Abstract
Anaphylaxis is caused by a variety of triggers including Food and Drug Administration (FDA)-approved antibiotics, contrast media and neuromuscular blocking drugs (NMBDs). Traditionally, drug-induced anaphylaxis was thought to result mainly from IgE-mediated histamine release from mast cells. Recently, a G protein-coupled receptor known as MRGPRX2 has been identified and shown to be highly expressed on human skin but not lung mast cells. The demonstration that many NMBDs induce degranulation in human mast cells via MRGPRX2 led to the idea that this receptor contributes to NMBD-induced hypersensitivity reactions. However, other studies have raised doubts regarding its role in drug-induced hypersensitivity. This review discusses the current status and controversy on MRGPRX2’s role on NMBD-induced hypersensitivity.
Introduction
Anaphylaxis is an acute systemic hypersensitivity reaction that is evoked by a number of triggers including food, drugs and stinging insects. The definition of anaphylaxis is not uniform [1], but according to The American Academy of Allergy Asthma and Immunology it is a serious allergic response that often involves swelling, hives, lowered blood pressure and in severe cases, shock. If anaphylactic shock is not treated immediately, it can be fatal. Anaphylaxis to Food and Drug Administration (FDA)-approved antibiotics, opioids, contrast media and neuromuscular blocking drugs (NMBDs) have increased in recent years [2]. Many of these agents contain quaternary amino groups, which are considered as the main antigenic epitope [3]. NMBDs such mivacurium, atracurium, cisatracurium and rocuronium are used routinely in surgery to reduce unwanted muscle movement and to allow intratracheal intubation for mechanical ventilation but are responsible for about 60% of allergic reactions in surgical settings [4]. Based on classical concepts, most individuals that develop anaphylaxis to these drugs generate IgE antibodies, which bind to their cell surface high affinity receptors (FcεRI) mainly on mast cells (MCs, and basophils). Subsequent administration of the drug results in cross-linking of FcεRI-bound IgE leading to massive histamine release, which is responsible for the manifestations of anaphylaxis (Figure 1) [5,6••,7••].
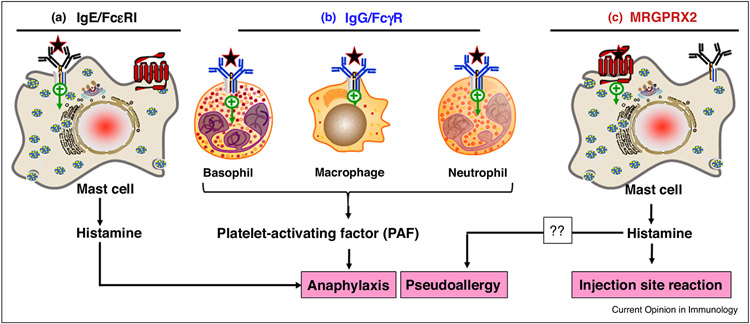
Figure 1.
Potential mechanism for NMBD–mediated anaphylaxis. (a) Most individuals that develop anaphylaxis to NMBDs generate IgE antibodies, which bind to their cell surface high affinity receptors (FcεRI) mainly on mast cells (also basophils not shown). Subsequent administration of the drug results in the cross-linking of FcεRI-bound IgE leading to massive histamine release, which is responsible for the manifestations of anaphylaxis. (b) IgG antibodies are also be generated in response to certain NMBDs. These antibodies bind to FcγRI on the surface of basophils, macrophages and neutrophils. Subsequent exposure to the drug leads to immune complex formation and activation of FcγRI, resulting in the generation of PAF. This pathway could aggravate IgE-mediated anaphylaxis or could form the underlying mechanism of anaphylaxis in the absence of IgE [6••]. (c) It has been proposed that activation of cutaneous MCs by NMBDs via MRGPRX2 and the subsequent mediator release leads to pseudoallergy. Whether or not this pathway plays an important role in NMBD-induced anaphylaxis remains to be confirmed. NMBDs that activate cutaneous MCs via MRGPRX2 likely cause local erythema and swelling (injection site reaction) due to histamine release.
The clinical diagnosis of anaphylaxis to NMBDs is based on the presence of drug-specific IgE and biological signs of IgE-dependent cell activation (elevated tryptase, positive skin test, and positive basophil activation test, BAT; which is based on the upregulation of cell surface CD63 and CD203c following ex vivo incubation with the suspected drug) [7••,8,9]. Up to 15% of patients who encounter drug-induced reactions are allergic in nature but many do not exhibit biological signs of IgE-dependent immune cell activation [10,11], indicating the involvement of other potential mechanisms. IgG-mediated release of platelet-activating factor (PAF) from macrophages and basophils are thought to be involved in certain forms of anaphylaxis [12-14]. Jonsson et al. [6••], recently showed that concentrations of anti-NMBD IgG, neutrophil activation and PAF release correlate with anaphylaxis severity [6••]. Neutrophil activation is also observed in patients lacking evidence of classical IgE-dependent anaphylaxis [6••]. Thus, drug-induced anaphylaxis could reflect collective immune response involving IgE-mediated MC degranulation and IgG-mediated PAF release from basophils, macrophages and neutrophils (Figure 1).
