Abstract
Enteropathogenic Escherichia coli (EPEC) interacts with intestinal epithelial cells, activating host signaling pathways leading to cytoskeletal rearrangements and ultimately diarrhea. In this study, we demonstrate that EPEC interacts with the macrophage-like cell line J774A.1 to inhibit phagocytosis by these cells. Antiphagocytic activity was also observed in cultured RAW macrophage-like cells upon EPEC infection. The EPEC antiphagocytic phenotype was dependent on the type III secretion pathway of EPEC and its secreted proteins, including EspA, EspB, and EspD. Intimin and Tir mutants displayed intermediate antiphagocytic activity, suggesting that intimate attachment mediated by intimin-Tir binding may also play a role in antiphagocytosis. Tyrosine dephosphorylation of several host proteins was observed following infection with secretion-competent EPEC but not with secretion-deficient mutants. Dephosphorylation was detectable 120 min after infection with EPEC, directly correlating with the onset of the antiphagocytic phenotype. Inhibition of protein tyrosine phosphatases by pervanadate treatment increased the number of intracellular wild-type EPEC organisms to levels seen with secretion-deficient mutants, suggesting that dephosphorylation events are linked to the antiphagocytic phenotype. No tyrosine phosphatase activity was detected with the EPEC-secreted proteins, suggesting that EPEC induces antiphagocytosis via a different mechanism than Yersinia species. Taken together, the present findings demonstrate a novel function for EPEC-secreted proteins in triggering macrophage protein tyrosine dephosphorylation and inhibition of phagocytosis.
Enteropathogenic Escherichia coli (EPEC), a human pathogen, is a leading cause of infantile diarrhea in developing countries, killing several hundred thousand children per year worldwide (21). Despite the significance of this disease, the mechanisms by which EPEC causes diarrhea remain poorly characterized. It has been demonstrated both in vitro and in vivo that EPEC organisms initially adhere nonintimately in microcolonies to host intestinal epithelial cells via bundle-forming pili (Bfp) (13). Upon initial adherence, several bacterial proteins are secreted by a type III secretion pathway, of which at least three, EspA, EspB, and EspD, are involved in activating host signals (10, 14–16, 20). Induction of host signaling events leads to rearrangement of the host cytoskeleton to form the characteristic attaching and effacing lesion, resembling a pedestal structure upon which the organism resides (25). The signaling events include induction of host inositol triphosphate and Ca2+ fluxes, as well as host protein phosphorylation (3, 8, 9, 16, 24). Tir is a bacterially secreted protein which is inserted into host cell membranes and tyrosine phosphorylated (18). Binding of Tir to the EPEC outer membrane protein intimin leads to intimate binding of the pathogen to the host cell and pedestal formation (25, 18).
Tyrosine dephosphorylation of host proteins also occurs within the host epithelial cell following EPEC infection, but its role in the attachment process remains undefined (16). This dephosphorylation occurs independently of intimin. Furthermore, inhibition of tyrosine dephosphorylation does not prevent pedestal formation, suggesting that it may play another role in the infection process (16). Pathogenic Yersinia species induce tyrosine dephosphorylation of macrophage proteins to paralyze host phagocytic activity (26, 27). This event requires a bacterial tyrosine phosphatase, YopH, which is translocated into the host cell (4, 2).
The rabbit form of EPEC, RDEC-1, inhibits its uptake by membranous (M) cells on intestinal surfaces, but the mechanism mediating antiphagocytosis remains undefined (12). This pathogen is very similar to EPEC, secreting EspA, EspB, and Tir and producing attaching and effacing lesions (1). M cells are specialized epithelial cells which engulf many bacteria (except RDEC-1), bacterial components, and other antigens from the lumen transporting them into contact with the underlying antigen-presenting cells of the gut-associated lymphoid tissue (GALT) (30). An antiphagocytic phenotype may be advantageous in that it allows the bacteria to colonize the intestinal lining and M cells without being transported to the GALT, thereby delaying an elicited immune response.
Given the antiinvasive phenotype of RDEC-1 and the occurrence of tyrosine dephosphorylation events upon EPEC infection of epithelial cells, we investigated the possible relationship between EPEC-induced dephosphorylation and anti-phagocytic activity.
