Abstract
Spleen and peritoneal macrophages obtained from innately resistant A/J mice released low levels of interleukin 18 (IL-18) upon infection with Salmonella typhimurium C5 RP4. Incubating the cells with recombinant gamma interferon (rIFN-γ) enhanced IL-18 production. A/J mice treated in vivo with anti-IL-18 antibodies showed impaired resistance to infection, with increased bacterial loads in the liver and spleen. Administration of rIL-18 could protect A/J mice from challenge with a lethal dose of virulent salmonellae, with a dramatic reduction in bacterial numbers in the tissues. rIL-18 administration did not ameliorate the disease in IFN-γ-R−/− mice. IL-18 proved to be required for IFN-γ production by mouse splenocytes from conventional, scid, and rag-1−/− mice; in vivo IL-18 neutralization caused a decrease in circulating IFN-γ levels. Thus, IL-18 is a key factor in early host resistance to Salmonella and probably acts via IFN-γ.
A better knowledge of the mechanisms of pathogenesis and immunity operating in Salmonella infections is needed for a more rational approach to the treatment of the disease as well as for the development of improved vaccines.
The mouse model is widely used for the study of systemic Salmonella infections. In mice, host-adapted salmonellae can invade and multiply in the tissues of the reticuloendothelial system (RES), with the severity and outcome of the disease depending on the infecting dose, on the virulence of the bacterial strain, and on the genetic background of the animal (15).
In mice, early resistance is under control of the innate resistance Nramp (Ity) gene, which is expressed by macrophages (10). In lethal infections, bacterial growth in the tissues progresses unrestrained until large bacterial numbers are reached (ca. 108 CFU), causing death. In sublethal infections, survival requires an early host response that controls the growth of the organisms in the tissues (5, 12). This early response does not require functional T cells, depends on the presence of bone marrow-derived cells (mononuclear cells) (5), and coincides with the formation of granulomas in the infected tissues (18, 26). Most of the salmonellae in the spleen and liver of the infected animal are localized within the phagocytes present in the focal lesions (26).
Several cytokines and soluble factors play a crucial role in early resistance to mouse typhoid. Tumor necrosis factor alpha (TNF-α)-interleukin 12 (IL-12), gamma interferon (IFN-γ), and nitric oxide derivatives (NO) are all required for the control of Salmonella growth by the infected host (8, 13, 14, 16–18, 20, 21, 30). TNF-α is needed for granuloma formation (18); IL-12 is required for IFN-γ production by NK cells (16, 24, 25); IFN-γ is a key factor in the enhancement of Salmonella killing by macrophages (6).
IL-18 is an 18- to 19-kDa cytokine produced by several cell types, including activated mononuclear cells and epidermal cells, in response to bacterial and inflammatory stimuli (22, 27). IL-18 specific mRNA can be detected in a wide range of cell types (29). The cytokine is produced as a precursor polypeptide, which is cleaved by caspase 1 to yield a 157-amino-acid biologically active monomer (4). IL-18 has multiple biological activities including induction of IFN-γ from NK cells and antigen- or mitogen-stimulated Th1 cells, upregulation of IL-2R on T cells (9, 28), enhancement of Fas ligand-mediated cytotoxicity of murine T-helper cells (3), and augmentation of NK cell cytotoxicity (28). IL-18 and IL-12 have a synergistic effect on the induction of IFN-γ from T cells, probably due to the upregulation of IL-18 receptors by IL-12 (1, 28). IL-18 has been recently shown to induce TNF-α from human NK and T cells (23).
IL-18 plays a role in host resistance to infection and in lipopolysaccharide-induced histopathology. The cytokine enhances host resistance to infection with virulent Cryptococcus neoformans (31) and is involved in LPS-mediated liver injury in animals exposed to Propionibacterium acnes (22). Recently, IL-18 has been shown to play a crucial role in the control of Yersinia enterocolitica infection in mice (2).
In the present study, we investigated the role of IL-18 in host resistance to virulent salmonellae by using the mouse typhoid model. We assessed whether IL-18 is induced in response to Salmonella; we studied the effect of administration of anti-IL-18 antibodies and recombinant IL-18 (rIL-18) to Salmonella-infected mice; and we evaluated the involvement of IL-18 in IFN-γ production from mouse splenocytes. Finally, we wished to assess whether IL-18 acts via IFN-γ induction.
MATERIALS AND METHODS
Animals.