NMBDs can also induce anaphylaxis in patients without previous exposure to the drugs [15]. Thus, drug-induced anaphylaxis can occur in drug-naïve patients either by immune-cross sensitization or via a non-immune mechanism [7••]. Immune-independent reactions or pseudoallergy can occur following intravenous injection of drugs at high doses leading to symptoms such as angioedema, urticaria, bronchospasm, gastrointestinal problems and hypotension [16]. These reactions are known as anaphylactoid reactions when the symptoms are severe. A novel G protein-coupled receptor (GPCR) known as Mas-related GPCR-X2 (MRGPRX2) has recently been shown to be expressed at high levels in skin human MCs, but low levels in lung and gut MCs [17••,18-20]. It is not found in normal macrophages and neutrophils but its status in basophils and eosinophils is the subject of controversy [17••,21,22••,23,24•,25]. McNeil et al. [10] found that of the 22 murine Mrg coding genes, mouse peritoneal MCs (PMCs) and skin MCs express MrgprB2, which has been designated as the mouse ortholog of human MRGPRX2, despite the fact that there is only ~53% overall sequence similarity between the two receptors [26]. NMBDs and other cationic drugs activate murine MCs both in vitro and in vivo via MrgprB2 [10,27,28]. Based on these findings, it was proposed that activation of MRGPRX2 contributes to drug-induced pseudoallergy in humans (Figure 1).
Since the original identification of MrgprB2 as the mouse counterpart of human MRGPRX2, there has been an explosion in research related to its involvement in pseudoallergy and inflammatory diseases. Most of these studies have been performed with a human MC line (LAD2 cells) that endogenously express MRGPRX2, mouse PMCs and MrgprB2−/− mice. In addition, a number of studies with human subjects have supported the idea that MRGPRX2 contributes to NMBD-induced hypersensitivity [29,30•,31-34], whereas others have questioned its role [7••,35]. Most of these controversies resulted from studies with rocuronium. This article will first describe the available evidence for MRGPRX2’s role in mivacurium, atracurium and cisatracurium-induced hypersensitivity and then conclude with a discussion of the controversy related to rocuronium.
Mivacurium
Mivacurium is a short acting muscle relaxant that is widely used for surgical procedures particularly in infants and children with no increase in plasma histamine level [36]. However, intradermal administration of mivacurium leads to dose-dependent histamine release, causing vasodilatation, itch and pain [37,38]. Mivacurium induces degranulation in mouse PMCs and human LAD2 cells via MrgprB2 and MRGPRX2, respectively [10,28]. Che et al. [28] showed that mivacurium-induced paw edema and hypothermia are substantially reduced in MrgprB2−/− mice when compared to wild-type (WT) mice [28]. Based on these findings it was proposed that mivacurium causes pseudo-allergic reactions in humans via MRGPRX2 [28]. However, mivacurium can be injected in humans safely at a concentration of 2 mg/mL, which is higher than the concentration required to activate MRGPRX2 and MrgprB2 [10,28]. The reason for this difference is not clear but unlike many other MRGPRX2 agonists, mivacurium does not induce chemokine production in LAD2 cells [28]. It was proposed that the lack of cytokine generation by mivacurium in MCs reflects its relative safety profile in humans [28]. However, given that anaphylaxis is an acute reaction mostly mediated via the release of histamine and PAF from immune cells [5,6••], it is unclear how the lack of cytokine generation is associated with the safety profile of this drug. It is noteworthy that while all MCs are characterized via the expression of FcεRI, only cutaneous MCs express MRGPRX2 at high levels [17••,18,19]. It is therefore possible that local skin reaction induced by mivacurium reflects the activation of MRGPRX2 in cutaneous MCs without involving pseudo-allergic reactions [37,38]. Thus, caution should be exercised in translating the ability of mivacurium to cause paw edema and hypothermia in mice in vivo through MrgprB2 to pseudo-allergic reactions in humans via MRGPRX2 [28].