MATERIALS AND METHODS
Cell culture and bacterial growth.
The mouse phagocytic cell lines J774A.1 (22) and RAW (23) were grown in Dulbecco’s minimal Eagle medium (DMEM) supplemented with 10% fetal calf serum at 37°C in a humidified atmosphere with 5% CO2. EPEC and Yersinia pseudotuberculosis strains used in this study are listed in Table 1. EPEC strains were cultured in Luria-Bertani broth overnight at 37°C without shaking. Y. pseudotuberculosis strains were grown in brain-heart infusion broth at 26°C overnight on a rotary shaker. These cultures were diluted to 108 bacteria/ml (optical density at 550 nm, 0.1) and further incubated at 26°C for 1 h and then at 37°C for 2 h prior to infection of J774 cells.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype or description | Reference |
|---|---|---|
| Strains | ||
| EPEC | ||
| E2348/69 | Wild type | 6 |
| UMD864 | E2348/69 with espB deletion | 11 |
| UMD872 | E2348/69 with espA deletion | 15 |
| UMD870 | E2348/69 with espD deletion | 20 |
| CVD206 | E2348/69 with eaeA deletion | 7 |
| E2348/69Δtiz | E2348/69 with tiz deletion | 18 |
| cfm 14-2-1(1) | cfm::TnPhoA | 6 |
| 31-6-1 | bfpA::TnPhoA | 13 |
| Y. pseudotuberculosis | ||
| YPIII(pIB102) | Wild type | 5 |
| YPIII(p−) | Plasmid cured | 5 |
| Plasmid | ||
| pCVD450 | Encodes the per regulon | 11 |
Determination of bacterial uptake by J774 cells by immunofluorescence.
Two days prior to infection, J774 cells were seeded onto coverslips (11-mm diameter; 5 × 104 cells/well) in a 24-well microtiter plate. Monolayers were routinely infected with EPEC (multiplicity of infection, 50) for 180 min (except for time course experiments). Bacterial uptake was stopped by placing the microtiter plates on ice. Intra- and extracellular bacteria were determined as described previously for Y. pseudotuberculosis (2, 26). Briefly, to stain extracellular bacteria, coverslips were washed and incubated with rabbit anti-EPEC antibodies (1:100 dilution) (11) and incubated for 30 min at 4°C. Monolayers were washed four times with phosphate-buffered saline (PBS) and fixed with ice-cold methanol for 90 s. After methanol removal, extracellular bacteria were labeled with Texas red-conjugated goat anti-rabbit antiserum (1:200 dilution; Jackson Laboratories) for 20 min at 37°C, followed by four washes in PBS. Both intra- and extracellular bacteria were then stained by incubation of coverslips for 1 h at 37°C with anti-EPEC antisera and were washed four times in PBS. Coverslips were then incubated for 20 min at 37°C with fluorescein isothiocyanate-conjugated goat anti-rabbit antiserum (1:200 dilution; Jackson Laboratories). After four washes in PBS, coverslips were mounted in Mowiol mounting medium on a glass slide. Extracellular bacteria were detected by excitation at 596 nm, and total cell-associated bacteria were detected at 490 nm. For each experiment, 50 cells per coverslip with 10 to 20 cell-associated bacteria were randomly selected, and the numbers of extracellular and total cell-associated bacteria were determined. To determine the effects of bacterial numbers on antiphagocytosis, 50 cells per coverslip were counted with either 0 to 5, 5 to 15, or 15 to 50 bacteria per cell.
Preparation of cell lysates for Western blotting.
J774 monolayers were cultured to confluency in 60-mm plates and infected with an overnight EPEC culture for 3 h (multiplicity of infection, 50) in serum-free DMEM. The monolayers were washed three times in PBS and resuspended directly in boiling Laemmli sample buffer (19). Protein samples were resolved by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis (SDS-PAGE) (19), and the proteins were transferred to nitrocellulose as described elsewhere (29). Blots were blocked in 4% bovine serum albumin BSA in PBS prior to incubation with antiphosphotyrosine (4G10 [1:2,000 dilution]; Upstate Biotechnology Inc.), and phosphotyrosine proteins were detected by alkaline phosphatase-conjugated secondary antibodies, as described previously (24).
