Abstract
Background
Lagopsis supina (Steph. ex Willd.) Ikonn.-Gal. has been a traditional Chinese medicine (TCM) for the treatment of blood stasis, inflammation, and diuresis. Moreover, Huo Xue Li Shui theory was an important TCM theory that used to treat many ailments. Nevertheless, the scientific connotation of this theory has not been clearly elucidated so far.
Aim of the study
The aim of this study was to explore the scientific connotation of Huo Xue Li Shui with promoting blood circulation and removing blood stasis (PBCRBS), anti-inflammatory and diuretic effects in trauma-induced blood stasis model (TBSM) rats, taking microporous adsorption resin with water (LSB) and 30% ethanol (LSC) elution fractions from L. supina as a classical demonstration.
Materials and methods
48 rats were randomly assigned into six groups (n = 8/group): the control group, the model group, and model groups treatment with LSB or LSC. The biochemical parameters and protein expression were measured using kit method and Western blot assay, respectively.
Results
Both LSB and LSC were effective in elevating body weight, food consumption, and water intake in model rats. In PBCRBS efficacy evaluation, LSB and LSC remarkably improved histopathological tissues. On the other hand, LSB and LSC prominently decreased the contents of plasma viscosity, platelet aggregation rate, thrombin time, prothrombin time, activated partial thromboplastin time (APTT), fibrinogen, thromboxane B2, thromboxane B2/6-keto-prostaglandin F1α, urokinase-type plasminogen activator (u-PA), plasminogen activator inhibitor-1(PAI-1), PAI-1/tissue-type plasminogen activator (t-PA), and PAI-1/u-PA, while significantly enhanced the contents of antithrombin III, 6-keto-prostaglandin F1α, and t-PA. In parallel, LSB and LSC obviously down-regulated the levels of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8, and remarkably up-regulated the level of IL-10. In determining diuretic activities, LSB and LSC prominently elevated urinary excretion volume and the level of atriopeptin, and remarkably reduced the levels of angiotensin II, anti-diuretic hormone, aldosterone, aquaporin 1 (AQP1), AQP2, and AQP3. In addition, LSB and LSC clearly suppressed protein expressions of AQP1, AQP2, and AQP3. Finally, LSB and LSC did not caused urinary pH, Na+, and Cl− electrolytes and had minor effects on K+ and Ca2+ concentrations.
Conclusions
LSB and LSC exhibited prominent PBCRBS, anti-inflammatory, and diuretic effects in TBSM rats, thereby supported the traditional folk use of L. supina. This study successfully provided an experimental basis for the scientific connotation of Huo Xue Li Shui.
Keywords: Lagopsis supina, Trauma-induced blood stasis model, PBCRBS effect, Anti-inflammatory effect, Diuretic effect, Huo Xue Li Shui
Graphical abstract
Lagopsis supina; Trauma-induced blood stasis model; PBCRBS effect; Anti-inflammatory effect; Diuretic effect; Huo Xue Li Shui.
1. Introduction
For thousands of years in Asian countries, such as China, Japan, and Korea, traditional Chinese medicine (TCM) has been a complex mixture of multi-component mechanisms that used to prevent and treat diseases [1, 2, 3]. For a long period of time, TCM research has applied the methodology of Western medical experience and achieved certain goals in the screening of bio-active phytochemicals [4]. Nevertheless, the integrity of TCM and the connection of TCM theories had been somewhat overlooked. As a result, appropriate strategies to explore the integrality of TCM and its scientific connotations under the guidance of TCM theory was a crucial challenge that needs to be solved urgently.
From the perspective of TCM theory, an excess of changes in the imbalance of Qi-Blood in the human body could lead to blood stasis when Qi overwhelms Blood, which refers to the blood circulation being turbulent or stagnant in the body [1, 2]. Huo Xue Li Shui (Chinese: 活血利水) theory was an ancient and vital TCM theory that applied to the treatment of blood stasis, edema and other diseases [1, 5, 6]. The clinical manifestations of Huo Xue Li Shui theory refer to the two categories of promoting blood circulation and removing blood stasis (PBCRBS), and promoting blood circulation and diuresis, whereby the former regulated the balance of Qi-Blood, boosts blood flow and excretes excess water via metabolism, while the latter egested water out through urination [1, 6, 7, 8]. Although good clinical applications had been achieved, the theory of Huo Xue Li Shui has not been adequately illustrated, which severely restricted its clinical application, and the modern biological effect still needs an in-depth explanation. Moreover, the problems of efficacy and mechanism remained the main obstacles to the clinical application of TCMs. In addition, trauma-induced blood stasis model (TBSM) rat was one of blood stasis model, and commonly used to evaluate the PBCRBS and diuretic effects in vivo [1, 6, 7]. For this reason, the TBSM rat was frequently used as a classical rodent model to explain the Huo Xue Li Shui theory of TCMs [1].