Atracurium and cisatracurium
Intradermal injection of atracurium in healthy volunteers results in wheal and flare responses, which are associated with degranulation of human skin MCs via an unknown mechanism [39,40]. It was later shown that atracurium activates human MCs via MRGPRX2 [10,34]. Additionally, injection of atracurium in WT mice leads to paw edema formation, which is substantially reduced in MrgprB2−/− mice [27]. Cisatracurium, a stereoisomer of atracurium, induces less histamine release and displays fewer allergy-like reactions than other NMBDs [41,42]. However, in some patients, cisatracurium may be associated with severe anaphylactic reactions possibly via IgE-mediated MC activation [43,44]. Cisatracurium also induces degranulation in LAD2 cells via MRGPRX2 and murine MCs via MrgprB2 [27,34]. Similar to mivacurium, cisatracurium does not induce chemokine generation despite causing robust degranulation [27,28]. It has recently been shown that MRGPRX2 activation by atracurium causes increased skin reactivity in patients with chronic spontaneous urticaria [24•]. This suggests that local skin reaction induced by atracurium and cisatracurium reflect the activation of skin MCs via MRGPRX2. However, any systemic effects of cisatracurium likely reflect IgE and IgG-mediated immune cell activation (Figure 1) [5,6••,43-45].
Rocuronium
In general, rocuronium has higher allergenic potential when compared to other NMBDs [45,46]. Not surprisingly, the mechanism of rocuronium-induced hypersensitivity has been studied most extensively. McNeil et al. [10] showed that rocuronium induces Ca2+ mobilization in transfected HEK293 cells expressing MrgprB2 and MRGPRX2 with EC50 values of 22.2 μg/mL and 263 μg/mL, respectively. The authors showed that rocuronium induces degranulation in mouse PMCs via MrgprB2 but they did not examine its effect on human MCs [10]. Based on mouse studies, it was proposed that MRGPRX2 contributes to rocuronium-induced hypersensitivity. However, Navinés-Ferrer et al. [34] showed that while cisatracurium at a concentration of 50 μg/mL induces significant degranulation in LAD2 cells via MRGPRX2, rocuronium at a concentration of up to 2 mg/mL has no effect. Other investigators also failed to demonstrate the ability of rocuronium to induce degranulation in RBL-2H3 cells stably expressing MRGPRX2 or human CD34+ cell-derived primary human MCs, despite the fact that it induces a robust response in mouse PMCs [29,30•,34,47].
The differences in the ability of rocuronium to activate MrgprB2 and MRGPRX2 is surprising and could reflect the fact that there is only ~53% sequence homology between the human and the mouse receptor [10]. Interestingly, compound 48/80, a polymer with a strong positive charge and multiple bulky hydrophobic moieties, is one of the most potent MRGPRX2 agonists known with a potency of ~130-fold higher than for MrgprB2 [10]. Although rocuronium has one quaternary amine and another amine that is mostly positively charged at pH 7.4, its hydrophobic steroidal backbone lacks the aromatic ring present in higher affinity MRGPRX2 agonists such as ZINC3573 and compound 48/80 [10,48,49]. Thus, in addition to positive charges, the hydrophobic moieties may be required for agonist binding to MRGPRX2 but it may interfere agonist-MrgprB2 interaction.
Given that rocuronium induces Ca2+ mobilization in HEK293 cells expressing MRGPRX2 and CD34+ cell-derived primary human MCs [10,47], it was surprising that it did not induce degranulation via MRGPRX2 [29,30•,47]. To resolve this discrepancy, Chompunud Na Ayudhya et al. [50••] conducted a comprehensive study using mouse PMCs, RBL-2H3 cells stably expressing MRGPRX2, LAD2 cells and human skin MCs. The authors found that, consistent with the previously reported EC50 value for Ca2+ mobilization in MrgprB2 transfected HEK293 cells [10], rocuronium at 20 μg/mL caused significant degranulation in mouse PMCs and that this response was abolished in PMCs derived from MrgprB2−/− mice. However, unlike previous reports, rocuronium was found to induce degranulation in transfected RBL-2H3 cells and LAD2 cells with an EC50 value of ~500 μg/mL and reaching maximal response at 2 mg/mL. Rocuronium also induced degranulation in human skin MCs but the magnitude of the response was much lower than that in LAD2 or transfected RBL-2H3 cells, which reflected lower level of MRGPRX2 expression. Thus, these findings provided the first demonstration that rocuronium induces degranulation in human MCs via MRGPRX2. Furthermore, the important difference between mouse MrgprB2 and human MRGPRX2 demonstrate the difficulty in translating findings from mice to humans.
