Abstract
Background
Labour dystocia is associated with a number of adverse maternal and neonatal outcomes. Augmentation of labour is a commonly used intervention in cases of labour dystocia. Misoprostol is an inexpensive and stable prostaglandin E1 analogue that can be administered orally, vaginally, sublingually or rectally. Misoprostol has proven to be effective at stimulating uterine contractions although it can have serious, and even life‐threatening side‐effects. Titration refers to the process of adjusting the dose, frequency, or both, of a medication on the basis of frequent review to achieve optimal outcomes. Studies have reported on a range of misoprostol titration regimens used for labour induction and titrated misoprostol may potentially be effective and safe for augmentation of labour.
Objectives
To examine the effects and safety of titrated oral misoprostol compared with placebo, oxytocin, other interventions, or no active treatment, in women with labour dystocia.
Search methods
The Trials Search Co‐ordinator of the Cochrane Pregnancy and Childbirth Group searched the Cochrane Pregnancy and Childbirth Group’s Trials Register; date of search: 29 May 2013. We also searched the reference lists of retrieved studies
Selection criteria
Randomised trials (including quasi‐randomised and cluster‐randomised trials) comparing titrated oral misoprostol with placebo, other interventions (e.g. oxytocin, other prostaglandins), or no treatment in women requiring augmentation of labour were eligible for inclusion.
Data collection and analysis
Two review authors independently assessed eligibility for inclusion, carried out data extraction and assessed risk of bias in included studies. Data were entered by one author and checked for accuracy.
Main results
We included two randomised trials with a total of 581 women each comparing different regimens of titrated oral misoprostol with intravenous oxytocin. One study compared 20 mcg doses of misoprostol dissolved in water (repeated every hour up to four hours, after which the dose was increased to 40 mcg per hour up to a maximum total dose of 1600 mcg), while the second study gave women 75 mcg doses (repeated after four hours provided there were no adverse effects observed).
Neither trial reported maternal death, severe maternal morbidity, or fetal/neonatal mortality outcomes, and only a few fetal/neonatal morbidity outcomes were considered, none of which were significantly different between groups. For several outcomes (such as maternal side‐effects, instrumental birth, maternal blood transfusion for hypovolaemia and epidural analgesia), the number of events was generally too low for sufficient statistical power to be achieved. Maternal satisfaction was not reported in either trial. One trial reported a slight reduction in the median duration of labour from the start of augmentation to vaginal delivery in the oxytocin group.
Neither trial reported significantly higher rates of caesarean section (CS) in the oral misoprostol group. Rates of vaginal delivery within 12 and 24 hours of commencing augmentation were not significantly different in the trial using a 20 mcg misoprostol dose. Neither trial had significantly higher rates of uterine hyperstimulation with fetal heart rate changes in the titrated oral misoprostol group. However, the rates of this outcome varied so greatly between the two studies as to suggest that other factors were at play. The only significant differences between groups related to uterine hyperstimulation (without fetal heart rate changes), and results were not consistent in the two trials. In the trial examining the higher dose of misoprostol, more women in the misoprostol group experienced hyperstimulation of labour measured over a 10‐minute period compared with those receiving oxytocin (risk ratio (RR) 1.17, 95% confidence interval (CI) 1.02 to 1.35, 350 women). In the study examining the lower titrated dose of misoprostol, there was a lower incidence of tachysystole when labour was augmented with titrated oral misoprostol than with oxytocin (RR 0.39, 95% CI 0.17 to 0.91, 231 women) with no occurrences of hypertonus in either group of women.
Authors' conclusions
Important uncertainties still exist on the safety and acceptability of titrated oral misoprostol compared with intravenous oxytocin regimens in women with dystocia following spontaneous onset of labour. Although in facilities where electronic oxytocin infusion is not available, low‐dose titrated misoprostol may offer a better alternative to an uncontrolled oxytocin infusion to avoid hyperstimulation. Further research is needed in both high‐ and low‐resource settings More trials should be conducted to evaluate the effect of a standard titration oral misoprostol regimen, both following spontaneous labour and labour induction. Comparisons with other augmentation methods are also warranted, as are any effects on women's birth experiences.
Keywords: Female; Humans; Pregnancy; Administration, Oral; Cervical Ripening; Cervical Ripening/drug effects; Cervical Ripening/physiology; Delivery, Obstetric; Delivery, Obstetric/statistics & numerical data; Injections, Intravenous; Misoprostol; Misoprostol/administration & dosage; Misoprostol/adverse effects; Oxytocics; Oxytocics/administration & dosage; Oxytocics/adverse effects; Oxytocin; Oxytocin/administration & dosage; Oxytocin/adverse effects; Pregnancy Outcome; Randomized Controlled Trials as Topic
Plain language summary
Adjusted doses of oral misoprostol for treating slow progress in labour
Abnormally slow progress in labour (labour dystocia) may lead to serious complications including death for women and their babies. Drugs to increase the frequency and strength of contractions have often been used in such births. Misoprostol is an inexpensive and stable drug that stimulates uterine contractions, but it can have serious and even life‐threatening side‐effects and so the dose has to be carefully adjusted. Titration refers to the process of adjusting the dosing of a medication on the basis of frequent monitoring to achieve the best outcomes. Titrated misoprostol could be effective in treating delayed progress in labour and as an alternative to oxytocin which is harder to store and is given intravenously by infusion.
We identified two randomised controlled trials with 581 women requiring augmentation, each looking at different doses of oral misoprostol compared with oxytocin. One study gave 20 mcg doses of misoprostol every hour up to four hours, after which the dose was increased; the second gave women 75 mcg doses, repeated after four hours provided there were no adverse effects observed. Neither trial reported on the important safety outcomes of maternal or neonatal deaths, or severe maternal ill health.
One trial measured duration of labour from the start of augmentation, which was slightly shorter with intravenous oxytocin (5.20 hours compared with 5.22 hours). The number of vaginal deliveries within 12 and 24 hours, and caesarean section rates were similar. Neither trial reported clearly higher rates of uterine hyperstimulation with worrying fetal heart rate changes in the titrated oral misoprostol group. The rates of this outcome in the two studies varied greatly however. The evidence on uterine hyperstimulation without fetal heart rate changes was not consistent.
The number of women reporting nausea, vomiting, shivering and pyrexia was low with both misoprostol and oxytocin. Maternal satisfaction was not reported in either trial.
Important uncertainties still exist on the safety and acceptability of titrated oral misoprostol for labour dystocia, and further research is needed before it can be recommended as an alternative to oxytocin. However, in facilities that do not have access to electronic oxytocin infusion, lower doses of titrated misoprostol may be a better alternative to avoid hyperstimulation.
Background
Description of the condition
Labour dystocia (or difficult labour) is characterised by abnormally slow progress of labour. It can be due to one or more causes, including abnormalities of uterine contractility or the maternal bony pelvis and soft tissues, as well as abnormal fetal presentation, position or development (Neilson 2003). A slow labour does not necessitate a diagnosis of labour dystocia.
Labour dystocia is associated with a number of adverse maternal and neonatal outcomes. It can result in a traumatic birth experience (Nystedt 2005), and may lead to fetal distress requiring operative birth, either by emergency caesarean section (CS) or vaginal instrumental birth (Neilson 2003; Selin 2008). It may result in perinatal or maternal morbidity or mortality (Kjaergaard 2009), especially in settings where emergency CS is not widely available (Buchmann 2006; McClure 2006). There is no universally accepted definition of labour dystocia and criteria often vary between studies; however, studies in high‐resource countries report a high incidence of births complicated by dystocia (e.g. 37% in a sample of 2810 healthy, term nulliparas with no indication for induction or elective CS (Kjaergaard 2008), and 21% in a sample of 1480 deliveries (Selin 2008)).
Of the 3.2 million stillbirths that occur annually, 32% (1.02 million) are intrapartum (occur during labour or delivery) (Yakoob 2010), and intrapartum complications, such as malpresentation or obstructed labour, are by far the most predictive factors for perinatal mortality, and may increase the risk for perinatal death by up to a factor of 85 in low‐ and middle‐ income countries (Lawn 2009). McClure 2006 et al reported that in six countries with low availability of CS, the most important cause of stillbirth was obstructed or prolonged labour with associated asphyxia, infection and birth injury.
Inadequate uterine contractions, fetal malposition, or cephalopelvic disproportion (baby's head/body too large to fit through pelvis) may cause labour dystocia (Shields 2007). Nulliparous women have slower labour progression than multiparous women (Vahratian 2006), and those who are overweight experience a slower first and second stage of labour compared with women of normal weight (Vahratian 2004). Other factors associated with increased risk of prolonged labour include advanced maternal age (Kalogiannidis 2011; Sheiner 2002), especially older primagravidas (Kalogiannidis 2011); multiparity with no previous vaginal birth; gestational age over 42 weeks (Selin 2008); obesity (Vahratian 2004); epidural analgesia (Kjaergaard 2008; O'Hana 2008; Selin 2008; Sheiner 2002); female circumcision (Chibber 2011); vitamin D deficiency (Scholl 2012); infertility treatment; induction of labour; prelabour rupture of membranes; hypertensive disorders; hydramnios (too much amniotic fluid); gestational diabetes (Sheiner 2002); early admission to a labour unit (Kjaergaard 2008); macrosomia (large baby) (Hogberg 2000; Selin 2008); occipitoposterior (back‐to‐back) position (Shields 2007); short maternal stature (Piper 1991; Sheiner 2007); and high levels of stress hormones in the mother (Lederman 1978).
