Abstract
Objectives:
Interferons (IFNs) are one of the most important components of innate immunity against viruses, especially those carrying the RNA genomes such as influenza viruses. Upon viral infection, the IFNs are rapidly secreted, inducing the expression of several genes in the target cells and establishing an antiviral state. In this study, the effects of proteins encoded by some IFN-related genes on influenza A virus RNA-dependent RNA polymerase enzyme were investigated. We evaluated the importance of these proteins in the pathogenesis of different influenza A virus types.
Materials and Methods:
The IFN-related genes were amplified by polymerase chain reaction from the HEK293 cDNA library and cloned into pCHA expression vector. The expression of genes and subcellular localizations of the proteins were determined by Western blotting and immunofluorescence staining, respectively. The effects of IFNs-related proteins on virus RdRP enzyme were determined by influenza A virus mini-replicons.
Results:
The study revealed that the influenza A virus infections significantly altered the transcript level of the IFN-related CCL5, IFIT1, IFIT3, IFITM3, and OAS1 genes in HEK293 cells. It was determined that the alteration of the gene expression was also related to the virus type. The mini-replicon assays showed that the transient expression of CCL5, IFI27, OAS1, IFITM3, IFIT1, and IFIT3 have inhibitory effects on WSN and/or DkPen type virus RdRP enzymes. We observed that the proteins except OAS1 inhibited WSN type RdRP enzyme at a higher level than that of DkPen enzyme.
Conclusion:
It was concluded that influenza A virus infection significantly alters the IFN-related gene expression in the cells. Most of the proteins encoded from these genes showed an inhibitory effect on the virus RdRP enzymes in the HEK293 cells. The inhibition of the influenza virus RdRP with IFN-related proteins may be the result of direct or indirect interactions between the host proteins and the viral enzyme subunits.
Keywords: Influenza A virus interferon response, PCR array, influenza RdRP
INTRODUCTION
Influenza A viruses are enveloped viruses classified in the Orthomyxoviridae family. These viruses have a genome consisting of single-stranded, negative-sense RNA molecules. Influenza A viruses can be easily transmitted from person to person and cause upper respiratory disease. The high mutation rate and reassortment of the viral genome make it necessary to develop new prevention and treatment methods.1 In this context, it is critical to identify the viral and cellular protein factors that play a role in virus-host interactions. However, innate and acquired immunity are of great importance in terms of the severity of influenza A virus infections. Interferons (IFNs) are among the most important actors of innate immunity.2,3 The IFNs bind to specific receptors on the target cells, triggering several biochemical reactions within the cell and obstructing or completely inhibiting the virus replication. This occurs because of interactions between IFN-related host proteins and viral replication processes. The IFNs secreted from the infected cells stimulate the transcription of the genes carrying the ISRE control elements by transmitting signals to other cells through both paracrine and endocrine pathways. These proteins, which are synthesized because of the stimulation of IFNs, act as barriers to prevent viral replication in the cells by different mechanisms.
The influenza A virus RNA polymerase enzyme, consisting of PB2, PB1, and PA subunits, is the target of some host cellular proteins stimulated by IFNs. Among these proteins, Mx proteins inhibit influenza A virus RNA polymerase enzymes by direct interaction or indirect mechanisms.4,5 Additionally, the viral RNA polymerase enzyme plays an important role in controlling the host’s anti-viral mechanisms by binding to cellular proteins such as IPS-1 or inhibiting IFNβ production.6 In this study, how the expression levels of some genes related to the IFN response were affected in cells infected with influenza A viruses was evaluated. The effects of the proteins encoded by these genes on the viral RdRP enzyme activity were investigated with a mini-replicon assay.5
MATERIALS AND METHODS
Cells
Human cervical cancer cells (HeLa) and human embryonic kidney cells (HEK293) were used for viral infections and transient transfection assays. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) medium supplemented with 10% of heat-inactivated fetal calf serum (Gibco Laboratories, Gaithersburg, MD, USA, #11573397), 100 IU/mL penicillin, 100 µg/mL streptomycin, and 2 mM glutamine. The cultures were incubated at 37°C in an atmosphere of more than 95% humidity and 5% carbon dioxide.
