Abstract
Objective:
Identifying associations among circulating proteins, dietary intakes, and clinically-relevant indicators of cardiometabolic health during weight loss may elucidate biologically-relevant pathways affected by diet, allowing us to incorporate precision nutrition approaches when designing future interventions. We hypothesized that plasma proteins would be associated with diet and cardiometabolic health indicators within a behavioral weight loss intervention.
Methods:
This secondary data analysis included participants (n=20, age: 40.1±9.5 years, BMI: 34.2±4.0 kg/m2) who completed a 1-year behavioral weight loss intervention. Cardiovascular disease (CVD)-related plasma proteins, diet, and cardiometabolic health indicators were evaluated at baseline and 3 months. Associations were determined via linear regression and integrated networks created using Visualization Of LineAr Regression Elements.
Results:
Sixteen plasma proteins were associated with ≥1 diet or health indicator at baseline (p<0.001); changes in 42 proteins were associated with changes in diet or health indicators from baseline to 3 months (p<0.005). Baseline TNFRSF10C was associated with intakes of dark green vegetables (r=−0.712) and FABP4 with unsweetened coffee (r=−0.689). Changes in refined grain intakes were associated with changes in CD163 (r=0.725), IL1R-T1 (r=0.624), insulin (r=0.656), and triglycerides (r=0.648).
Conclusions:
Circulating CVD-related proteins are associated with diet and cardiometabolic health indicators prior to and in response to weight loss.
Keywords: behavioral weight management, diet quality, healthy eating index, cardiovascular disease III panel, network analysis
Graphical Abstract
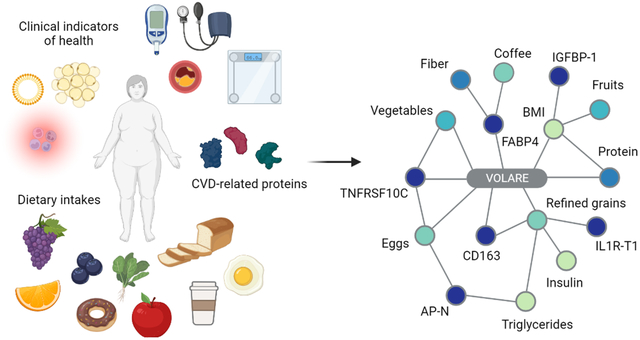
Introduction
Cardiometabolic health can be improved with changes in lifestyle behaviors independent of weight loss (1, 2). However, while many adults with overweight or obesity experience improvements in biomarkers of cardiometabolic function after weight loss, others do not similarly respond to intervention. Thus, it is imperative to increase our understanding of the influence of modifiable behaviors, such as dietary intake patterns, on changes in health indicators in order to mitigate risk factors for metabolic diseases including cardiovascular disease (CVD) (3) and type 2 diabetes mellitus (T2DM) (4). Identifying which factors are associated with clinical and biochemical indicators of cardiometabolic health, including circulating plasma proteins, may help to elucidate biologically-relevant pathways, and therefore, allow us to move towards precision nutrition approaches when designing future interventions (5).
There is significant evidence that dietary patterns directly and indirectly influence the risk for development, progression, and mortality from cardiometabolic diseases such as T2DM and CVD (6). Higher overall diet quality and consumption of specific foods, such as fruits and vegetables, have been associated with reduction in metabolic disease risk in multiple epidemiologic cohorts (7, 8). Indeed, U.S. adults exhibiting high diet quality have a 14% to 28% lower risk of CVD-related mortality when compared to those with low diet quality (9). Consequently, diet is an attractive target for behavioral modification for both disease prevention and management.
Circulating proteins offer a promising approach for studying changes in host biology in response to weight loss, as the proteome is responsive to environmental cues such as diet (10). These proteins are readily measurable in serum and plasma and can be interrogated with multi-plex platforms (11, 12). High throughput targeted proteomic arrays have been developed to measure proteins that have been associated with CVD risk. These arrays, coupled with appropriate clinical, biochemical, and dietary data, can be used to evaluate changes associated with behavioral modification or weight loss. Previous studies, including a secondary analysis of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial have employed such arrays, reporting that several plasma proteins were cross-sectionally and longitudinally associated with body weight (13). Such studies indicate robust changes in the proteome following alterations in dietary intake; yet, these previous works have not identified discrete dietary features (e.g., specific nutrients, food groups, or components of diet quality) that are associated with plasma proteomic profiles.
The aim of this pilot study was to identify baseline cross-sectional and longitudinal associations between CVD-related plasma proteins, dietary intakes, and clinically-relevant indicators of cardiometabolic health following 3 months of a behavioral weight loss intervention. We hypothesized that baseline CVD-related proteins would be associated with dietary intakes and clinical indicators of cardiometabolic health (e.g., weight, blood pressure, lipid panel, glucose, insulin). Further, we hypothesized that changes in CVD-related proteins would be associated with changes in both dietary intakes and clinical indicators.
Methods
Participants
This study evaluated baseline and 3-month data from 20 healthy individuals with overweight or obesity (age 18–55 years, BMI 27–45 kg/m2) participating in an ongoing 2-arm randomized controlled trial (ClinicalTrials.gov NCT03411356) comparing the efficacy of weight loss achieved through daily caloric restriction (DCR) versus intermittent fasting (IMF). All participants were provided a comprehensive behavioral weight loss intervention based upon evidence-based guidelines and instructed to consume an energy-restricted diet, increase physical activity, and attend group-based support sessions (14). Both groups targeted a weekly caloric deficit of ~34% compared to baseline-estimated weight maintenance energy requirements (15). Participants who enrolled in 2018–2019 and completed the 1-year intervention (n=20) were included in this secondary analysis (Figure S1). Proteomics, dietary, and clinical data were collected at baseline and 3 months. Selected participants were frequency matched on sex, BMI, and intervention arm. Because the parent trial is blinded and remains in progress, between-group differences were not assessed. All study procedures were approved by the Colorado Multiple Institutional Review Board, and participants provided written informed consent. Details regarding inclusion and exclusion criteria and complete study procedures were published previously (16).
Dietary Assessment
Dietary intakes were assessed via 7-day diet records at each timepoint. Participants were directed by study staff to record all foods and beverages consumed over 7 days, including information on portion size, brands, and preparation methods. Data were entered into Nutrition Data System for Research (NDSR, University of Minnesota Nutrition Coordinating Center, Minneapolis, MN) for dietary analyses using standard protocols by the University of Colorado Clinical and Translational Sciences Institute (CCTSI) Nutrition Core personnel. Three individual days of intake across all diet records and time points that were not representative of usual intake (e.g., participant report of illness) were excluded from analyses.