Spoerl et al. [32] reported three cases of rocuronium-induced hypersensitivity; these patients had no increase in serum IgE and displayed a negative BAT. The authors found that undiluted rocuronium (10 mg/mL) induces small and variable skin reaction. This effect likely reflects the direct activation of MRGPRX2 in cutaneous MCs by rocuronium [50••]. Sugammadex is a γ-cyclodextrin that encapsulates NMBDs such as rocuronium, preventing its interaction with the nicotinic receptor, and thereby reversing neuromuscular blockade [32,51]. Sugammadex inhibits both rocuronium-induced MRGPRX2-mediated signaling in MCs in vitro and irritative skin reactions in vivo [29,32]. Based on these findings, it was proposed that MRGPRX2 plays an important role in drug-induced pseudoallergy and that this reaction should be re-classified as type A adverse reaction [32,33].
Consistent with findings discussed above, Suzuki et al. [30•] reported a case of hypersensitivity reaction to rocuronium; intradermal skin testing with undiluted rocuronium (10 mg/mL) resulted in a positive reaction but total IgE and specific IgE to rocuronium were negative. Based on these findings, this patient was diagnosed with non-IgE-mediated allergic reaction. Moreover, sequence analysis of genomic DNA of this patient revealed three amino acid mutations (M196I, L226P and L237P) in MRGPRX2 [30•]. These mutations are located in MRGPRX2’s 5th and 6th transmembrane (TM) domains in close proximity to its ligand binding pocket (Figure 2) [48,52,53]. The authors suggested that these mutations would enhance the affinity of MRGPRX2 for rocuronium, thus providing genetic evidence for the role of MRGPRX2 on rocuronium-induced hypersensitivity [30•]. Lansu et al. [48] identified an MRGPRX2 mutation in its ligand binding pocket (E164D) that increases the receptor affinity for certain drugs. Sequence alignment predicts that MrgprB2’s E171 is likely the residue that ‘sits’ in the MRGPRX2 E164 position [52]. Thus, it is quite possible that MRGPRX2 mutations found in a patient with rocuronium hypersensitivity [30•] may allosterically modify the receptor for ligand binding/signalling so that the receptor functions similar to mouse MrgprB2 for enhanced responsiveness to rocuronium and other NMBDs.
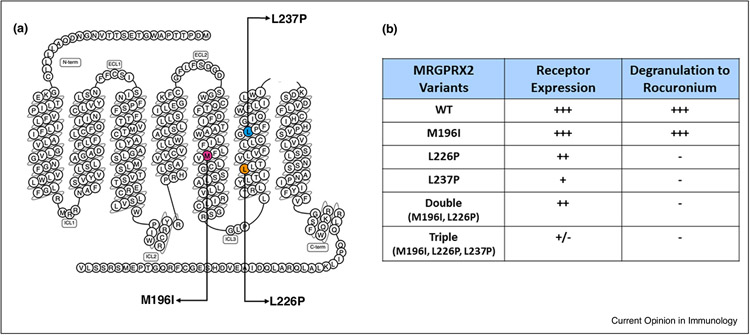
Figure 2.
Missense MRGPRX2 variants and their responsiveness to rocuronium. (a) Snake diagram of MRGPRX2 indicating three missense mutations identified in a patient diagnosed with non-IgE-mediated rocuronium hypersensitivity [30•]. (b) Data summarizing expression of wild-type (WT), single, double and triple variants of MRGPRX2 in transiently transfected RBL-2H3 cells and their responsiveness to rocuronium for degranulation [50••].
Chompunud Na Ayudhya et al. [50••] constructed cDNAs encoding MRGPRX2 variants M196I, L226P and L237P and generated separate transient transfectants expressing each variant in RBL-2H3 cells. They found that while M196I and L226P variants were expressed on the cell surface at a level similar to WT MRGPRX2, L237P variant displayed reduced expression (Figure 2). Surprisingly, cells expressing L226P and L237P variants showed loss-of-function phenotype for MC degranulation in response to rocuronium, while cells expressing M196I variant responded similarly to the WT receptor. Cells expressing double (M196I, L226P) or triple variants (M196I, L226P and L237P) were also hypo-responsive to rocuronium (1 mg/mL) for degranulation (Figure 2) [50••]. These findings suggest that the weak erythema response to intradermal injection of 10 mg/mL rocuronium results from low level of degranulation through the activation of the mutated MRGPRX2 and does not support its role in hypersensitivity reaction observed in this patient [30•,50••]. In addition to rocuronium, this patient was also exposed to remifentanil, latex and chlorhexidine, and the possibility that the hypersensitivity reaction in this patient could have resulted from one or more of these agents was not considered [30•]. Furthermore, a negative rocuronium-specific IgE result does not rule out an IgE-mediated rocuronium allergy [30•,54]. Thus, it is possible that hypersensitivity reaction to rocuronium reported by Suzuki et al. [30•] may be unrelated to MRGPRX2 activation.