A/J, C57BL/6, C57BL/6 rag-1−/−, 129 sv, and 129 sv IFN-γ-R−/− mice were purchased from BαK Universal Ltd. C.B.-17 and C.B.-17 scid mice were purchased from Charles River U.K. Ltd. Age- and sex-matched groups were used when older than 8 weeks.
Bacteria.
S. typhimurium C5 is a virulent strain with an intravenous (i.v.) 50% lethal dose for A/J and 129 sv mice of ca. 104.5 CFU (reference 16 and our unpublished observations). Bacteria were grown at 37°C as stationary overnight cultures in Luria-Bertani (LB) broth (Difco). Aliquots were snap-frozen and stored in liquid nitrogen. The inoculum was diluted in phosphate-buffered saline (PBS) and injected in a lateral tail vein. The dose was further checked by pour plating. For infection of macrophage cultures, the ampicillin-resistant S. typhimurium C5 RP4 was grown as above in LB broth containing 50 μg of ampicillin per ml. The mouse virulence of S. typhimurium C5 RP4 and S. typhimurium C5 are very similar in terms of 50% lethal dose and growth curves in innately susceptible and resistant mice (our unpublished observations). The bacteria were diluted at the appropriate concentration in RPMI 1640 containing 10% fresh mouse serum and incubated at 37°C for 50 min before being added to the macrophage cultures. In a series of preliminary experiments, we found that a bacterium-to-macrophage ratio of 5:1 gave the highest intracellular bacterial penetration of S. typhimurium C5 in murine peritoneal and splenic macrophages.
Bacterial enumeration in organ homogenates.
Mice were sacrificed by cervical dislocation. Spleens and livers were aseptically removed and homogenized in a Colworth stomacher in 10 ml of distilled water (16). Viable counts were performed with pour plates of LB agar.
Anti-IL-18 antibodies and rIL-18.
Neutralizing anti-IL-18 antiserum was prepared from sera of rabbits immunized with murine rIL-18. A 200-μg dose of anti-IL-18 antibody completely blocked the IFN-γ-inducing activity of 50 ng of IL-18 in spleen cells stimulated with concanavalin A (ConA) (22).
rIL-18 was prepared as described elsewhere (22). The endotoxin content was <0.9 ng/mg of protein as assessed by the Limulus amebocyte lysate assay (Seikagaku Kogyo, Tokyo, Japan).
Preparation of peritoneal and splenic macrophages.
Mice were sacrificed by cervical dislocation. The peritoneal cavity was injected with 5 ml of cold RPMI 1640 (Sigma, Poole, United Kingdom) containing 10 U of heparin (Sigma) per ml. The fluid-distended peritoneal cavity was massaged, and the cells were collected, washed three times by centrifugation at 290 × g for 7 min, resuspended in RPMI 1640 supplemented with 2 mM glutamine, 1 mM HEPES (Sigma), 50 μg of ampicillin (Sigma) per ml, and 10% heat-inactivated fetal calf serum (FCS) (Sigma), and dispensed in 24-well plates (Corning, Corning, N.Y.) at 106 cells per well.
Splenic macrophages were prepared by a modification of the method described by Lissner et al. (11). Briefly, spleens were aseptically removed and mashed through a stainless steel sieve in RPMI 1640. The supernatant was collected. Gross pieces were allowed to sediment before being treated with 0.075% collagenase and 0.001% DNase I in Ca2+- and Mg2+-free Dulbecco modified phosphate-buffered saline (D-PBS) for 45 min at 37°C. The supernatant and the collagenase-treated sediment were pooled and incubated in Gey’s solution to lyse erythrocytes and the cells were washed three times at 290 × g for 7 min, resuspended in RPMI 1640 supplemented as described above, and dispensed in 24-well plates at 106 cells/well. After a 3-h incubation, the plates containing splenic and peritoneal cells were vigorously washed and incubated overnight in RPMI 1640 supplemented as described above. Different sets of wells were incubated for 16 h with medium alone or with medium containing recombinant murine IFN-γ (8,000 to 100 U/ml) (kindly provided by G. R. Adolf, Bender, Vienna, Austria).
Adherent cells were washed once and exposed to 500 μl of the opsonized S. typhimurium C5 RP4 (time zero) at a bacterium-to-cell ratio of approximately 5:1 for 1 h at 37°C. The supernatant was removed, the wells were washed three times with RPMI 1640, and the cells were further cultured in RPMI 1640 supplemented with 2 mM glutamine, 1 mM HEPES, 50 μg of ampicillin per ml, 5 μg of gentamicin (Sigma) per ml, and 10% heat-inactivated FCS. Supernatants were removed at intervals thereafter and stored at −70°C until use.
Splenocyte cultures.