Daily intake of energy, macronutrients, fiber, micronutrients, and food group serving counts were exported from NDSR, and mean intakes over the 7 days were calculated. Total and component Healthy Eating Index (HEI) scores were calculated using previously published methods and publicly available SAS code (17). To determine mean total HEI and individual HEI component scores, a simple HEI scoring algorithm using all 7 days of intake was employed. Total scores represent energy-adjusted intakes of key food groups with recommendations for adequacy (9 components) or moderation (4 components) and range from 0 to 100; higher scores indicate greater adherence to the U.S. Dietary Guidelines for Americans (17). Dietary intake is reported as nutrients, food groups, and HEI scores, offering three different, but technically correlated quantifications of the diet records (Table S1).
Anthropometry and Cardiometabolic Health Indicators
Anthropometry measures, blood collections and processing, and circulating cardiometabolic assays were performed by trained study personnel at the University of Colorado Anschutz Health and Wellness Center (CU-AHWC) and University of Colorado Clinical and Translational Research Center (CTRC), respectively, and described in detail in Supporting Information.
Proteomics Assay
The Olink® Target 96 CVD III panel was performed by Olink Proteomics using proximity extension assay (PEA) technology, described in detail in Supporting Information (11, 12). The CVD III panel covers 92 proteins related to CVD and inflammation, providing normalized protein expression (NPX) data, with higher values indicating higher protein concentrations but not absolute quantifications. The 92 proteins are enriched in biological pathways such as angiogenesis, blood vessel morphogenesis, inflammatory response, and platelet activation and have been associated with clinical indicators of cardiovascular risk in several studies (18–20). For the completed array, the mean intra-assay coefficient of variation was 13% among all samples and assays. All 92 proteins were detected with none failing quality control checks. No proteins exhibited ≥90% of samples below the limit of detection or greater than 10% missingness, and therefore none were excluded from analyses.
Statistical Analyses
Descriptive statistics were generated for all outcome variables. Variables that were non-normally distributed were log transformed prior to analyses to account for heteroscedasticity, and changes from baseline to 3 months were determined using paired t-tests for each outcome of interest. For data that remained non-normal after log transformation, non-parametric Wilcoxon signed rank tests were used to assess changes from baseline to 3 months. Significance was determined a priori at α = 0.05.
VOLARE Network Analysis
To assess relationships between proteins from the CVD III panel, dietary intakes, and clinical indicators of health, we applied Visualization Of LineAr Regression Elements (VOLARE) to create integrated networks as previously described (16, 21). VOLARE provides visual representation of top-table pairwise associations and their associated regression elements to aid in interpretation of complex networks among features of interest (http://aasix.cytoanalytics.com/volare/). A combined dataset including baseline values for proteomics (number of features, p=92), dietary intakes (p=220), and clinical indicators of health (p=10) was created, as detailed in Figure 1. Dietary intake variables were limited to those with nonzero values in greater than 50% of the participants. To determine pairwise relationships between all features across all three data categories (proteomics, diet, clinical), a linear model of the form FeatureA ~ FeatureB (e.g., IGFBP-1 expression ~ BMI) was fit for each cross-category pair (clinical:diet, clinical:proteomics, proteomics:diet) to identify associations at baseline. The top results (unadjusted p-value < 0.001) were included in a VOLARE network. A second analysis was performed using changes from baseline to 3 months, including only those relationships with an unadjusted p-value < 0.005. To identify whether longitudinal relationships differed by sex, we used a linear model of the form FeatureA ~ sex + FeatureB + sex × FeatureB. All analyses were performed in R version 4.1.0.
Figure 1.
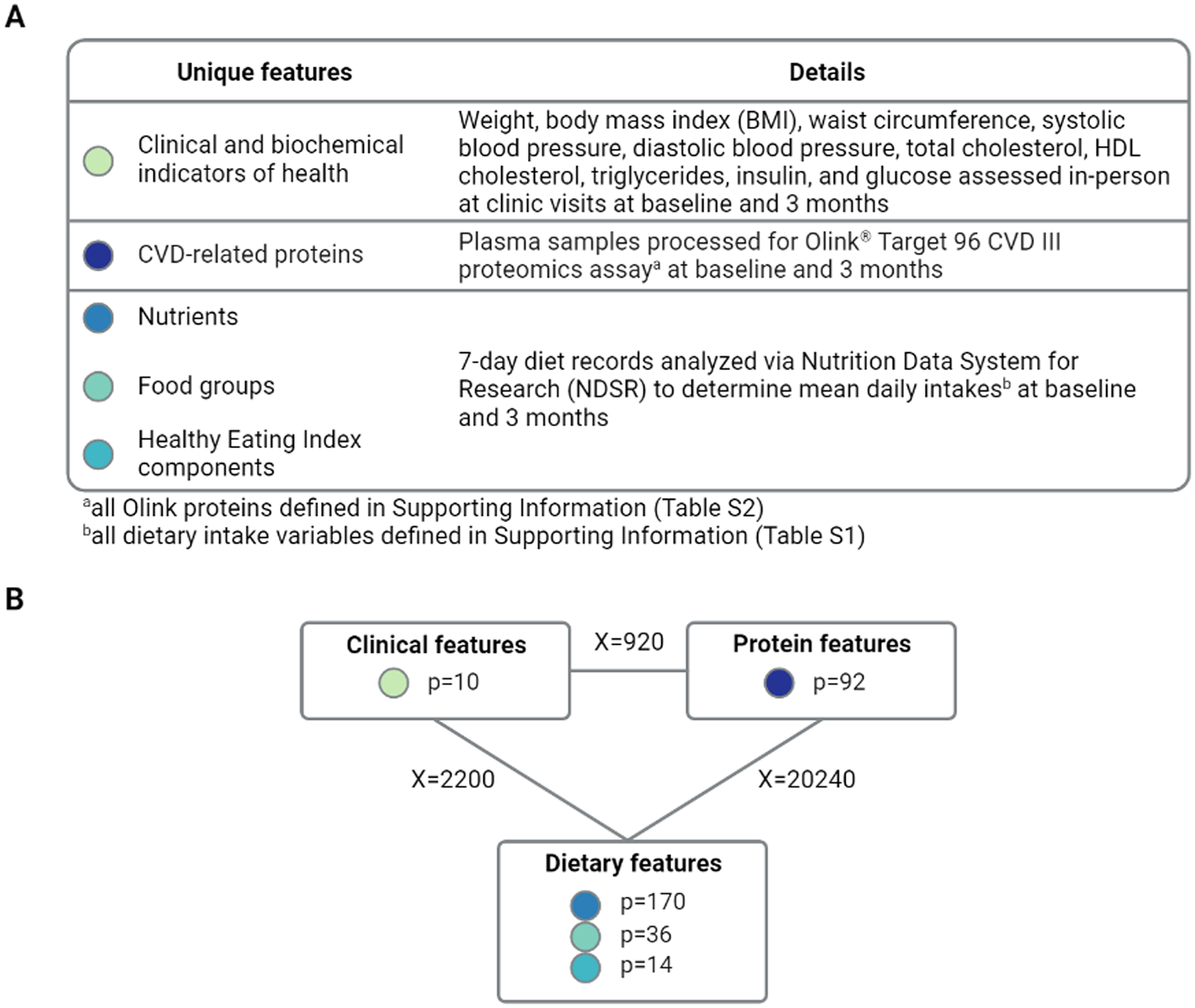
Creation of VOLARE network
(A) Outcomes were assessed at baseline and 3 months for all participants and grouped into three categories: proteins, dietary intakes, and clinical indicators of health. For calculating change, data were required at both baseline and 3 months. (B) Pairwise regression models were fit across the three data categories for both baseline and change analyses. The number of features in each data category are shown in rectangles, while the number of pairwise comparisons across categories are indicated on the lines connecting the categories. The top models per category pair based on p<0.001 for baseline associations and p<0.005 for baseline to 3-month changes were then included in a VOLARE network.