The most convincing evidence that rocuronium does not contribute to rocuronium hypersensitivity in most patients came from Van Gasse et al. [7••] who conducted a study with 140 patients suspected of hypersensitivity to rocuronium. The authors utilized a diagnostic approach that included measurement of serum tryptase level, quantification of specific IgE antibodies to rocuronium, intradermal skin test (50 μg/mL rocuronium) and BAT. Using this approach it was concluded that rocuronium-induced hypersensitivity in most patients resulted from IgE-mediated MC activation.
Conclusions
The seminal observation by McNeil et al. [10] that NMBDs induce degranulation in mouse PMCs via MrgprB2 led to the notion that these drugs induce hypersensitivity reactions in humans via MRGPRX2. Although a number of studies with human subjects supported this conclusion, others have not [7••,29,30•,31-34]. In this context, it is noteworthy that of the NMBDs tested, rocuronium has the least potency for inducing degranulation in human MCs via MRGPRX2 but has the highest allergenic potential [7••,45,46]. It now appears that the mechanism of rocuronium-induced hypersensitivity in most patients involves IgE-mediated MC-degranulation and possibly IgG-mediated PAF release from basophils, macrophages and neutrophils (Figure 1) [5,6••,7••,35]. In addition, MRGPRX2 mutations in a patient with rocuronium hypersensitivity are not associated with gain-of-function phenotype for MC degranulation in vitro [30•,50••].
Peptidergic drugs such as icatibant, cetrorelix, leuprolide, octreolide and sermorelin that induce injection site swelling, pain or pruritus in humans activate murine PMCs via MrgprB2 [10] but none of these drugs are associated with anaphylaxis. Given that MRGPRX2 is expressed predominantly in human skin MCs, this raises the interesting possibility that injection site reaction such as erythema and swelling observed following intradermal administration of high concentration of rocuronium (up to 10 mg/mL) [30•,32] likely reflects skin MC degranulation via MRGPRX2 [50••].
While MRGPRX2 is expressed predominantly in skin MCs, it is found at low levels in lung and gut MCs and whether or not it is expressed in normal basophils is the subject of current controversy [21,22••,23,24•,25]. It is therefore possible that gain-of-function mutations in MRGPRX2 or its increased expression in skin, lung and gut MCs and basophils could lead to pseudoallergy or anaphylactoid reaction in certain individuals. It is important to note that anaphylaxis is a highly complex disease with intrinsic factors such as higher age, male sex, concomitant mastocytosis and extrinsic factors including vigorous exercise, psychological burden and drugs (β-blockers and angiotensin-converting enzyme inhibitors) may modulate the severity of the disease [55-57]. In addition, there is a tremendous variability in the individual responsiveness of cutaneous MCs to MRGPRX2 agonists [58,59]. Therefore, further studies will be required to more precisely delineate the roles of MRGPRX2 on the activation and modulation of drug-induced hypersensitivity reactions.
Acknowledgements
I am grateful to my lab members (Chalatip Chompunud Na Ayudhya, Saptarshi Roy, Shaswati Chaki and Monica Thapaliya) for proof reading the article. I also thank Chalatip Chompunud Na Ayudhya for providing Figure 2. This work was supported by National Institutes of Health grants R01-AI124182, R01-AI143185 and R01-AI149487.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Bilo MB, Martini M, Tontini C, Corsi A, Antonicelli L: Anaphylaxis. Eur Ann Allergy Clin Immunol 2021, 53:4–17. [DOI] [PubMed] [Google Scholar]
- 2.Yu RJ, Krantz MS, Phillips EJ, Stone CA Jr: Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA Adverse Event Reporting System (FAERS). J Allergy Clin Immunol Pract 2021, 9:819–829.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldo BA, Fisher MM: Substituted ammonium ions as allergenic determinants in drug allergy. Nature 1983, 306:262–264. [DOI] [PubMed] [Google Scholar]
- 4.Mertes PM, Alla F, Trechot P, Auroy Y, Jougla E, Groupe d’Etudes des RéactionsAnaphylactoïdes Peranesthésiques: Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol 2011, 128:366–373. [DOI] [PubMed] [Google Scholar]
- 5.Reber LL, Hernandez JD, Galli SJ: The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017, 140:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.••. Jonsson F, de Chaisemartin L, Granger V, Gouel-Cheron A, Gillis CM, Zhu Q, Dib F, Nicaise-Roland P, Ganneau C, Hurtado-Nedelec M et al. : An IgG-induced neutrophil activation pathway contributes to human drug-induced anaphylaxis. Sci Transl Med 2019, 11. This publication showed that concentrations of anti-NMBD IgG, neutrophil activation and PAF release correlate with anaphylaxis severity. Neutrophil activation is also observed in patients lacking evidence of classical IgE-dependent anaphylaxis.