Splenocytes were prepared as described elsewhere (16). Briefly, mice were sacrificed by cervical dislocation and single-cell suspensions were prepared. The cells were washed once in RPMI 1640 medium and incubated in Gey’s solution to lyse the erythrocytes. Leukocytes were washed twice more and resuspended in RPMI 1640 supplemented with 100 U of penicillin per ml, 100 μg of streptomycin (Sigma) per ml, 2 mM glutamine, 2 × 10−5 M β-mercaptoethanol, 1 mM HEPES, and 10% heat-inactivated FCS. For stimulation with whole bacteria (105 CFU of S. typhimurium C5), cells were dispensed at a concentration of 106/well in flat-bottom 96-well plates (Corning) in 200 μl. For IFN-γ measurements, the supernatants were harvested at 48 h, aliquoted, and stored at −70°C.
IL-18 ELISA.
IL-18 was measured by capture enzyme-linked immunosorbent assay (ELISA). The antibodies used in the ELISA react with mature IL-18 in Western blots (our observations). We coated 96-well ELISA plates (Maxisorp Immunoplates, Nunc, Roskilde, Denmark) at room temperature (RT) for 3 h with 100 μl of capture monoclonal antibody 74 (rat splenocyte/Y3Ag1.2.3. rat myeloma) per well in D-PBS at 20 μg/ml. After blocking with 250 μl of D-PBS containing 1% bovine serum albumin (Sigma) for 30 min at RT, 50 μl of D-PBS containing 1% bovine serum albumin, 5% FCS, and 1 M NaCl was added to each well. Samples and standard dilutions of recombinant murine IL-18 ranging between 1 and 0.1 ng/ml (in RPMI 1640 plus 10% FCS) were loaded in 50 μl in triplicate, and the plates were incubated at RT for 3 h. After being washed, the plates were incubated at RT for 3 h with 100 μl of the horseradish peroxidase-conjugated detection monoclonal antibody 93-10C–HRP (rat splenocytes/SP2/O mouse myeloma) in D-PBS containing 5% FCS, 0.15 M NaCl, and 0.1% 3-[(β-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS; Sigma) at 0.4 μg/ml. o-Phenylenediamine (OPD) (1 mg/ml in 0.2 M Na2HPO4–0.1 M citrate buffer) in the presence of H2O2 was used to develop the plates. The reaction was stopped by adding 15 μl of 3 M H2SO4 per ml. The optical density was read at 490 nm. IL-18 concentrations (in picograms per milliliter) were determined by comparison with the standard curve. The lower detection limit for the IL-18 ELISA was 120 pg/ml.
IFN-γ ELISA.
IFN-γ was measured by capture ELISA with antibody pairs and rIFN-γ purchased from PharMingen (Becton Dickinson UK, Ltd.). We coated each well of 96-well ELISA plates at 37°C for 2 h with 50 μl of a capture rat anti-mouse IFN-γ immunoglobulin G1 monoclonal antibody (clone R4-6A2) in 0.1 M NaHCO3 buffer (pH 8.2) at 2 μg/ml and incubated the plates overnight at 4°C. After blocking at 37°C for 1 h with RPMI 1640 supplemented with 10% FCS (RPMI-FCS), samples (diluted 1/2 in RPMI-FCS) were loaded at 50 μl in triplicate and the plates were incubated at 37°C for 2 h. Serial twofold dilutions of rIFN-γ ranging from 60 ng/ml to 40 pg/ml were included as standards. Use of 100 μl of the biotinylated rat anti-mouse IFN-γ immunoglobulin G1 monoclonal antibody (clone XMG1.2) at 1 μg/ml in PBS–10% FCS (1 h at 37°C) per well was followed by 100 μl of peroxidase-labelled streptavidin at 2.5 μg/ml (Sigma) in PBS–10% FCS (45 min at RT) per well. The reaction was developed and stopped as described for the IL-18 ELISA. IFN-γ concentrations (in nanograms per milliliter) were determined by comparison with the standard curve. We considered 100 pg/ml to be the lower limit of sensitivity of our ELISA.
Statistical analysis.
Student’s t test was used to determine the significance of differences between controls and experimental groups. We consider differences between experimental groups statistically significant for P < 0.05.
RESULTS
IL-18 production by macrophages infected with S. typhimurium.