Results
Participant characteristics and changes in plasma proteins, dietary intakes, and clinical indicators over intervention
Participants in this study were predominately non-Hispanic Caucasian, with a mean age of 40.1 ± 9.5 years (Table 1). Mean body mass index (BMI) was significantly reduced at 3 months (p<0.001, Table 2). Similar changes were noted for other anthropometrics, including body weight (p<0.001) waist circumference (p<0.001), and several clinical outcomes (Table 2). Dietary intake data are presented in Table 3. Individuals reduced total energy intake by over 400 kcal/day from baseline to 3 months, with reductions in percent of energy from fat and increases in percent of energy from protein (all p<0.001). While participants improved adherence to dietary guidelines for intakes of both total fruits (p=0.003) and total vegetables (p=0.015), mean total HEI scores were not significantly changed over this time period. Several plasma proteins also changed from baseline to 3 months (Table S2).
Table 1.
Baseline sociodemographic characteristics of individuals participating in a behavioral weight loss intervention (n=20)
| Characteristic | n (%) or Mean ± SD | |
|---|---|---|
| Age | 40.1 ± 9.5 | |
| Sex | Male | 10 (50.0) |
| Female | 10 (50.0) | |
| Race | Black or African American | 1 (5.0) |
| White or Caucasian | 18 (90.0) | |
| One or more races | 1 (5.0) | |
| Ethnicity | Hispanic | 4 (20.0) |
| Non-Hispanic | 16 (80.0) | |
Table 2.
Baseline and 3-month clinical outcomes of individuals participating in a behavioral weight loss intervention (n=20)
| Clinical Outcome | Baseline Mean ± SD |
3-Month Mean ± SD |
Mean Change | p-value |
|---|---|---|---|---|
| Body weight (kg) | 102.8 ± 15.8 | 93.1 ± 15.6 | −9.7 | <0.001 |
| BMI (kg/m2) | 34.2 ± 4.0 | 30.9 ± 4.0 | −3.2 | <0.001 |
| Waist circumference (cm)b | 113.5 ± 10.0 | 102.1 ± 9.4 | −11.4 | <0.001 |
| Systolic blood pressure (mmHg) | 120.7 ± 14.7 | 117.4 ± 12.8 | −3.3 | 0.312 |
| Diastolic blood pressure (mmHg) | 77.9 ± 5.9 | 76.5 ± 8.8 | −1.4 | 0.597 |
| Total cholesterol (mg/dL)a,b | 171.7 ± 23.5 | 154.2 ± 23.9 | −17.5 | 0.002 |
| HDL cholesterol (mg/dL)a,b | 43.5 ± 10.0 | 43.6 ± 12.7 | 0.1 | 0.964 |
| Triglycerides (mg/dL)a,b | 133.3 ± 62.6 | 103.9 ± 43.3 | −29.4 | 0.024 |
| Glucose (mg/dL)a,b | 91.6 ± 9.4 | 85.3 ± 6.0 | −6.3 | 0.002 |
| Insulin (uIU/mL)a,b | 10.3 ± 5.5 | 6.9 ± 3.4 | −3.5 | <0.001 |
n=19 at baseline due to missing blood draw
data natural log-transformed prior to analysis due to heteroscedasticity
Table 3.
Baseline and 3-month dietary intakes for features of interest from individuals participating in a behavioral weight loss intervention (n=20)
| Dietary Outcome | Baseline Mean ± SD |
3-Montha Mean ± SD |
Mean Change | p-value | |
|---|---|---|---|---|---|
| Nutrients | |||||
| Energy (kcal/day) | 1896 ± 433 | 1457 ± 384 | −438 | <0.001 | |
| Total protein (% kcal) | 16.6 ± 3.1 | 20.8 ± 4.4 | 4.2 | <0.001 | |
| Total fat (% kcal) | 38.2 ± 5.2 | 34.7 ± 5.8 | −3.5 | <0.001 | |
| Total carbohydrate (% kcal) | 42.4 ± 6.6 | 43.3 ± 7.7 | 0.8 | 0.605 | |
| Total dietary fiber (g/day) | 17.9 ± 7.9 | 15.4 ± 7.3 | −2.4 | 0.139 | |
| Soluble dietary fiber (g/day) | 6.4 ± 2.9 | 5.0 ± 2.2 | −1.5 | 0.020 | |
| Total folate (mcg/day) | 366 ± 149 | 292 ± 127 | −74.2 | 0.039 | |
| Beta-carotene (mcg/day) | 2542 ± 1999 | 2789 ± 1829 | 246 | 0.638 | |
| Food Groups b | |||||
| Dark green vegetables (servings) | 0.6 ± 0.6 | 0.5 ± 0.5 | −0.1 | 0.754 | |
| Other vegetables (servings) | 0.6 ± 0.4 | 0.8 ± 0.5 | 0.2 | 0.343 | |
| Legumes (servings) | 0.2 ± 0.2 | 0.2 ± 0.3 | 0.0 | 0.936 | |
| Refined grain cookies, cakes, pies (servings) | 0.4 ± 0.7 | 0.2 ± 0.2 | −0.2 | 0.920 | |
| Refined grains, flours, dry mixes (servings) | 1.4 ± 1.0 | 0.7 ± 0.5 | −0.7 | 0.043 | |
| Eggs (servings) | 0.8 ± 0.7 | 0.7 ± 0.8 | −0.1 | 0.316 | |
| Healthy Eating Index Components c | Max Score | ||||
| Total fruit | 5 | 1.2 ± 1.3 | 2.3 ± 1.9 | 1.1 | 0.003 |
| Whole fruit | 5 | 1.8 ± 1.8 | 2.9 ± 2.1 | 1.0 | 0.007 |
| Total vegetables | 5 | 2.9 ± 1.1 | 3.5 ± 1.1 | 0.6 | 0.015 |