Viruses
Influenza A/WSN/33/H1N1 (WSN) and low pathogenic avian influenza A/Duck/Pennsylvania/10,218/84/H5N2 (DkPen) viruses were used to infect the HEK293 cells. The viruses were obtained from infectious biology (virology) laboratory of Tsukuba University (Japan). Viruses were propagated in Madin-Darby canine kidney cells and titrated with a standard plaque formation assay.7
Plasmid vectors
Some plasmids were constructed within the scope of this work whereas others were constructed in previous studies. For protein expression in transiently transfected HEK293 and HeLa cells, the IFN-related chemokine (C-C motif) ligand 5 (CCL5), IFN, alpha-inducible protein 27 (IFI27), IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), IFN-induced protein with tetratricopeptide repeats 3 (IFIT3), IFN-induced transmembrane protein 3 (IFITM3), and 2’-5’-oligoadenylate synthetase 1 (OAS1) genes were cloned in pCHA plasmid8 derived from pCAGGS.9 The cDNAs were prepared with reverse transcription of the HEK293 cell RNA. A 500 ng of RNA and 50 pmol oligo-dT primer were added to a 1.5 mL plastic tube, and the volume was increased to 12.5 µL with nuclease free-water. After denaturation of the RNA molecules at 65°C for 10 min, 4 µL 5x reaction buffer, 0.5 µL (40 U/µL) RNase inhibitor (Biotechrabbit GmbH, Hennigsdorf, Germany, #BR0400901), 2 µL (10 mM) dNTP mixture, and 1 µL (200 U/µL) reverse transcriptase enzyme (Biotechrabbit GmbH, Hennigsdorf, Germany, #BR0400601) were added to the samples. The reaction was carried out at 45°C for 60 min and then held at 80°C for 10 min to inactivate the reaction. The cDNAs of the CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 genes were amplified by polymerase chain reaction (PCR) by using gene-specific primer pairs phosphorylated with T4 polynucleotide kinase (New England Biolabs Ltd., Hitchin, UK, #M0201S). The primers given in Table 1 were designed from reference gene sequences of the genes (CCL5/NCBI reference sequence: NM_002985.3, IFI27-v.1/NCBI reference sequence: NM_001130080.3, IFIT-v.1/NCBI reference sequence: NM_001548.5, IFIT3/NCBI reference sequence: NM_001549.6, IFITM3 -v.1/NCBI reference sequence, and OAS1/NCBI reference sequence: NM_016816.4). The PCR-amplified cDNAs were cloned into pCHA plasmid digested by EcoRV (New England Biolabs Ltd., Hitchin, UK, #R095S), and the resultant plasmid vectors were designated as pCHA-CCL5, pCHA-IFI27, pCHA-IFIT1, pCHA-IFIT3, pCHA-IFITM3, and pCHA-OAS1.
Table 1. Oligonucleotide primers used in PCR amplification of human CCL5, IFI27, IFIT1, IFIT3, IFIM3, and OAS1 genes.
The construction of plasmids sets of the influenza A virus mini-replicon (pCAGGS-PB2/WSN, pCAGGS-PB1/WSN, pCAGGS-PA/WSN, pCAGGS-NP/WSN, pCAGGS-PB2/DkPen, pCAGGS-PB1/DkPen and pCAGGS-PA/DkPen, pCAGGS-NP/DkPen and pHH21-vNS-Luc) has been described previously.5,10 For normalization of firefly luciferase, the pRL (Promega, Madison, USA, #E289B) plasmid encoding Renilla reniformis luciferase was used.
Infections of HEK293 cells with viruses and total RNA extraction
The HEK293 cells were seeded in 12 well plates (5 x 105 cells/well) and incubated in standard culture conditions (37°C, 5% CO2, and >95% humidity) for 24 h. After incubation, the media of the cultures were removed, and the cells were washed with serum-free DMEM medium. 200 µL of influenza A virus (WSN or DkPen) samples diluted at an average of 1 moi in 1% BSA were added to the cell monolayers. Plates were kept for 30 min at 37°C with gentle mixing at 5 minute intervals for virus attachment. The virus suspensions were removed, and 1 mL of OPTI-MEM (Gibco Laboratories, Gaithersburg, MD, USA, #31600-083) was added to the cells. Infected cultures were incubated for 8 h, and then the RNA was extracted from the cells. The total RNA extraction was carried out with a commercial RNA extraction kit (New England Biolabs Ltd., Hitchin, UK, #T2010S), according to the manufacturer’s instructions.
Quantification of IFN-related gene transcripts in virus - infected cells with qPCR
The cDNAs of IFN-related genes from the total RNA of virus-infected cells were prepared as described above. The 96 well “RT2 Profiler PCR Array” (PAHS-016ZD-6) plates developed by Qiagen (Qiagen, Hilden, Germany) was used for quantitation of CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 gene transcripts in the cDNAs with quantitative real-time qPCR. Equal amounts of ×2 SYBR Green Master Mix (Roche, Mannheim, Germany, #4673484001) were added to each PCR tube containing 2.5 µL cDNA and 5 µL primer mix. The cycle conditions were applied as an initial denaturation step at 95°C for 10 min, followed by 45 cycles of amplification for 15 s at 95°C and 1 min at 60°C. The relative quantities of the transcripts were normalized by the amount of signal transducer and activator of transcription 3 transcript level. The relative expression values of each gene were revealed, and the results were given as a bar graph and heat map.
Transfection
Polyethyleneimine (PEI) was used for transfection of plasmid DNA into the HEK293 cells.11 The cells were seeded in 12 well culture plates (1x105 cells/well) and incubated at standard culture conditions for 24 h. The plasmid DNAs diluted in Opti-MEM (Gibco, USA, #31600-083) at a concentration of 20 ng/µL were mixed with equal volumes of the PEI solution (50 ng/ µL). The mixtures were maintained at room temperature for 5-10 minutes for complex formation and added to the cultures. Forty-eight hours after transfection, the cells were harvested for Western blotting or luciferase enzyme assays.