Cell viability assays.
Trypan blue (0.08% final volume; Gibco) was added to wells containing infected monolayers of J774 cells (200 μl/ml of DMEM) for 10 min, followed by four washes in PBS. Cells were visualized by phase-contrast microscopy and the percentage of blue cells was determined.
Inhibition of protein tyrosine phosphatases by pervanadate.
Hydrogen peroxide (2 mM) was added to 0.1 mM vanadate (VO4; Sigma) to make pervanadate in DMEM. This solution was added to infected J774 cells following 90 min of EPEC infection, and cells were incubated for an additional 60 min. Control experiments with untreated cells or cells treated with H2O2 or VO4 only were performed in parallel. After treatment, cells were prepared for fluorescence microscopy or for Western analysis as described above.
Detection of protein tyrosine phosphatase activity.
To detect phosphatase activity of EPEC-secreted proteins and of Y. pseudotuberculosis Yop proteins in vitro, a tyrosine phosphatase assay kit (Boehringer Mannheim) was used according to the manufacturer’s instructions. EPEC was grown under optimal conditions for secretion of Esp proteins, as described elsewhere (17). Briefly, EPEC organisms transformed with plasmid CVD450 were grown standing overnight at 37°C in Luria-Bertani broth supplemented with tetracycline (25 μg/ml, final concentration). The culture was then diluted 1:100 in M9 medium supplemented with 0.45% glucose, 0.2% Casamino Acids, and 0.4% (wt/vol) NaHCO3. EPEC cultures were grown to an optical density at 600 nm of 0.6 and centrifuged (16,000 RCF) for 2 min to pellet the bacteria. M9 supernatant containing the secreted proteins was concentrated with a Centricon-10 (Amicon). To collect Y. pseudotuberculosis Yop proteins, the bacteria were grown as described above and concentrated in the Centricon-10.
RESULTS
Phagocytosis of EPEC by J774 cells.
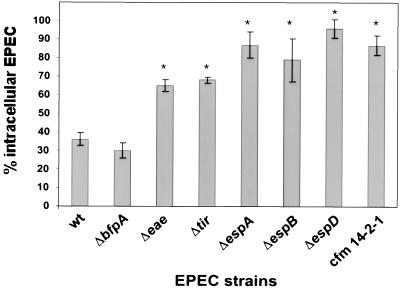
To examine whether EPEC exhibited any antiphagocytic activity, cultured J774 cells were infected with wild-type EPEC and a type III secretion-deficient mutant, cfm, followed by immunofluorescence to determine the numbers of extracellular and total cell-associated bacteria. Wild-type EPEC inhibited its own uptake into J774 cells (only 36% of cell-associated bacteria were intracellular), whereas cfm was internalized in significantly greater numbers (87% intracellular [P < 0.01]) (Fig. 1). Similar results were obtained with RAW cells, with 19% of wild-type EPEC being intracellular compared to 66% of the cfm strain being internalized. To determine the potential roles of different EPEC gene products in mediating antiphagocytosis, several EPEC mutants were examined by immunofluorescence. The Bfp mutant inhibited its own uptake into J774 cells at levels comparable to that seen by wild-type EPEC (30% intracellular). In contrast, the type III secretion- and signaling-defective ΔespA, ΔespB, and ΔespD mutants were internalized in significantly greater numbers than the parental strain (P < 0.01), with 87, 79, and 96% of cell-associated bacteria found inside the cell, respectively. The Tir and intimin (eae) mutants showed intermediate phenotypes, with 65 and 68% intracellular bacteria, respectively.
FIG. 1.
EPEC inhibits its own uptake by J774 cells. J774 monolayers were infected with various EPEC strains for 3 h and prepared for fluorescence microscopy as described in Materials and Methods. Data are averages and standard errors of the means for three independent experiments performed in duplicate. ∗, significant difference from the wild type (wt) at P of <0.05.
It is possible that the lack of intracellular EPEC could be due simply to EPEC inducing J774 cell death and not preventing uptake at all. To examine this possibility, EPEC-infected macrophages were assayed for membrane integrity by trypan blue exclusion. No significant J774 membrane permeability was observed during the 3-h infection period with any of the EPEC strains.