| Greens and beans | 5 | 3.2 ± 1.7 | 3.8 ± 1.7 | 0.5 | 0.208 |
| Whole grains | 10 | 5.3 ± 3.3 | 4.2 ± 3.1 | −1.1 | 0.284 |
| Dairy | 10 | 5.4 ± 2.9 | 5.7 ± 2.7 | 0.3 | 0.551 |
| Total protein foods | 5 | 4.8 ± 0.6 | 4.9 ± 0.3 | 0.1 | 0.469 |
| Seafood and plant proteins | 5 | 3.2 ± 2.0 | 2.9 ± 1.8 | −0.3 | 0.706 |
| Fatty acids | 10 | 4.7 ± 3.0 | 4.3 ± 2.5 | −0.5 | 0.495 |
| Refined grains | 10 | 6.2 ± 3.0 | 6.1 ± 3.8 | −0.1 | 0.610 |
| Sodium | 10 | 4.8 ± 2.5 | 4.4 ± 2.9 | −0.3 | 0.294 |
| Added sugars | 10 | 8.5 ± 1.8 | 8.9 ± 1.3 | 0.4 | 0.193 |
| Saturated fats | 10 | 4.3 ± 2.7 | 5.1 ± 3.0 | 0.8 | 0.258 |
| Total HEI | 100 | 56.3 ± 12.6 | 59.0 ± 13.0 | 2.7 | 0.294 |
n=19 due to missing diet data
Food Groups variables natural log-transformed prior to analyses due to heteroscedasticity
Healthy Eating Index scores were assessed via nonparametric Wilcoxon signed rank test due to non-normality
Associations between plasma proteins, dietary intakes, and clinical indicators at baseline
Of the 92 tested proteins, 16 were significantly associated with at least 1 clinical or dietary intake variable at baseline. The final VOLARE network for baseline-to-baseline associations contained 51 nodes and 40 edges, with edges representing linear relationships across pairs (Figure 2A). Nodes with more than 2 neighbors were identified as hubs; 9 hubs were present within this network. The hubs with the greatest number of edges included 3 proteins: fatty acid binding protein 4 (FABP4), tumor necrosis factor receptor superfamily member 10C (TNFRSF10C), and C-X-C motif chemokine 16 (CXCL16), with 13, 6, and 6 edges, respectively. FABP4 was inversely associated with several dietary features, including total fiber (interquartile range effect size (IQRe) = −0.417 g/day, r = −0.706, p<0.001), soluble fiber (IQRe = −0.551 g/day, r = −0.784, p<0.001), folate (IQRe = −0.403 mcg/day, r = −0.699, p<0.001), beta-carotene (IQRe = −0.390 mcg/day, r = −0.679, p=0.001), and unsweetened coffee (IQRe = −0.644 servings/day, r = −0.689, p<0.001), as seen in Figure 2B–2C. The IQR effect size represents the predicted change in the dependent variable associated with a change in the independent variable from the 25th percentile to the 75th percentile (22). TNFRSF10C was likewise inversely associated with intakes of dark green vegetables (IQRe = −0.245 servings/day, r = −0.712, p<0.001) and HEI component scores for both total vegetables (IQRe = −0.450 points, r = −0.751, p<0.001) and greens and beans (IQRe = −0.571 points, r = −0.751, p<0.001). CXCL16 was inversely associated with total saturated fatty acid intakes (IQRe = −0.184 g/day, r = −0.703, p<0.001) as well as intakes of palmitic acid, stearic acid, and trans-octadecadienoic acid (IQRe = −0.229 g/day, r = −0.719; IQRe = −0.258 g/day, r = −0.759; and IQRe = −0.352 g/day, r = −0.722, respectively, all p<0.001).
Figure 2.
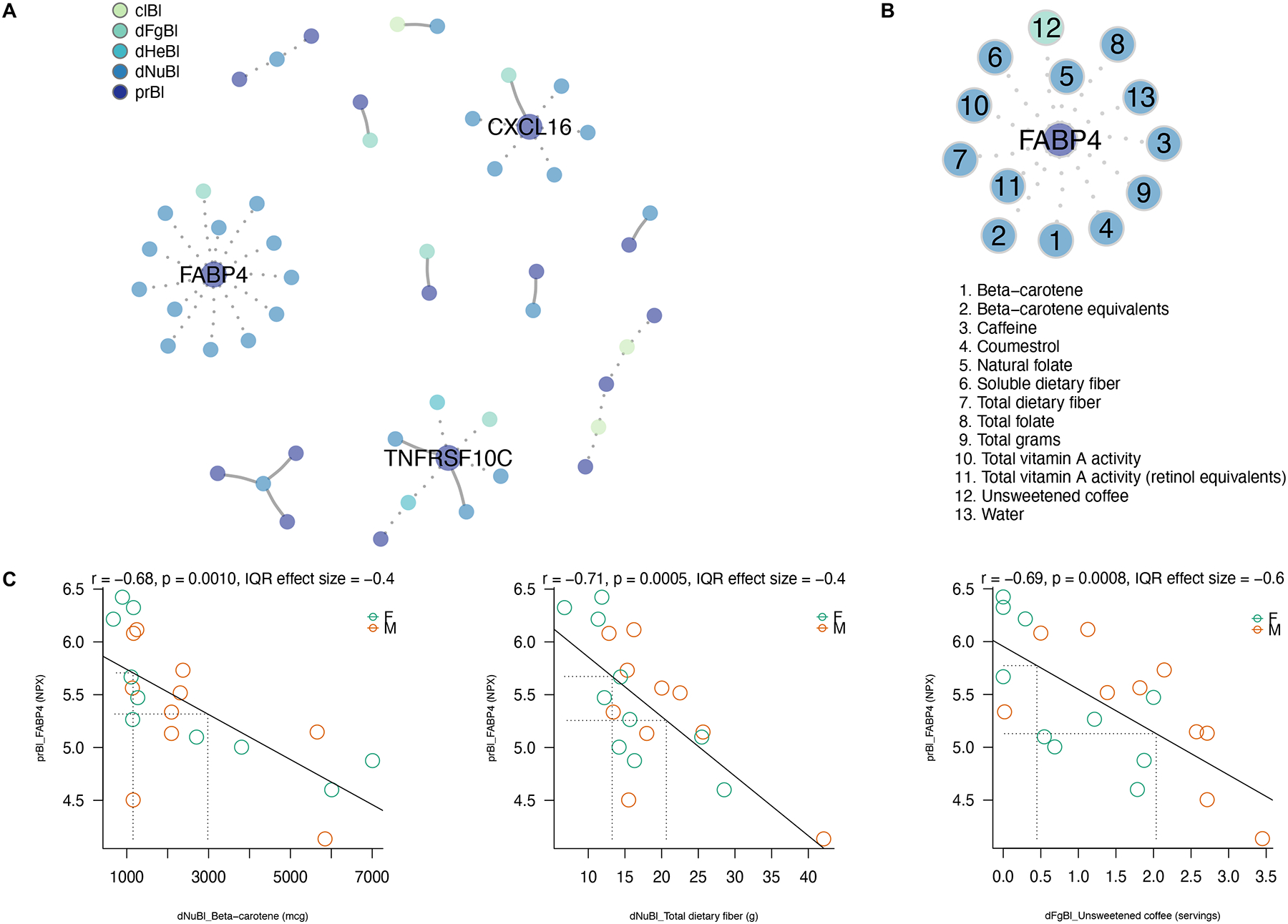
Cross-sectional VOLARE network analysis at baseline
(A) The VOLARE network for associations at baseline shows 51 total nodes connected by 40 edges, with edges representing linear relationships across pairs. Solid lines represent positive associations, while dotted lines indicate inverse associations. Nine hubs, representing nodes with at least two neighbors, are present. The categories are color-coded according to the legend (clBl = clinical baseline; dFgBl = diet food groups baseline; dHeBl = diet Healthy Eating Index baseline; dNuBl = diet nutrients baseline; prBl = proteins baseline). (B) One hub, for the protein fatty acid binding protein 4 (FABP4) depicts 13 individual nodes and the directionality of the significant pairwise associations with FABP4. All 13 nodes were inversely associated with FABP4 at baseline. (C) Detailed plots showing the underlying data and interquartile range effect sizes for three select pairwise associations from the FABP4 hub: beta carotene (1), total dietary fiber (7) and unsweetened coffee (12). Each circle represents one study participant colored by sex (green = female, brown = male). The fitted regression lines show inverse associations with FABP4 for each detailed plot.