Description of the intervention
Augmentation of labour is a commonly used intervention globally in cases of labour dystocia; Lovold 2008 identified augmentation rates of 2.5% to 32.9% in provinces of 11 lower‐income countries. In high‐income settings, labour dystocia is generally managed using a titrated intravenous oxytocin regimen via a regulatory infusion pump with continuous fetal monitoring (Lovold 2008). However, many facilities in low‐income settings face significant hurdles in the availability of appropriately trained staff, adequate patient supervision, fetal monitoring and the availability and use of clinical practice guidelines (Simpson 2003). The quality of oxytocin may be poor (Stanton 2012), or may be administered without the aid of infusion pumps or fetal monitoring (Lovold 2008). Oxytocin misuse increases the risk of ruptured uterus, fetal asphyxia and fetal death (Konje 1990; Lovold 2008).
Misoprostol is an inexpensive and stable prostaglandin E1 analogue that can be administered orally, vaginally, sublingually or rectally (Weeks 2005). It has proven to be effective at stimulating uterine contractions (Norman 1991), even at low doses (Kundodyiwa 2009). As a prostaglandin analogue, misoprostol has ubiquitous effects on many organ systems (Hofmeyr 2011). The side‐effects of misoprostol include pyrexia (fever) and shivering, hyperpyrexia (which may be life‐threatening), decrease in heart rate, uterine hyperstimulation and uterine rupture (Hofmeyr 2010; Hofmeyr 2013; Tang 2007).
While originally marketed for the prevention and treatment of gastric ulcers, misoprostol has a wide range of obstetric applications (Blanchard 2002). The low cost, heat stability, ease of administration and storage of misoprostol are advantageous in many low‐resource settings over other oxytocics (Blanchard 2002). The obstetric applications of misoprostol include induction and augmentation of labour, prevention and treatment of postpartum haemorrhage, evacuation of the uterus after pregnancy failure and induced abortion (Hofmeyr 2011; Weeks 2007). Despite this, only a small number of countries have licensed misoprostol for obstetric use (Weeks 2005). There is no guidance provided by manufacturers regarding safe and appropriate dosage in obstetric situations (Friedman 2001).
Administered orally or vaginally, misoprostol is effective for the induction of labour (Margulies 1992), though it poses certain risks (Hofmeyr 1999; Kundodyiwa 2009). A Cochrane review of misoprostol use for cervical ripening and labour induction concluded that it is more effective than conventional methods (Hofmeyr 2010); however, limitations in the evidence mean that the possibility of uncommon serious adverse effects cannot be excluded. Specifically, the studies reviewed were not large enough to conclude whether misoprostol use could cause an unacceptably high number of serious adverse events including uterine rupture and asphyxial fetal deaths. Oral administration of misoprostol has been shown to have similar safety and efficacy to vaginally‐administered misoprostol for induction of labour (Abbassi 2008; Uludag 2005), but oral misoprostol performed better in terms of treatment interval and number of doses required (Abbassi 2008), and is preferred by women (Nassar 2007).
Titration refers to the process of adjusting the dose, frequency ‐ or both ‐ of a medication on the basis of frequent review to achieve optimal outcomes. Titration regimens have the advantage of potentially avoiding adverse outcomes, by using smaller doses, and dosing only as required. Titration of oral misoprostol for labour induction using a misoprostol solution was a pioneered by Hofmeyr and colleagues (Hofmeyr 2001a), and several studies have reported on a range of misoprostol titration regimens for labour induction (Bricker 2008; Cheng 2006; Cheng 2008; Cheng 2010; Ho 2010; Matonhodze 2003; Souza 2010; Thaisomboon 2012; Zvandasara 2008). Given the efficacy of oral misoprostol for augmentation of labour (Bleich 2011), efforts have been made to identify a safe and effective titration regimen for using misoprostol for this purpose (Ho 2010; Villano 2011).
Why it is important to do this review
There is already evidence about other pharmacological and non‐pharmacological methods of augmenting labour. Methods vary in effectiveness, and some methods may be more appropriate for use in high‐ or low‐resource settings, either because of cost, availability or resources. As an inexpensive drug that does not need refrigeration and can be administered orally, misoprostol may offer an alternative to other commonly‐used augmentation agents. Titration regimens using oral misoprostol have now been examined in trials, so this review fills a gap in the evidence.
Objectives
To examine the effects and safety of titrated oral misoprostol compared with placebo, oxytocin, other interventions for augmentation of labour, or no active treatment, in women with dystocia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials (including quasi‐randomised trials where allocation is not truly random, for example, alternative allocation).
We planned to include trials with either individual‐ or cluster‐randomisation. In this version of the review we did not identify any cluster‐randomised trials. We planned to include studies reported in published abstracts provided that there was sufficient information to assess risk of bias, and results were reported by randomisation group.
Types of participants
Women in labour with dystocia requiring labour augmentation (as defined by trialists). We included trials including nulliparous or multiparous women, or both. We did not plan to exclude trials that included women with pregnancy complications recruited as part of broader populations, but we have not included trials focusing specifically on high‐risk groups.
Types of interventions
We planned to include trials comparing titrated oral misoprostol with:
placebo;
other pharmacological or non‐pharmacological interventions (e.g. oxytocin, other prostaglandins);
no treatment.
If data were available, we also planned to compare titrated oral misoprostol with other regimens of misoprostol but no such trials were identified in this version of the review. If, in future updates, we identify trials where titrated misoprostol is used with other interventions (e.g. amniotomy), including induction of labour regimens, we will include the trial, provided women in both the intervention and control arms received the same co‐intervention.
Types of outcome measures
Primary outcomes
Maternal death or severe morbidity (e.g. ruptured uterus, admission to intensive care unit, septicaemia, organ failure).
Uterine hyperstimulation with fetal heart rate (FHR) changes.
Perinatal death (stillbirth and neonatal death).
Secondary outcomes
Maternal
Maternal mortality.
Serious maternal morbidity (e.g. ruptured uterus, admission to intensive care unit, septicaemia, organ failure).
Duration of labour.
Caesarean section.
Caesarean section for fetal distress.
Instrumental birth.
Caesarean section for prolonged labour.
Duration of first stage of labour.
Duration of second stage of labour.
Postpartum haemorrhage (as defined by trialists).
Hyperstimulation of labour.
Diagnosis of chorioamnionitis (inflammation of the fetal membranes).
Episiotomy.
2nd or 3rd degree perineal tear.
Breastfeeding.
Maternal satisfaction with care in labour.
Postnatal depression.
Vaginal birth not achieved within 24 hours of onset of labour.
Fetal/neonatal
Fetal intrapartum death.
Early neonatal death.
Birth trauma (e.g. Erb's palsy, fractured skull, cephalhaematoma, fractured clavicle).
pH of arterial cord blood less than 7.0.
Neonatal morbidity, excluding malformations (e.g. seizures, birth asphyxia, neonatal encephalopathy, infection requiring antibiotics).
Need for intubation at birth.
Childhood disability.
Apgar score less than seven at five minutes.
Admission to neonatal intensive care unit (NICU).
Duration of hospital stay.
Search methods for identification of studies
Electronic searches
We asked the Trials Search Co‐ordinator of the Cochrane Pregnancy and Childbirth Group to search the Cochrane Pregnancy and Childbirth Group’s Trials Register. Date of search: 29 May 2013.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
In addition, we searched the reference lists of reports identified by the search.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors assessed eligibility for inclusion by independently examining all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
We created a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2012), and checked for accuracy.
If information regarding any of the above was unclear, we planned to contact authors of the original reports to request that they provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we describe the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we describe the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we describe the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study and each outcome or class of outcomes, we describe the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we planned to re‐include missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we describe how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; or study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study, we describe any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias and assess each as:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we present the mean difference with 95% confidence intervals. In this version of the review, we have not pooled any findings from trials but in future updates we will use the mean difference to combine findings from trials provided outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods to do so.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials in this version of the review. In future updates, if such trials are identified, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Higgins 2011), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over studies were not eligible for inclusion; this is not a suitable design for this type of intervention in labour.