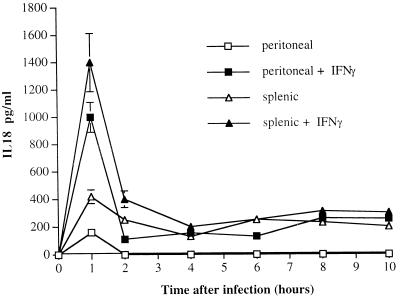
Peritoneal and splenic macrophages were cultured overnight in the presence of medium alone or medium supplemented with 800 U of rIFN-γ per ml. Thereafter, the cells were infected with S. typhimurium C5 RP4 and supernatants were collected for IL-18 measurements. Salmonella infection induced the release of low levels of IL-18 from untreated (no rIFN-γ) peritoneal and splenic macrophages 1 h after infection. Thereafter, low IL-18 levels were detectable in splenic macrophages cultures but not in supernatants from peritoneal macrophages (Fig. 1).
FIG. 1.
Peritoneal and splenic macrophages were cultured overnight with medium alone or in the presence of 800 U of rIFN-γ per ml. The cells were infected with S. typhimurium C5 RP4 (0 h) at a bacterium-to-cell ratio of 5:1. Supernatants for IL-18 measurements were collected at the indicated times thereafter. The results are expressed as the concentration of IL-18 from triplicate cultures (mean and standard deviation).
rIFN-γ-treated macrophages (both splenic and peritoneal) released higher levels of IL-18 than did untreated macrophages, with a peak being reached 1 h after infection and the cytokine being detectable in the supernatants throughout the experiment (10 h). The differences in IL-18 production between rIFN-γ-treated and untreated macrophages were statistically significant 1 and 2 h after infection (Fig. 1). The experiment in Fig. 1 is representative of three similar experiments.
A statistically significant enhancement of IL-18 production from peritoneal and splenic macrophages was observed over a wide range of IFN-γ concentrations (100 to 8,000 U/ml) (results not shown). No IL-18 was detected in uninfected cultures.
Thus, resident peritoneal and splenic macrophages release IL-18 in response to Salmonella. IFN-γ stimulation of mononuclear cells greatly increases IL-18 production.
Effect of IL-18 neutralization on host resistance to Salmonella.
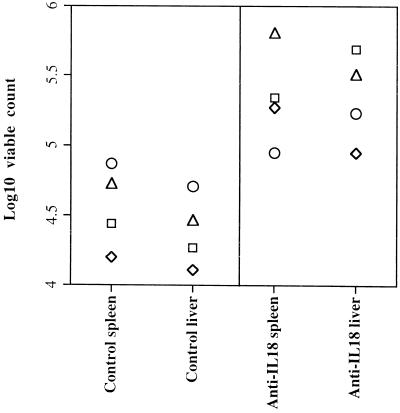
A/J mice were sublethally infected with ca. 103 CFU of the virulent S. typhimurium C5. One group of mice received two i.v. injections of 0.25 mg of anti-IL-18 neutralizing globulins 2 h before challenge and on day 3 of the infection; controls received a similar amount of normal rabbit globulins (NRG). On days 1 and 3, bacterial counts in the spleen and liver were similar in control and anti-IL-18-treated animals. Conversely, on day 7, bacterial counts in the spleen and liver were significantly higher in anti-IL-18-treated mice than in NRG-treated controls, indicating a clear exacerbation of the infection (Fig. 2). A repeat experiment with a larger amount (0.5 mg per dose) of anti-IL-18 antibodies gave similar results (data not shown).
FIG. 2.
A/J mice were sublethally infected with ca. 103 CFU of the virulent S. typhimurium C5. One group of mice received two i.v. injections of 0.25 mg of anti-IL-18 neutralizing globulins 2 h before challenge and on day 3 of the infection; controls received similar amounts of NRG. Bacterial counts in the spleen and liver were measured on day 7 of the infection. The results are expressed as log10 viable counts on four individual mice.
Thus, IL-18 is required for the control of bacterial growth in mice infected intravenously with a virulent S. typhimurium strain.
Effect of rIL-18 administration on a lethal Salmonella infection.
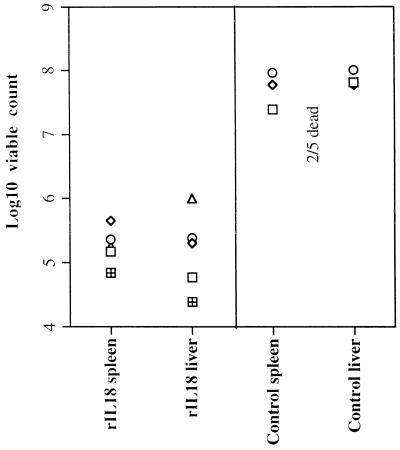
A/J mice were infected with a lethal dose (ca. 106 CFU) of S. typhimurium C5. One group of mice was intraperitoneally injected daily with 1.25 μg of rIL-18 starting 2 days prior to infection. Control mice were similarly treated with diluent (PBS).