- 7.••. Van Gasse AL, Elst J, Bridts CH, Mertens C, Faber M, Hagendorens MM, De Clerck LS, Sabato V, Ebo DG: Rocuronium hypersensitivity: does off-target occupation of the MRGPRX2 receptor play a role? J Allergy Clin Immunol Pract 2019, 7:998–1003. The authors utilized four assays; serum tryptase level, quantification of specific IgE antibodies to rocuronium, intradermal skin test and BAT to conclude that rocuronium-induced hypersensitivity in most patients tested resulted from IgE-mediated MC activation.
- 8.Ebo DG, Faber M, Elst J, Van Gasse AL, Bridts CH, Mertens C, De Clerck LS, Hagendorens MM, Sabato V: In vitro diagnosis of immediate drug hypersensitivity during anesthesia: a review of the literature. J Allergy Clin Immunol Pract 2018, 6:1176–1184. [DOI] [PubMed] [Google Scholar]
- 9.Kalangara J, Vanijcharoenkarn K, Lynde GC, McIntosh N, Kuruvilla M: Approach to perioperative anaphylaxis in 2020: updates in diagnosis and management. Curr Allergy Asthma Rep 2021, 21:4. [DOI] [PubMed] [Google Scholar]
- 10.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X: Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausmann O, Schnyder B, Pichler WJ: Etiology and pathogenesis of adverse drug reactions. Chem Immunol Allergy 2012, 97:32–46. [DOI] [PubMed] [Google Scholar]
- 12.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD: Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol 2002, 109:658–668. [DOI] [PubMed] [Google Scholar]
- 13.Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC: IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol 2020, 11:603050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause K, Gimenez-Arnau A, Martinez-Escala E, Farre-Albadalejo M, Abajian M, Church MK, Maurer M: Platelet-activating factor (PAF) induces wheal and flare skin reactions independent of mast cell degranulation. Allergy 2013, 68:256–258. [DOI] [PubMed] [Google Scholar]
- 15.Fisher MM, Munro I: Life-threatening anaphylactoid reactions to muscle relaxants. Anesth Analg 1983, 62:559–564. [PubMed] [Google Scholar]
- 16.Nel L, Eren E: Peri-operative anaphylaxis. Br J Clin Pharmacol 2011, 71:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.••. Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald HR: Human mast cell proteome reveals unique lineage, putative functions, and structural basis for cell ablation. Immunity 2020, 52:404–416.e5. The authors used flow cytometry to show that human fat and skin MCs express MRGPRX2 at high levels but the expression of this receptor is low on colon and lung MCs. Using the same procedure it was shown that human dendritic cells, B cells, T cells, monocytes, neutrophils, eosinophils and basophils do not express cell surface MRGPRX2.
- 18.Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T et al. : Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123:e58–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T et al. : Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol 2014, 134:622–633.e9. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer DF, Barrett NA, Austen KF, The Immunological Genome Project Consortium: Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol 2016, 17:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabato V, Elst J, Van Houdt M, Bridts C, Mertens C, Ebo DG: Surface expression of MRGPRX2 on resting basophils: an area of controversy. Allergy 2020, 75:2421–2422. [DOI] [PubMed] [Google Scholar]
- 22.••. Wedi B, Gehring M, Kapp A: The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: expression and function. Allergy 2020, 75:2229–2242. Using flow cytometry, immunocytochemistry, immunofluorescence, Western blot, and RT-PCR the authors showed that MRGPRX2 is significantly expressed by basophils and eosinophils but not neutrophils. However, ciprofloxacin, which induces degranulation in human MCs via MRGPRX2 does not activate human basophils.
- 23.Wedi B, Gehring M, Kapp A: Reply to Sabato V et al. "Surface expression of MRGPRX2 expression on resting basophils: an area of controversy". Allergy 2020, 75:2424–2427. [DOI] [PubMed] [Google Scholar]
- 24.•. Shtessel M, Limjunyawong N, Oliver ET, Chichester K, Gao L, Dong X, Saini SS: MRGPRX2 activation causes increased skin reactivity in patients with chronic spontaneous urticaria. J Invest Dermatol 2021, 141:678–681.e2. MRGPRX2 expression is increased in skin MCs of patients with chronic spontaneous urticaria (CSU, Ref. [19]). In this paper, the authors showed that wheal reaction to intradermal injection of atracurium is signicanlty greater in CSU skin when compared to healthy control. Although anti-IgE causes basophil activation actracurium does not. This suggests that skin reaction to NBMD administration is mediated by MRGPRX2 on MCs not via IgE/FcεRI.