Other unit of analysis issues
If we had identified any trials with more than two arms (multi‐arm trials), we planned to include as many data as are relevant to the scope of the review. If such trials are identified when we update the review, where appropriate, we will combine arms (using the methods described in the Handbook (Higgins 2011)) to create a single pair‐wise comparison. If it is not appropriate to combine experimental arms, we will present results separately for each arm, sharing results for the control arm between each to avoid double counting (for dichotomous outcomes we will divide the number of events and total sample by two; for continuous outcomes we will assume the same mean and standard deviation but halve the control sample size for each comparison).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis; in this version of the review we did not carry out any meta‐analysis and only two trials were included so we did not carry out this planned sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
If we had combined trials in the meta‐analysis, we planned to assess statistical heterogeneity using the T², I² and Chi² statistics. If we carry out meta‐analysis in updates, we will regard heterogeneity as substantial if an I² is greater than 30% and either the T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If in future updates there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2012). If in future updates we carry out any meta‐analysis we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged to be sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary where an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects, and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If in future updates we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful and, if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
by parity (primiparous versus multiparous);
by dose (low‐dose versus higher‐dose titration);
by whether titrated oral misoprostol is used as part of a labour induction regimen versus women in spontaneous labour.
We will examine only our primary outcomes in subgroup analysis.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
If we had included cluster‐randomised trials in the review, we planned to carry out sensitivity analysis. We also planned sensitivity analysis according to trial quality; temporarily excluding trials at high risk of bias due to inadequate allocation concealment to explore whether this has any impact on the direction or size of the effect estimate. In this version of the review we did not carry out any sensitivity analysis as insufficient data were available and we did not carry out meta‐analysis. If more data are included in updates, we will carry out planned sensitivity analysis using our primary outcomes only.
Results
Description of studies
Results of the search
The search strategy identified four reports for possible inclusion in the review. We examined the reference lists of these four reports and did not identify any further studies for inclusion. After assessing eligibility, we included two trials and excluded one. One report was a trial registration for an ongoing study (Aalami‐Harandi 2009) and we have contacted the authors for more information. Details of all trials identified by the search are set out in tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies).
Included studies
Two trials examined oral misoprostol to augment labour.
In a trial carried out in Taiwan between 2008 and 2009, 231 women were randomised to receive either titrated oral misoprostol or usual care (intravenous (IV) oxytocin) according to the hospital protocol (Ho 2010). Women recruited to the trial were in spontaneous labour with inadequate uterine contractions requiring augmentation. The misoprostol was prepared by dissolving a 200 microgram (mcg) tablet in tap water. Women in the misoprostol group were randomised to receive an initial dose of 20 mcg of misoprostol (in 20 mL of water) repeated every hour up to four hours, after which the dose was increased to 40 mcg per hour up to a maximum total dose of 1600 mcg. Women in both arms of the trial were monitored for signs of fetal distress or uterine hyperstimulation. All but three of the women randomised received the intended treatment. The primary outcomes in this study were duration of labour from the start of augmentation, vaginal delivery within 12 and 24 hours, incidence of tachysystole, uterine hyperstimulation, and non‐reassuring fetal heart rate.
In the second trial, carried out in the USA between 2008 and 2011, 350 mainly Hispanic women were recruited (Bleich 2011). All women were in spontaneous labour with ruptured membranes requiring labour augmentation for slow progress. Women were randomised to receive either oral misoprostol or usual care (IV oxytocin). In this study the dose was not strictly titrated as in the Ho 2010 trial, however, in view of the paucity of data on oral misoprostol for augmentation, we decided to include results from this trial in the review. In the Bleich 2011 study, lower doses of misoprostol were achieved by cutting up 100 mcg tablets. Women were initially given 75 mcg of oral misoprostol which was repeated after four hours provided no adverse effects were observed. Primary outcomes in this trial were incidence of tachysystole, hypertonus, or both in a 10‐minute period, and women in both arms of the trial were closely monitored for signs of fetal distress or uterine hyperstimulation. There was considerable deviation from the protocol in this study although there was both intention‐to‐treat and as‐treated analyses for some outcomes (in the misoprostol group 136/176 (77.3%) received the study drug while in the control group 143/174 (82.2%) women received the control drug as intended).
Excluded studies
One study identified by the search was excluded (Patel 2000). The reason for exclusion was that in this trial IV oxytocin was compared with vaginal (rather than oral) misoprostol.
Risk of bias in included studies
Please see Figure 1.
1.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both included studies were assessed as being at low risk of bias for random sequence generation and allocation concealment. Both studies used computer‐generated randomisation sequences and allocations were concealed in opaque envelopes.
Blinding
Blinding of women, staff and outcome assessors was not attempted in either study and this may have introduced bias. The impact of lack of blinding on most of the outcomes measured was not clear. It is possible that lack of blinding may have led to changes in subsequent clinical assessments and decision‐making which may have affected some outcomes.
Incomplete outcome data
One of the studies did not appear to have serious sample attrition (Ho 2010). In the study by Bleich 2011, there was some deviation from protocol which meant that results were more difficult to interpret: 20.30% of study participants overall (22.70% in experimental group and 17.80% in control group) did not receive study drugs and, while reasons were reported, the authors did not explain why the proportion attributable to progression to 8 cm prior to initiation of augmentation differed between groups (18/40 women in experimental group and 30/31 in control group). It was also unclear how many women randomised to the misoprostol arm eventually received oxytocin due to drug failure, as per study protocol.
Selective reporting
Both studies were assessed as unclear for outcome reporting bias.
Other potential sources of bias
Neither study had other obvious sources of bias. We have set out summaries of our 'Risk of bias' decisions in Figure 1.
Effects of interventions
Titrated oral misoprostol versus intravenous oxytocin (2 studies with 581 women)
The studies in the review compared different regimens of titrated oral misoprostol with intravenous oxytocin. Ho 2010 used 20 microgram (mcg) doses of misoprostol (repeated every hour up to four hours, after which the dose was increased to 40 mcg per hour up to a maximum total dose of 1600 mcg), while Bleich 2011 gave women 75 mcg doses (repeated after four hours provided there were no adverse effects observed).
As the two trials examined different interventions, we did not pool results in meta‐analyses.
Primary outcomes
Maternal outcomes
Maternal death or severe morbidity (e.g. ruptured uterus, admission to intensive care unit, septicaemia, organ failure)
This outcome was not reported in either of the included trials.
Uterine hyperstimulation with fetal heart rate (FHR) changes
Both studies reported hyperstimulation with changes in FHR; neither trial showed clear differences between groups (Ho 2010 (20 mcg dose): risk ratio (RR) 0.96, 95% confidence interval (CI) 0.14 to 6.68, 231 women, Analysis 1.1; Bleich 2011 (75 mcg dose): RR 1.26, 95% CI 0.88 to1.80, 350 women Analysis 2.1).
1.1. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 1 Uterine hyperstimulation with non‐reassuring heart rate changes.
2.1. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 1 Uterine hyperstimulation (tachysystole, hypertonus or both) associated with fetal heart changes.
Overall, the rates of uterine hyperstimulation with FHR changes were very different in the two studies. A much higher proportion of women in the study carried out by Bleich 2011 experienced hyperstimulation with FHR changes (29% of women receiving misoprostol and 23% of women receiving oxytocin) than those enrolled in Ho 2010 (1.70% of women receiving misoprostol and 1.80% of women receiving oxytocin). These differences in background rates of hyperstimulation suggest that factors other than the dosing regimen differed between studies, such as the measurement of this outcome or other aspects of women's care.
Neonatal/fetal outcomes
Perinatal death (stillbirth and neonatal death)
This outcome was not reported in either of the included trials.
Secondary outcomes
Maternal outcomes
Maternal mortality, or serious maternal morbidity (e.g. ruptured uterus, admission to intensive care unit, septicaemia, organ failure)
These outcomes were not reported in either of the included trials.
Duration of labour
This outcome was not reported in either of the included trials in a way that allowed us to enter data into data and analyses tables. Ho 2010 reported the median and interquartile range (IQR) for the duration of labour from augmentation to vaginal delivery; the median time to delivery was 5.22 hours (IQR 3.77 to 8.58) in the misoprostol group compared with 5.20 hours (IQR 3.23 to 6.50) in the oxytocin group (P = 0.019) (data not shown). Bleich 2011 reported no significant difference in the median time between start of augmentation and delivery (median 306 minutes, IQR 252 to 408 in the misoprostol group versus 276 minutes IQR 162 to 462 in the oxytocin group, P = 0.29 (data not shown)).
Caesarean section (CS)
Both studies reported this outcome, and neither study showed any significant difference for rates of CS for women who had experienced augmentation with titrated oral misoprostol versus oxytocin (Ho 2010 (20 mcg dose): RR 0.88, 95% CI 0.42 to 1.85, 231 women, Analysis 1.3; Bleich 2011 (75 mcg dose): RR 1.04, 95% CI 0.57 to 1.92, 350 women, Analysis 2.3). Bleich 2011 also provided data for CS for non‐reassuring FHR and prolonged labour; the difference between groups was not statistically significant for either indication (CS for fetal distress: RR 1.58, 95% CI 0.53 to 4.74, 350 women, Analysis 2.6; CS for prolonged labour: RR 0.84, 95% CI 0.39 to 1.82, 350 women, Analysis 2.8).