On day 7, two of five control mice (injected with PBS) were dead and the remaining control animals showed signs of serious illness, while all the rIL-18-treated animals appeared healthy. Bacterial counts in the spleens and livers of the infected mice were measured. Control mice showed the expected high premortem bacterial loads, while fewer bacteria were present in the tissues of mice treated with rIL-18 (Fig. 3). A repeat experiment gave similar results.
FIG. 3.
A/J mice were given a lethal i.v. dose (ca. 106 CFU) of S. typhimurium C5. One group of mice was injected (i.p.) daily with 1.25 μg of rIL-18 starting 2 days prior to infection. Control mice were similarly treated with diluent (PBS). Bacterial counts in the spleen and liver were measured on day 7 of the infection. The results are expressed as log10 viable counts on five individual mice.
Thus, treatment with rIL-18 confers protection against a lethal bacterial challenge with virulent salmonellae.
IL-18 produced in response to Salmonella triggers IFN-γ release by spleen cells from T-cell-deficient mice.
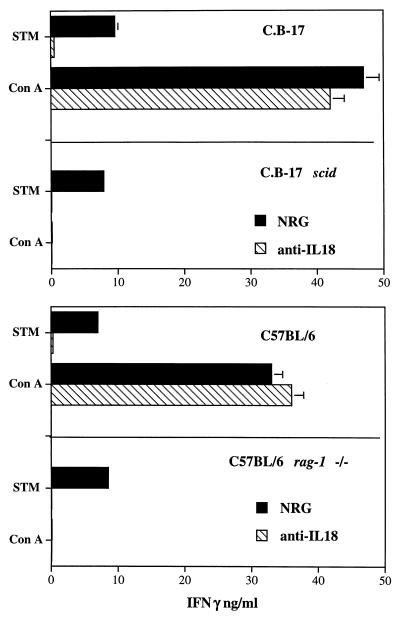
Splenocytes from uninfected C.B.-17 scid and C57BL/6 rag-1−/− mice (T- and B-cell deficient) and wild-type control mice were stimulated in vitro with 105 CFU of S. typhimurium C5 or with 5 μg of ConA per ml in the presence of 40 μg of anti-IL-18 neutralizing rabbit globulins per ml. Parallel cultures were stimulated in the presence of a similar amount of control rabbit globulins. IFN-γ was measured in the supernatants 48 h after stimulation. Cells from conventional mice of either strain produced significant levels of IFN-γ in response to both ConA and S. typhimurium; a dramatic and statistically significant reduction in IFN-γ release in response to Salmonella but not to ConA was seen in the cultures treated with anti-IL-18 antibodies compared to untreated cultures. Spleen cells from scid and rag-1−/− mice released IFN-γ in response to S. typhimurium but not in response to ConA. Addition of anti-IL-18 antibodies to the cultures eliminated IFN-γ release from spleen cells of scid and rag-1−/− mice (Fig. 4).
FIG. 4.
Spleen cells from C.B-17 and C.B-17 scid mice (top graph) and from C57BL/6 and C57BL/6 rag-1−/− mice (bottom graph) were stimulated in vitro with 105 CFU of S. typhimurium C5 (STM) or with 5 μg/ml of ConA in the presence of 50 μg of anti-IL-18 neutralizing antibodies (hatched bars) or NRG (solid bars) per ml. IFN-γ was measured in the supernatants at 48 h. The results are expressed as the concentration of IFN-γ in triplicate cultures (mean and standard deviation).
A statistically significant reduction in IFN-γ release in response to S. typhimurium but not to ConA was seen when using spleen cells from A/J mice (data not shown).
Thus, IL-18 neutralization reduces or eliminates IFN-γ release from spleen cells of conventional mice and scid and rag-1−/− (T- and B-cell-deficient) mice.
Effect of IL-18 neutralization on IFN-γ levels in the sera of infected mice.
A/J mice were infected and treated as in the experiment in Fig. 2. IFN-γ was measured in serum samples obtained on day 7 of the infection. IFN-γ levels were significantly lower in the sera of anti-IL-18-treated mice (300 ± 40 pg/ml) than in the sera of NRG-treated controls (1,250 ± 300 pg/ml).
Thus, in vivo neutralization of IL-18 causes a reduction in circulating IFN-γ levels.
