 32.12 (18/56) |
| ybts | 69.23 (9/13) | 40.91 (18/44) | 48.21 (27/56) |
| EntB | 100 (13/13) | 79.55 (35/44) | 85.71 (48/56) |
Hv KP, hypermucovicosity K. pneumoniae; iucA, aertobactin A; iroN, salmochelin N; ybts, yersiniabactin; EntB: enterobactin B.
3.2. Siderophore iucA enhances oxidative stress in K. pneumoniae
To determine whether siderophore iucA affected oxidative stress in K. pneumonieae, we used DCFH-DA methods to detect ROS level of the bacterial strains. Results indicated that the ROS level of ΔiucA-hvKP852 (32.27 ± 3.82) was significantly lower than wild type strain hvKP852 (50.73 ± 8.29) (P = 0.024) (Fig. 3A). In the K. pneumoniae clinical isolates, the ROS level of the iucA negative strains also showed lower (70.29 ± 25.41) than that of positive clinical isoaltes (121.26 ± 52.69) (P = 0.0001) (Fig. 3B). Further ROS and siderophore production of strains indicated positive relationship (P < 0.05) (Fig. 3E). However, the biofilm formation of iucA positive and negative strains was not statistically significant. (ΔiucA-hvKP852 vs KP852: 0.93 ± 0.14 vs 0.81 ± 0.18; iucA (−) vs iucA (−): 0.544 ± 0.12 vs 0.57 ± 0.14, P = 0.6857, P = 0.6651, respectively) (Fig. 3C–D).
Fig. 3.
The effect of iucA on oxidative stress levels of K. pneumoniae. A-B. Comparison of ROS levels in iucA positive and negative strains; C-D. the biofilm formation of iucA positive and negative strains; E. correlation between siderophore production and ROS levels in 56 K. Pneumoniae clinical isolates.
3.3. Siderophore iucA an important virulence factor of hypermucoviscosity K. pneumoniae and related with liver abcess formation during infection
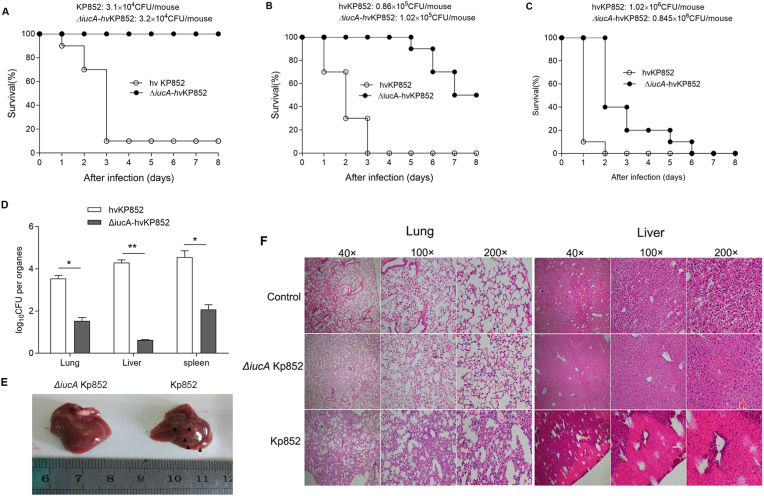
To investigate the effect of siderophore iucA on the pathogenicity of mice, we used ΔiucA-hvKP852 strain administered intraperitoneally to BALB/C female mice (6–8 weeks). The data showed that when mice were infected with 103 CFU/mouse and 105 CFU/mouse, after infection seven days. the survival rate of ΔiucA-hvKP852 infected mice was 100%, 50%, respectively, which was significantly higher than that of wild-type hvKP852-infected mice (10% and 0%) (Fig. 4A and B). At high doses (106 CFU/mouse), there was no significant difference for the virulence between mice infected with ΔiucA-hvKP852 and wild-type hvKP852 (Fig. 4C). At 24 h after infection, the number of bacteria in the lung, liver and spleen was significantly lower in the mice infected with ΔiucA-hvKP852 than that infected with wild-type strain hvKP852 (4 × 103 CFU per mice) (Fig. 4D). The multiple of abscess lesions were observed in the liver of wild-type hvKP852 infected mice (9 of 10 mice, 90%) (Fig. 4E). In the results of H&E staining of liver tissue, a large area of necrotic lesions was also observed (Fig. 4F). In the ΔiucA-hvKP852 infected mice, only 2 of 10 mice (20%) developed a single liver abscess. However, there was no abscess in the lung tissue (Fig. 4F).