Insulin-like growth factor binding proteins 1 and 2 (IGFBP-1 and IGFBP-2) were inversely associated with BMI (IQRe = −3.92 kg/m2, r = −0.727 and IQRe = −4.64 kg/m2, r = −0.734, both p<0.001). IGFBP-2 and paraoxonase 3 (PON3) were inversely associated with insulin (IQRe = −6.61 uIU/mL, r = −0.757 and IQRe = −4.01 uIU/mL, r = −0.701, both p<0.001). When evaluating pairwise relationships between food groups of interest and CVD-related proteins, intakes of refined grain cakes, cookies, pies, and pastries and refined grain flours were positively associated with plasma concentrations of scavenger receptor cysteine-rich type 1 protein M130 (CD163) and matrix extracellular phosphoglycoprotein (MEPE), respectively.
Associations between changes in plasma proteins, dietary intakes, and clinical indicators from baseline to 3 months
Changes in 42 proteins were associated with changes in at least 1 clinical or dietary intake feature. The final VOLARE network included 72 nodes with 65 edges (Figure 3A). Five hubs with at least 4 nodes were identified and included 2 food groups (eggs and refined grain flours), 1 nutrient (myristoleic acid), 1 clinical indicator of health (triglycerides), and 1 protein (IGFBP-2). Select associations are described to highlight examples of interest. Increase in percent of energy from protein was significantly associated with decrease in BMI (IQRe = −1.07 kg/m2, r = −0.635, p=0.004), as seen in Figure 3B. Change in whole fruit HEI component score was the only other dietary feature associated with a change in BMI (IQRe = −1.10 kg/m2, r = −0.618, p=0.005). Decreases in dietary intakes of refined grain flours were directly associated with reductions in triglycerides (IQRe = 24.5 mg/dL, r = 0.648, p=0.004) and insulin (IQRe = 1.61 uIU/mL, r = 0.656, p=0.003) as well as reductions in circulating CD163 (IQRe = 0.264 NPX, r = 0.725, p<0.001) and interleukin-1 receptor type 1 (IL1R-T1, IQRe = 0.203 NPX, r = 0.624, p=0.004) proteins (Figure 3C). Reductions in plasma triglycerides were likewise associated with decreases in several circulating proteins (Figure 3C). Decreased dietary intake of eggs was significantly and moderately positively associated with decreases in 21 proteins, including many that were associated with other dietary intakes or clinical indicators at baseline and over intervention such as TNFRSF10C and Aminopeptidase N (AP-N, Figure 3D). When evaluating longitudinal differences by sex, there were 3 relationships out of 65 (carboxypeptidase A1, carboxypeptidase B, and matrix metalloproteinase-3 versus dietary intakes of eggs) in which the sex-adjusted model was significantly different than the unadjusted model and the difference in slopes between males and females was significantly different (Figure S2).
Figure 3.

Longitudinal VOLARE network analysis of change from baseline to 3 months
(A) The VOLARE network depicting associations for changes from baseline to 3 months shows 72 nodes with 65 edges, with edges representing linear relationships across pairs. Solid lines represent positive associations, while dotted lines indicate inverse associations. Five hubs with at least four nodes were identified. The categories are color-coded according to the legend (clCh = clinical change from baseline to 3 months; dFgCh = diet food groups change from baseline to 3 months; dHeCh = diet Healthy Eating Index change from baseline to 3 months; dNuCh = diet nutrients change from baseline to 3 months; prCh = proteins change from baseline to 3 months). (B) Detailed data for the % calories from protein hub are depicted, showing inverse associations between changes in % calories from protein and BMI (IQRe = −1.1 kg/m2, r = −0.64, p=0.0035) and weight (IQRe = −3.1 kg, r = −0.65, p=0.0029). (C) Detailed data for the overlapping refined grains flours and triglycerides hubs are depicted. Decreased intakes of refined grains were positively associated with decreases in plasma triglycerides (IQRe = 24.5 mg/dL, r = 0.65, p=0.0036). The protein IL-1RT1 was shared between hubs, demonstrating a positive association with both intakes of refined grain flours (IQRe = 0.2 NPX, r = 0.62, p=0.0043) and plasma triglycerides (IQRe = 48.9 mg/dL, r = 0.68, p=0.0012). (D) Detailed data for the dietary intake of eggs hub are depicted, showing positive associations with 21 plasma proteins.
Discussion
In this study, we explored novel relationships among targeted plasma proteins, diet, and clinical indicators of cardiometabolic health at baseline and following 3 months of a behavioral weight loss intervention in men and women with overweight or obesity. Our primary findings indicate several aspects of diet are associated with circulating proteins and cardiometabolic health, providing insight into potential biologically-relevant pathways related to inflammation and metabolic regulation. Thus, diet was associated with changes in CVD risk during weight loss, which suggests relationships to investigate in future mechanistic and prognostic work. Some associations were persistent following weight loss, including those between dietary intakes of refined grains and circulating plasma proteins, though there were also several discrete differences between baseline and change analyses. Importantly, while other studies have examined changes in proteomic profiles during weight loss interventions, ours is the first to evaluate how dietary intakes of specific nutrients and food groups as well as established measures of diet quality may relate to targeted proteins that can provide more information about the underlying pathways contributing to the pathophysiology of obesity.