- 25.Elst J, Sabato V, Hagendorens MM, van Houdt M, Faber MA, Bridts CH, Ebo DG, Mertens C: Measurement and functional analysis of the mas-related G protein-coupled receptor MRGPRX2 on human mast cells and basophils. Methods Mol Biol 2020, 2163:219–226. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian H, Gupta K, Ali H: Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol 2016, 138:700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Che D, Rui L, Cao J, Wang J, Zhang Y, Ding Y, Zhao T, Ma P, An H, Gao Z et al. : Cisatracurium induces mast cell activation and pseudo-allergic reactions via MRGPRX2. Int Immunopharmacol 2018, 62:244–250. [DOI] [PubMed] [Google Scholar]
- 28.Che D, Wang J, Ding Y, Liu R, Cao J, Zhang Y, Hou Y, An H, Gao Z, Zhang T: Mivacurium induce mast cell activation and pseudo-allergic reactions via MAS-related G protein coupled receptor-X2. Cell Immunol 2018, 332:121–128. [DOI] [PubMed] [Google Scholar]
- 29.Fernandopulle NA, Zhang SS, Soeding PF, Mackay GA: MRGPRX2 activation in mast cells by neuromuscular blocking agents and other agonists: modulation by sugammadex. Clin Exp Allergy 2020, 00:1–11. [DOI] [PubMed] [Google Scholar]
- 30.•. Suzuki Y, Liu S, Kadoya F, Takasaki Y, Yorozuya T, Mogi M: Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Br J Anaesth 2020, 125:e446–e448. In this paper, the authors reported a case of a patient with hypersensitivity to rocuronium. Based on skin testing and specific IgE level, the patient was diagnosed with non-IgE-mediated allergy. They also identified a number of mutations in MRGPRX2 and proposed that these mutations contributed to the patient hypersensitivity to rocuronim.
- 31.Spoerl D: MRGPRX2, pseudo-allergies reloaded: a step forward and two backwards. Rev Med Suisse 2020, 16:679–682. [PubMed] [Google Scholar]
- 32.Spoerl D, D’Incau S, Roux-Lombard P, Harr T, Czarnetzki C: Non-IgE-dependent hypersensitivity to rocuronium reversed by sugammadex: report of three cases and hypothesis on the underlying mechanism. Int Arch Allergy Immunol 2016, 169:256–262. [DOI] [PubMed] [Google Scholar]
- 33.Spoerl D, Nigolian H, Czarnetzki C, Harr T: Reclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: IgE-mediated, pharmacological adverse reaction or "innate hypersensitivity"? Int J Mol Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navines-Ferrer A, Serrano-Candelas E, Lafuente A, Munoz-Cano R, Martin M, Gastaminza G: MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Rep 2018, 8:11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebo DG, Van der Poorten ML, Elst J, Van Gasse AL, Mertens C, Bridts C, Garvey LH, Horiuchi T, Sabato V: Immunoglobulin E cross-linking or MRGPRX2 activation: clinical insights from rocuronium hypersensitivity. Br J Anaesth 2021, 126:e27–e29. [DOI] [PubMed] [Google Scholar]
- 36.Zeng R, Liu X, Zhang J, Yin N, Fei J, Zhong S, Hu Z, Hu M, Zhang M, Li B et al. : The efficacy and safety of mivacurium in pediatric patients. BMC Anesthesiol 2017, 17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppert W, Blunk JA, Petersen LJ, Skov P, Rentsch K, Schmelz M: Different patterns of mast cell activation by muscle relaxants in human skin. Anesthesiology 2001, 95:659–667. [DOI] [PubMed] [Google Scholar]
- 38.Mertes PM, Moneret-Vautrin DA, Leynadier F, Laxenaire MC: Skin reactions to intradermal neuromuscular blocking agent injections: a randomized multicenter trial in healthy volunteers. Anesthesiology 2007, 107:245–252. [DOI] [PubMed] [Google Scholar]
- 39.Levy JH, Adelson D, Walker B: Wheal and flare responses to muscle relaxants in humans. Agents Actions 1991, 34:302–308. [DOI] [PubMed] [Google Scholar]
- 40.Veien M, Szlam F, Holden JT, Yamaguchi K, Denson DD, Levy JH: Mechanisms of nonimmunological histamine and tryptase release from human cutaneous mast cells. Anesthesiology 2000, 92:1074–1081. [DOI] [PubMed] [Google Scholar]
- 41.Doenicke A, Soukup J, Hoernecke R, Moss J: The lack of histamine release with cisatracurium: a double-blind comparison with vecuronium. Anesth Analg 1997, 84:623–628. [DOI] [PubMed] [Google Scholar]
- 42.Wastila WB, Maehr RB, Turner GL, Hill DA, Savarese JJ: Comparative pharmacology of cisatracurium (51W), atracurium, and five isomers in cats. Anesthesiology 1996, 85:169–177. [DOI] [PubMed] [Google Scholar]