Fig. 4.
Formation of Liver abscess rarelly seen in the mice infected with iucA deleted hvKP852strain. The suspension (0.5 mL) of △iucA-hvKP and hvKP852 strains were injected i.p. into Female BALB/C (ten per group) at different dose(A-C)to observe the survival rate of mice after 7day infection; D. Bacterial load in organisms in the hvKP infected mice after infection 24 h. E. formation of liver abscess seen in the hvKP852 infected mice, but not in the ΔiucA-hvKP852 infected mice. Abscess represented by black solid triangle; F. H&E staining of lung and liver in mice after infection. H&E stained liver and lung sections are shown at × 40, × 100 and × 200 original magnification.
3.4. Siderophore iucA enhances oxidative stress in liver infected with hypermucoviscosity K. pneumoniae
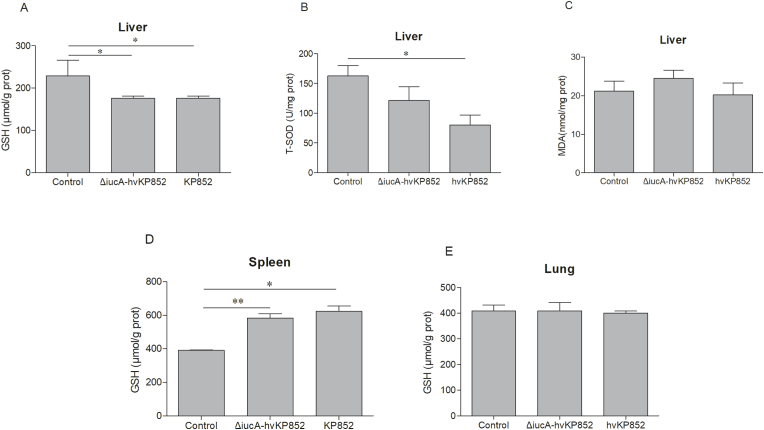
ROS are key signaling molecules in the various signalling pathways and ROS induce apoptosis and necrosis of cells through oxidative stress [27]. To investigate the effect of siderophore iucA on oxidative stress levels, we examined ROS levels in K. pneumoniae-infected tissues. The results showed that ROS levels in the liver of wild-type hvKP852-infected mice were significantly higher than ΔiucA-hvKP852 (P < 0.01) (Fig. 5A), while there was no statistically significant difference in ROS levels between serum, lung and spleen (Fig. 5B–D). Both ΔiucA-hvKP852 and hvKP852 strains decreased (Fig. 6A–B) or remains unchanged (Fig. 6C) liver antioxidant factors; GSH, T-SOD and MDA levels. Nevertheless, antioxidant factors of strains infected spleen and lung indicated higher or unchanged (Fig. 6D–E).
Fig. 5.
ROS levels in the mice infected with ΔiucA-hvKP852 and wild type hvKP852 strains. The ROS levels indicated per milligram of tissue protein, except serum (A. serum; B. Liver; C. spleen; D. lung).
Fig. 6.
Antioxidant levels in tissue of mice infected with ΔiucA-hvKP852 and wild type hvKP852 strains. GSH(A. liver; D. spleen; E. lung) and T-SOD (B. liver) and MDA (C. liver) of tissues were detected by chemiluminescence. The results showed fluorescence values per milligram of tissue protein.