Western blotting
The expression of human CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 genes in transiently transfected HEK293 cells was analyzed by Western blotting. Plasmid DNA (1-2 µg) was transfected into HEK293 cells as described above. After 48 h, the cells were harvested in an SDS-sample loading buffer. The proteins in the cell lysates were separated with SDS-PAGE and transferred to the polyvinylidene difluoride membrane. After blocking with 5% skim milk, the membrane was first treated with mouse monoclonal anti-HA (Santa Cruz, #sc-7392) followed by horseradish peroxidase-conjugated second antibody [anti-mouse IgG-HRP (invitrogen, #31420). The proteins were visualized with an ECL detection kit (GE Healthcare, Italy, #RPN3004).
Immunofluorescence assay
The HeLa cells were seeded on coverslips in a 12 well palate (7.5x104 cells/well) and incubated in standard culture conditions for 24 h. Then the cells were transfected with 1.5 µg of the plasmid DNA encoding CCL5, IFI27, IFIT1, IFIT3, IFITM3 or OAS1 proteins as described above. After 40 h transfection, the cells were rinsed in PBS and fixed with 3% para-formaldehyde. The cells were permeabilized with 0.1% NP-40, washed in PBS, treated with 1% skim milk for 30 min, and incubated with mouse monoclonal anti-HA for 60 min. After washing with PBS, the cells were treated with Alexa-488-conjugated goat anti-mouse IgG (at 1:300 dilutions in 1% skim milk) for 60 min. The nuclei of the cells were stained with DAPI. The coverslips were mounted in a medium (0.1% p-phenylenediamine and 80% glycerol), and the cells were visualized using a fluorescence microscope.
RdRP activity assay
The effects of IFNs-related proteins on the influenza A virus RdRP enzyme were investigated with the influenza A virus mini-replicon system.5 The HEK293 cells were seeded in 24 well plates (5x104 cells/well) and incubated under standard culture conditions for 24 h. The plasmids encoding IFNs-associated proteins were mixed with a set of mini-replicon plasmids (pHH21-vNS-luc, pCAGGS-PB1, pCAGGS-PB2, pCAGGS-PA, and pCAGGS-NP). The pRL plasmid was used for normalization. The total amount of plasmid DNA for each well was adjusted to 250 ng with the pCAGGS plasmid. Then, the plasmid DNAs were transfected with PEI as described above. After 48 h transfection, the cells were lysed in a lysis buffer (Promega, Madison, USA, # E1531), and luciferase activity was detected with commercial kits (for firefly luciferase, #E1483; for Renilla luciferase, #Z3051, Promega, Madison, USA) according to the manufacturer’s instructions.
Statistical analysis
The statistical significance of differences between experimental groups was evaluated using analysis of variance (one-way ANOVA with Newman-Keuls post-test) in the SPSS. P values less than 0.05 were considered statistically significant.
RESULTS
Quantifications of IFN-related gene transcript in virus-infected cells
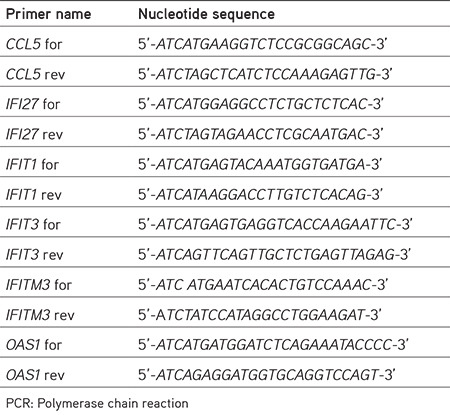
In our previous study, the expression profiles of 84 IFN and IFN-related genes were determined in the cells infected with influenza A viruses.12 Here, the CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 genes, which are thought to be important in viral replication, and the effects of proteins encoded by these genes on influenza A viral RdRP enzymes were evaluated. The transcript levels of the genes in HEK293 cells infected with two types of influenza A virus were determined by the qPCR technique (Figure 1).
Figure 1.
Expression profiles of interferon-related genes in HEK293 cells infected with influenza A viruses (WSN or DkPen) and uninfected HEK293 cells
The results showed that CCL5 gene expression is up-regulated in the HEK293 cells infected with both influenza A virus types (p<0.01). It was observed that up-regulation was more pronounced in the cells infected with WSN type viruses compared with the DkPen type viruses. No significant change was detected in the IFI27 gene transcript level in the virus-infected cells. The profiles of the IFIT1 and OAS1 gene transcripts in the virus-infected cells were found to be similar to the changes in the CCL5 gene transcript levels. A significant increase in the transcript level of these two genes was detected in the cells infected with WSN type viruses compared with uninfected cells (p<0.01). In contrast, IFIT3 gene was up-regulated in WSN-type virus-infected cells, while it was significantly down-regulated in the cells infected with DkPen type viruses. While some decrease in IFITM3 gene transcript was observed in DkPen type virus-infected cells, there was no statistically significant change in the IFITM3 gene transcript level in WSN virus-infected cells.