- 43.Krombach J, Hunzelmann N, Koster F, Bischoff A, Hoffmann-Menzel H, Buzello W: Anaphylactoid reactions after cisatracurium administration in six patients. Anesth Analg 2001, 93:1257–1259 table of contents. [DOI] [PubMed] [Google Scholar]
- 44.Linauskiene K, Grinceviciene G, Malinauskiene L, Blaziene A, Chomiciene A: Severe anaphylactic reaction to cisatracurium during anesthesia with cross-reactivity to atracurium. Open Med (Wars) 2020, 15:384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petitpain N, Argoullon L, Masmoudi K, Fedrizzi S, Cottin J, Latarche C, Mertes PM, Gillet P, The French Network of Regional Pharmacovigilance Centres: Neuromuscular blocking agents induced anaphylaxis: results and trends of a French pharmacovigilance survey from 2000 to 2012. Allergy 2018, 73:2224–2233. [DOI] [PubMed] [Google Scholar]
- 46.Reddy JI, Cooke PJ, van Schalkwyk JM, Hannam JA, Fitzharris P, Mitchell SJ: Anaphylaxis is more common with rocuronium and succinylcholine than with atracurium. Anesthesiology 2015, 122:39–45. [DOI] [PubMed] [Google Scholar]
- 47.Elst J, Sabato V, Faber MA, Bridts CH, Mertens C, Van Houdt M, Van Gasse AL, Hagendorens MM, Van Tendeloo V, Maurer M et al. : MRGPRX2 and immediate drug hypersensitivity: insights from cultured human mast cells. J Investig Allergol Clin Immunol 2020, 0. [DOI] [PubMed] [Google Scholar]
- 48.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J et al. : In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 2017, 13:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNeil BD: Minireview: mas-related G protein-coupled receptor X2 activation by therapeutic drugs. Neurosci Lett 2021, 135746:1–10. [DOI] [PubMed] [Google Scholar]
- 50.••. Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Oskeritzian CA, Ali H: MRGPRX2 activation by rocuronium: insights from studies with human skin mast cells and missense variants. Cells 2021, 10. The authors showed that rocuronium activates murine MCs (MrgprB2) with a much higher affinity than human MCs (MRGPRX2). Furthermore, they showed that MRGPRX2 mutations reported in Ref. [30] do not result in enhanced activation by rocuronium thus questioning the role of this receptor in NMBD-induced hypersensitivity.
- 51.Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, MacLean EJ, Muir AW, Palin R, Rees DC et al. : A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl 2002, 41:266–270. [DOI] [PubMed] [Google Scholar]
- 52.Reddy VB, Graham TA, Azimi E, Lerner EA: A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J Allergy Clin Immunol 2017, 140:1726–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alkanfari I, Gupta K, Jahan T, Ali H: Naturally occurring missense MRGPRX2 variants display loss of function phenotype for mast cell degranulation in response to substance p, hemokinin-1, human beta-defensin-3, and icatibant. J Immunol 2018, 201:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elst J, Sabato V, Mertens C, Garvey LH, Ebo DG: Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Br J Anaesth 2020, 125:e448–e450. [DOI] [PubMed] [Google Scholar]
- 55.Nassiri M, Babina M, Dolle S, Edenharter G, Rueff F, Worm M: Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015, 135:491–499. [DOI] [PubMed] [Google Scholar]
- 56.Worm M, Francuzik W, Renaudin JM, Bilo MB, Cardona V, Scherer Hofmeier K, Kohli A, Bauer A, Christoff G, Cichocka-Jarosz E et al. : Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy 2018, 73:1322–1330. [DOI] [PubMed] [Google Scholar]
- 57.Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, Lockey RF, El-Gamal YM, Brown SG, Park HS et al. : 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J 2015, 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babina M, Wang Z, Roy S, Guhl S, Franke K, Artuc M, Ali H, Zuberbier T: MRGPRX2 is the codeine receptor of human skin mast cells: desensitization through beta-arrestin and lack of correlation with the FcepsilonRI pathway. J Invest Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Babina M, Guhl S, Artuc M, Zuberbier T: Allergic FcepsilonRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73:256–260. [DOI] [PubMed] [Google Scholar]