1.3. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 3 Caesarean section.
2.3. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 3 Caesarean section.
2.6. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 6 Caesarean for non‐reassuring fetal heart rate (i.e. fetal distress).
2.8. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 8 Caesarean section for dystocia (i.e. prolonged labour).
Instrumental birth
Bleich 2011 reported the number of forceps deliveries in the two groups; overall, the number of events was low and the difference between groups was not significant (RR 1.98, 95% CI 0.37 to 10.66, 350 women, Analysis 2.7).
2.7. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 7 Forceps delivery.
Duration of first and second stages of labour
These outcomes were not reported in either of the included trials.
Postpartum haemorrhage (as defined by trialists)
This outcome was not reported in either of the included trials.
Hyperstimulation of labour
Results from the two studies regarding uterine hyperstimulation without FHR changes were measured in different ways, and findings were not consistent. Rates of tachysystole and hypertonus were reported by Ho 2010, who found a significantly lower incidence of tachysystole when labour was augmented with titrated oral misoprostol than with oxytocin (RR 0.39, 95% CI 0.17 to 0.91, 231 women, Analysis 1.4), and recorded no occurrences of hypertonus in either group of women (Analysis 1.5). On the other hand, Bleich 2011 reported the incidence of uterine tachysystole, hypertonus, or both, in a 10‐minute period (hyperstimulation of labour) and more women whose labours had been augmented with titrated oral misoprostol experienced this outcome (RR 1.17, 95% CI 1.02 to 1.35, 350 women, Analysis 2.9). However, Bleich 2011 also reported uterine tachysystole in a 20‐minute interval and for this outcome the difference between groups was not statistically significant (RR 1.21, 95% CI 0.84 to 1.72, 350 women, Analysis 2.10)
1.4. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 4 Incidence of tachysystole.
1.5. Analysis.
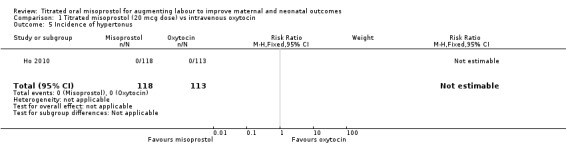
Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 5 Incidence of hypertonus.
2.9. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 9 Uterine tachysystole, hypertonus, or both in 10‐minute period (hyperstimulation of labour).
2.10. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 10 Uterine tachysystole in a 20‐minute interval.
Diagnosis of chorioamnionitis
Bleich 2011 reported the number of women with a diagnosis of chorioamnionitis; the difference between groups was not statistically significant (RR 0.87, 95% CI 0.55 to 1.37, 350 women, Analysis 2.11).
2.11. Analysis.
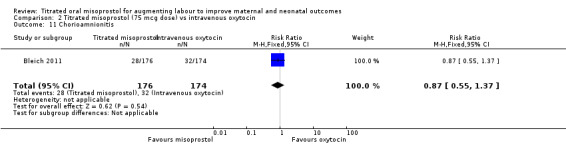
Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 11 Chorioamnionitis.
Other secondary outcomes
Several of our secondary maternal outcomes were not reported in either trial (episiotomy, perineal trauma, breastfeeding, maternal satisfaction with care in labour, vaginal birth not achieved in 24 hours and postnatal depression).
Ho 2010 reported vaginal delivery within 12 hours of commencement of augmentation (RR 0.91, 95% CI 0.80 to 1.03, 231 women, Analysis 1.6) and vaginal delivery within 24 hours of commencement of augmentation (RR 1.02, 95% CI 0.93 to 1.11, 231 women, Analysis 1.7). Neither of these outcomes showed a statistically significant difference between the two interventions.
1.6. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 6 Vaginal delivery within 12 hours of commencement of augmentation.
1.7. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 7 Vaginal delivery within 24 hours of commencement of augmentation.
Neonatal/Fetal outcomes
Most of our pre‐specified neonatal outcomes were not reported in either trial (fetal intrapartum death; early neonatal death; birth trauma (e.g. Erb's palsy, fractured skull, cephalhaematoma, fractured clavicle); neonatal morbidity, excluding malformations (e.g. seizures, birth asphyxia, neonatal encephalopathy, infection requiring antibiotics); need for intubation at birth; duration of infant hospital stay; or childhood disability).
pH of arterial cord blood less than 7.0
Bleich 2011 reported umbilical cord artery pH less than 7.1, however, the number of events was low in both groups, and the difference was not statistically significant (RR 0.74, 95% CI 0.17 to 3.26, data for 350 infants, Analysis 2.12).
2.12. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 12 Umbilical cord artery pH < 7.1.
Apgar score less than seven at five minutes
Ho 2010 recorded no events for this outcome in either group (Analysis 1.8).
1.8. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 8 Apgar < 7 at 5 minutes.
Admission to neonatal intensive care unit (NICU)
Bleich 2011 and Ho 2010 both reported admissions to NICU; the differences between groups were not statistically significant in either study (Bleich 2011: RR 2.97, 95% CI 0.12 to 72.31, data for 350 infants, Analysis 2.13; Ho 2010: RR 2.39, 95% CI 0.47 to 12.09, data for 231 infants, Analysis 1.9). Few babies were admitted to NICUs in these studies (seven out of 231 in Ho 2010, one out of 350 in Bleich 2011), so these studies did not have sufficient power to demonstrate differences for this outcome.
2.13. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 13 Admission to neonatal intensive care unit (NICU).
1.9. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 9 Admission to neonatal intensive care unit (NICU).
Non pre‐specified outcomes
Rate of failure to progress
Ho 2010 reported failure to progress in labour; the difference between groups was not significant (RR 0.80, 95% CI 0.36 to 1.77, 231 women, Analysis 1.10).
1.10. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 10 Non pre‐specified outcome: Rate of failure to progress.
Maternal side‐effects: nausea, vomiting, diarrhoea, shivering and pyrexia
Ho 2010 reported data for several side‐effects commonly associated with augmentation of labour using misoprostol and/or oxytocin. The prevalence of these side‐effects was very low and there were no significant differences between groups in the number of women experiencing nausea, vomiting, shivering or pyrexia: nausea (RR 2.87 95% CI 0.12 to 69.82, 231 women, Analysis 1.11), vomiting (RR 2.87 95% CI 0.12 to 69.82, 231 women, Analysis 1.12), shivering (RR 2.87, 95% CI 0.12 to 69.82, 231 women, Analysis 1.14), and pyrexia (RR 2.87 95% CI 0.12 to 69.82, 231 women, Analysis 1.15). No experience of diarrhoea was reported (Analysis 1.13). The low number of events means that there was insufficient evidence to reach conclusions about the prevalence of side‐effects for misoprostol versus oxytocin.
1.11. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 11 Non pre‐specified outcome: Maternal side‐effects: nausea.
1.12. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 12 Non pre‐specified outcome: Maternal side‐effects: vomiting.
1.14. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 14 Non pre‐specified outcome: Maternal side‐effects: shivering.
1.15. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 15 Non pre‐specified outcome: Maternal side‐effects: pyrexia.
1.13. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 13 Non pre‐specified outcome: Maternal side‐effects: diarrhoea.
Apgar score less than seven at one minute
Ho 2010 reported on Apgar score less than seven at one minute; however, the number of events was very low and the difference between groups was not statistically significant (RR 4.79, 95% CI 0.23 to 98.69, data for 231 infants, Analysis 1.17).
1.17. Analysis.

Comparison 1 Titrated misoprostol (20 mcg dose) vs intravenous oxytocin, Outcome 17 Non pre‐specified outcome: Apgar score < 7 at 1 minute.
Birthweight of newborn (g)
Bleich 2011 provided data on birthweight. the difference between groups was not statistically significant (mean difference (MD) ‐22.00 g, 95% CI ‐117.96 to 73.96, data for 350 infants, Analysis 2.17).
2.17. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 17 Non pre‐specified outcome: Birthweight.
Maternal blood transfusion for hypovolaemia
Bleich 2011 reported the number of women requiring blood transfusions for hypovolaemia; however, the number of events was low, and the difference between groups was not significant (RR 2.97, 95% CI 0.61 to 14.49, 350 women, Analysis 2.5).
2.5. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 5 Maternal blood transfusion for hypovolemia.
Apgar score less than four at five minutes
No babies born to women recruited by Bleich 2011 had an Apgar score less than at five minutes (Analysis 2.14).
2.14. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 14 Non pre‐specified outcome: Apgar score < 4 at 5 minutes.
Spontaneous vaginal delivery
Bleich 2011 showed no clear differences in the rate of spontaneous vaginal delivery between women who received titrated oral misoprostol versus oxytocin (RR 0.98, 95% CI 0.91 to 1.06, 350 women, Analysis 2.15).