Expression and subcellular localization of the IFN-related proteins
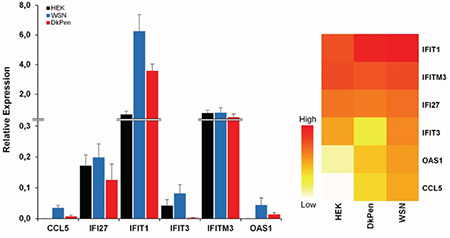
To identify the effects of proteins encoded by the IFN-related genes on the influenza A virus RdRP enzymes, the genes were cloned into the pCHA mammalian expression vector. Expression of the genes and subcellular localization of the encoded proteins were determined by Western blotting and immunofluorescence techniques, respectively (Figures 2 and 3). Western blotting revealed that the genes cloned into the pCHA plasmid were expressed at different levels in the HEK293 cells. The expected band sizes were detected based on the size of the protein: ~46 kDa for OAS1, ~55 kDa for IFIT1, ~56 kDa for IFIT3, ~15 kDa for IFITM3, and ~12 kDa for IFI27 (Figure 2A). The CCL5 protein (~10 kDa) could not be detected with Western blotting in transiently transfected HEK393 cells. The expression levels of OAS1, IFIT1, and IFIT3 proteins were found to be higher than that of IFITM3 and IFI27 proteins in transiently transfected HEK293 cells (Figure 2B).
Figure 2.
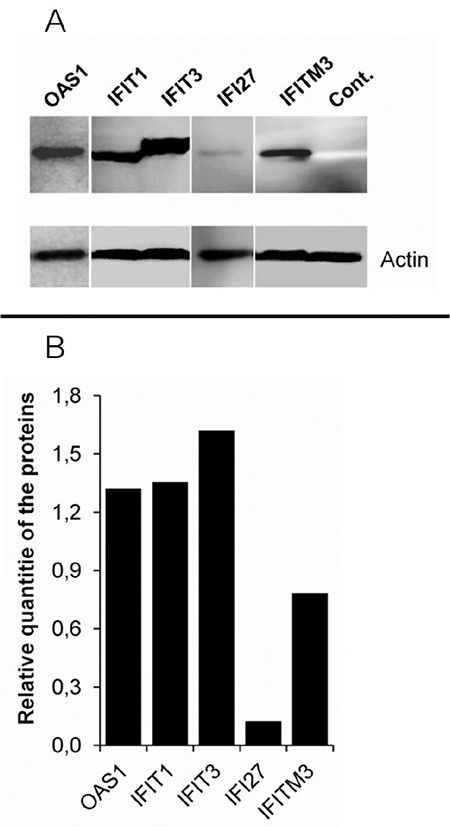
Western blot analysis of the interferon-related proteins encoded from the plasmid vectors in the HEK293 cells transiently transfected. A) The bands of OAS1, IFIT1, IFIT3, IFI27, IFITM3 and actin beta proteins. B) Relative quantity of the proteins compared to the actin beta. A total of 15 μL cell lysate was loaded into each well of 10% polyacrylamide gel. Electrophoresis was performed under a constant voltage of 40 V/gel for 15 minutes and then 80 V/gel for 75 minutes. The relative quantity of the proteins was determined by using ImageJ software. Cont: Untransfected-HEK293 cell lysate
Figure 3.