In this context, Lagopsis supina (Chinese: 夏至草), which belongs to the Labiatae family, was an effective and widely used TCM associated with Huo Xue Li Shui theory, has been first recorded in Shennong Bencao Jing and commonly applied as a remedy for against blood stasis, inflammation, diuresis, and edema [1]. Our previous studies had demonstrated that the crude extract of L. supina together with its microporous adsorption resin with water (LSB) and 30% ethanol (LSC) elution fraction may be involved in PBCRBS and diuretic effects in rats [9, 10, 11]. However, due it is still unclear to date due to the indeterminate relationship between the efficacy of LSB/LSC and the Huo Xue Li Shui theory. Therefore, there was an urgent need to figure out the efficacy of Huo Xue Li Shui associated with PBCRBS, anti-inflammatory, and diuretic effects.
Therefore, this work took TBSM rats as the integral animal to explore the scientific connotation with PBCRBS, anti-inflammatory, and diuretic effects of LSB and LSC, and to tentatively discuss the application of Huo Xue Li Shui.
2. Materials and methods
2.1. Chemicals and drugs
Pentobarbital sodium, heparin, Dextran 500 and sodium carboxymethyl cellulose (CMC-Na) were manufactured by Shanghai Rongbai Biological Technology Co., Ltd. (Shanghai, China), Shanghai Solarbio Bioscience and Technology Co., Ltd. (Shanghai, China) and Sigma Co., Ltd. (St. Louis, Mo, USA), respectively. Furthermore, Tween 20, ponceau, acrylamide and sodium lauryl sulfate were purchased from Solon Co., Ltd. (OH, USA).
Radio Immunoprecipitation Assay lysis buffer and hematoxylin-eosin staining were produced by Beyotime Biotechnology Co., Ltd. (Shanghai, China). Polyvinylidene fluoride membranes and pre-stained protein markers were purchased from Millipore Co., Ltd. (Schwalbach, Germany) and Green BioResearch Co., Ltd. (LA, USA), respectively. Assay kits for detection Na+, K+, Cl−, and Ca2+ concentrations were produced by Nanjing Jiancheng Institute of Biotechnology Co., Ltd. (Nanjing, China). Enzyme linked immunosorbent assay (ELISA) kits for the detection of tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), IL-8, IL-10, angiotensin II, anti-diuretic hormone, aldosterone, atriopeptin, antithrombin-III, thromboxane B2, 6-keto-prostaglandin F1α, urokinase-type plasminogen activator (u-PA), tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), aquaporin 1 (AQP1), AQP2, and AQP3 were manufactured by Chuzhou Shinuoda Biological Technology Co., Ltd. (Anhui, China). Anti-AQP1 and anti-AQP3 antibodies were produced by Abcam Co., Ltd. (Cambridge, UK), whereas the anti-AQP2 antibody was purchased from CST Co., Ltd. (Boston, USA).
2.2. Plant materials and fractions
The whole plant of L. supina (No. XZC201606) and its fractions (LSA∼D) were carried out in our previous papers [10]. In brief, the whole plants of L. supina were collected from Tongliao City, Inner Mongolia, China, in June 2016, and authenticated by prof. Guoyue Zhong of our team.
The dried powders of L. supina (38.0 kg) were extracted with 95% ethanol (300 L × 3) and then 50% ethanol (300 L × 3) by maceration for 7 consecutive days. These solvents were evaporated to acquire the crude extract (LS, 8.7 kg, 22.90 %), which was then suspended in water and successfully extracted with petroleum ether (LSA, 1320 g). Subsequently, the water portion was subjected to macroporous resin column chromatography, eluted with water (LSB, 6148 g, 16.18%), 30% ethanol (LSC, 669 g, 1.76%), 60% ethanol (LSD), and 95% ethanol (LSE) consecutively to obtain targeted fractions. Moreover, the fractions of LSB and LSC were dissolved in 0.3% CMC-Na and stored at −20 °C.
2.3. Animals
Specific pathogen free male Sprague-Dawley rats (No. SYXK 2013-0032, 200 ± 20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China) and maintained in metabolism with free access to food and water in cages, at 23 ± 2 °C under a 12 h/12 h light/dark cycles. The experiment was approved by the Animal Care and Research Committee of Jiangxi University of Chinese Medicine (JZLLSC20210028).
2.4. Experimental design
Forty-eight rats were randomly assigned into 6 groups (n = 8/group): Control (normal rats, 0.3% CMC-Na), Model (TBSM rats, 0.3% CMC-Na), LSB-H (TBSM + LSB-H rats, high dose of LSB), LSB-L (TBSM + LSB-L rats, low dose of LSB), LSC-H (TBSM + LSC-H rats, high dose of LSC) and LSC-L (TBSM + LSC-L rats, low dose of LSC), administered orally once daily for 7 consecutive days. The doses of LSB and LSC were based on previous experiments and TCM clinical application [10] and were listed in Table 1.
Table 1.