4. Discussion
HvKp is the predominant bacterial responsible for pyogenic liver abscess worldwide, especially in Asian countries [4]. Tracing back to previous studies, the siderophore production of hvKP and cKP is different. The general trend is that hvKP produce more siderophore than cKP [10].
Genome sequence analysis of K. pneumoniae causing liver abscess found that hvKP is different from cKP which carry four siderophore related genes iucA, iroN, bts and enterobactin [12]. In order to clarify the genes involved in the production of siderophores, we collected 56 K. pneumoniae clinical isolates using the CAS assay to measure the production of siderophores. The results showed that iucA-positive clinical isolates produce more siderophores than iucA-negative clinical isolates, and the siderophore related iroN and ybtS were not related to the yield of the siderophore. Similar results also were seen in the ΔiucA-hvKP852 which reduced siderophore production compared with wild-type strain (Fig. 2), further confirming that iucA is a major factor in the production of siderophores [10].
Research has shown that the main difference between the variant hvKP and cKP tend to spontaneously form abscesses, especially liver abscesses even in healthy human adults [3]. The siderophore iucA is an important virulence factor for hvKP (Fig. 4). However, it is unclear whether it plays a role in the formation of liver abscess. Animal experiments indicated that the number of liver abscess (about 90%) observed in mice infected with wild-type hvKP852 strain was higher compared with ΔiucA-hvKP852 (iucA deleted strain). This indicated that iucA may play a critical role in hvKP liver abscess formation and liver cell death. In our study, there are 20% (2/10) of mice infected with ΔiucA-hvKP852 strain also showing liver abscess. Lin and colleague [21] demonstrated that EREM-1 (triggering receptor expressed on myeloid cells) promotes K. pneumonae liver abscess in mice, suggest that host factor also involved in the formation of liver abscess.
When body have a bacterial infection, high activity molecules such as reactive oxygen species are produced. When the oxidation level exceeds the scavenging ability of the body, this would lead to neutrophil infiltration and the production of a large number of oxidative intermediates. Thus ROS is favorable for bacterial infection [28]. Kamaladevi A et al., established that K. pneumoniae can induce oxidative stress in host cells and cause sudden oxidative cell death in infected host [29]. To clarify the role of siderohpre iucA in this regard, we examined the ROS levels of animal tissues infected with hvKP. The results showed that the ROS level in liver infected with hvKP852 strain was significantly higher than that of ΔiucA-hvKP852 strain infected mice. However, the levels of anti-oxidant factors GSH, T-SOD, MDA were not changed in the hvKP852 and ΔiucA-hvKP852 strain infected mice liver and other organs. In fact, These antioxidants were lower than the uninfected mice. Our data strongly suggest that during the infection of hypermucoviscosity K. pneumoniae, iucA promotes siderophore production, which stimulates strain proliferation and colonization in the liver, leading to the production of more free radicals, and the imbalance in the production of oxidative and antioxidant elements in the liver. Collectively, these changes contribute to liver inflammatory lesions and damage (Fig. 7).
Fig. 7.
Schematic illustration of the mechanism of siderophore iucA related liver abscess formation during hypermucoviscous K. pneumoniae infection. After infection with hypermucoviscous K. pneumoniae, the iucA of K. pneumoniae promotes the production of siderophore and the proliferation and colonization in the liver and further enhances ROS production. Nevertheless, anti-oxidant elements, such as T-SOD and GSH decreased or did not changed. Then excessive ROS leads to extensive damage and necrosis of hepatocytes in some way, and the formation of abscesses. iucA, aerobactin; iucA (+), iucA positive strains; iucA (−), iucA negative strains; hv, hypermucoviscosity; “↑”, increaced; “↓”, decreaced; “-”, unchanged; ROS, reactive oxygen; GSH, glutathione; T-SOD, total superoxide dismutase.