Effect of rIL-18 treatment in IFN-γ-R−/− mice infected with virulent salmonellae.
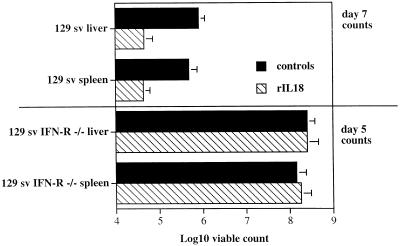
129 sv IFN-γ-R−/− mice and 129 sv controls were infected with ca. 105 CFU of S. typhimurium C5. One group of mice from each strain was injected daily i.p. with 1.5 μg of rIL-18 starting 2 days before infection. Control mice were treated with PBS. On day 5, both rIL-18-treated and PBS-treated 129 sv IFN-γ-R−/− mice showed serious signs of illness, and viable counts revealed similarly high bacterial loads in both groups (no statistical significance between groups), indicating that rIL-18 had exerted no protective effects. At the challenge dose used in this experiment, the infection appeared to be milder in conventional 129 sv mice, which were not ill on day 5. Nevertheless, day 7 viable counts in rIL-18-treated 129 sv mice were significantly lower than in the mice treated with PBS (Fig. 5).
FIG. 5.
129 sv IFN-γ-R−/− mice and conventional 129 sv mice were infected i.v. with 105 CFU of S. typhimurium C5. One group of mice from each strain was injected i.p. daily with 1.5 μg of rIL-18 neutralizing globulins starting 2 days prior to infection. Control mice from each strain received PBS only. Bacterial counts in the spleen and liver were measured on day 5 or 7 of the infection. The results are expressed as log10 viable count (mean and standard deviation) on groups of four mice.
In a similar experiment, mice were challenged with ca. 6 × 104 CFU of S. typhimurium C5. Viable counts were performed in both 129 sv mice and 129 sv IFN-γ-R−/− mice on day 6 of the infection. Once again, counts in rIL-18 treated 129 sv mice were significantly lower than in the mice treated with PBS, while the rIL-18 treatment had no effect in the IFN-γ-R−/− mice (data not shown).
Thus, IFN-γ is required for the protective effects of rIL-18 administration during salmonellosis.
DISCUSSION
In the present report, we show that IL-18 (IGIF) is involved in host resistance to virulent salmonellae.
Splenic and peritoneal macrophages released IL-18 upon infection with salmonellae. In vivo administration of anti-IL-18 antibodies exacerbated the course of the infection, while rIL-18 could ameliorate the disease in conventional but not in IFN-γ-R−/− mice. We also show that IL-18 positively modulates IFN-γ production from mouse splenocytes and enhances IFN-γ production in vivo.
The early mechanisms of immunity to Salmonella require the balanced interaction of a multiplicity of factors. We and others have previously shown that resistance to Salmonella in the mouse model requires granuloma formation as well as the action of cytokines and soluble factors including TNF-α, IL-12, IFN-γ, and nitric oxide (5, 6, 8, 12–18, 20, 21, 26, 30). We found that TNF-α is needed for granuloma formation in the RES (18); TNF-α neutralization prevents the formation of macrophage-rich focal lesion in the tissues and leads to unrestrained growth and dissemination of the bacteria in the organs. Others have found that nitric oxide is also involved in resistance and granuloma formation (30). In vivo and in vitro evidence indicates that IFN-γ plays a key role in macrophage activation by enhancing the ability of mononuclear cells to restrain bacterial growth in the tissues (6, 20, 21).
Increasing interest has been focused on cytokine networks in bacterial infections. Efforts have been made to elucidate the mechanisms that regulate IFN-γ, due to the importance of this cytokine in early resistance and in the development of Th1-type long-term immunity to disease. IL-12 had been identified as the main IFN-γ inducer in response to products of bacterial origin. We and others previously reported that IL-12 is absolutely required for host resistance and IFN-γ production in responses to salmonellae (8, 16).
In the present report, we conclusively show that IL-18 is crucial for resistance to virulent salmonellae and for IFN-γ production in vitro and in vivo. We found that mice injected with anti-IL-18 antibodies cannot efficiently control the growth of virulent salmonellae in the RES. Similar results obtained with the C. neoformans and Y. enterocolitica mouse models showed that in vivo neutralization of IL-18 impairs host resistance to infection (2, 7, 31). Therefore, it appears that a common mechanism involving IL-18 exists for the control of different pathogens. This can probably be explained considering the absolute requirement for IFN-γ production in host resistance to pathogens whose growth is controlled by macrophage activation, although IFN-γ-independent immune system mechanisms cannot be completely disregarded.