Our results suggest that dietary intakes are associated with circulating plasma proteins and clinical indicators of cardiometabolic health both before and during weight loss. A related study by Geyer et al. examined over 1,200 plasma proteins in 43 men and women with obesity who underwent an 8-week caloric restriction intervention of 800 kcal/day followed by 1 year of weight loss maintenance (10). The authors reported robust changes in the proteome with altered proteins in classes that were related to systemic inflammation, insulin resistance, and apolipoproteins. Another group found similar proteome alterations in response to DCR (23). These studies suggest weight loss leads to favorable changes in proteins that may be associated with reduction in future CVD risk. However, they did not assess nor account for specific changes in dietary intakes, which may influence proteomic profiles. Our work, which also evaluated early-intervention changes in plasma proteins, begins to fill this gap and provides relevant insight and potential targets for dietary modification that are in line with current evidence, such as increases in fruit and vegetable intakes and reduction in refined grain intakes, giving further support for weight loss strategies focusing on improved adherence to established dietary recommendations.
At baseline, dietary intakes of foods high in fiber, folate, and carotenoids such as vegetables were inversely associated with proteins including FABP4 and TNFRSF10C. Fatty acid binding proteins are expressed in adipocytes and macrophages and through their role in lipid transport and the inflammatory response they may contribute to insulin resistance, chronic inflammation, and obesity. In addition, FABP4 deficiency has been shown to protect against the development of these conditions in murine models (24, 25). Studies also suggest higher levels of FABP4 are related to increased major cardiovascular events and CVD-related mortality in several large cohorts (26, 27). Preclinical studies have demonstrated flavonoids such as quercetin and carotenoids such as beta-carotene, which are found primarily in fruits and vegetables, are associated with lower levels of circulating FABP4, suppression of inflammatory cytokines, and improved insulin sensitivity, suggesting intakes of foods high in these phytochemicals may serve as therapeutic agents for obesity and obesity-induced inflammation (28, 29). While no studies have directly evaluated TNFRSF10C concentrations in response to dietary intakes, research indicates elevated expression of TNFRSF10C is associated with greater senescent vascular smooth muscle cells, which are one of the key proinflammatory cell populations implicated in atherosclerotic development (30). Thus, greater intakes of specific food groups providing key beneficial nutrients and phytochemicals may translate to a reduction in future CVD risk through reductions in circulating proteins such as FABP4 and TNFRSF10C.
Associations between changes in dietary intakes and changes in clinical and proteomic variables yielded similar results. We observed slight increases in circulating FABP4 and reductions in dietary intakes of fiber and folate at 3 months, aligning with the inverse associations identified at baseline. However, our longitudinal VOLARE network analysis suggests this change in FABP4 was not significantly related to change in any other protein, diet, or clinical variable. Despite this, findings suggest it remains prudent to help individuals minimize reductions in dietary intakes of beneficial food components, such as fiber, when implementing future dietary interventions focused on overall reduction in caloric intake.
Reductions in refined grain intakes over 3 months were linearly associated with reductions in plasma triglycerides, insulin, and 2 circulating CVD proteins. Previous work indicates replacement of refined grains with whole grains can improve lipid profiles and indicators of metabolic function (31–33). Further, circulating CD163 and IL1R-T1, which are linked to oxidative stress and proinflammatory cytokines, have been significantly associated with unhealthy dietary patterns high in refined grains and added sugars in large cohorts (34). Notably, the relationship between CD163 and refined grain intakes was consistent between our baseline and change analyses and provides insight into potential mechanisms by which reductions in refined grain intakes may lead to cardiovascular benefits.
Several direct associations were observed between changes in dietary intakes of eggs and changes in circulating proteins, including TNFRSF10C and AP-N. Changes in AP-N were also associated with changes in triglycerides. Similar to TNFRSF10C, AP-N has been implicated in CVD, though its mechanism of action is hypothesized to be related to cholesterol uptake (35). It is possible that slight reductions in egg intakes over the intervention may have led to decreases in several proteins related to varying biological mechanisms underlying CVD development. However, the relative effect sizes for these observed associations were small, suggesting a shift in dietary intakes may only have a modest effect on circulating proteins. Further, it is difficult to isolate the effect of eggs from frequently-paired foods, such as bacon or sausage (36). Controversy surrounding the consumption of eggs and meat continues, with recent suggestions that constituents apart from cholesterol and saturated fat may be responsible for the observed impacts on cardiovascular health (37). Interestingly, three of the observed associations between circulating plasma proteins and dietary intakes of eggs differed by sex, however, dietary intakes of eggs did not demonstrate any significant associations with clinical indicators of health, warranting follow-up investigation to further explore the observed relationships.
Participants reduced energy intake by over 400 calories per day, and we observed corresponding reductions in BMI and improvements in clinical indicators of health. Our novel network approach allowed us to explore other dietary relationships to identify discrete factors significantly associated with change in weight, such as percent of energy from protein. This aligns with current recommendations to maintain adequate protein intakes during weight loss (38). Increases in whole fruit HEI scores were likewise associated with a decreased BMI. Coupled with observed decreases in refined grains, our data suggest participants may have replaced calorically-dense sweets or other refined-grain desserts with nutrient-dense choices such as fruit, which may have led to favorable impacts on weight loss efforts.
Lastly, several plasma proteins were associated with clinical indicators of cardiometabolic health. Insulin and BMI were inversely related to circulating IGFBP-1 and IGFBP-2 proteins at baseline. These proteins have been associated with improved insulin sensitivity and regulation of adiposity in humans and preclinical models (39). Our results are consistent with several other studies, including those demonstrating inverse associations between IGFBP-1 and IGFBP-2 with BMI in both surgical and dietary weight loss interventions as well as incident metabolic syndrome and T2DM in epidemiologic cohorts (13, 40, 41). These results provide confidence in our approach and suggest consistent associations between proteomic profiles and important clinical indicators that are highly relevant for the management of obesity and its sequalae.
Strengths and limitations
Strengths of this study include the application of our novel bioinformatics visualization tool, VOLARE, to evaluate interrelationships among several variables of interest. In addition, repeated measurement of plasma proteins, clinical indicators, and dietary intake allowed for both baseline and change analyses. Use of the established, targeted CVD III proteomics array also allowed for direct comparison to previously published works. Despite its self-report nature, perhaps the most critical strength was the inclusion of detailed dietary intake data, assessed via rigorous methods and analyzed with the most comprehensive nutrient database available, allowing for the first in-depth evaluation of relationships among diet, circulating proteins, and cardiometabolic indicators of health.