In conclusion, iucA is considered as the main factor of siderophore production in hypermucoviscosity K. pneumoniae. It is an important virulence factor involved in liver damage induced by oxidative stress. Further study is required to confirm the interaction of siderophore iucA and host factors, and to investigate additional factors that may contribute to liver abscess induced by hypermucoviscosity K. pneumoniae.
Funding
This work was supported by grants of National Natural Science Foundation of China (NSFC) (81373145).
Author contributions
J Wu and J chen: performed experiments, analysis and interpretaion of data, Y Wang: Analysis and interpretation of data, Qingtai Meng: Analysis and interpretation of data, Jizi Zhao, Analysis, Validation, Supervision, wrote the manuscript.
Declaration of competing interest
All authors declare that they have no competing interests.
Data availability
The data that has been used is confidential.
References
- 1.Shon A.S., Bajwa R.P., Russo T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marr C.M., Russo T.A. Hypervirulent Klebsiella pneumoniae: a new public health threat. Expert Rev. Anti Infect. Ther. 2019;17:71–73. doi: 10.1080/14787210.2019.1555470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Yan Y., Xue X., Wang K., Shen D. Comparison of pyogenic liver abscesses caused by hypermucoviscous Klebsiella pneumoniae and non-Klebsiella pneumoniae pathogens in Beijing: a retrospective analysis. J. Int. Med. Res. 2013;41:1088–1097. doi: 10.1177/0300060513487645. [DOI] [PubMed] [Google Scholar]
- 4.Siu L.K., Yeh K.M., Lin J.C., Fung C.P., Chang F.Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 5.Rahimian J., Wilson T., Oram V., Holzman R.S. Pyogenic liver abscess: recent trends in etiology and mortality. Clin. Infect. Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 6.Kashani A.H., Eliott D. The emergence of Klebsiella pneumoniae endogenous endophthalmitis in the USA: basic and clinical advances. J. Ophthal. Inflamm. Infect. 2013;3:28. doi: 10.1186/1869-5760-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W.L., Ko W.C., Cheng K.C., Lee C.C., Lai C.C., Chuang Y.C. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn. Microbiol. Infect. Dis. 2008;62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J., Chen J., Zhao M., Qiu X., Chen X., Zhang W., Sun R., Ogutu J.O., Zhang F. Multilocus sequence types and virulence determinants of hypermucoviscosity-positive Klebsiella pneumoniae isolated from community-acquired infection cases in Harbin, north China. Jpn. J. Infect. Dis. 2016;69:357–360. doi: 10.7883/yoken.JJID.2015.321. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y.C., Lu M.C., Tang H.L., Liu H.C., Chen C.H., Liu K.S., Lin C., Chiou C.S., Chiang M.K., Chen C.M., Lai Y.C. Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain. BMC Microbiol. 2011;11:50. doi: 10.1186/1471-2180-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo T.A., Olson R., Macdonald U., Metzger D., Maltese L.M., Drake E.J., Gulick A.M. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect. Immun. 2014;82:2356–2367. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden V.I., Breen P., Houle S., Dozois C.M., Bachman M.A. Klebsiella pneumoniae siderophores induce inflammation, bacterial dissemination, and HIF-1alpha stabilization during pneumonia. mBio. 2016;7 doi: 10.1128/mBio.01397-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu K.M., Li L.H., Yan J.J., Tsao N., Liao T.L., Tsai H.C., Fung C.P., Chen H.J., Liu Y.M., Wang J.T., Fang C.T., Chang S.C., Shu H.Y., Liu T.T., Chen Y.T., Shiau Y.R., Lauderdale T.L., Su I.J., Kirby R., Tsai S.F. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J. Bacteriol. 2009;191:4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh P.F., Lin T.L., Lee C.Z., Tsai S.F., Wang J.T. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect. Dis. 2008;197:1717–1727. doi: 10.1086/588383. [DOI] [PubMed] [Google Scholar]