The doses of different groups.
| Groups | n | Dose (mg/kg) | Drug |
|---|---|---|---|
| Control | 8 | − | − |
| Model | 8 | − | − |
| LSB-H (high dose of LSB) | 8 | 323.6 | LSB |
| LSB-L (low dose of LSB) | 8 | 80.9 | LSB |
| LSC-H (high dose of LSC) | 8 | 35.2 | LSC |
| LSC-L (low dose of LSC) | 8 | 8.8 | LSC |
The TBSM rat used in this experiment has been previously reported by our team [1]. Briefly, anesthetized rat was fixed and knocked by a 500 g weight, which dropped from 58 cm. As a result, the right hind limb midsection of rat was injured, indicated the TBSM rat was established. Then, the injured muscle tissue of rat was harvested and store at −80 °C for the subsequent experiments. Daily body weight, food consumption (expressed in g/100g), water intake (expressed in mL/100g), urinary volume, and urinary pH were measured in all rats.
2.5. Biochemical assay
2.5.1. Effects of LSB and LSC on histological examination
Histopathological observation of injured muscle tissue was reported in our previous paper [1, 12]. In brief, damaged muscle tissue was fixed in 4% paraformaldehyde. After 24 h, the graded ethanol dehydration, xylene and liquid paraffin series, specimens were embedded in paraffin wax. Subsequently, sections (4 μm) were paraffin embedded, deparaffinized, rehydrated and stained with hematoxylin and eosin (HE). Mounted sections were examined using a BX53 microscope (Olympus Corporation, Japan).
2.5.2. Effects of LSB and LSC on hemorheological indices
Hemorheological indexes of plasma viscosity and platelet aggregation rate contents were analyzed by Sysmex XN1000 (Sysmex corporation, Hyogo, Japan) and LBY-NJ4A (Beijing Pilisheng Instrument corporation, Beijing, China), respectively. Briefly, the platelet-rich plasma was obtained from blood sample after anti-coagulated and centrifuged (1000 g, 10 min, 4 °C). Then, the platelet-rich plasma was further to centrifuge (3000 g, 10 min, 4 °C) and the sample of platelet-poor plasma. Subsequently, the plasma viscosity and platelet aggregation rate of the platelet-poor plasma were measured by an automatic blood analyzer (Sysmex XN1000, Sysmex Corporation™, Hyogo, Japan) and an automatic platelet aggregation analyzer (LBY-NJ4A, Beijing Pilisheng Instrument Co. Ltd. Beijing, China), respectively.
2.5.3. Effects of LSB and LSC on coagulation function parameters
Anti-coagulation parameters for thrombin time, prothrombin time, APTT and fibrinogen contents were measured by an automatic coagulation analyzer (CoaLAB1000, LAbor BioMedical Technologies GmbH, Ahrensburg, Germany). Moreover, the blood sample was direct to centrifuge (3000 g, 10 min, 4 °C) and to obtain the serum sample, which was used to detect the level of antithrombin III using an ELISA kit.
2.5.4. Effects of LSB and LSC on thromboxane B2 and 6-keto-prostaglandin F1α levels in serum
The serum levels of thromboxane B2 and 6-keto-prostaglandin F1α were detected using ELISA kits according to the manufacturer’s instructions.
2.5.5. Effects of LSB and LSC on t-PA, u-PA and PAI-1 levels in serum
The serum levels of t-PA, u-PA, and PAI-1 were detected using ELISA kits according to the manufacturer’s instructions.
2.5.6. Effects of LSB and LSC on urinary electrolyte concentrations
The urinary electrolyte concentrations of Na+, K+, Cl−, and Ca2+ were determined using colorimetric methods according to the manufacturer’s instructions.
2.5.7. Effects of LSB and LSC on angiotensin II, anti-diuretic hormone, aldosterone, atriopeptin, AQP1, AQP2, and AQP3
The serum levels of angiotensin II, anti-diuretic hormone, aldosterone and atriopeptin, AQP1, AQP2, and AQP3 were analyzed by ELISA kits according to the manufacturer’s instructions.
2.5.8. Western blot assay
The protein expressions of AQPs-1, 2, and 3 in injured muscle tissues were reported in our previous work [1]. Briefly, the injured muscle tissue was homogenized in ice-cold RIPA lysis buffer for 30 min with occasional rocking. The lysates were clarified by centrifuging at 13,201 g for 5 min at 4 °C. The protein concentration of each supernatant was determined using the BCA kit (Beyotime Biotechnology., Ltd, Shanghai, China). Analysis was performed with 10 % SDS-PAGE separation gel and 50 μg proteins were transferred to the PVDF membrane. The membrane was blocked using blocking buffer for 1.0 h at room temperature, and incubated overnight at 4 °C with specific primary antibodies (AQP-1 for 1:2000, AQP2, AQP3 and GAPDH for 1:1000). Subsequently, the membrane was washed five times with TBST buffer and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000) for 1 h at 37 °C, and washed extensively before detection. The membranes were subsequently developed using an enhanced chemiluminescence reagent and exposed to a film according to the manufacturer’s protocol. GAPDH was used as an internal reference.