Kinetics of EPEC-induced antiphagocytosis.
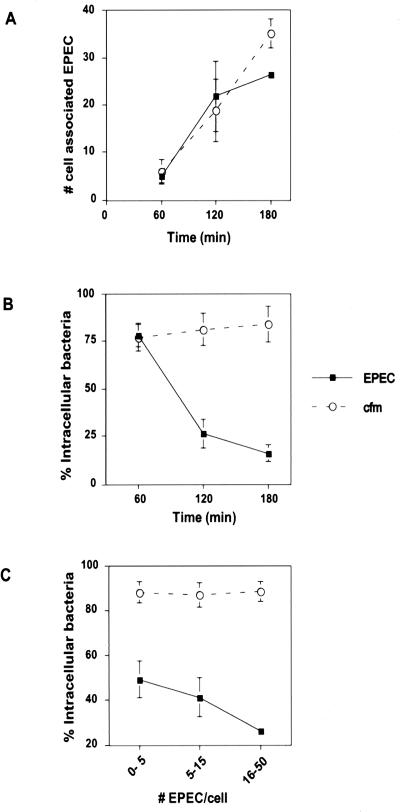
To further characterize the antiphagocytic effect of EPEC on J774 cells, the numbers of cell-associated EPEC (wild type and cfm) and the percentages of those that were intracellular were determined at 60, 120, and 180 min postinfection (Fig. 2A and B). EPEC exhibited increasing adherence and antiphagocytosis (i.e., decreasing numbers of intracellular bacteria) with increasing time. At 60 min after bacterial addition, J774 cells had approximately five EPEC associated per cell, 78% of which were intracellular. At 120 and 180 min after bacterial addition, EPEC adherence to J774 cells increased significantly relative to 60 min of infection (P < 0.05). Correlated with the increased adherence was a significant increase in antiphagocytic activity (26 and 16% of EPEC were intracellular at 120 and 180 min, respectively). This was in contrast to cfm, as increased adherence did not result in increased antiphagocytic activity. The percentages of intracellular cfm remained similar at 60, 120, and 180 min after bacterial addition.
FIG. 2.
EPEC-mediated antiphagocytosis is evident after 120 min of infection of J774 cells and correlates with increasing numbers of cell-associated bacteria. J774 cells were infected with EPEC or cfm for 60, 120, or 180 min. Monolayers were prepared for fluorescence microscopy as described in Materials and Methods. (A) Numbers of total cell-associated bacteria determined as a function of time. (B) Percentages of intracellular cell-associated bacteria determined at 60, 120, and 180 min of infection. (C) Percentages of intracellular bacteria determined for cells with 0 to 5, 5 to 15, or 15 to 50 associated bacteria per cell at 180 min after bacterial addition to macrophages. Data are averages and standard errors of the means for three independent experiments performed in duplicate.
The above results correlate antiphagocytic activity with increased numbers of adherent secretion-competent EPEC. To further examine this relationship, J774 cells were infected for 180 min with wild-type EPEC and cfm, and the percentages of intracellular bacteria were determined for cells with 0 to 5, 5 to 15, or 15 to 50 cell-associated bacteria. The antiphagocytic effect of wild-type EPEC was increased significantly (P < 0.05) with increasing numbers of bacteria per J774 cell (Fig. 2C). This differed from cfm, as the percentage of intracellular bacteria remained independent of the number of cell-associated bacteria.
Phosphorylation profiles of EPEC-infected J774 cells.
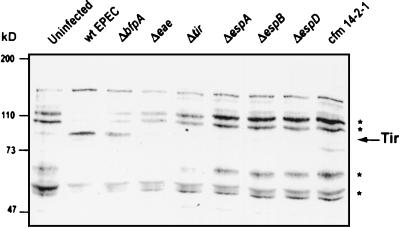
To determine whether EPEC induced tyrosine dephosphorylation in J774 cells, similar to that seen with HeLa cells (16), J774 cells were infected with wild-type and mutant EPEC, and protein extracts were resolved by SDS-PAGE. Phosphotyrosine profiles were visualized by Western blot analysis with antiphosphotyrosine antibodies (see Materials and Methods). Wild-type EPEC and the Bfp mutant induced tyrosine dephosphorylation of a triplet of proteins migrating around 50 kDa, a 60-kDa protein, a 100-kDa protein, and a 110-kDa protein (Fig. 3). The eae and tir mutants induced dephosphorylation of the 60-kDa protein as well as the 50, 100, and 110-kDa proteins, although to a lesser extent. The secretion-deficient mutants (espA, espB, and espD mutants and cfm) did not cause tyrosine dephosphorylation of host proteins relative to the uninfected control. The strains that caused dephosphorylation were the same as those that blocked bacterial uptake, correlating dephosphorylation with the observed antiphagocytic phenotype. Interestingly, the eae and tir mutants, which demonstrated intermediate antiphagocytic effects, induced intermediate levels of dephosphorylation.