Limitations of this study include that other circulating proteins not present in the targeted assay may be influential during weight loss, cardiometabolic health, and responsive to changes in dietary intakes. The size and homogeneity of the sample size limit generalizability and therefore findings should be validated using the full cohort and expanded to other populations. Further, our analyses were not adjusted for potential confounders nor multiplicity of testing due to the sample size and exploratory nature of this study. Because the parent study is ongoing and we were blinded to intervention group assignment, it is also possible that there are intervention factors that may be relevant. Thus, results should be interpreted with caution and should be used to generate hypotheses to be tested in follow-up study. Exploration of the relationships among dietary intake variables and untargeted proteomic assays may also provide additional insight and should be included in future work.
Conclusion
Herein, we combined dietary intakes (computationally derived from self-reported food records), common clinical measurements, and plasma proteins from a multi-plex research assay to identify and illustrate interrelationships among these three modalities in the context of a behavioral weight loss intervention. Our study offers biological insight into how dietary intakes may influence CVD risk in those with obesity through the elucidation of several associations with circulating proteins and cardiometabolic health indicators prior to and in response to weight loss. This provides an initial step into the investigation of how targeted changes in specific aspects of diet may be used to improve outcomes during weight loss interventions. This approach provides a cohesive perspective that can be appreciated by dietitians, clinicians, and biomedical researchers and supports the inclusion of evidence-based strategies focused on improvements in dietary patterns to help individuals with overweight and obesity and improve cardiometabolic health.
Supplementary Material
Study Importance Questions.
What is already known?
Targeted plasma proteomics profiles, dietary intakes, and clinical indicators of cardiometabolic health are associated with future cardiovascular disease (CVD) risk.
Weight loss may improve both plasma proteomics profiles and cardiometabolic health, and these relationships may be influenced by changes in modifiable factors such as dietary intakes.
What does this study add?
Dietary intakes of discrete foods and food groups contributing to greater diet quality, computationally derived from self-reported diet records, are strongly associated with circulating plasma proteins and clinical outcomes such as BMI and triglycerides, providing additional evidence for biologically-relevant pathways that may connect diet with future CVD risk.
How might these results change the direction of research or the focus of clinical practice?
Integrated networks can be used to visualize complex associations between related variables to study factors contributing to the pathophysiology of obesity and CVD.
Identified associations can aid in generating follow-up hypothesis-oriented analyses to further investigate predictive relationships between plasma proteomics profiles, dietary intakes, and cardiometabolic health.
Acknowledgments
We thank the study participants and the clinical research team at the CU-AHWC for their time, effort, and participation in this study and Purevsuren Jambal for organizing biospecimens. The graphical abstract and Figure 1 for this manuscript were created with BioRender.com.
Funding:
This work was made possible by the support of Olink Proteomics and the CU Anschutz Genomics and Microarray Core for a pilot and feasibility grant (SJB), the American Heart Association 18IPA34170317 (SJB), the National Institutes of Health (NIH) R01 DK111622 (VAC), T32 DK007658 (EBH), F32 DK122652 (DMO), and U54 AG062319 (PSM). Additionally, the Colorado Nutrition and Obesity Research Center (NORC) P30 DK048520 and the Colorado Clinical and Translational Sciences Institute (CCTSI) NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 provided resources and support related to outcomes measured in this study. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The funding sources were not involved in the study design; data collection, analysis, or interpretation; or in the writing and submitting of this work.
Footnotes
Disclosure: The authors declared no conflict of interest.
Clinical trial registration: ClinicalTrials.gov NCT03411356
Data sharing statement
Upon publication and with no end date, individual deidentified participant data that underlie the results reported in this article will be available to investigators upon reasonable request. Relevant data will be made publicly available for the National Heart, Lung, and Blood Institute’s ADOPT Core Measures Project. Requests should be directed to Sarah.Borengasser@cuanschutz.edu and Vicki.Catenacci@cuanschutz.edu.
References
- 1.Herrmann SD, Willis EA, Honas JJ, Lee J, Washburn RA, Donnelly JE. Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The Midwest Exercise Trial 2. Obesity. 2015;23(8):1539–49. doi: 10.1002/oby.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefan N, Häring H-U, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? The Lancet Diabetes & Endocrinology. 2018;6(3):249–58. doi: 10.1016/S2213-8587(17)30292-9. [DOI] [PubMed] [Google Scholar]
- 3.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams J, Garvey WT. Weight Loss and the Prevention and Treatment of Type 2 Diabetes Using Lifestyle Therapy, Pharmacotherapy, and Bariatric Surgery: Mechanisms of Action. Current Obesity Reports. 2015;4(2):287–302. doi: 10.1007/s13679-015-0155-x. [DOI] [PubMed] [Google Scholar]
- 5.Aleksandrova K, Mozaffarian D, Pischon T. Addressing the Perfect Storm: Biomarkers in Obesity and Pathophysiology of Cardiometabolic Risk. Clinical Chemistry. 2018;64(1):142–53. doi: 10.1373/clinchem.2017.275172. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation. 2021;144(23). doi: 10.1161/cir.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 7.Onvani S, Haghighatdoost F, Surkan PJ, Larijani B, Azadbakht L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: a meta-analysis of observational studies. J Hum Nutr Diet. 2017;30(2):216–26. Epub 2016/09/14. doi: 10.1111/jhn.12415. [DOI] [PubMed] [Google Scholar]
- 8.Wang DD, Li Y, Bhupathiraju SN, Rosner BA, Sun Q, Giovannucci EL, et al. Fruit and Vegetable Intake and Mortality: Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation. 2021;143(17):1642–54. Epub 2021/03/02. doi: 10.1161/CIRCULATIONAHA.120.048996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, et al. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145(3):393–402. Epub 2015/03/04. doi: 10.3945/jn.114.205336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geyer PE, Wewer Albrechtsen NJ, Tyanova S, Grassl N, Iepsen EW, Lundgren J, et al. Proteomics reveals the effects of sustained weight loss on the human plasma proteome. Molecular Systems Biology. 2016;12(12):901. doi: 10.15252/msb.20167357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4):e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39(15):e102. Epub 2011/06/08. doi: 10.1093/nar/gkr424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figarska SM, Rigdon J, Ganna A, Elmståhl S, Lind L, Gardner CD, et al. Proteomic profiles before and during weight loss: Results from randomized trial of dietary intervention. Scientific Reports. 2020;10(1). doi: 10.1038/s41598-020-64636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation. 2014;129(25_suppl_2):S102–S38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Agriculture Organization of the United Nations. Human energy requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome, Italy: 2004. [Google Scholar]