2.6. Statistical analysis
All data were conducted at least in triplicated and expressed as mean ± standard deviation (SD) and performed via one-way analysis of variance (ANOVA), followed by Tukey’s test for different groups. The p-values less than 0.05 or 0.01 were indicated as statistically significant and were labeled with a single #/& and double ##/&&, respectively.
3. Results
3.1. LSB and LSC increased the body weight, food consumption and water intake
To determine the effects of LSB and LSC on TBSM rats, the body weight, food consumption, and water intake of all rats were detected once daily for 6 consecutive days. Compared to the control group, model rats showed a significant decrease in body weight (from 3rd to 6th day) and food consumption (from 1st to 6th day), while water intake (from 3rd to 6th day) increased remarkably (Figure 1, p < 0.01 or p < 0.05). Meanwhile, the body weight, food consumption and water intake were significantly increased after oral administration of LSB and LSC compared to those of in the model rats (p < 0.01).
Figure 1.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on body weight (A), food consumption (B), and water intake (C) in rats during the treatment period. #p < 0.05 and ##p < 0.01 compared with control group, &p < 0.05 and &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.2. LSB and LSC possessed PBCRBS effects
3.2.1. LSB and LSC ameliorated histopathological lesions
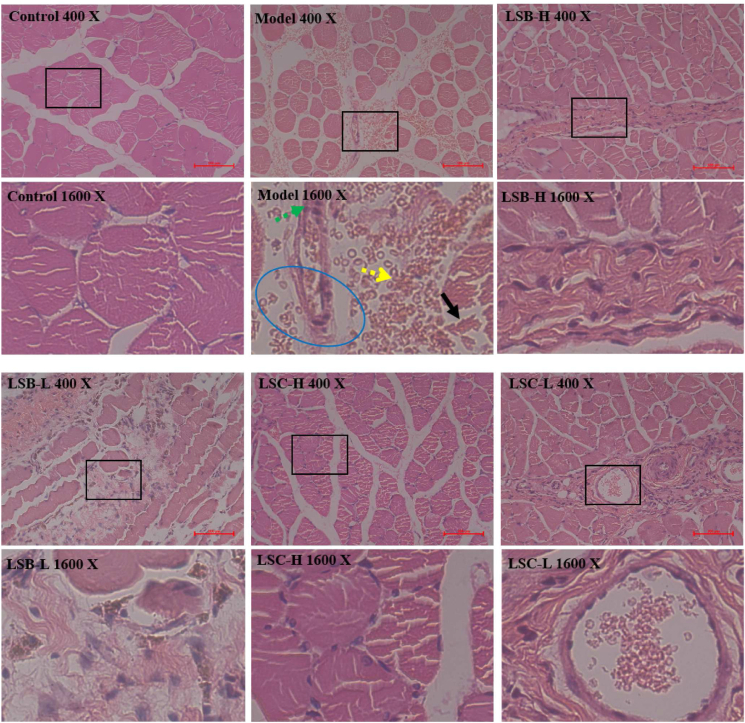
Injured muscle tissues of model rats were determined by histopathological examinations (Figure 2). Compared with the control rats, model rats displayed massive mononuclear cells and neutrophil granulocyte filtration (yellow arrow), congestive edema (blue circle), muscle fiber rearrangement (black arrow), and blood stasis (green arrow). However, as expected, these histopathological lesions were ameliorated by after treatment with LSB and LSC. Thus, LSB and LSC play pivotal roles in the treatment of blood stasis, inflammatory response, and edema.
Figure 2.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on injured muscle in rats (hematoxylin eosin staining).
3.2.2. LSB and LSC improved hemorheological indices
The effects of LSB and LSC on hemorheological functional indices were evaluated by assessing of plasma viscosity and platelet aggregation rate in the plasma of rats. As illustrated in Figure 3, the levels of plasma viscosity and platelet aggregation rate were significantly increased in the model group compared to the control group (p < 0.01). Moreover, with treatments of LSB and LSC, the levels of these factors in model rats were clearly decreased in a dose-dependent manner (p < 0.01 or p < 0.05). Therefore, LSB and LSC may play important roles in the improvement of abnormal hemorheology.
Figure 3.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on plasma viscosity (A) and platelet aggregation rate (B) levels in rats. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.2.3. LSB and LSC improved blood coagulation function
The effects of LSB and LSC on blood coagulation function were determined by assessing the levels of thrombin time, prothrombin time, APTT, fibrinogen, and antithrombin III. As showed in Figure 4, thrombin time, prothrombin time, APTT and fibrinogen levels were remarkably elevated (p < 0.01), while the antithrombin III level was markedly decreased in the model group compared to the control group (p < 0.05). Under LSB and LSC treatments, thrombin time, prothrombin time and APTT were prominently shortened (p < 0.01 or p < 0.05), and the level of fibrinogen was obviously down-regulated compared to the model group (p < 0.01 or p < 0.05). Furthermore, LSC-H treatment significantly elevated the level of antithrombin III (p < 0.05). Therefore, LSB and LSC possessed remarkable dose-dependent effects on anti-coagulant activities in TBSM rats.