FIG. 3.
Signaling-competent EPEC induces tyrosine dephosphorylation in J774 cells. J774 cells were infected with various EPEC strains for 3 h prior to isolation of J774 cell proteins as described in Materials and Methods. Samples were resolved by SDS–8% PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies. Molecular masses are indicated on the left. ∗, proteins that are tyrosine dephosphorylated in response to signaling-competent EPEC infection. The arrow indicates the phosphorylated form of Tir. wt, wild type.
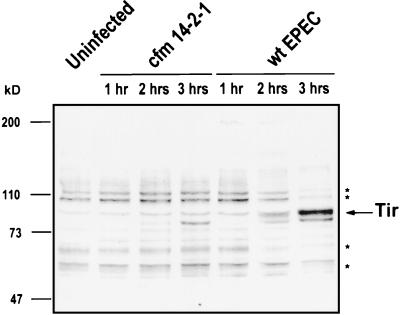
Tyrosine dephosphorylation kinetics of EPEC-infected J774 cells.
To correlate the onset of antiphagocytosis with tyrosine dephosphorylation, tyrosine phosphorylation profiles were determined for J774 cells infected with wild-type EPEC and cfm for 60, 120, and 180 min (Fig. 4). At 60 min of infection, both EPEC- and cfm-infected cells showed tyrosine phosphorylation profiles similar to the uninfected control. However, at 120 and 180 min of infection, significant tyrosine dephosphorylation of the 110, 100, 60, and 50-kDa proteins had occurred in the EPEC-infected samples relative to the uninfected control. No such dephosphorylation was observed with the cfm-infected cells at 180 min. Tir phosphorylation was observed at 120 min and remained at 180 min. These data further suggest a role for tyrosine dephosphorylation in EPEC-mediated antiphagocytosis.
FIG. 4.
EPEC-induced tyrosine dephosphorylation is evident after 120 min of infection. Monolayers were left uninfected or were infected with wild-type (wt) EPEC or cfm for 60, 120, and 180 min prior to isolation of J774 cell proteins as described in Materials and Methods. Samples were resolved by SDS–8% PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies. Molecular masses of proteins are indicated on the left. ∗, proteins that are tyrosine dephosphorylated in response to signaling-competent EPEC infection. The arrow indicates the phosphorylated form of Tir.
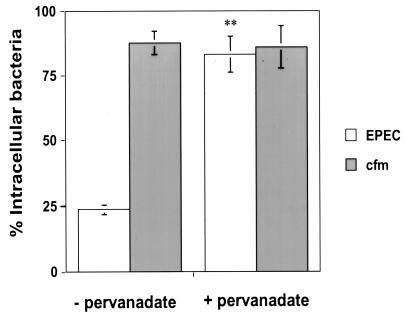
Inhibition of protein tyrosine phosphatases by pervanadate.
To determine if the antiphagocytic phenotype was dependent upon tyrosine dephosphorylation, pervanadate was used to inhibit protein tyrosine phosphatases in J774 cells. Monolayers were infected with EPEC for 90 min prior to the addition of pervanadate for the remaining 60 min of infection, as described in Materials and Methods. No significant loss of membrane integrity was observed during these experiments, as determined by trypan blue staining. EPEC-infected J774 cells treated with pervanadate showed a 3.5-fold increase in the percentage of total cell-associated EPEC organisms that were intracellular (83%) relative to cells that received no treatment (24%) (Fig. 5). However, the cfm EPEC-infected cells maintained similar numbers of intracellular bacteria with or without pervanadate treatment (88 and 86%, respectively). The Y. pseudotuberculosis wild-type strain was used as a control to demonstrate the ability of pervanadate to inhibit Y. pseudotuberculosis antiphagocytic activity (15% of Y. pseudotuberculosis organisms were intracellular without pervanadate treatment; 60% of the bacteria were intracellular with pervanadate treatment [data not shown]). Additionally, the tyrosine phosphorylation profile of EPEC-infected cells with pervanadate showed strong inhibition of dephosphorylation compared to the untreated control (data not shown).