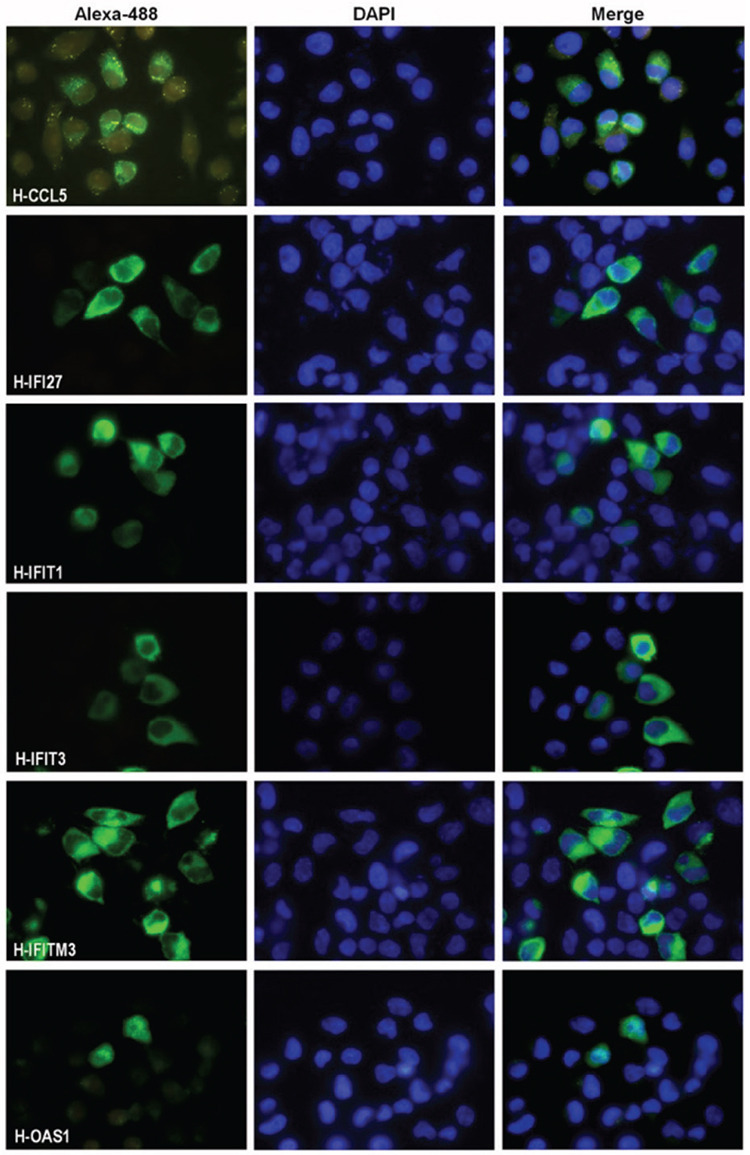
Subcellular localizations of interferon-related proteins encoded by plasmids in HeLa cells. HeLa cells were transiently transfected with plasmids encoding HA-tagged CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 proteins. The cells were fixed 44 h after transfection and stained with mouse monoclonal anti-HA antibodies. Alexa 488 conjugated anti-mouse IgG antibody (1/300 dilution) was used as the secondary antibody. The cell nuclei were stained with DAPI
HeLa: Human cervical cancer cell, IgG: Immunoglobulin
We have examined the subcellular localizations of the IFN-related proteins in the HeLa cells with an immunofluorescence assay. HeLa cells were transiently transfected with plasmids coding the HA-tagged proteins. The cells were fixed 44 h after transfection and stained with mouse monoclonal anti-HA antibodies (Figure 3). The results showed that all genes cloned into the pCHA plasmid were expressed in the cells. It was observed that all proteins except the OAS1 were dominantly localized in the cytosol. The presence of CCL5 and IFITM3 proteins as granules in the cytoplasm indicated that these proteins were localized in the organelles. The OAS1 protein was observed more intensely in the cell nucleus. However, OAS1 protein was found to be present in significant amounts in the cytoplasm.
Effects of IFN-related proteins on the influenza A virus RNA polymerase enzyme
The influenza A virus RdRP enzyme consists of three subunits-PB2, PB1, and PA-that performs transcription and replication of the viral genome. This enzyme, which is vital for virus replication, interacts with many cellular events and proteins. These interactions may be necessary for the functions of the RdRP enzyme or may cause the enzyme inhibition. Therefore, the effects of CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 proteins, which are thought to be important for influenza A virus replication, were tested in the viral RdRP enzymes in the HEK293 cells by using the mini-replicon assays.5,10 The HEK293 cells were co-transfected with a certain number of minireplicon plasmids and increasing number of plasmids encoding IFNs-related proteins. The reporter luciferase activity normalized with Renilla luciferase in transfected HEK293 cells is shown in Figure 4. The results showed that the proteins, except OAS1, negatively affected both types of influenza A virus RdRP enzymes at different levels. We observed that the proteins had a higher inhibition on the WSN type virus RdRP enzyme than that of DkPen in the HEK293 cells. In contrast, the OAS1 protein did not have a significant effect on the WSN type virus RdRP in the cells while it had a negative effect on the DkPen type virus enzyme.
Figure 4.