Figure 4.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on thrombin time (A), prothrombin time (B), activated partial thromboplastin time (C), fibrinogen (D) and antithrombin III (E) levels in rats. #p < 0.05 and ##p < 0.01 compared with control group, &p < 0.05 and &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.2.4. LSB and LSC inhibited platelet aggregation
As illustrated in Figure 5, the thromboxane B2 level and thromboxane B2/6-keto-prostaglandin F1α ratio were significantly higher in model rats than in the control group (p < 0.01), while the level of 6-keto-prostaglandin F1α was remarkably lower model rats than in the control group (p < 0.01). LSB and LSC treatments were able to reduce the thromboxane B2 level and thromboxane B2/6-keto-prostaglandin F1α ratio, as well raise the level of 6-keto-prostaglandin F1α (p < 0.01). These findings suggest that LSB and LSC can perform anti-platelet aggregation activities with a dose-dependent relationship in TBSM rats.
Figure 5.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on thromboxane B2 (A), 6-keto-prostaglandin F1α (B), and thromboxane B2/6-keto-prostaglandin F1α (C) levels in rats. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.2.5. LSB and LSC suppressed fibrinolysis
The serum levels of t-PA, u-PA, PAI-1, PAI-1/t-PA, and PAI-1/u-PA in model rats were determined to evaluate the functions of the fibrinolytic system (Figure 6). Compared to normal rats, model rats showed markedly lower levels of t-PA (p < 0.01) and higher levels of u-PA, PAI-1, PAI-1/t-PA, and PAI-1/u-PA (p < 0.01). Under LSB and LSC treatments, the levels of u-PA, PAI-1, PAI-1/t-PA, and PAI-1/u-PA were reduced in a dose-dependent behavior compared to those of the model group (p < 0.01 or p < 0.05). Meanwhile, the level of t-PA increased only after treatment with LSB-H (p < 0.05). Therefore, LSB and LSC play crucial roles in inhibited effects on fibrinolysis in TBSM rats.
Figure 6.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on tissue-type plasminogen activator (t-PA), urokinase-type plasminogen activator (u-PA, B), plasminogen activator inhibitor-1 (PAI-1, C), PAI-1/t-PA (D) and PAI-1/u-PA (E) levels in rats. #p < 0.05 and ##p < 0.01 compared with control group, &p < 0.05 and &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
In short, LSB and LSC possessed remarkable PBCRBS effects via anti-coagulation, anti-platelet activation, and anti-fibrinolysis pathways in TBSM rats.
3.3. LSB and LSC exhibited anti-inflammatory effects
The levels of TNF-α, IL-6, IL-8, and IL-10 in the serum of rats were examined to evaluate the severity of inflammation. As showed in Figure 7, the serum levels of TNF-α, IL-6, and IL-8 were obviously up-regulated in the model group rats compared to the control group rats (p < 0.01). In contrast, the serum level of IL-10 was significantly down-regulated in the model group rats compared to normal rats (p < 0.01). Following LSB and LSC treatment, these high serum levels of TNF-α, IL-6, and IL-8 were remarkably reduced, while low serum level of IL-10 was obviously increased (p < 0.01 or p < 0.05). Thus, LSB and LSC can suppressed and palliated the symptoms of inflammatory response in a dose-dependent manner in TBSM rats.
Figure 7.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on tumor necrosis factor alpha (TNF-α, A), interleukin-6 (IL-6, B), IL-8 (C) and IL-10 (D) levels in rats. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.4. LSB and LSC possessed diuretic effects
3.4.1. LSB and LSC increased urinary excretion volume
As illustrated in Figure 8A, the urinary output volumes increased in model rats compared to normal rats, but the increase did not reach statistical significance (p > 0.05). However, oral administration of LSB and LSC prominently increased urinary excretion volumes from day 1 to day 6 compared to model rats (p < 0.01 or p < 0.05). Therefore, LSB and LSC exhibited vigorous diuretic effects in TBSM rats.
Figure 8.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on urine output (A) and urine pH (B) in rats. &p < 0.05 and &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.4.2. LSB and LSC did not affect urinary pH
Treatment with LSB and LSC did not alter urinary pH values from day 1 to day 6 compared to the model group (Figure 8B, p > 0.05).
3.4.3. LSB and LSC minor affect the urinary electrolyte concentrations of K+ and Ca2+
To determine whether LSB and LSC affect urinary electrolyte balance, urinary concentrations of Na+, K+, Cl−, and Ca2+ were measured for 6 consecutive days. As depicted in Figure 9, LSB and LSC did not cause Na+ and Cl− concentrations, and had certain effects on K+ and Ca2+ concentrations compared to the model group.