FIG. 5.
EPEC-mediated antiphagocytosis is dependent upon tyrosine dephosphorylation. J774 cells were infected with EPEC or cfm for 90 min, prior to a 60-min incubation with 0.1 mM VO4 and 2 mM H2O2. Data are averages and standard errors of the means for three independent experiments performed in duplicate. ∗∗, significant difference from the wild type at P of <0.01, as obtained by Student t tests.
Tyrosine phosphatase activity of EPEC-secreted proteins.
To determine if the observed tyrosine dephosphorylation was caused by one of the EPEC-secreted proteins, phosphatase activity was monitored (Table 2). No significant decrease in absorbance was detected when the EPEC-secreted proteins were added to a tyrosine-phosphorylated substrate in vitro compared to the substrate alone, suggesting that tyrosine dephosphorylation did not occur under these conditions. However, very significant phosphatase activity (P < 0.01) was detected with the Y. pseudotuberculosis-secreted proteins, as would be expected due to the presence of YopH. The addition of pervanadate caused a significant increase in absorbance with Y. pseudotuberculosis (P < 0.01), suggesting that pervanadate inhibited phosphatase activity of the Yop proteins. No effect was seen with the EPEC-secreted proteins upon pervanadate addition.
TABLE 2.
EPEC-secreted proteins have no detectable protein tyrosine phosphatase activity in vitro
| Sample | Absorbance (405 nm)a |
|---|---|
| None | 1.190 ± 0.063 |
| Yop proteins from Y. pseudotuberculosis | 0.334 ± 0.023* |
| Yop proteins from Y. pseudotuberculosis + pervanadate | 0.715 ± 0.164* |
| EPEC-secreted proteins | 1.223 ± 0.032 |
| EPEC-secreted proteins + pervanadate | 1.258 ± 0.010 |
Values are means ± standard errors of the means (three samples per group). *, significantly different (P < 0.01) from value for control group with no sample, as calculated by Student t tests.
DISCUSSION
This study demonstrates that EPEC inhibits its own phagocytosis into cultured macrophage-like cells. This ability is dependent upon the EPEC type III secretion system and its secreted proteins (EspA, EspB, and EspD), which mediate effects on tyrosine-phosphorylated proteins within the host cell. Two lines of evidence suggest the involvement of the secreted proteins. Mutations in the secretion apparatus, as seen in the cfm strain, resulted in the inability of EPEC to inhibit phagocytosis by J774 cells. EPEC strains lacking EspA, EspB, or EspD also lost any antiphagocytic capacity, indicating a role in mediating this process, either directly as effector molecules or indirectly as part of a transport delivery apparatus. Time course data indicate that EPEC-mediated antiphagocytosis does not occur within the first hour of infection but is evident after two hours. Protein secretion and significant bacterial attachment do not occur until 90 min after infection (14). Indeed, only low-level adherence to J774 cells was observed until 120 min after bacterial addition, and as bacterial adherence increased, antiphagocytosis also increased, correlating EPEC attachment and protein secretion/transfer with antiphagocytic activity.