Our results show that IL-18 is required for IFN-γ production in response to Salmonella. In fact, addition of anti-IL-18 neutralizing antibodies to splenocyte cultures causes a dramatic reduction in IFN-γ release upon exposure to whole bacteria. These in vitro results are matched and further supported by our in vivo observations showing a reduction in circulating IFN-γ levels in mice treated with anti-IL-18 globulins. IL-18 plays a role in IFN-γ release from mouse splenocytes exposed to Y. enterocolitica (2), although the effect is not as strong as that observed upon IL-12 neutralization. We observed a dramatic reduction in IFN-γ production by mouse splenocytes after addition of either anti-IL-12 or anti-IL-18 antibodies to the cultures (reference 16 and see above). This discrepancy seems to indicate that the relative importance of IL-12 and IL-18 in the regulation of IFN-γ production can be different depending on the invading pathogen. IL-18 also induces an increase in IFN-γ levels in serum when exogenously administered to mice infected with C. neoformans (31). Furthermore, P. acnes-treated IL-18−/− mice show reduced IFN-γ levels in serum upon LPS administration (28), supporting the observation that IL-18 contributes to IFN-γ release in response to gram-negative bacterial products.
In vitro evidence indicates that CD4−NK1.1+ cells (NK cells) release IFN-γ in response to Salmonella (24, 25). Furthermore, IFN-γ can be detected in the circulation of Salmonella-infected nude, scid, and rag-1−/− (T-cell-deficient) mice infected with salmonellae (our unpublished observations). NK cells seem to be the main (although possibly not the only) target of the joint action of IL-12 and IL-18 in the early (T-cell-independent [5]) stages of murine salmonellosis. In fact, in the present study we found that neutralization of IL-18 reduces IFN-γ production from spleen cells of scid and rag-1−/− mice. These mice lack T cells, and therefore NK cells are the primary source of IFN-γ. Our findings therefore indicate that IL-18 production is triggered by Salmonella and that at least in T-cell-deficient mice, it induces IFN-γ production by acting presumably on NK cells. Nevertheless, our in vitro data, obtained with T-cell-deficient mice, cannot exclude that other IFN-γ-producing cells are also targeted by IL-18 in conventional animals. Furthermore, the lack of effect of IL-18 neutralization on IFN-γ release by splenocytes in response to ConA seems to indicate that naive T cells (within the total splenocyte population) do not require IL-18 to release IFN-γ in response to mitogenic stimuli. With the present data, we neither show nor infer that IL-18 would not act on immune T cells specifically stimulated with antigen.
IFN-γ seems to be required for the protective effects exerted by IL-18. In fact, administration of rIL-18 resulted in significant protection and lower bacterial loads in the RES in conventional but not in IFN-γ-R−/− mice. The in vivo and in vitro reduction or elimination of IFN-γ production, paralleled by the lack of protective effect of rIL-18 in IFN-γ-R−/− mice seems to indicate that IL-18 contributes to host resistance to Salmonella by triggering the production and release of IFN-γ. Similarly, recent evidence indicates that IL-18 protects mice against pulmonary and disseminated C. neoformans infections, acting mainly via IFN-γ induction (31). Conversely, administration of rIL-18 has been recently reported to have no protective effect in Y. enterocolitica-infected mice (2).
It is becoming increasingly clearer that in Salmonella infections, the expression of the bactericidal or bacteriostatic activity of the RES is achieved via IFN-γ induction and requires at least two distinct signals provided by the combined action of IL-12 and IL-18. Neither cytokine is dispensable, and no redundancy occurs. At present, we cannot exclude that IL-18 also mediates host resistance to Salmonella by inducing cytokines different from IFN-γ. Interestingly, a recent report indicates that IL-18 is required for TNF production by human NK cells and T cells (23).
IFN-γ appears to positively modulate IL-18 production from macrophages. A cytokine network can be envisaged in which Salmonella-induced IL-18 (in conjunction with IL-12), triggers IFN-γ production, which then enhances IL-18 release by mononuclear cells (see Results), ultimately resulting in the amplification of the initial response. This interpretation is in line with our in vitro data showing increased IL-18 production by macrophages cultured in the presence of IFN-γ and with recent observations showing that IL-18-induced IFN-γ production is reduced in IFN-γ-R−/− mice (2). Nevertheless, it remains to be established whether IFN-γ modulates IL-18 production in vivo.
The results shown in this paper allow us to conclude that IL-18 is a key factor in host resistance to virulent salmonellae by acting as a potent inducer of IFN-γ.