2.15. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 15 Non pre‐specified outcome: Spontaneous vaginal delivery.
Epidural analgesia
Bleich 2011 reported on use of epidural analgesia. There was a trend towards lower use of epidural analgesia among women with labours augmented using misoprostol, although this difference did not reach statistical significance (RR 0.92, 95% CI 0.84 to 1.01, 350 women, Analysis 2.16)
2.16. Analysis.

Comparison 2 Titrated misoprostol (75 mcg dose) vs intravenous oxytocin, Outcome 16 Non pre‐specified outcome: Epidural analgesia.
Discussion
Summary of main results
The two trials included in this review used different oral misoprostol regimens to augment labour, complicating comparisons of outcomes. Neither trial reported maternal death, severe maternal morbidity, or fetal/neonatal mortality outcomes, and only a few fetal/neonatal morbidity outcomes were considered, none of which were significantly different between groups. For several outcomes (such as maternal side‐effects, instrumental birth, maternal blood transfusion for hypovolaemia and epidural analgesia), the number of events was generally too low for sufficient statistical power to be achieved, and maternal satisfaction was not used as an outcome in either trial. The duration of labour from the start of augmentation to delivery in the Ho 2010 trial was slightly less in the oxytocin group compared with the misoprostol group (median 5.20 hours compared with 5.22). Bleich 2011 reported no significant difference between groups in the interval between start of augmentation and delivery. Neither trial had significantly higher rates of uterine hyperstimulation with fetal heart rate (FHR) changes in the titrated oral misoprostol group. However, the rates of this outcome varied so greatly between the two studies to suggest that other factors were at play.
Neither trial reported significantly higher rates of CS in the oral misoprostol group, nor CS for non‐reassuring FHR or prolonged labour in the trial using a 75 mcg misoprostol dose (Bleich 2011). Rates of vaginal delivery within 12 and 24 hours of commencing augmentation were not significantly different in the trial using a 20 mcg misoprostol dose (Ho 2010). The only significant differences in these two trials related to hyperstimulation and the results from the trials were not consistent. Following titrated oral misoprostol in the Bleich 2011 trial, significantly more women experienced hyperstimulation of labour (tachysystole, hypertonus or both) in a 10‐minute interval compared to those receiving oxytocin. However, we find it noteworthy that Ho 2010 reported a significantly lower incidence of tachysystole when labour was augmented with titrated oral misoprostol compared to oxytocin (via an electronic infusion pump), and recorded no occurrences of hypertonus in either group. This suggests that lower doses of misoprostol for augmentation are a credible alternative to an uncontrolled oxytocin infusion in order to avoid hyperstimulation.
Overall completeness and applicability of evidence
Titrated oral misoprostol use was not associated with lower rates of vaginal delivery or higher rates of CS compared to intravenous (IV) oxytocin regimens in women with dystocia following spontaneous onset of labour. The association between misoprostol use and uterine hyperstimulation (Bleich 2011) is not a new finding; however, the higher oral misoprostol dose (75 mcg) used in the Bleich 2011 trial (compared with the 20 mcg dose in Ho 2010) may have been a contributing factor. We did not identify any trials examining other comparisons (such as oral misoprostol against placebo or other augmentation methods), nor did we identify trials examining differences in outcomes following induction, rather than in women who labour spontaneously.
Quality of the evidence
Both of the trials included in the review were of fairly good methodological quality. Both trials used methods of randomisation which we assessed as low risk of bias, and overall rates of sample attrition were low. Blinding women, staff and outcome assessors for these types of interventions is not simple as both groups would have needed either placebo IV infusions or oral drugs; the impact of lack of blinding on outcomes is uncertain. The results of the Bleich 2011 study were more difficult to interpret because women in both groups did not receive the intended treatment, and the reasons for this did not appear to be the same in the two arms of the trial; on the other hand, both an intention‐to‐treat and as‐treated analyses were presented for selected outcomes.
Potential biases in the review process
We are aware that the review process itself may be subject to bias, and we attempted to minimise this in a number of ways. Assessment of eligibility and data extraction were carried out independently by two review authors (J Vogel and H West). Assessment of risk of bias was also carried out independently by two review authors and a consensus reached through discussion; we have provided detailed information in Characteristics of included studiesRisk of Bias tables to explain our decisions, but it is possible a different review team may have made slightly different assessments.
Agreements and disagreements with other studies or reviews
There are no other reviews of titrated oral misoprostol regimens for augmentation. However, a Cochrane review (Alfirevic 2006) of oral misoprostol for induction of labour contained a subgroup analysis of oral misoprostol versus IV oxytocin for induction. In the eight trials including 1026 women for this comparison, the only overall difference in outcomes was an increase in meconium‐stained liquor in women with ruptured membranes in the oral misoprostol group. There was not a significant difference in hyperstimulation of labour outcomes (with or without FHR changes).
Authors' conclusions
Implications for practice.
Important uncertainties still exist on the safety and acceptability of titrated oral misoprostol compared to IV oxytocin regimens in women with dystocia following spontaneous onset of labour. In addition, there is no consistent definition of a titrated oral misoprostol regimen, thus complicating interpretation. The available evidence suggests titrated oral misoprostol for women with labour dystocia (following spontaneous onset of labour) is not significantly different to IV oxytocin in terms of vaginal delivery within 12 or 24 hours or rates of CS. The evidence on uterine hyperstimulation is not clear; compared with oxytocin (via electronic pump infusion), hyperstimulation may be increased with higher doses of misoprostol, whereas with lower doses it may be reduced. To avoid hyperstimulation, lower doses of misoprostol may be a good alternative in facilities where an uncontrolled oxytocin infusion is the only other option. In addition, clinically important outcomes, such as severe maternal and fetal/newborn morbidity and mortality, as well as maternal adverse effects and acceptability, have not been explored.
Implications for research.
Further research is needed before titrated oral misoprostol regimens can be considered a safe, effective and acceptable alternative to oxytocin regimens for augmentation in labour dystocia. More trials should be conducted to evaluate the effect of a standard titration oral misoprostol regimen, both following spontaneous labour and labour induction. Comparisons with other augmentation methods are also warranted, as are any effects on women's birth experiences.
Acknowledgements
This review is financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), the Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
The World Health Organization and Therese Dowswell and Helen West retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication. As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Titrated misoprostol (20 mcg dose) vs intravenous oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with non‐reassuring heart rate changes | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.14, 6.68] |
| 2 Duration of labour (e.g.: 3 cm dilatation to delivery), in minutes | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.42, 1.85] |
| 4 Incidence of tachysystole | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.17, 0.91] |
| 5 Incidence of hypertonus | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Vaginal delivery within 12 hours of commencement of augmentation | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 7 Vaginal delivery within 24 hours of commencement of augmentation | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 8 Apgar < 7 at 5 minutes | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Admission to neonatal intensive care unit (NICU) | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.47, 12.09] |
| 10 Non pre‐specified outcome: Rate of failure to progress | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.36, 1.77] |
| 11 Non pre‐specified outcome: Maternal side‐effects: nausea | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 69.82] |
| 12 Non pre‐specified outcome: Maternal side‐effects: vomiting | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 69.82] |
| 13 Non pre‐specified outcome: Maternal side‐effects: diarrhoea | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Non pre‐specified outcome: Maternal side‐effects: shivering | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 69.82] |
| 15 Non pre‐specified outcome: Maternal side‐effects: pyrexia | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 69.82] |
| 16 Non pre‐specified outcome: Total dosage of oxytocin or misoprostol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Non pre‐specified outcome: Apgar score < 7 at 1 minute | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.79 [0.23, 98.69] |
| 18 Non pre‐specified outcome: Active phase interval | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Non pre‐specified outcome: Birthweight of newborn | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Titrated misoprostol (75 mcg dose) vs intravenous oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation (tachysystole, hypertonus or both) associated with fetal heart changes | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.88, 1.80] |
| 2 Duration of labour (from start of augmentation to delivery), in minutes | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.57, 1.92] |
| 4 Duration of second stage | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal blood transfusion for hypovolemia | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.61, 14.49] |
| 6 Caesarean for non‐reassuring fetal heart rate (i.e. fetal distress) | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.53, 4.74] |
| 7 Forceps delivery | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.37, 10.66] |
| 8 Caesarean section for dystocia (i.e. prolonged labour) | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.82] |
| 9 Uterine tachysystole, hypertonus, or both in 10‐minute period (hyperstimulation of labour) | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 10 Uterine tachysystole in a 20‐minute interval | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.72] |
| 11 Chorioamnionitis | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 12 Umbilical cord artery pH < 7.1 | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.17, 3.26] |
| 13 Admission to neonatal intensive care unit (NICU) | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.12, 72.31] |
| 14 Non pre‐specified outcome: Apgar score < 4 at 5 minutes | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Non pre‐specified outcome: Spontaneous vaginal delivery | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 16 Non pre‐specified outcome: Epidural analgesia | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.84, 1.01] |
| 17 Non pre‐specified outcome: Birthweight | 1 | 350 | Mean Difference (IV, Random, 95% CI) | ‐22.0 [‐117.96, 73.96] |
| 18 Non pre‐specified outcome: Maximum number of Monteovideo units | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Non pre‐specified outcome: Maximum oxytocin dose | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Non pre‐specified outcome: Admission to study drug (minutes) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bleich 2011.