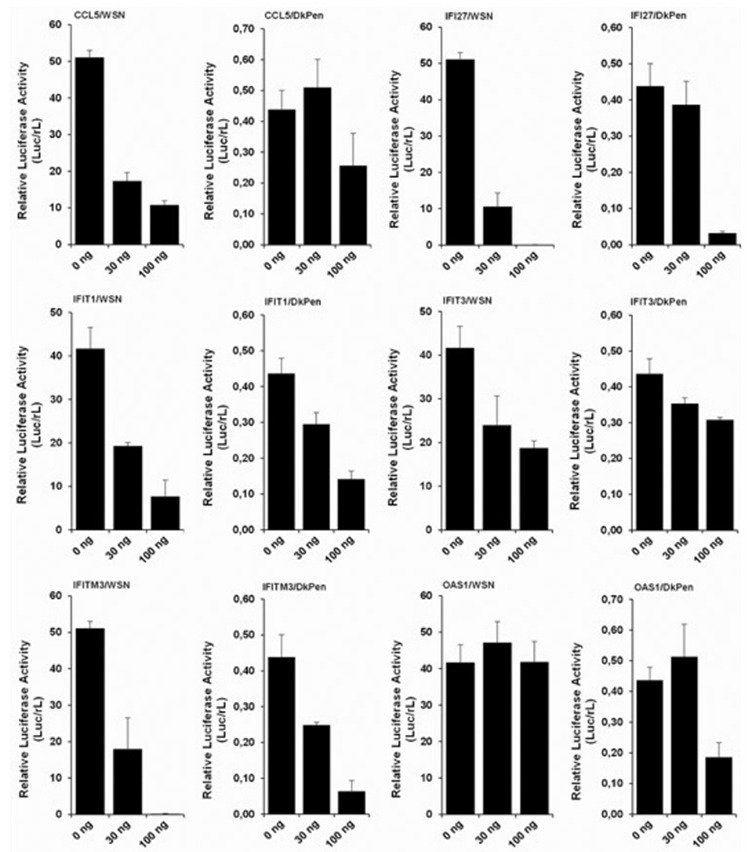
The effects of interferon-related CCL5, IFI27, IFIT1, IFIT3, IFITM3, and OAS1 proteins on WSN- and DkPen-type virus RdRP enzymes in the HEK-293 cells. The cells were grown in 24 well plates, and co-transfected with a protein coding-plasmid (30/well or 100 ng/well), and a certain amount of minireplicon plasmids [WSN: pCAGGS-PB2/W (3 ng/well), pCAGGS-PB1/W (3 ng/well), pCAGGS-PA/W (1 ng/well), pCAGGS-NP/W (10 ng/well), pHH21-vNS-luc (5 ng/well), pRL (2 ng/well), DkPen: pCAGGS-PB2/D (10 ng/well), pCAGGS-PB1/D (10 ng/well), pCAGGS-PA/D (3 ng/well), pCAGGS-NP/D (10 ng/well), pHH21-vNS-luc, (5 ng/well) pRL (2 ng/well)]. After 48 hours transfection, the cells were lysed, and reporter luciferase and Renilla luciferase activities were measured with luciferase assay kits
DISCUSSION
Among the host defense mechanisms against the viral infections, the IFN-related pathways of host cells are of great importance. Influenza infection causes rapid synthesis and secretion of type I IFNs after the appearance of viral components in infected cells. The binding of these proteins to the specific receptors on the cell surface causes up-regulation of hundreds of IFN-stimulated genes, which creates an “antiviral state” that will limit the further proliferation and spread of the viruses.13 In this study, the expression profiles of some genes related to the IFN pathway in virus-infected cells and the effects of the proteins encoded from these genes on the influenza A virus RNA polymerase enzyme were investigated. Several proteins in the IFN pathway interact with influenza A viruses, some of which have negative regulatory effects on viral replication and some of which have stimulating effects.5,14,15,16,17,18,19 However, the expression profiles of various genes in the cells infected with the viruses are altered at different levels and in different directions. It was observed that IFIT3 gene expression was up-regulated in cells infected with WSN-type virus but down-regulated in DkPen-type virus-infected cells (Figure 1). Genes other than IFI27 were more up-regulated in the cells infected with WSN-type viruses than those of DkPen. The results showed that the transcript levels of genes except IFI27 were higher in the cells infected with WSN type viruses. In some studies, it has been reported that DkPen-type viruses have a lower replication efficiency in the mammalian cells.20,21,22 Although this seems to contradict the fact that the DkPen-type virus infection up-regulates the expression of some IFN-related genes less than WSN type viruses; this may be a result of the DkPen virus RdRP enzyme inhibiting the host cell gene expression more than the WSN type virus enzyme.12
The influenza A virus RdRP enzymes are targets of some IFN-related proteins. In this study, the HEK293 cells were co-transfected with certain number of minireplicon plasmids and increasing number of the plasmids encoding IFN-related proteins, and the viral RdRP enzyme activities were measured via the luciferase reporter enzyme activity. The results showed that the expression of IFN-related proteins CCL5, IFI27, IFITM3, IFIT1, and IFIT3 have inhibitory effects on both WSN and DkPen-type virus RdRP enzymes. OAS1 protein showed a negative effect only on the DkPen virus RdRP (Figure 4). The CCL5 gene encodes a G-protein-coupled receptor protein carrying 7 transmembrane domains. The CCL5 protein, along with the CCL3 and CCL4 receptors, belongs to the C-C chemokine family.23 It was reported that the CCL5 gene, whose transcript level is up-regulated in HEK293 cells infected with influenza A viruses, negatively affects the virus replication, and mutant mice with this gene were found to be very susceptible to virus infection.24 In this study, it was determined that the CCL5 gene transcript level increased in HEK293 cells infected with both the WSN- and DkPen-type viruses. Furthermore, the CCL5 gene expression was increased approximately 5 times more with the WSN-type virus infection compared to the DkPen viruses. This difference may be important for influenza A virus host specificity.