ACKNOWLEDGMENTS
This work was supported by grants from the EC, The Wellcome Trust, and the BBSRC.
REFERENCES
- 1.Ahn H J, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 2.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, Heeseman J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of bacterial infections in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 3.Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H. Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T-helper 1 cells. Cell Immunol. 1996;173:230–235. doi: 10.1006/cimm.1996.0272. [DOI] [PubMed] [Google Scholar]
- 4.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Hallen H. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 5.Hormaeche C E, Mastroeni P, Arena A, Uddin J, Joysey H S. T cells do not mediate the initial suppression of a salmonella infection in the RES. Immunology. 1990;70:247–250. [PMC free article] [PubMed] [Google Scholar]
- 6.Kagaya K, Watanabe K, Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989;57:609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFNγ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 8.Kincy-Cain T, Clements J D, Bost L. Endogenous and exogenous interleukin-12 augment protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 10.Lang T, Prina M, Sibithorpe D, Blackwell J M. Nramp1 transfection transfers Ity/Lsh/Bcg related pleiotropic effects on macrophage activation: influence on antigen processing and presentation. Infect Immun. 1997;65:380–386. doi: 10.1128/iai.65.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lissner C R, Swanson R N, O’Brien A D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- 12.Maskell D J, Hormaeche C E, Harrington K E, Joysey H S, Liew F Y. The initial suppression of bacterial growth in a Salmonella infection is mediated by a localised rather than a systemic response. Microb Pathog. 1987;2:295–305. doi: 10.1016/0882-4010(87)90127-6. [DOI] [PubMed] [Google Scholar]
- 13.Mastroeni P, Arena A, Costa G B, Liberto M C, Bonina L, Hormaeche C E. Serum TNFα in mouse typhoid and enhancement of a Salmonella infection by anti-TNFα antibodies. Microb Pathog. 1991;11:33–38. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- 14.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Effect of late administration of anti-TNFα antibodies on a Salmonella infection in the mouse model. Microb Pathog. 1993;14:473–480. doi: 10.1006/mpat.1993.1046. [DOI] [PubMed] [Google Scholar]
- 15.Mastroeni P, Harrison J A, Hormaeche C E. Natural resistance and acquired immunity to Salmonella. Fundam Clin Immunol. 1994;2:83–95. [Google Scholar]
- 16.Mastroeni P, Harrison J A, Chabalgoity J A, Hormaeche C E. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastroeni P, Harrison J A, Robinson J H, Clare S, Khan S, Maskell D J, Dougan G, Hormaeche C E. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastroeni P, Skepper J N, Hormaeche C E. Effect of anti-tumor necrosis factor alpha antibodies on hystopathology of primary Salmonella infections. Infect Immun. 1995;63:3674–3682. doi: 10.1128/iai.63.9.3674-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer Zum Buschenfelde C, Cramer S, Trumpfheller C, Fleisher B, Frosch S. Trypanosoma cruzi induces strong IL12 and IL18 gene expression in vivo: correlation with interferon-γ (IFNγ) production. Clin Exp Immunol. 1997;110:378–385. doi: 10.1046/j.1365-2249.1997.4471463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muotiala A, Makela P H. The role of IFNγ in murine Salmonella typhimurium infection. Microb Pathog. 1990;8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 21.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamura H, Tsutsui H, Komasu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning a new cytokine that induces IFNγ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 23.Puren A J, Fantuzzi G, Gu Y, Su M S, Dinarello C A. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1beta via TNFα production from non-CD14+ human blood mononuclear cells. J Clin Investig. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramarathinam L, Niesel D W, Klimpel G R. Ity influences the production of IFN-γ by murine splenocytes stimulated in vitro with Salmonella typhimurium. J Immunol. 1993;150:3965–3972. [PubMed] [Google Scholar]
- 25.Ramarathinam L, Niesel D W, Klimpel G R. Salmonella typhimurium induces IFN-γ production in murine splenocytes. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 26.Richter-Dahlfors A, Buchan A M J, Finlay B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoll S, Muller G, Kurimoto M, Saloga J, Tanimoto T, Yamauchi H, Okamura H, Knop J, Enk A H. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J Immunol. 1997;159:298–302. [PubMed] [Google Scholar]
- 28.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 29.Tone M, Thompson S A J, Tone Y, Fairchild P J, Waldman H. Regulation of IL18 (IFNγ inducing factor) gene expression. J Immunol. 1997;159:6156–6163. [PubMed] [Google Scholar]
- 30.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin 12 (IL-12) and IL18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]