| Methods | Randomised trial, two‐arm, individual randomisation stratified as either nulliparous or multiparous. Analysed by both intention‐to‐treat, and as‐treated for selected outcomes. | |
| Participants |
Setting: Single facility in Dallas County, USA. Period of data collection: December 2008 ‐ January 2011. Inclusion criteria: Women with spontaneous labour that reached the active phase of labour (defined as cervical dilation of 4 cm or more) but did not progress, maternal age 16 years or older, gestational age 36 weeks or more, singleton, cephalic presentation, reassuring FHR, cervical dilation between 4 and 8 cm, and English or Spanish speaking. Eligible individuals were required to have ruptured membranes with an intrauterine pressure catheter in place confirming the presence of fewer than 200 Montevideo units of uterine activity, the absence of tachysystole, defined as 6 or more contractions in a single 10‐min period, and the absence of uterine hypertonus, defined as a single contraction lasting longer than 120 seconds. Exclusion criteria: non‐reassuring FHR pattern, meconium‐stained amniotic fluid, previous uterine incision, maternal fever (defined as temperature 38°C or higher), known fetal anomalies, placenta previa or unexplained vaginal bleeding, estimated fetal weight 4500 g or more, abnormal bony pelvis, and parity of 6 or more. Participants: 439 women met inclusion criteria, of which 350 (79.7%) consented and were randomised. Women recruited were mostly Hispanic (88% Hispanic, 5% White, 7% African American). |
|
| Interventions |
Experimental group: 75 mcg of oral misoprostol, for up to 2 doses 4 hrs apart. This was prepared by cutting generic 100 mcg misoprostol tablets into one‐half and one‐quarter tablets. Women were eligible for a second dose of misoprostol if they had a reassuring FHR tracing and did not experience uterine tachysystole or hypertonus after the first dose. All women in the misoprostol arm were assessed for drug failure 2 hrs after administration of the second dose. If they experienced less than 200 Montevideo units and minimal cervical change, IV oxytocin was initiated. If women randomised to the misoprostol arm developed a non‐reassuring fetal heart rate (defined as tachycardia, prolonged or late decelerations, moderate to severe variable decelerations (nadir of up to 90 beats per min for more than 30 seconds or a duration of 60 seconds or longer), or any combination of these), uterine tachysystole, or hypertonus before the administration of misoprostol, the study drug was withheld and labour augmented with IV oxytocin. 176 (50.3%) women were randomised to the experimental group, of which 136 (77.30%) received the study drug and 40 (22.7%) did not. Control group: Women received titrated IV oxytocin according to a hospital protocol of a 6 milliunits/min starting dose, followed by incremental increases of 6 milliunits/min at 40‐min intervals, up to a maximum dose of 42 milliunits/min. The protocol also specified use of 3‐milliunit/min or 1‐milliunit/min doses if tachysystole or hypertonus were encountered. The oxytocin was prepared as a 20 units/L solution in isotonic saline and administered by using calibrated infusion pumps. 174 (49.7%) randomised to the control group, of which 143 (82.2%) women received the control drug and 31 (17.8%) did not. |
|
| Outcomes |
Primary outcome: incidence of uterine tachysystole, hypertonus, or both in a 10‐min period. Secondary outcomes:
Also reported on other outcomes:
|
|
| Notes | Several continuous outcomes were reported as median (interquartile range) rather than mean (standard deviation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | On enrolment, an opaque envelope corresponding to the participant’s enrolment number was opened. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women and care staff were not blinded to treatment allocation once it has been assigned, suggesting a high risk of bias. However, it is unclear what (if any) effect this may have had on most outcomes, but the risk of bias is likely to be high for the epidural analgesia outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were blinded for judgements of FHR traces and uterine hyperstimulation ‐ low risk of bias for these outcomes only. However, outcome assessment was not blinded for other outcomes but it is unclear what (if any) effect this may have had. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20.3% of study participants overall (22.7% in experimental group and 17.8% in control group) did not receive study drugs ‐ reasons were reported, however the authors did not explain why the proportion attributable to progression to 8 cm prior to initiation of augmentation differed between groups (18/40 women in experimental group and 30/31 in control group). It is also unclear how many women randomised to misoprostol arm eventually received oxytocin due to drug failure, as per study protocol. |
| Selective reporting (reporting bias) | Unclear risk | The study reported on 5 additional secondary outcomes (uterine tachysystole in a 20‐min interval, birthweight, elapsed time from the start of labour augmentation to delivery, maximum number of Montevideo units, diagnosis of non‐reassuring FHR) compared to the outcomes stated in the clinical trial protocol registration, and did not report on one of the stated clinical trial protocol outcomes (maternal metritis). The reason for these changes was not stated and it is unclear what effect this may have had on conclusions. |
| Other bias | Unclear risk | 20% of women declined participation when approached; the reason for this is unclear. |
Ho 2010.
| Methods | Randomised trial with two arms and individual randomisation. Analysed by intention‐to‐treat. |
|
| Participants |
Setting: single facility in Taiwan, China. Period of data collection: March 2008‐December 2009 Inclusion criteria:
Exclusion criteria:
Participants: 827 women met the inclusion criteria, of which 712 (86.1%) consented. Of these, 231 (32.4%) developed inadequate uterine contractions and were randomised. |
|
| Interventions |
Experimental group: One 200‐mcg tablet of misoprostol was completely dissolved in 200 mL of tap water with stirring bar in a medicine bottle by the duty nurse. The misoprostol solution was stored in a medicine bottle at the nurses’ station and used completely within 24 hrs after preparation or discarded. Women were given 1 basal unit of 20 mL of misoprostol solution (1 mcg/mL, 20 mcg total). Misoprostol was initially administered at a dose of 20 mcg/h, until adequate uterine contractions (defined as 3 or more in 10‐min over 30‐min windows) were achieved. If contractions did not occur after 4 hrs (4 doses), the dosage was increased to 40 mcg and repeated every hr until uterine contractions occurred. Nothing by mouth, except medication, was allowed during the active phase of labour. Once uterine activity was adequate over 1 hr, no further misoprostol was given. If contractions subsequently became inadequate, hourly doses of misoprostol solution were started at 10 mcg/h and could be increased to 20 mcg/h and to as much as 40 mcg/h based on uterine responsiveness. This process was repeated until adequate uterine contractions occurred. A maximum cumulative dose of 1,600 mcg of misoprostol was permitted. 118 women (51.10%) were randomised to receive the titrated oral misoprostol regimen. Control group: Women received IV oxytocin by pump initially set to deliver 1 milliunit/min for 20 mins, and then increase the rate by 1 milliunit/min every 20 mins until adequate uterine contractions were attained. Maximum dosing rate permitted was 20 milliunits/min of oxytocin. 113 (48.9%) women were randomised to receive titrated IV oxytocin. Women in both groups had FHR and uterine activity monitored continuously throughout augmentation. IV magnesium sulfate (4 g over 30 mins) could be given at the discretion of the physician if uterine hyperstimulation occurred. Cesarean delivery was offered to all patients after failure of labour to progress or when non‐reassuring FHR occurred. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Also reported on other outcomes:
|
|
| Notes | Several continuous outcomes were reported as median (interquartile range) rather than mean (standard deviation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment arm allocation was determined using a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Low risk | The randomisation assignments were placed into opaque, sealed envelopes. When inadequate uterine contractions occurred, the envelope was opened by the patient’s obstetrician to determine the treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors stated it was not possible to blind the study participants and personnel from knowledge of which intervention a participant received because the two methods were clearly different. However, it is unclear to what extent this may have affected outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above. It is unclear to what extent this may have affected outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women did not receive intended treatment (2 in experimental group ‐ 1 woman asked for epidural and 1 underwent emergency caesarean section due to non‐reassuring FHR; 1 in control group, due to non‐reassuring FHR). They were included in the analysis by intention‐to‐treat. Appears that all participants were followed up; however, this is not explicitly stated. |
| Selective reporting (reporting bias) | Unclear risk | Trial data were recent and all declared outcomes were reported, even if insignificant. However, Apgar scores less than 7 at 1 min and 5 mins were both reported, despite only Apgar score less than 7 at 5 mins being prespecified. The study also reported on an additional primary outcome (interval from the start of augmentation to vaginal delivery) and several additional secondary outcomes, compared to the original clinical trial protocol. The reasons for these changes were not reported. There were no outcomes reported on maternal mortality/severe morbidity, nor on perinatal mortality. |
| Other bias | Unclear risk | No obvious other bias. |
FHR: fetal heart rate g: gram hr(s): hour(s) IV: intravenous mcg: microgram min(s): minute(s) NICU: neonatal intensive care
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Patel 2000 | Women were randomised to receive IV oxytocin or vaginal (rather than oral) misoprostol every 4 hrs. The inclusion criteria included women who had been induced, rather than women in spontaneous labour only. |
hrs: hours IV: intravenous
Characteristics of ongoing studies [ordered by study ID]
Aalami‐Harandi 2009.