No significant change was detected in the transcript level of the IFI27 gene in virus-infected HEK293 cells. However, the high inhibition of influenza RdRP enzymes by the IFI27 protein in HEK293 cells reveals the importance of this protein for viral replication. Remarkable increases in the expression level of this gene in virus-infected organisms have been reported.25 Tang et al.26 demonstrated that IFI27 is an important diagnostic marker in the differentiating of influenza and bacterial infections.
One of the IFN-related genes whose transcript levels were increased in HEK293 cells infected with the influenza A virus is the OAS1. The OAS1 gene expression in WSN-infected cells was also found to be higher than that of DkPen viruses. The human OAS1 gene belongs to the OAS gene family, which includes the OAS2, OAS3, and OASL genes.27 These genes encode the 2’-5’ oligoadenylate synthetase enzymes that catalyzing the formation of 2’-5’ oligoadenylate, which activates RNase L enzymes.28,29 The active RNase L enzyme inhibits the protein synthesis by breaking down both viral and cellular RNA molecules.30,31,32 Although there was a significant increase in the OAS1 transcript level in cells infected with influenza A viruses, it was observed that this protein did not cause an inhibition of the WSN-type virus RdRP enzyme (Figure 4). In contrast, it was observed that the OAS1 expression partially inhibited the DkPen virus RdRP enzyme. The negative effect of OAS1 on the DkPen virus RdRP enzyme can be considered a reason for the slow replication of this virus type in the mammalian cells.
IFITM3 belongs to the IFITM. These proteins get the fusion of viral and endosomal membranes difficult and prevent the release of virus genomes inside the cell.14,15,17 No significant change in the transcription of this gene was observed in the WSN virus-infected cells (Figure 1). However, both viral RdRP enzymes were strongly inhibited by IFITM3 expression (Figure 4).
IFIT proteins (IFIT1 and IFIT3) related to the IFNs defense mechanism have negative regulatory effects on viral replication. These proteins specifically inhibit the viral replication by binding directly to the viral nucleic acids or disrupting the function of eukaryotic initiation factor 3.33 Both of these proteins showed a negative effect on the influenza A virus RdRP enzyme. However, while the IFIT3 gene transcription was up-regulated in WSN virus-infected cells, the down-regulation of the cells infected with DkPen viruses remains to be elucidated.
CONCLUSION
In conclusion, most IFN-related proteins focused on in this study showed inhibitory effects on the virus RdRP enzymes in the HEK293 cells. However, the possible interactions of these proteins with the RdRP enzyme subunits have not been considered. Therefore, it is uncertain that these proteins directly inhibit influenza A virus RdRP enzymes in minireplicons by direct interaction with the subunits. The inhibition of the influenza virus RdRP enzymes with IFN-related proteins in transfected HEK293 cells may also be the result of the indirect interactions.
Footnotes
Ethics
Ethics Committee Approval: Not necessary.
Informed Consent: Not necessary.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Concept: E.Ç., K.T., Design: E.Ç., K.T., Data Collection or Processing: E.Ç., K.T., Analysis or Interpretation: E.Ç., K.T., Literature Search: E.Ç., K.T., Writing: E.Ç., K.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This work was supported by a grant from the Marmara University Research Foundation (grant no: SAG-C-DRP-250919-0291).
References
- 1.Chao DL, Bloom JD, Kochin BF, Antia R, Longini IM Jr. The global spread of drug-resistant influenza. J R Soc Interface. 2012;9:648–656. doi: 10.1098/rsif.2011.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitini V, Arrigo C, Altavilla G. How cells respond to interferons. J Clin Oncol. 2010;28:e439–author reply e440. doi: 10.1200/JCO.2010.28.9603. [DOI] [PubMed] [Google Scholar]
- 3.Stark GR. How cells respond to interferons revisited: from early history to current complexity. Cytokine Growth Factor Rev. 2007;18:419–423. doi: 10.1016/j.cytogfr.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhelst J, Parthoens E, Schepens B, Fiers W, Saelens X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. 2012;86:13445–13455. doi: 10.1128/JVI.01682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turan K, Mibayashi M, Sugiyama K, Saito S, Numajiri A, Nagata K. Nuclear MxA proteins form a complex with influenza virus NP and inhibit the transcription of the engineered influenza virus genome. Nucleic Acids Res. 2004;32:643–652. doi: 10.1093/nar/gkh192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwai A, Shiozaki T, Kawai T, Akira S, Kawaoka Y, Takada A, Kida H, Miyazaki T. Influenza A virus polymerase inhibits type I interferon induction by binding to interferon beta promoter stimulator 1. J Biol Chem. 2010;285:32064–32074. doi: 10.1074/jbc.M110.112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turan K, Nagata K, Kuru A. Antiviral effect of Sanicula europaea L. leaves extract on influenza virus-infected cells. Biochem Biophys Res Commun. 1996;225:22–26. doi: 10.1006/bbrc.1996.1125. [DOI] [PubMed] [Google Scholar]