- 16.Siebert JC, Stanislawski MA, Zaman A, Ostendorf DM, Konigsberg IR, Jambal P, et al. Multiomic Predictors of Short-Term Weight Loss and Clinical Outcomes During a Behavioral-Based Weight Loss Intervention. Obesity (Silver Spring). 2021;29(5):859–69. Epub 2021/04/04. doi: 10.1002/oby.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. Epub 2018/08/28. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoogeveen RM, Pereira JPB, Nurmohamed NS, Zampoleri V, Bom MJ, Baragetti A, et al. Improved cardiovascular risk prediction using targeted plasma proteomics in primary prevention. Eur Heart J. 2020;41(41):3998–4007. Epub 2020/08/19. doi: 10.1093/eurheartj/ehaa648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimczak-Tomaniak D, de Bakker M, Bouwens E, Akkerhuis KM, Baart S, Rizopoulos D, et al. Dynamic personalized risk prediction in chronic heart failure patients: a longitudinal, clinical investigation of 92 biomarkers (Bio-SHiFT study). Sci Rep. 2022;12(1):2795. Epub 2022/02/20. doi: 10.1038/s41598-022-06698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurmohamed NS, Belo Pereira JP, Hoogeveen RM, Kroon J, Kraaijenhof JM, Waissi F, et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. European Heart Journal. 2022. doi: 10.1093/eurheartj/ehac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siebert JC, Neff CP, Schneider JM, Regner EH, Ohri N, Kuhn KA, et al. VOLARE: visual analysis of disease-associated microbiome-immune system interplay. BMC Bioinformatics. 2019;20(1). doi: 10.1186/s12859-019-3021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE Jr.. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Switzerland: Springer International Publishing; 2015. 2015. [Google Scholar]
- 23.Bruderer R, Muntel J, Müller S, Bernhardt OM, Gandhi T, Cominetti O, et al. Analysis of 1508 Plasma Samples by Capillary-Flow Data-Independent Acquisition Profiles Proteomics of Weight Loss and Maintenance. Mol Cell Proteomics. 2019;18(6):1242–54. Epub 2019/04/06. doi: 10.1074/mcp.RA118.001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141(9):3388–96. Epub 2000/08/31. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 25.Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clinical Medicine Insights: Cardiology. 2014;8s3:CMC.S17067. doi: 10.4137/cmc.S17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höbaus C, Herz CT, Pesau G, Wrba T, Koppensteiner R, Schernthaner G-H. FABP4 and Cardiovascular Events in Peripheral Arterial Disease. Angiology. 2018;69(5):424–30. doi: 10.1177/0003319717728226. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Ding M, Chiuve SE, Rimm EB, Franks PW, Meigs JB, et al. Plasma Levels of Fatty Acid–Binding Protein 4, Retinol-Binding Protein 4, High-Molecular-Weight Adiponectin, and Cardiovascular Mortality Among Men With Type 2 Diabetes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(11):2259–67. doi: 10.1161/atvbaha.116.308320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo MJ, Lee YJ, Hwang JH, Kim KJ, Lee BY. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J Nutr Biochem. 2015;26(11):1308–16. Epub 2015/08/19. doi: 10.1016/j.jnutbio.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Kameji H, Mochizuki K, Miyoshi N, Goda T. β-Carotene accumulation in 3T3-L1 adipocytes inhibits the elevation of reactive oxygen species and the suppression of genes related to insulin sensitivity induced by tumor necrosis factor-α. Nutrition. 2010;26(11):1151–6. doi: 10.1016/j.nut.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Stojanović SD, Fuchs M, Kunz M, Xiao K, Just A, Pich A, et al. Inflammatory Drivers of Cardiovascular Disease: Molecular Characterization of Senescent Coronary Vascular Smooth Muscle Cells. Frontiers in Physiology. 2020;11. doi: 10.3389/fphys.2020.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall S, Petocz P, Duve E, Abbott K, Cassettari T, Blumfield M, et al. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. Journal of the Academy of Nutrition and Dietetics. 2020;120(11):1859–83.e31. doi: 10.1016/j.jand.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(8):837–44. doi: 10.1016/j.numecd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Sawicki CM, Jacques PF, Lichtenstein AH, Rogers GT, Ma J, Saltzman E, et al. Whole- and Refined-Grain Consumption and Longitudinal Changes in Cardiometabolic Risk Factors in the Framingham Offspring Cohort. The Journal of Nutrition. 2021;151(9):2790–9. doi: 10.1093/jn/nxab177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warensjö Lemming E, Byberg L, Stattin K, Ahmad S, Lind L, Elmståhl S, et al. Dietary Pattern Specific Protein Biomarkers for Cardiovascular Disease: A Cross-Sectional Study in 2 Independent Cohorts. Journal of the American Heart Association. 2019;8(11). doi: 10.1161/jaha.118.011860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, et al. Intestinal cholesterol transport proteins: an update and beyond. Current Opinion in Lipidology. 2007;18(3):310–8. doi: 10.1097/MOL.0b013e32813fa2e2. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation. 2021. doi: 10.1161/cir.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 37.Spence JD, Srichaikul K, Jenkins DJA. Cardiovascular Harm From Egg Yolk and Meat: More Than Just Cholesterol and Saturated Fat. Journal of the American Heart Association. 2021;10(7). doi: 10.1161/jaha.120.017066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raynor HA, Champagne CM. Position of the Academy of Nutrition and Dietetics: Interventions for the Treatment of Overweight and Obesity in Adults. J Acad Nutr Diet. 2016;116(1):129–47. Epub 2016/01/01. doi: 10.1016/j.jand.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 39.Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Molecular Metabolism. 2019;19:86–96. doi: 10.1016/j.molmet.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah RV, Hwang S-J, Yeri A, Tanriverdi K, Pico AR, Yao C, et al. Proteins Altered by Surgical Weight Loss Highlight Biomarkers of Insulin Resistance in the Community. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(1):107–15. doi: 10.1161/atvbaha.118.311928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceccarini G, Pelosini C, Ferrari F, Magno S, Vitti J, Salvetti G, et al. Serum IGF-binding protein 2 (IGFBP-2) concentrations change early after gastric bypass bariatric surgery revealing a possible marker of leptin sensitivity in obese subjects. Endocrine. 2019;65(1):86–93. doi: 10.1007/s12020-019-01915-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon publication and with no end date, individual deidentified participant data that underlie the results reported in this article will be available to investigators upon reasonable request. Relevant data will be made publicly available for the National Heart, Lung, and Blood Institute’s ADOPT Core Measures Project. Requests should be directed to Sarah.Borengasser@cuanschutz.edu and Vicki.Catenacci@cuanschutz.edu.