| Trial name or title | Comparison of the effect of oral misoprostol versus oxytocin on labour augmentation in pregnant women. |
| Methods | Randomised, non‐blinded trial, two arms. |
| Participants |
Setting: Shohada Tajrish Hospital, Islamic Republic Of Iran. Period of data collection: January 2009 to January 2011. Inclusion criteria: ‐ Pregnancy between 36 and 42 weeks of gestation. ‐ A live singleton fetus in cephalic presentation. ‐ No history of uterine surgery. ‐ Spontaneous onset of active labour with regular contractions. ‐ An effaced cervix dilated between 3 cm and 9 cm. ‐ A reassuring fetal heart rate pattern. ‐ Maternal age 16 to 45 years of age. Exclusion criteria: ‐ Non‐reassuring fetal heart rate pattern. ‐ Parity greater than 5. ‐ Any contraindication to labour or vaginal delivery or both. ‐ Epidural analgesia. ‐ Significant maternal cardiac, renal or hepatic disease. ‐ Hypersensitivity to misoprostol or prostaglandin analogues. |
| Interventions |
Experimental group: In the misoprostol group, a tablet of 200 mcg misoprostol dissolved in 200 cc of water and 25 cc was administered every 2 hrs for up to 24 hrs. The maximum dose was 300 mcg. Fetal heart monitoring and uterine contraction were recorded. Adequate uterine contractions were defined as 3 or more in 10 mins over 30‐min windows. Once uterine activity was adequate over 1 hr, no further misoprostol was given. Control group: In the oxytocin group, infusion rate of 2 mIU/min was prescribed for induction and gradually increased by 2 mIU/min every 15 mins to a maximum dose of 36 mIU/min. In presence of any tachysystole (5 contractions in a 10‐min interval) or hypertonus (single contractions lasting 2 mins or longer), or changes in fetal heart rate associated with tachysystole or hypertonus, infusion rate was decreased or stopped |
| Outcomes |
Primary outcomes: ‐ The time from augmentation to vaginal delivery, 12 and 24 hrs during labour. ‐ Mode of delivery, 12 and 24 hrs during labour. Secondary outcomes: ‐ Fetal and neonatal status in 'peri labor phase'. ‐ Maternal complications in 'peri labour phase'. |
| Starting date | 1st August, 2008 |
| Contact information | Assistant Professor Rezvan Aalami‐Harandi Department of Obstetrics and Gynecology, Shahid Beheshti University of Medical Science Tehran, Islamic Republic of Iran mahmaz2002@yahoo.com Dr Seyed Jalaledin Khoshnevis Shahid Beheshti University of Medical Science Tehran, Islamic Republic of Iran jkhosh20012002@yahoo.com Dr Aida Moeini Department of Obstetrics and Gynecology, Shahid Beheshti University of Medical Science Tehran, Islamic Republic of Iran a.moeini64@gmail.com Study co‐ordinators emailed on 29th May, 2013 for request for further information on trial and outcomes. |
| Notes |
hr(s): hour(s) mcg: microgram min(s): minute(s)
Contributions of authors
For the protocol: Helen West (HW) produced the first draft. Joshua Vogel (JV) and Therese Dowswell commented upon, and revised the text.
For the review: HW and JV carried out data extraction and entered data. All three authors contributed to the text.
Sources of support
Internal sources
University of Liverpool, UK.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), the Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Helen West: no known conflicts of interest. Joshua Vogel: no known conflicts of interest. Therese Dowswell: no known conflicts of interest.
New
References
References to studies included in this review
Bleich 2011 {published data only}
- Bleich AT, Villano KS, Lo JY, Alexander JM, McIntire DD, Leveno KJ. Oral misoprostol for labor augmentation: a randomized controlled trial. Obstetrics and Gynecology 2011;118(6):1255‐60. [DOI] [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho M, Cheng SY, Li TC. Titrated oral misoprostol solution compared with intravenous oxytocin for labor augmentation: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):612‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Patel 2000 {published data only}
- Patel A, Giles JM, Moffett D, Mahram R, Diro M, Burkett G. Can misoprostol be interchanged with oxytocin for augmentation of labor? [abstract]. Obstetrics & Gynecology 2000;95(4 Suppl):10S. [Google Scholar]
References to ongoing studies
Aalami‐Harandi 2009 {published data only}
- Aalami‐Harandi R. Comparison the effect of oral misoprostol versus oxytocin on labor augmentation in pregnant women. Iranian Registry of Clinical Trials 2009.
Additional references
Abbassi 2008
- Abbassi RM, Sirichand P, Rizvi S. Safety and efficacy of oral versus vaginal misoprostol use for induction of labour at term. Journal of the College of Physicians & Surgeons ‐ Pakistan: JCPSP 2008;18(10):625‐9. [DOI] [PubMed] [Google Scholar]
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Blanchard 2002
- Blanchard K, Clark S, Winikoff B, Gaines G, Kabani G, Shannon C. Misoprostol for women's health: a review. Obstetrics & Gynecology 2002;99(2):316‐32. [DOI] [PubMed] [Google Scholar]
Bricker 2008
- Bricker L, Peden H, Tomlinson AJ, Al‐Hussaini TK, Idama T, Candelier C, et al. Titrated low‐dose vaginal and/or oral misoprostol to induce labour for prelabour membrane rupture: a randomised trial. BJOG: an International Journal of Obstetrics & Gynaecology 2008;115(12):1503‐11. [DOI] [PubMed] [Google Scholar]
Buchmann 2006
- Buchmann EJ, Pattinson RC. Babies who die from labour‐related intrapartum hypoxia: a confidential enquiry in South African public hospitals. Tropical Doctor 2006;36(1):8‐10. [DOI] [PubMed] [Google Scholar]
Cheng 2006
- Cheng SY, Chen TC. Pilot study of labor induction with titrated oral misoprostol. Taiwanese Journal of Obstetrics & Gynecology 2006;45(3):225‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2008
- Cheng SY, Ming H, Lee JC. Titrated oral compared with vaginal misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 2008;111(1):119‐25. [DOI] [PubMed] [Google Scholar]
Cheng 2010
- Cheng SY, Hsue CS, Hwang GH, Chen W, Li TC. Comparison of labor induction with titrated oral misoprostol solution between nulliparous and multiparous women. Journal of Obstetrics & Gynaecology Research 2010;36(1):72‐8. [DOI] [PubMed] [Google Scholar]
Chibber 2011
- Chibber R, El‐Saleh E, Harmi J. Female circumcision: obstetrical and psychological sequelae continues unabated in the 21st century. Journal of Maternal‐Fetal & Neonatal Medicine 2011;24(6):833‐6. [DOI] [PubMed] [Google Scholar]
Friedman 2001
- Friedman MA. Manufacturer's earning regarding unapproved uses of misoprostol. New England Journal of Medicine 2001;344(1):61. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 1999
- Hofmeyr G J, Gulmezoglu A M, Alfirevic Z. Misoprostol for induction of labour: a systematic review. British Journal of Obstetrics and Gynaecology 1999;106(8):798‐803. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2001a
- Hofmeyr GJ, Alfirevic Z, Matonhodze B, Brocklehurst P, Campbell E, Nikodem VC. Titrated oral misoprostol solution for induction of labour: a multi‐centre, randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2001;109(9):952‐9. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2011
- Hofmeyr GJ, Gülmezoglu AM, Novikova N, Lawrie TA. Postpartum misoprostol for preventing maternal mortality and morbidity. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008982] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2013
- Hofmeyr GJ, Gülmezoglu AM, Novikova N, Lawrie TA. Postpartum misoprostol for preventing maternal mortality and morbidity. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008982.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogberg 2000
- Hogberg U, Lekas Berg M. Prolonged labour attributed to large fetus. Gynecologic and Obstetric Investigation 2000;49(3):160‐4. [DOI] [PubMed] [Google Scholar]
Kalogiannidis 2011
- Kalogiannidis I, Margioula‐Siarkou C, Petousis S, Masoura S, Goutzioulis A, Traianos A, et al. Parity affects pregnancy outcomes in women 35 and older. Clinical & Experimental Obstetrics & Gynecology 2011;38(2):146‐9. [PubMed] [Google Scholar]