Figure 9.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on the concentrations of Na+ (A), K+ (B), Cl− (C) and Ca2+ (D) in rats. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.4.4. LSB and LSC suppressed RAAS and up-regulated atriopeptin release
To explore whether LSB and LSC are involved in the renin-angiotensin-aldosterone system (RAAS), serum levels of angiotensin II, anti-diuretic hormone, and aldosterone, together with atriopeptin level in rats were analyzed. As illustrated in Figure 10, compared with the control group, the levels of angiotensin II, anti-diuretic hormone and aldosterone in the model group were significantly increased (p < 0.01). By contrast, these indexes were prominently down-regulated after treatment with LSB and LSC (p < 0.01 or p < 0.05). Meanwhile, the high doses of LSB and LSC remarkably up-regulated the level of atriopeptin compared with that in the model group (p < 0.01). The results indicated that LSB and LSC had dose-dependent effects on suppressing RAAS and up-regulating atriopeptin release in TBSM rats.
Figure 10.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on angiotensin II (A), anti-diuretic hormone (B), aldosterone (C) and atriopeptin (D) levels in rats. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.4.5. LSB and LSC reduced serum levels of AQPs-1, 2 and 3
To investigate whether LSB and LSC play roles in the excretion of AQPs-1, 2, and 3, the levels of these parameters were measured in the serum of rats. As showed in Figure 11, these parameters were remarkably enhanced in the model group compared to the control group (p < 0.01), and prominently down-regulated after treatment with LSB and LSC in a dose-dependent behavior (p < 0.01 or p < 0.05). Based on these results, we hypothesized that LSB and LSC can target AQPs proteins and lead to increase urinary excretion volume in model rats. Moreover, this hypothesis will need to be further verified by Western blot analysis in subsequent experiments.
Figure 11.
Effects of LSB and LSC (H and L represent high and low doses, respectively) on aquaporin 1 (AQP1, A), AQP2 (B) and AQP3 (C) levels in rats. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
3.4.6. LSB and LSC suppressed protein expressions of AQPs-1, 2 and 3
To further evaluate the effects of AQPs protein expression by oral administration of LSB and LSC in model rats, the protein expression of AQPs-1, 2, and 3 was also determined by Western blot analysis in the present study. Other than that, LSB and LSC also suppressed the protein expression of AQPs-1, 2, and 3 in a dose-dependent relationship in model rats (p < 0.01, Figure 12).
Figure 12.
LSB and LSC (H and L represent high and low doses, respectively) decreased the protein expression of aquaporins (AQPs)-1, 2 and 3 of injured muscle tissue in rats were lysed and subjected to immunoblotting with the indicated antibodies. ##p < 0.01 compared with control group, &&p < 0.01 compared with model group. One-way ANOVA followed by Tukey’s test with GraphPad Prism 6.
Summarized above evidences, LSB and LSC exhibited prominent diuretic effects through blocking AQPs and RAAS, as well as up-regulating atriopeptin in TBSM rats.
4. Discussion
According to TCM theory, Huo Xue Li Shui refers to PBCRBS and diuretic effects, and applied to treat blood stasis, edema, and other diseases [1, 5, 6]. In addition, the inflammatory response was positively related to PBCRBS effect [13, 14]. Despite the fact that the theory of Huo Xue Li Shui with PBCRBS, anti-inflammatory, and diuretic effects has been well practiced in TCM and achieved satisfactory clinical outcomes, it has not been adequately reported. For this reason, it is very important to explore the efficacy of Huo Xue Li Shui associated with PBCRBS, anti-inflammatory, and diuretic effects in clinical application.
Lagopsis supina is a well-known TCM with Huo Xue Li Shui, and widely used to treat blood stasis, inflammation, diuresis, and other diseases [1]. Our previous study demonstrated that LSB and LSC fractions from L. supina exhibited significant PBCRBS effect in Dextran 500-induced acute blood stasis model rats [10]. Meanwhile, LSB and LSC also possessed prominent diuretic effects in saline-loaded rats [9, 11]. As a result, the present study used LSB and LSC as a classical demonstration to explore the scientific connotation of Huo Xue Li Shui theory with the PBCRBS, anti-inflammatory, and diuretic effects in TBSM rats, which is a holistic rodent model that can be used to clarify the integrality scientific connotation of Huo Xue Li Shui theory [1]. In addition, male rats were widely chosen for the TBSM experiment [1, 6, 7]. Furthermore, the crude extract of Salvia miltiorrhiza Bunge roots (“Dan-Shen” in chinese) possessed the PBCRBS and diuretic effects in TBSM rats based on the Huo Xue Li Shui theory [6, 7]. Additionally, the application of Huo Xue Li Shui theory in the treatment of ocular diseases using TCMs and their prescription had achieved good clinical effects [15].