- 14.Russo T.A., Shon A.S., Beanan J.M., Olson R., MacDonald U., Pomakov A.O., Visitacion M.P. Hypervirulent K. pneumoniae secretes more and more active iron-acquisition molecules than "classical" K. pneumoniae thereby enhancing its virulence. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Zhang Y., Wang X., Ding F., Fu Y., Zhao J., Song W., Opiyo O.J., Zhang F., Chen X. Siderophores in clinical isolates of Klebsiella pneumoniae promote ciprofloxacin resistance by inhibiting the oxidative stress. Biochem. Biophys. Res. Commun. 2017;491:855–861. doi: 10.1016/j.bbrc.2017.04.108. [DOI] [PubMed] [Google Scholar]
- 16.Peralta D.R., Adler C., Corbalan N.S., Paz Garcia E.C., Pomares M.F., Vincent P.A. Enterobactin as part of the oxidative stress response repertoire. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharm. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cichoz-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh P.F., Liu J., Pan Y., Wu M., Lin T., Huang Y., Wang J. Klebsiella pneumoniae peptidoglycan-associated lipoprotein and murein lipoprotein contribute to serum resistance, antiphagocytosis, and proinflammatory cytokine stimulation. J. Infect. Dis. 2013;208:1580–1589. doi: 10.1093/infdis/jit384. [DOI] [PubMed] [Google Scholar]
- 20.Chen N., Jin T.T., Liu W.N., Zhu D.Q., Chen Y.Y., Shen Y.L., Ling Z.X., Wang H.J., Zhang L.P. Gastric Microbiota Alteration in Klebsiella pneumoniae caused liver abscesses in mice. Pol. J. Microbiol. 2019;68:247–254. doi: 10.33073/pjm-2019-026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y.T., Tseng K.Y., Yeh Y.C., Yang F.C., Fung C.P., Chen N.J. TREM-1 promotes survival during Klebsiella pneumoniae liver abscess in mice. Infect. Immun. 2014;82:1335–1342. doi: 10.1128/IAI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Han, M Niu, S Liu, J Mao, Y Yu, Y Du, The effect of siderophore virulence genes entB and ybtS on the virulence of Carbapenem-resistant Klebsiella pneumoniae, Microb. Pathog., 171(2022):105746. [DOI] [PubMed]
- 23.Liu X., Chen G., DU G., Pan Y., Song W., Jiang T., Liu Hai. Berbamine ameliorates ethanol-induced liver injury by inhibition of hepatic inflammation in mice. Chin. J. Nat. Med. 2020;18:186–195. doi: 10.1016/S1875-5364(20)30020-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y., Zhao J., Ding F., Wang B., Zhang W., Gu J., Huang Y., Fu Y., Zhang F. The bla(CTX-M) gene independently enhances drug resistance level to ampicillin in clinical isolates of Klebsiella pneumoniae. J. Antibiot. (Tokyo) 2012;65:479–481. doi: 10.1038/ja.2012.44. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A., Khan A.K., Anwar A., Ali S.A., Shah M.R. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumoniae. Microb. Pathog. 2016;98:50–56. doi: 10.1016/j.micpath.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2019;38 doi: 10.15252/embj.2019101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T., Naguro I., Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1398–1409. doi: 10.1016/j.bbagen.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Yin Y., Zhang W., Li H., Wang T., Yin H., Sun L., Su C., Zhang K., Xu H. Reactive oxygen species scavenging and inflammation mitigation enabled by biomimetic prussian blue analogues boycott atherosclerosis. J. Nanobiotechnol. 2021;19:161. doi: 10.1186/s12951-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamaladevi A., Balamurugan K. Global proteomics revealed Klebsiella pneumoniae induced autophagy and oxidative stress in Caenorhabditis elegans by inhibiting PI3K/AKT/mTOR pathway during infection. Front. Cell. Infect. Microbiol. 2017;7:393. doi: 10.3389/fcimb.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.