The intermediate antiphagocytic phenotype observed for the Tir and intimin mutants correlated with intermediate dephosphorylation of the same host proteins that are dephosphorylated following infection with both wild-type and Bfp mutant strains. Tir and intimin mutants thus seem to be less efficient at inducing antiphagocytosis although they are still signal-competent, unlike the EspA, EspB, EspD, or cfm mutants. This argues for a role of intimate attachment in antiphagocytosis. Prolonged intimate attachment of EPEC to the host cell leads to cytoskeletal rearrangements and pedestal formation. The role of tyrosine dephosphorylation in pedestal formation remains unclear. Prolonged treatment of EPEC-infected J774 cells with pervanadate leads to cell death (data not shown), and as a consequence it cannot be determined if pedestals are formed in the presence of this phosphatase inhibitor. Therefore, it cannot be ruled out that cytoskeletal rearrangements are not involved in the EPEC-mediated antiphagocytic process. Another explanation would be that intimate attachment enhances translocation of bacterial effectors required for inducing antiphagocytosis, since it has been shown that EspB is less efficiently translocated into HeLa cells by the intimin mutant than by the wild-type strain (31).
The number of cell-associated bacteria also affected the levels of antiphagocytic activity, probably through a dose effect on the signaling events triggered by the secreted proteins. It might be expected, therefore, that microcolony formation, mediated by Bfp, would be important in the process by increasing the number of adherent bacteria and thereby delivering a stronger antiphagocytic signal. However, there were no differences in levels of uptake between the wild type and the bfp mutant, suggesting that this was not true. Microcolony formation could possibly function in antiphagocytosis by interconnecting large numbers of bacteria to the macrophage surface. However, the secretion mutants are still capable of microcolony formation, and very few bacteria are found extracellularly on J774 cells, suggesting that this is probably not the case.
Antiphagocytic activity was correlated with tyrosine dephosphorylation events, and the presence of pervanadate specifically inhibited the antiphagocytic activity of wild-type EPEC without hindering the uptake of the signaling mutant, cfm. Pervanadate also inhibited the antiphagocytic activity of Y. pseudotuberculosis, as previously reported (2). Since Y. pseudotuberculosis transfers a phosphatase into host cells that leads to the activity, we hypothesized that EPEC may also inject a phosphatase. However, no tyrosine phosphatase activity was detected with the secreted proteins with an in vitro assay system. This suggests that EPEC, unlike Y. pseudotuberculosis, does not secrete a functional phosphatase under these conditions. It is possible that EPEC may secrete a phosphatase that requires a eukaryotic cofactor for activity. Alternatively, the secreted proteins may trigger the activation of a mammalian tyrosine phosphatase as part of their signal activation.
Taken together, the present findings demonstrate that EPEC blocks its own uptake into phagocytic cells by a mechanism associated with tyrosine dephosphorylation of host proteins. The EPEC-induced dephosphorylation events are dependent upon the secretion of several bacterial proteins, whose specific functions remain undefined. Research is ongoing to further elucidate the mechanism by which EPEC mediates its antiphagocytic phenotype.
ACKNOWLEDGMENTS
We thank Rebekah DeVinney for helpful discussions and technical advice.
D.L.G. was supported by a Canadian Natural Sciences and Engineering Research Council Post-Graduate Scholarship, J.C. was supported by a postdoctoral fellowship from the Fondation pour la Recherche Medicale, and B.K. was supported by a postdoctoral fellowship from the Human Frontiers Program. This work was supported by a Howard Hughes International Research Scholar Award and operating grants from the Medical Research Council of Canada to B.B.F.
REFERENCES
- 1.Abe A, Kenny B, Stein M, Finlay B B. Characterization of two virulence proteins secreted by rabbit enteropathogenic Escherichia coli, EspA and EspB, whose maximal expression is sensitive to host body temperature. Infect Immun. 1997;65:3547–3555. doi: 10.1128/iai.65.9.3547-3555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson K, Carballeira N, Magnusson K E, Persson C, Stendahl O, Wolf-Watz H, Fallman M. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signaling associated with phagocytosis. Mol Microbiol. 1996;20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliska J B, Guan K L, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Kaper J B. Construction of an eaeA deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dytoc M, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 9.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inman L R, Cantey J R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer’s patch in Escherichia coli diarrhea in the rabbit. J Clin Investig. 1983;71:1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic E. coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transduction signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 16.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B, Abe A, Stein M, Finlay B B. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those found in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic Escherichia coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lai L C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine M M, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;162:1285–1292. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- 22.Ralph P, Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975;257:393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- 23.Raschke W C, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 24.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal exchange between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell protein to initiate cytoskeletal rearrangment and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 26.Rosqvist R, Bolin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Siebers A, Finlay B B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 31.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]