- 8.Nagata K, Saito S, Okuwaki M, Kawase H, Furuya A, Kusano A, Hanai N, Okuda A, Kikuchi A. Cellular localization and expression of template-activating factor I in different cell types. Exp Cell Res. 1998;240:274–281. doi: 10.1006/excr.1997.3930. [DOI] [PubMed] [Google Scholar]
- 9.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 10.Pham PTV, Turan K, Nagata K, Kawaguchi A. Biochemical characterization of avian influenza viral polymerase containing PA or PB2 subunit from human influenza A virus. Microbes Infect. 2018;20:353–359. doi: 10.1016/j.micinf.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Longo PA, Kavran JM, Kim MS, Leahy DJ. Transient mammalian cell transfection with polyethylenimine (PEI) Methods Enzymol. 2013;529:227–240. doi: 10.1016/B978-0-12-418687-3.00018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Çağlayan E, Turan K. Expression profiles of interferon-related genes in cells infected with influenza A viruses or transiently transfected with plasmids encoding viral RNA polymerase. Turk J Biol. 2021;45:88–103. doi: 10.3906/biy-2005-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 14.Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, Ryan BJ, Weyer JL, van der Weyden L, Fikrig E, Adams DJ, Xavier RJ, Farzan M, Elledge SJ. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai TM, Marin M, Chin CR, Savidis G, Brass AL, Melikyan GB. IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog. 2014;10:e1004048. doi: 10.1371/journal.ppat.1004048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller O, Arnheiter H, Lindenmann J, Gresser I. Host gene influences sensitivity to interferon action selectively for influenza virus. Nature. 1980;283:660–662. doi: 10.1038/283660a0. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Markosyan RM, Zheng YM, Golfetto O, Bungart B, Li M, Ding S, He Y, Liang C, Lee JC, Gratton E, Cohen FS, Liu SL. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9:e1003124. doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlovic J, Haller O, Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992;66:2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao H, Killip MJ, Staeheli P, Randall RE, Jackson D. The human interferon-induced MxA protein inhibits early stages of influenza A virus infection by retaining the incoming viral genome in the cytoplasm. J Virol. 2013;87:13053–13058. doi: 10.1128/JVI.02220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slepushkin VA, Staber PD, Wang G, McCray PB Jr, Davidson BL. Infection of human airway epithelia with H1N1, H2N2, and H3N2 influenza A virus strains. Mol Ther. 2001;3:395–402. doi: 10.1006/mthe.2001.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wille M, Holmes EC. The Ecology and evolution of influenza viruses. Cold Spring Harb Perspect Med. 2020;10:a038489. doi: 10.1101/cshperspect.a038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Hale BG, Xu K, Sun B. Viral and host factors required for avian H5N1 influenza A virus replication in mammalian cells. Viruses. 2013;5:1431–1446. doi: 10.3390/v5061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med. 2005;11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Jiao B, Yao M, Shi X, Zheng Z, Li S, Chen L. ISG12a inhibits HCV replication and potentiates the anti-HCV activity of IFN-α through activation of the Jak/STAT signaling pathway independent of autophagy and apoptosis. Virus Res. 2017;227:231–239. doi: 10.1016/j.virusres.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Tang BM, Shojaei M, Parnell GP, Huang S, Nalos M, Teoh S, O’Connor K, Schibeci S, Phu AL, Kumar A, Ho J, Meyers AFA, Keynan Y, Ball T, Pisipati A, Kumar A, Moore E, Eisen D, Lai K, Gillett M, Geffers R, Luo H, Gul F, Schreiber J, Riedel S, Booth D, McLean A, Schughart K. A novel immune biomarker IFI27 discriminates between influenza and bacteria in patients with suspected respiratory infection. Eur Respir J. 2017;49:1602098. doi: 10.1183/13993003.02098-2016. [DOI] [PubMed] [Google Scholar]
- 27.Hovanessian AG. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: the 2’-5’oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 2007;18:351–361. doi: 10.1016/j.cytogfr.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Kodym R, Kodym E, Story MD. 2’-5’-Oligoadenylate synthetase is activated by a specific RNA sequence motif. Biochem Biophys Res Commun. 2009;388:317–322. doi: 10.1016/j.bbrc.2009.07.167. [DOI] [PubMed] [Google Scholar]
- 29.Melchjorsen J, Kristiansen H, Christiansen R, Rintahaka J, Matikainen S, Paludan SR, Hartmann R. Differential regulation of the OASL and OAS1 genes in response to viral infections. J Interferon Cytokine Res. 2009;29:199–207. doi: 10.1089/jir.2008.0050. [DOI] [PubMed] [Google Scholar]
- 30.Castelli JC, Hassel BA, Wood KA, Li XL, Amemiya K, Dalakas MC, Torrence PF, Youle RJ. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J Exp Med. 1997;186:967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarti A, Ghosh PK, Banerjee S, Gaughan C, Silverman RH. RNase L triggers autophagy in response to viral infections. J Virol. 2012;86:11311–11321. doi: 10.1128/JVI.00270-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou A, Paranjape J, Brown TL, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman RH. Interferon action and apoptosis are defective in mice devoid of 2’,5’-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]