Kjaergaard 2008
- Kjaergaard H, Olsen J, Ottesen B, Nyberg P, Dykes AK. Obstetric risk indicators for labour dystocia in nulliparous women: a multi‐centre cohort study. BMC Pregnancy and Childbirth 2008;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergaard 2009
- Kjaergaard H, Olsen J, Ottesen B, Dykes AK. Incidence and outcomes of dystocia in the active phase of labor in term nulliparous women with spontaneous labor onset. Acta Obstetricia et Gynecologica Scandinavica 2009;88(4):402‐7. [DOI] [PubMed] [Google Scholar]
Konje 1990
- Konje JC, Odukoya OA, Ladipo OA. Ruptured uterus in Ibadan—a twelve year review. International Journal of Gynecology and Obstetrics 1990;32(4):207‐13. [DOI] [PubMed] [Google Scholar]
Kundodyiwa 2009
- Kundodyiwa TW, Alfirevic Z, Weeks AD. Low‐dose oral misoprostol for induction of labor: a systematic review. Obstetrics & Gynecology 2009;113(2 Pt 1):374‐83. [DOI] [PubMed] [Google Scholar]
Lawn 2009
- Lawn JE, Lee AC, Kinney M, Sibley L, Carlo WA, Paul VK, et al. Two million intrapartum‐related stillbirths and neonatal deaths: where, why, and what can be done?. International Journal of Gynecology and Obstetrics 2009;107 Suppl 1:S5‐18, S19. [DOI] [PubMed] [Google Scholar]
Lederman 1978
- Lederman RP, Lederman E, Work BA Jr, McCann DS. The relationship of maternal anxiety, plasma catecholamines, and plasma cortisol to progress in labor. American Journal of Obstetrics & Gynecology 1978;132(5):495‐500. [DOI] [PubMed] [Google Scholar]
Lovold 2008
- Lovold A, Stanton C, Armbruster D. How to avoid iatrogenic morbidity and mortality while increasing availability of oxytocin and misoprostol for PPH prevention?. International Journal of Gynecology & Obstetrics 2008;103(3):276‐82. [DOI] [PubMed] [Google Scholar]
Margulies 1992
- Margulies M, Campos Pérez G, Voto LS. Misoprostol to induce labour. Lancet 1992;339(8784):64. [DOI] [PubMed] [Google Scholar]
Matonhodze 2003
- Matonhodze B, Hofmeyr GJ, Levin J. Labour induction at term‐‐a randomised trial comparing Foley catheter plus titrated oral misoprostol solution, titrated oral misoprostol solution alone, and dinoprostone. South African Medical Journal. Suid‐Afrikaanse Tydskrif Vir Geneeskunde 2003;93(5):375‐9. [PubMed] [Google Scholar]
McClure 2006
- McClure EM, Nalubamba‐Phiri M, Goldenberg RL. Stillbirth in developing countries. International Journal of Gynecology and Obstetrics 2006;94(2):82‐90. [DOI] [PubMed] [Google Scholar]
Nassar 2007
- Nassar AH, Awwad J, Khalil AM, Abu‐Musa A, Mehio G, Usta IM. A randomised comparison of patient satisfaction with vaginal and sublingual misoprostol for induction of labour at term. BJOG: an International Journal of Obstetrics & Gynaecology 2007;114(10):1215‐21. [DOI] [PubMed] [Google Scholar]
Neilson 2003
- Neilson JP, Lavender T, Quenby S, Wray S. Obstructed labour. British Medical Bulletin 2003;67:191‐204. [DOI] [PubMed] [Google Scholar]
Norman 1991
- Norman JE, Thong KJ, Baird DT. Uterine contractility and induction of abortion in early pregnancy by misoprostol and mifepristone. Lancet 1991;338(8777):1233‐6. [DOI] [PubMed] [Google Scholar]
Nystedt 2005
- Nystedt A, Högberg U, Lundman B. The negative birth experience of prolonged labour: a case–referent study. Journal of Clinical Nursing 2005;14(5):579‐86. [DOI] [PubMed] [Google Scholar]
O'Hana 2008
- O'Hana H P, Levy A, Rozen A, Greemberg L, Shapira Y, Sheiner E. The effect of epidural analgesia on labor progress and outcome in nulliparous women. Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(8):517‐21. [DOI] [PubMed] [Google Scholar]
Piper 1991
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. American Journal of Obstetrics and Gynecology 1991;165(4 Pt 1):976‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Scholl 2012
- Scholl TO, Chen X, Stein P. Maternal vitamin D status and delivery by cesarean. Nutrients 2012;4(4):319‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selin 2008
- Selin L, Wallin G, Berg M. Dystocia in labour ‐ risk factors, management and outcome: a retrospective observational study in a Swedish setting. Acta Obstetricia et Gynecologica Scandinavica 2008;87(2):216‐21. [DOI] [PubMed] [Google Scholar]
Sheiner 2002
- Sheiner E, Levy A, Feinstein U, Hallak M, Mazor M. Risk factors and outcome of failure to progress during the first stage of labor: a population‐based study. Acta Obstetricia et Gynecologica Scandinavica 2002;81(3):222‐6. [PubMed] [Google Scholar]
Sheiner 2007
- Sheiner E, Levy A, Katz M, Mazor M. Short stature ‐ an independent risk factor for Caesarean delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;120(2):175‐8. [DOI] [PubMed] [Google Scholar]
Shields 2007
- Shields SG Ratcliffe SD Fontaine P Leeman L. Dystocia in nulliparous Women. American Family Physician 2007;75(11):1671‐8. [PubMed] [Google Scholar]
Simpson 2003
- Simpson KR, Atterbury J. Trends and issues in labor induction in the United States: implications for clinical practice. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2003;32(6):767‐79. [DOI] [PubMed] [Google Scholar]
Souza 2010
- Souza AS, Scavuzzi A, Rodrigues DC, Oliveira RD, Feitosa FE, Amorim MM. Titrated oral solution of misoprostol for labour induction: a pilot study. Revista Brasileira de Ginecologia e Obstetricia 2010;32(5):208‐13. [DOI] [PubMed] [Google Scholar]
Stanton 2012
- Stanton C, Koski A, Cofie P, Mirzabagi E, Grady BL, Brooke S. Uterotonic drug quality: an assessment of the potency of injectable uterotonic drugs purchased by simulated clients in three districts in Ghana. BMJ open 2012;2(3):e000431. [DOI: 10.1136/bmjopen-2011-000431] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2007
- Tang OS, Gemzell‐Danielsson K, Ho PC. Misoprostol: Pharmacokinetic profiles, effects on the uterus and side‐effects. International Journal of Gynecology & Obstetrics 2007;99 Suppl 2:S160‐S167. [DOI] [PubMed] [Google Scholar]
Thaisomboon 2012
- Thaisomboon A, Russameecharoen K, Wanitpongpan P, Phattanachindakun B, Changnoi A. Comparison of the efficacy and safety of titrated oral misoprostol and a conventional oral regimen for cervical ripening and labor induction. International Journal of Gynecology & Obstetrics 2012;116(1):13‐6. [DOI] [PubMed] [Google Scholar]
Uludag 2005
- Uludag S, Salihoglu Saricali F, Madazli R, Cepni I. A comparison of oral and vaginal misoprostol for induction of labor. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2005;122(1):57‐60. [DOI] [PubMed] [Google Scholar]
Vahratian 2004
- Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega‐Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstetrics & Gynecology 2004;104(5 Pt 1):943‐51. [DOI] [PubMed] [Google Scholar]
Vahratian 2006
- Vahratian A, Hoffman MK, Troendle JF, Zhang J. The impact of parity on course of labor in a contemporary population. Birth 2006;33(1):12‐7. [DOI] [PubMed] [Google Scholar]
Villano 2011
- Villano KS, Lo JY, Alexander JM, McIntire DD, Leveno KJ. A dose‐finding study of oral misoprostol for labor augmentation. American Journal of Obstetrics and Gynecology 2011;204(6):560.e1‐5. [DOI] [PubMed] [Google Scholar]
Weeks 2005
- Weeks AD, Fiala C, Safar P. Misoprostol and the debate over off‐label drug use. BJOG: an International Journal of Obstetrics and Gynaecology 2005;112(3):269‐72. [DOI] [PubMed] [Google Scholar]
Weeks 2007
- Weeks A, Faundes A. Misoprostol in obstetrics and gynecology. International Journal of Gynecology and Obstetrics 2007;99 Suppl 2:S156‐S159. [DOI] [PubMed] [Google Scholar]
Yakoob 2010
- Yakoob MY, Lawn JE, Darmstadt GL, Bhutta ZA. Stillbirths: epidemiology, evidence, and priorities for action. Seminars in Perinatology 2010;34(6):387‐94. [DOI] [PubMed] [Google Scholar]
Zvandasara 2008
- Zvandasara P, Saungweme G, Mlambo J, Chidembo W, Madzivanzira N, Mwanjira C. Induction of labour with titrated oral misoprostol suspension. A comparative study with vaginal misoprostol. Central African Journal of Medicine 2008;54(9‐12):43‐9. [DOI] [PubMed] [Google Scholar]