Blood stasis is an important clinical manifestation, such as abnormal hemorheology, platelet aggregation, coagulation dysfunction, and abnormality of fibrinolytic system [10]. On the one hand, plasma viscosity and platelet aggregation rate are two essential parameters for evaluating the hemorheological function of PBCRBS effect in rats with blood stasis model [10, 16]. On the other hand, anti-coagulation (prothrombin time, APTT, thrombin time, fibrinogen, and antithrombin III), anti-platelet activation (thromboxane B2 and 6-keto-prostaglandin F1α), and anti-fibrinolysis (t-PA, u-PA, and PAI-1) are three typical pathways tightly involved in PBCRBS [3, 10, 13, 17, 18, 19]. In this study, LSB and LSC possessed prominent PBCRBS effects in TBSM rats, including prominently decreased the contents of prothrombin time, APTT, thrombin time, fibrinogen, thromboxane B2, u-PA, PAI-1, PAI-1/t-PA, and PAI-1/u-PA, while significantly increased AT-III, 6-keto-PGF1α, and t-PA levels when comparted with the model group. These results indicated that LSB and LSC showed PBCRBS effects through anti-coagulation, anti-platelet activation, and anti-fibrinolysis pathways, which are the three mainly underlying mechanism for PBCRBS effect [10].
Inflammation is a momentous and common regulatory process of the host defense system in response to tissue damage, harmful stimuli, physical stress, and other infections [12, 20]. Numerous studies revealed that the main cause of blood stasis is a massive inflammatory response due to obstructed blood circulation [13, 14, 21]. In the present study, LSB and LSC showed anti-inflammatory effect via down-regulated pro-inflammatory cytokines (TNF-α, IL-6, and IL-8) and up-regulated anti-inflammatory cytokine (IL-10) in TBSM rats.
It is well known that increased urinary output is the most intuitive manifestation of the diuretic effect of drugs [8, 9]. Secondly, angiotensin II, anti-diuretic hormone, and aldosterone are the three hormones in RAAS, which is an important system to reduce glomerular filtration rate. Besides, atriopeptin not only promotes urinary excretion, but also suppresses RAAS [11]. And lastly, AQPs-1, 2, and 3 are three intrinsic membrane proteins involved in the roles of urinary reabsorption and excretion [11]. In this work, LSB and LSC prominently possessed diuretic effects comprising reduced the production of angiotensin II, anti-diuretic hormone, aldosterone, AQP1, AQP2, and AQP3, while elevated atriopeptin level, indicated that LSB and LSC exerted diuretic effects through inhibiting AQPs and RAAS pathways and increasing atriopeptin in TBSM rats.
Our previous work demonstrated that phenylpropanoids were main constituents in LSB and LSC [10, 11]. In addition, stachysoside A (0.90 ± 0.01 mg/g L. supina) and acteoside (1.36 ± 0.01 mg/g L. supina), which belong to phenylpropanoids, were the two major phytochemicals in LSC and this plant [10]. Consequently, phenylpropanoids, especially stachysoside A and acteoside, may be responsible for the major bioactive phytochemicals in LSB and LSC with Huo Xue Li Shui effects in TBSM rats. However, further studies should be conducted with the bioactive phytochemicals from LSB and LSC of PBCRBS, anti-inflammatory, and diuretic effects in vivo.
5. Conclusion
In summary, LSB and LSC obtained from L. supina exhibited remarkable PBCRBS, anti-inflammatory, and diuretic effects in TBSM rats, thereby supporting the traditional folk use of this plant. This study successfully elucidated the scientific connotation of Huo Xue Li Shui theory and embodied the principles of its clinical application, and also provided a wonderful demonstration for the holistic evaluation of other TCMs.
Declarations
Author contribution statement
Xiaoyi Xia, Huilei Wang: Performed the experiments; Analyzed and interpreted the data.
Yelin Duan: Performed the experiments.
Li Yang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Junwei He: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Prof. Junwei He was supported by the Subject of Jiangxi Education Department [GJJ201221, China].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.He J.W., Zeng L.B., Wei R.R., Zhong G.Y., Zhu Y.Y., Xu T.T., et al. Lagopsis supina exerts its diuretic effect via inhibition of aquaporin-1, 2 and 3 expression in a rat model of traumatic blood stasis. J. Ethnopharmacol. 2019;231:446–452. doi: 10.1016/j.jep.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Pan M.H., Zhu S.R., Duan W.J., Ma X.H., Luo X., Liu B., et al. Shanghuo” increases disease susceptibility: modern significance of an old TCM theory. J. Ethnopharmacol. 2020;250 doi: 10.1016/j.jep.2019.112491. [DOI] [PubMed] [Google Scholar]
- 3.Yue S.J., Xin L.T., Fan Y.C., Li S.J., Tang Y.P., Duan J.A., et al. Herb pair Danggui-Honghua: mechanisms underlying blood stasis syndrome by system pharmacology approach. Sci. Rep-UK. 2017;7 doi: 10.1038/srep40318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P.L., Su W.W., Yun S., Liao Y.Q., Liu H., Li P.B., et al. Toward a scientific understanding of the effectiveness, material basis and prescription compatibility of a Chinese herbal formula Dan-hong injection. Sci. Rep-UK. 2017;7 doi: 10.1038/srep46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou J.W., Cao L.L., Zhang Q., Jiang D.J., Yao W.F., Bao B.H., et al. The toxicity and efficacy evaluation of different fractions of Kansui frybaked with vinegar on Walker-256 tumor-bearing malignant ascites effusion rats and normal rats. J. Ethnopharmacol. 2018;219:257–268. doi: 10.1016/j.jep.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Dong X.J., Yang Y., Ren T.Y., Xu J.Q., Li F., Chen X.L. Verifying the part nature of “promoting blood circulation and diuresis” by adjusting the AQP1’s characteristic expression with Salvia. J. Emerg. Tradit. Chin. Med. 2013;22:732–735. [Google Scholar]
- 7.Dong X.J., Guo L.F., Yao R., Xue S.Y., Li F. Relationship between regulation effect of salvia miltiorrhiza on AQP2 in kidney and promoting blood circulation and diuresis. China J. Chin. Mater. Med. 2014;39:3162–3165. [PubMed] [Google Scholar]
- 8.Liu Z.Y., Yang L., Li R.X., Wei R.R., Luo X.Q., Xu T.T., et al. Diuretic and anti-diuretic activities of ethanol extract and fractions of Lagopsis supina in normal rats. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/6927374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J.W., Yang L. Diuretic effect of Lagopsis supina fraction in saline-loaded rats is mediated through inhibition of aquaporin and renin-angiotensin-aldosterone systems and up-regulation of atriopeptin. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111554. [DOI] [PubMed] [Google Scholar]
- 10.Yang L., He J.W. Lagopsis supina extract and its fractions exert prophylactic effects against blood stasis in rats via anti-coagulation, anti-platelet activation and anti-fibrinolysis and chemical characterization by UHPLC-qTOF-MS/MS. Biomed. Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110899. [DOI] [PubMed] [Google Scholar]
- 11.Yang L., He Z.W., He J.W. The chemical profiling of aqueous soluble fraction from Lagopsis supina and its diuretic effects via suppression of AQP and RAAS pathways in saline-loaded rats. J. Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113951. [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Liu R.H., Fan A.G., Zhong G.Y., He J.W. Dendropanax dentiger (Harms) Merr. root and its major constituents exert therapeutic effect on adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2021;267 doi: 10.1016/j.jep.2020.113631. [DOI] [PubMed] [Google Scholar]
- 13.Dang X., Miao J.J., Chen A.Q., Li P., Chen L., Liang J.R. The antithrombotic effect of RSNK in blood-stasis model rats. J. Ethnopharmacol. 2015;173:266–272. doi: 10.1016/j.jep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Lv M., Wang T.Y., Tian X.X., Shi X.H., Fan G.W., Zhang Y., et al. Interaction of anti-thrombotic and anti-inflammatory activities of commonly used traditional Chinese medicine for promoting blood circulation and removing blood stasis revealed by network pharmacology analysis. Acta. Pharmaceut. 2015;50:1135–1141. [PubMed] [Google Scholar]
- 15.Zheng Z.C., Peng J., Tan H.Y., Yao X.L., Peng Q.H. Clinical research of activating blood and diuresis for ocular diseases in Chinese medicine. J. TCM. Univ. Hunan. 2010;30:74‒78. [Google Scholar]
- 16.Liu L., Duan J.A., Tang Y.P., Guo J.M., Yang N.Y., Ma H.Y., et al. Taoren–Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J. Ethnopharmacol. 2012;139:381–387. doi: 10.1016/j.jep.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Cao D., Xu C.C., Xue Y.Y., Ruan Q.F., Yang B., Liu Z.Q., et al. The therapeutic effect of Ilex pubescens extract on blood stasis model rats according to serum metabolomics. J. Ethnopharmacol. 2018;227:18–28. doi: 10.1016/j.jep.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Gong P.Y., Tian Y.S., Guo Y.J., Gu L.F., Li J.Y., Qi J., et al. Comparisons of antithrombosis, hematopoietic effects and chemical profiles of dried and rice wine-processed Rehmanniae Radix extracts. J. Ethnopharmacol. 2019;231:394–402. doi: 10.1016/j.jep.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J.J., Song Z.H., Han M.S., Yu B.X., Lv G.H., Han N., et al. Evaluation of the antithrombotic activity of Zhi-Xiong capsules, a traditional Chinese medicinal formula, via the pathway of anti-coagulation, anti-platelet activation and anti-fibrinolysis. Biomed. Pharmacother. 2018;97:1622–1631. doi: 10.1016/j.biopha.2017.11.135. [DOI] [PubMed] [Google Scholar]
- 20.Yang L., Liu R.H., Fang Y.W., He J.W. Anti-inflammatory effect of phenylpropanoids from Dendropanax dentiger in TNF-α-induced MH7A cells via inhibition of NF-κB, Akt and JNK signaling pathways. Int. Immunopharm. 2021;94 doi: 10.1016/j.intimp.2021.107463. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X.S., Li P.L., Hua Y.L., Ji P., Yao W.L., Ma Q., et al. Urinary metabolomics study the mechanism of Taohong Siwu Decoction intervention in acute blood stasis model rats based on liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Chromatogr. B. 2018;1074:51–60. doi: 10.1016/j.jchromb.2017.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.